Abstract
Here we report structure-activity relationship study of a novel hybrid series of compounds where structural alteration of aromatic hydrophobic moieties connected to the piperazine ring and bioisosteric replacement of the aromatic tetralin moieties were carried out. Binding assays were carried out with HEK-293 cells expressing either D2 or D3 receptors with tritiated spiperone to evaluate inhibition constants (Ki). Functional activity of selected compounds in stimulating GTPγS binding was assessed with CHO cells expressing human D2 receptors and AtT-20 cells expressing human D3 receptors. SAR results identified compound (−)-24c (D-301) as one of the lead molecules with preferential agonist activity for D3 receptor (EC50 (GTPγS); D3 = 0.52 nM; D2/D3 (EC50): 223). Compounds (−)-24b and (−)-24c exhibited potent radical scavenging activity. The two lead compounds (−)-24b and (−)-24c exhibited high in vivo activity in two Parkinson’s disease (PD) animal models, reserpinized rat model and 6-OH-DA induced unilaterally lesioned rat model. Future studies will explore potential use of these compounds in the neuroprotective therapy for PD.
Introduction
Parkinson’s Disease (PD) is a progressive neurodegenerative disorder characterized by degeneration of the nigrostriatal dopaminergic pathway. It is estimated that PD affects approximately 1 % of people older than 65 years of age. It is primarily a sporadic disorder although a rare subset of population (<10%) acquires this disease due to several genetic defects 1, 2. Some of the symptoms associated with PD involve rigidity, bradykinesia, resting tremor and postural instability along with cognitive and psychiatric complications 3–5. The neuropathological hallmark of PD is the presence of Lewy bodies (LB) in the surviving neurons of the substantia nigra 6, 7. The etiology of PD is not fully understood. Both oxidative stress and mitochondrial dysfunction have been strongly implicated in cell death 8–10. Recently, α-synuclein, a presynaptic protein involved in fibrilization, has been implicated in the pathogenesis of PD 7, 11. In a rare familial form of PD, a mutation in the α-synuclein gene has been linked to autosomal dominant PD 12, 13.
Levo-DOPA (L-DOPA) has proven to be one of the mainstay therapy for PD. However, prolong use of L-DOPA gives rise to motor fluctuations with dyskinesias and the decrease in duration of response to a given L-dopa dose 14. Prolong use of L-dopa also gives rise to “on” and “off” episodes resulting in additional complications. Dopaminergic agents have been used more extensively in the therapy for PD than any other class of drug molecules besides L-DOPA 15–19. The dopamine receptor belongs to the class of seven-member G-protein coupled receptors and has been divided into two main classes as D1-like and D2-like. D1-like receptors include D1 and D5 subtypes whereas D2, D3 and D4 subtype receptors fall in the D2-like category 20–26. This classification is based on distinct physiological and pharmacological properties of these two receptors as stimulation of the D1-type receptor leads to activation of adenylate cyclase, which promotes synthesis of cAMP, whereas D2-type receptor activation leads to inhibition of adenylate cyclase activity. In the CNS, D1-type receptors are located post-synaptically, whereas D2-type receptors are located both pre- and post-synaptically and have a high affinity for dopamine 27.
Due to the high degree of homology between D2 and D3 receptors, especially in the transmembrane domain binding regions for agonists, it has been challenging to develop agonist with highly selectivity for D3 receptors.28, 29 D3 preferring agonists have been shown to be neuroprotective in both cell culture and in vivo experiments.30–33 Some of the most selective agonist are pramipexole and ropinirole which are used as therapeutic agents in PD.34, 35 However, the neuroprotective property of pramipexole has been shown to be complex and mediated by both dopamine- and non-dopamine-dependent pathways.33, 36 Recently another D3 preferring agonist, [(+)-trans-3,4,4a,5,6,10b-hexahydro-9-carbamoyl-4-propyl-2H-naphth[1,2-b]-1,4-oxazine] (R,R-S32504) has been developed and shown to posses antiparkinsonian and neuroprotective effect.37 Interestingly, pramipexole was also shown to be a potent antidepressant agent in PD patient, speculated to be due to its interaction with the D3 receptor in the mesolimbic dopamine pathway which controls behavior and mood38, 39. Such antidepressant effect was found to be beneficial for PD patients. Besides developing agonists, there is now a growing interest in developing selective antagonist for the D3 receptor as therapeutic agents for psychiatric diseases and drugs of abuse. Consequently, a large number of antagonists have been developed so far which exhibited varying degree of selectivities.28, 40
It is increasingly evident that for a complex disease such as PD, a drug targeting only one target site will only partially address the therapeutic need of the disease. Thus it is hypothesized that multi-functional drugs having multiple pharmacological activities will be an effective disease modifying agent in case of PD.41 In our effort to produce such agent, we have undertaken one of our goals of introducing antioxidant property in our D2/D3 hybrid agonist template which we established earlier42, 43. Recently, we have reported the development of potent D3 preferring agonists, which exhibited potent in vivo activity in PD animal model experiments44, 45. It is believed that the neuroprotective property of pramipexole might originate from its preferential D3 agonist property coupled with its antioxidant activity, although some other mechanisms have also been implicated46, 47. We hypothesize that a combination of D3 preferential agonist property along with antioxidant activity in a compound enhances its neuroprotective effect as it will address an underlying oxidative stress in the Parkinsonian brain. The current SAR study expands this line of inquiry by including compounds having bulky hydrophobic N-aryl substitutions in the piperazine moiety to explore and map out the binding site interaction further. Our earlier report described the development of a 2-aminothiazole based, high-affinity D3 selective agonist 2 (D-264)44, which is currently undergoing in vivo evaluation to determine its neuroprotective effect. We introduced quinolines and isoquinolines in the hybrid structure as analogues of 2. Future studies will indicate whether these derivatives will have better pharmacokinetics properties compared to 2.
Chemistry
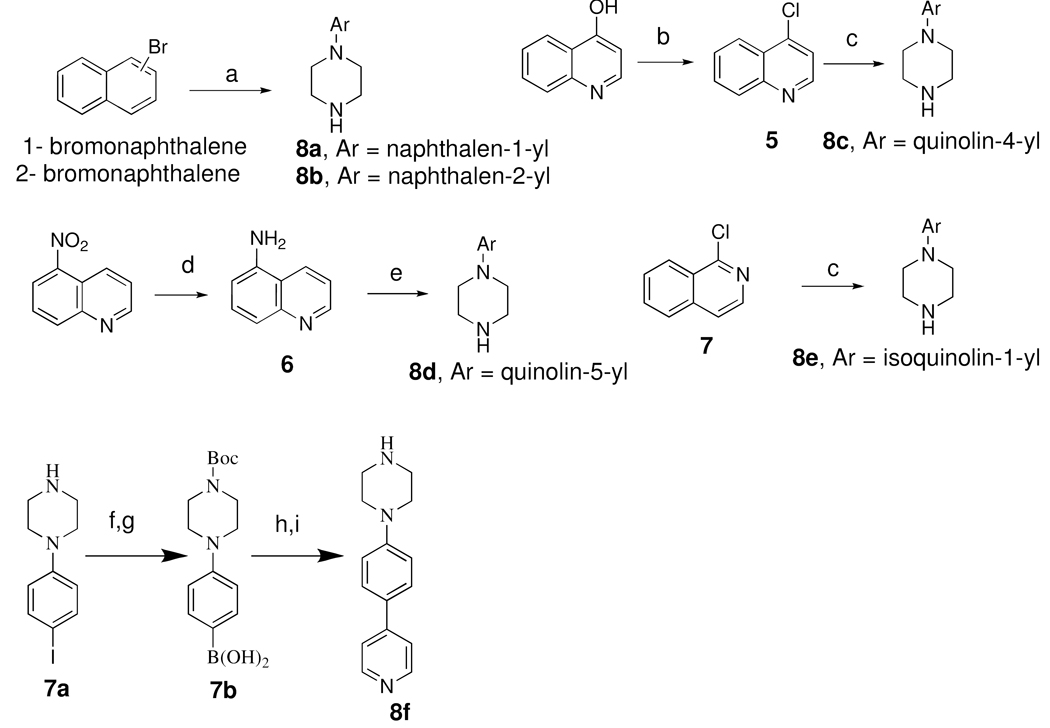
Scheme 1 outlines the syntheses of different aryl piperazines 8a-f used in the following schemes to synthesize the target compounds. Compound 8a and 8b were synthesized by palladium (II) catalyzed amination reaction with two corresponding aryl halides 1 or 2-bromonaphthalene with excess piperazine48. 8c was prepared from commercially available 4-hydroxyquinoline by chlorination reaction with POCl3 followed by reflux in i-propanol with excess piperazine for 4 h49. Compound 8d was synthesized from commercially available 5-nitroquinoline by reduction in presence of SnCl2 in ethanol under refluxing condition for 1.5 h50 followed by reaction of the amino compound 6 with bis-(2-chloroethyl)amine HCl in presence of K2CO3 under reflux for 48 h 51. 8e was synthesized from 1-chloroisoquinoline by i-propanol reflux in presence of excess piperazine. Compound 8f was synthesized by reaction of boronic acid intermediate 7b with 4-bromopyridine in presence of palladium catalyst.
Scheme 1a.
a Reagents and Conditions : a. 3–5 mol% PdCl2[P(o-tol)3]2, NaOt-Bu, Diglyme, reflux, 48h; b. POCl3, reflux, 2h; c. Piperazine, i-propanol, reflux, 4h; d. SnCl2,2H2O,ethanol,NaBH4, reflux 55 °C, 1.5h; e. bis-(2-chloroethyl)amine HCl, diglyme, K2CO3, reflux, 48h; f. Boc2O, EtOH; g. n-BuLi, trimethyl borate, −78 °C; h. 4-bromopyridine, Pd(PPh3)4, Na2CO3; i. TFA/CHCl3;
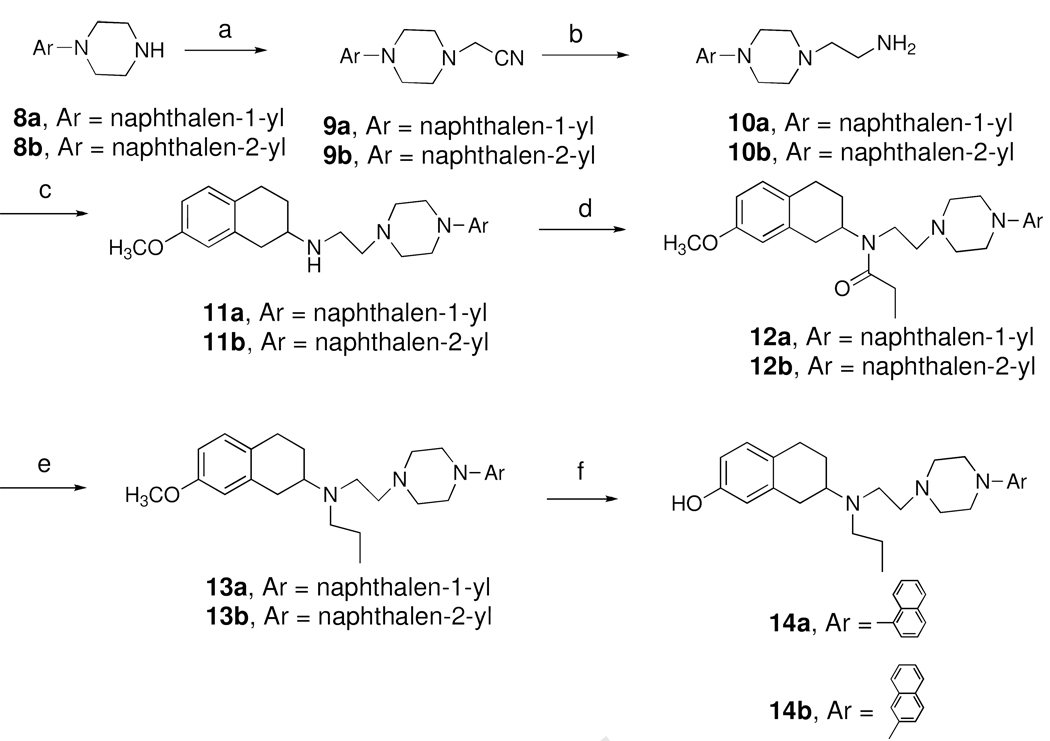
Scheme 2 depicts the synthesis of two target compounds 14a-b. Aryl piperazine 8a-b were N-alkylated with chloroacetonitrile under reflux in presence of K2CO3 as base to synthesize intermediate 9a-b, followed by raney nickel catalyzed hydrogenation to yield 10a-b which was subjected to reductive amination condition in presence of 7-methoxy-2-tetralone to yield intermediates 11a-b. N-alkylation of 11a-b with propionyl chloride in presence of triethyl amine as base yielded 12a-b which was reduced to the corresponding amines 13a-b using LiAlH4 as reducing agent. The target compounds 14a-b were obtained by demethylation of 13a-b using 1M solution of borontribromide in dichloromethane at −40 °C to room temperature for overnight.
Scheme 2a.
a Reagents and Conditions : a. Chloroacetonitrile, K2CO3, toluene, reflux, 3h; b. Raney nickel, H2, 60 psi, 8 h; c. 7-methoxy-2-tetralone, NaCNBH3, AcOH, dichloroethane, RT, overnight; d. propionyl chloride, Et3N, CH2Cl2, 0 °C to RT, 4h; e. LiAlH4, THF, reflux, 4 h; f. BBr3, CH2Cl2, −40 °C to RT, overnight.
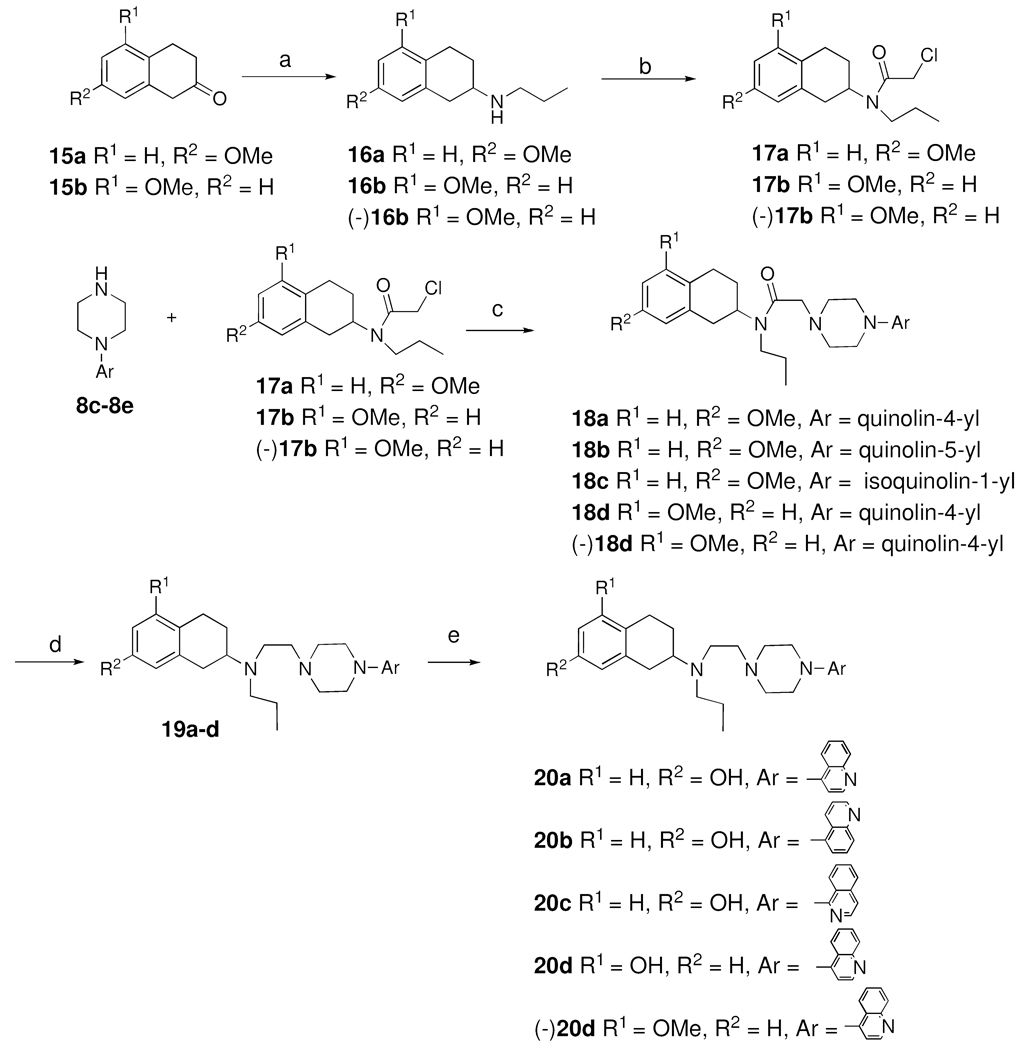
The syntheses of compounds 20a-d are shown in Scheme 3. 5- or 7- Methoxy 2-tetralone was reacted with n-propyl amine under reductive amination condition to yield 16a-b. Enantiomerically pure (−)-16b was obtained from racemic 16b using a synthetic chiral resolving agent following previously reported procedure52,45. N-alkylation of 16a-b and (−)-16b using chloroacetyl chloride in presence of triethyl amine produced intermediate 17a-b and (−)-17b which were coupled with different aryl piperazines 8c-e to yield 18a-d and (−)-18d in moderate to good yield. Reduction of the amide group of 18a-d using LiAlH4 followed by demethylation using boron tribromide at −40 °C yielded the target compounds 20a-d and (−)-20d.
Scheme 3a.
a Reagents and Conditions: a. n-Propylamine, NaCNBH3, CH3COOH, dichloroethane, RT, overnight; b. chloroacetyl chloride, TEA, dichloromethane, 0°C, 30 min; c. K2CO3, CH3CN, 80 °C, 2 h; d. LiAlH4, THF, reflux, 2 h; e. BBr3, −78° C, CH2Cl2, overnight
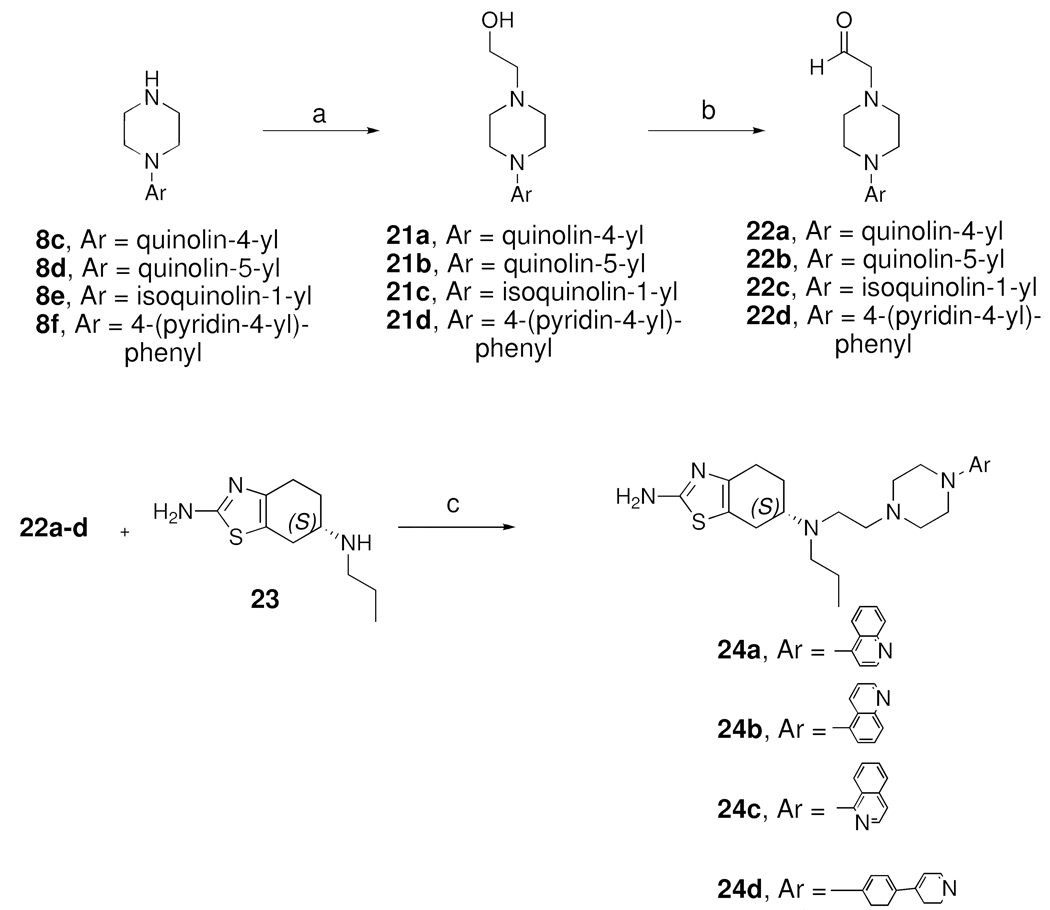
Scheme 4 depicts the synthesis of (S)-(—)-pramipexole hybrid analogs 24a-24d. Alkylation of 8c-f with 2-bromoethanol produced 21a-d, which were oxidized under Swern oxidation conditions to the arylpiperazine acetaldehydes 22a-d. S-(−)-Pramipexole (23) was further condensed with 22a-d under reductive amination conditions (NaCNBH3 as the reducing agent) to give hybrid analogs 24a-d.
Scheme 4a.
a Reagents and Conditions : a. 2-bromoethanol, K2CO3, AcCN, reflux; b. oxalyl chloride, DMSO, Et3N, −78 °C; c. NaCNBH3, AcOH, 1,2-dichloroethane
Discussion
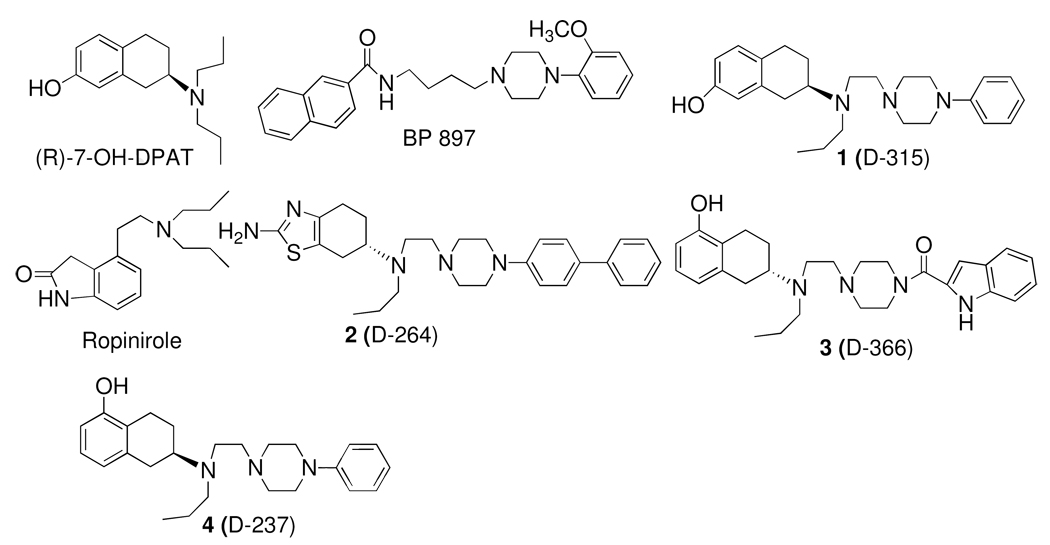
Our earlier SAR studies with hybrid compounds produced ligands which exhibited potent and selective agonist activity at D3 receptor. Our two starting piperazine hybrid compounds based on 7-OH-DPAT and 5-OH-DPAT were 1 (D-315)53 and 4 (D-237)45. These first generation hybrid compounds maintained similar affinities and selectivities for D2/D3 receptors as their parent non-hybrid counter part. Further expansion of SAR studies led to the development of compounds 2 and 3 (D-366)53 which exhibited high and selective affinity for the D3 receptor.44, 53 2 was among the most potent and selective agonist for the D3 receptor developed so far. Our SAR studies indicated that the N-piperazinyl substituent could be modified by introducting biphenyl and indole substituents without compromising affinity for the D3 receptor. It is important to point out here that for development of D3 antagonist one of the frequently used molecular template consists of amide-piperazine moiety separated via a linker where bulky aromatic substituents have been introduced mainly on the amide moiety.28 The compound BP 897 is one of the prototypical example of a compound developed from this template.
In our current SAR study we wanted to see the influence of different piperazynyl N-aryl or heteroaryl moieties on interaction with dopamine D2 and D3 receptors. Our previous report indicated that bulky hydrophobic substituents attached to the piperazine ring are well tolerated as 2 and 3 retained affinity and were selective for D3. Here we wanted to explore this further with naphthalene and quinoline substituents. Our initial design to incorporate naphthalalen-1yl and naphthalene-2yl substituents led to development of compounds 14a and 14b. Compound 14a showed a 3- to 4-fold increase in binding affinity for D2/D3 (Ki for D2 = 13.5 nM, Ki for D3 = 0.435 nM) accompanied by increased selectivity for D3 (D2/D3 = 31.0) compared to 1. Compound 14b exhibited a binding affinity (Ki for D2 = 8.85 nM, Ki for D3 = 0.685 nM) similar to that of 14a, however, its selectivity (D2/D3 = 12.9) was reduced compared to that of 1 and 14a. This result indicated that a bulkier N-4-substituted group in the piperazine ring is well tolerated by dopamine D2/D3 receptors. Next, we wanted to introduce a heteroatom in the naphthalene moiety producing different quinoline derivatives. Compound 20a with an N-quinoline-4yl substitution in the piperazine ring displayed no further increase in binding potency (Ki for D2 = 7.08 ± 0.34, Ki for D3 = 2.65 ± 0.65 nM) compared to 14a or 14b, but its binding potency was still greater than that of 1 although its selectivity for D3 over D2 was reduced (D2/D3 = 2.67). The other regioisomer 20b which is a quinoline-5yl derivative, exhibited comparable low nanomolar binding affinity for D2/D3 receptors (Ki for D2 = 5.22 nM, Ki for D3 = 1.27 nM). Both 20a and 20b were not highly selective for the D3 receptor. Another compound of this series was 20c, an isomeric isoquinoline-1yl derivative displaying subnanomolar binding potency at D3 receptors (Ki = 0.44 nM) while it was less potent at D2 receptors (Ki = 21.7 nM) compared to 20a and 20b. Consequently, 20c exhibited the highest selectivity for D3 receptors (D2/D3 = 49.2) in this series of compounds. Subsequently, the positional effect of an aromatic 5-hydroxyl functionality in the aminotetraline moiety was evaluated on binding affinity and selectivity. Thus, compounds 20d and its s(−) enantiomers (20e) were synthesized. Compound 20d showed increased binding potency at both D2 and D3 receptors (Ki for D2 = 4.89 nM, Ki for D3 = 0.40 nM) although its selectivity for D3 receptors (D2/D3 = 12.22) did not increase appreciably. The enantiomerically pure compound 20e exhibited increased potency at both D2 (Ki = 3.74 nM) and D3 (Ki = 0.19 nM) receptors compared with its racemic counter part, with comparable selectivity for D3 over D2 receptors (D2/D3 = 19.68).
In one of our earlier publications, we reported compound 2 as one of the most potent and selective compounds for the D3 receptor; this compound contains a 2-aminothiazolidium moiety as bio-isosteric replacement of the hydroxyl phenyl moiety in the aminotetraline fragment of the hybrid structure. In designing the next set of compounds 24a-d, we incorporated a 2-aminothiazolidinium moiety instead. In addition to probing the D3 receptor selectivity for these derivatives, we aimed for improved pharmacokinetic properties by the introduction of the more polar quinoline moiety. Specifically, the (−)-isomers of these compounds were made as we have shown previously that activity is increased in the (−)-isomers in the 2-aminothiazolidinium series of compounds 44. Compound 24a which is a quinoline-4yl analog, exhibited moderate binding potency for D2 receptors (Ki = 109 nM) while the binding affinity toward D3 (Ki = 2.61 nM) receptor was in the low nanomolar range with selectivity for D3 over D2 receptors (D2/D3 = 41.8) higher than that for the corresponding 20a. Compound 24c, an isoquinoline-1yl derivative, exhibited approximately 2-fold less potency at D2 (Ki = 269 nM) and identical affinity at D3 (Ki = 2.23 nM) receptors compared to 24a. Thus, compound 24c displayed the highest selectivity (D2/D3 = 121) for D3 receptor in the current series of compounds. The other thiazolidinium compound in this series was quinoline-5yl derivative 24b, which displayed high binding affinity for both D2 and D3 receptors (Ki for D2 = 57.7 nM, Ki for D3 = 1.21 nM) with appreciable selectivity for D3 over D2 receptors (D2/D3 = 47.7). Next we designed and synthesized compound 24d consisting of a linearly fused 4-(pyridine-4-yl)-phenyl moiety. This compound could retain its binding affinity at D2 and D3 receptors (Ki = 270 nM and 4.78 nM for D2 and D3 receptors, respectively) and was moderately selective for D3 over D2 receptors (D2/D3 = 56.5). Collectively, the current results are consonant with the existence of hydrophobic interactions resulting from bulky substitutions on the piperazine N-atom.
Following binding analysis, selected compounds 24c, 24b and 24a were subjected to the [35S]GTPγS functional assay for D2 and D3 receptors and compared with the full agonist dopamine. The assays were carried out with the cloned human D2 and D3 receptors expressed in CHO and AtT cells as described by us earlier45. All three compounds exhibited high potency for the D3 receptor in the subnanomolar range (EC50; 0.52, 0.52 and 0.49 nM, respectively) (Table 2). Similar to what was found in the D2 binding assays, isoquinoline derivative 24c exhibited the lowest potency for D2 receptors in the functional assays with [35S]GTPγS (EC50; 116 nM); it was also the most selective for D3 (D2/D3 (EC50 ratio) = 223) and this selectivity was comparable to our previously developed 2 (D2/D3 (EC50 ratio) = 248). Like compounds 24b and 24c, compound 24a was potent at D3 (EC50: 0.49 nM) and was less potent at D2 (EC50: 77 nM). Compound 24a was second best selective compound for D3 receptor. Compound 24b displayed the highest potency in activating D2 receptors (EC50; 13.5 nM) and was the least selective in activating D3 receptors. In comparison, the reference compound ropinirole displayed much less selectivity for and potency at D3 receptors. As demonstrated by plateaus of activation of 80–119% of maximal stimulation by DA, all compounds appeared to exert close to full agonism in the [35S]GTPγS assay (Table 2).
Table 2.
Stimulation of [35S]GTPγS binding to cloned human D2 receptor expressed in CHO cells and cloned human D3 receptor expressed in AtT-20 cells. EC50 is the concentration producing half-maximal stimulation; for each compound, maximal stimulation (Emax) is expressed as percent of the Emax observed with 1 mM (D2) or 100 µM (D3) of the full agonist DA (%Emax). Results are means ± SEM for 3–5 independent experiments each
| CHO-D2 | AtT-D3 | ||||
|---|---|---|---|---|---|
| Compound | EC50 (nM) [35S]GTPγS |
%Emax | EC50 (nM) [35S]GTPγS |
%Emax | D2/D3 |
| Dopamine | 209 (4) ± 29 | 100 | 8.53 ± 0.62 | 100 | |
| Ropinirole | 304 ± 11 | 83.9 ± 0.3 | 19.2 ± 5.1 | 91.6 ± 0.5 | 15.83 |
| 24c | 116± 16 | 88.4 ± 3.9 | 0.520 ± 0.16 | 95.1 ± 2.1 | 223 |
| 24b | 13.5 ± 4.3 | 104 ± 2 | 0.528 ± 0.077 | 101 ± 2 | 25 |
| 24a | 77.2 ± 8.3 | 118 ± 4 | 0.493 ± 0.12 | 96.8 ± 4.4 | 156 |
| 5-OH-DPAT | 41.2 ± 6.0 | 80.0 ± 4.4 | 1.23 ± 0.53 | 91.2 ± 1.0 | 33.49 |
| 2 | 19.9 ± 0.9 | 119 ± 6 | 0.085 ± 0.016 | 102 ± 19 | 248 |
Evaluation of free radical scavenging activity
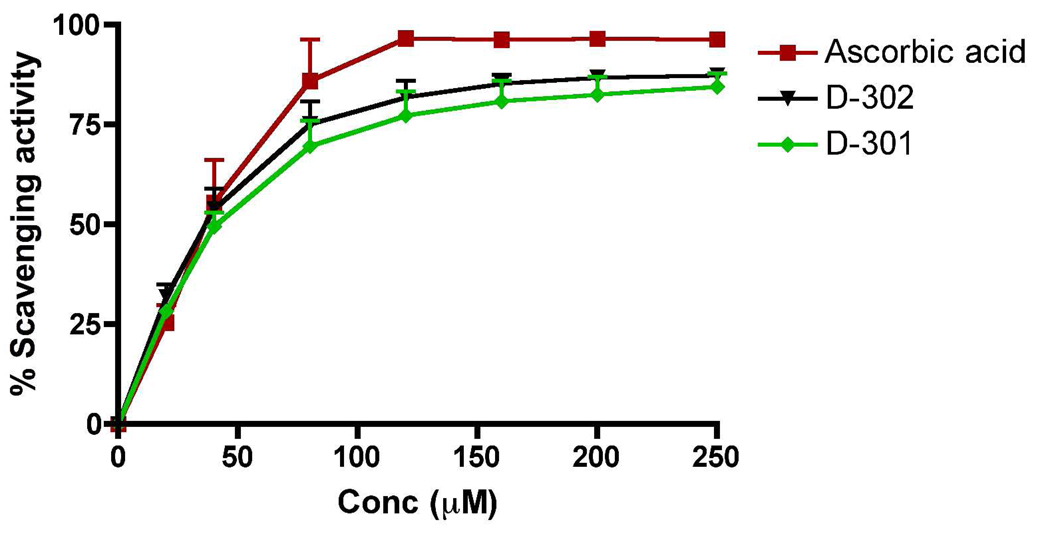
Scavenging of DPPH (1,1-Diphenyl-2-picryl-hydrazyl) radical by 24c, 24b and ascorbic acid was carried out based on published method and is shown in Figure 2.54 The scavenging effect is expressed as percent of control. As shown in Figure 2, all three compounds inhibited DPPH radical activity dose dependently. The standard compound ascorbic acid had an IC50 of 34.6 ± 3.6 µM in this assay procedure whereas the IC50 value for 24b was 39.67 ± 6.23 µM and for 24c 45.67 ± 6.89 µM (n=3 for all compounds). It is evident from the results that the two test compounds are as potent as potent known antioxidant ascorbic acid.
Figure 2.
DPPH radical scavenging activity by 24b, 24c and ascorbic acid.
Reversal of reserpine-Induced hypolocomotion in Rats by 24a, 24b, 24c and ropinirole
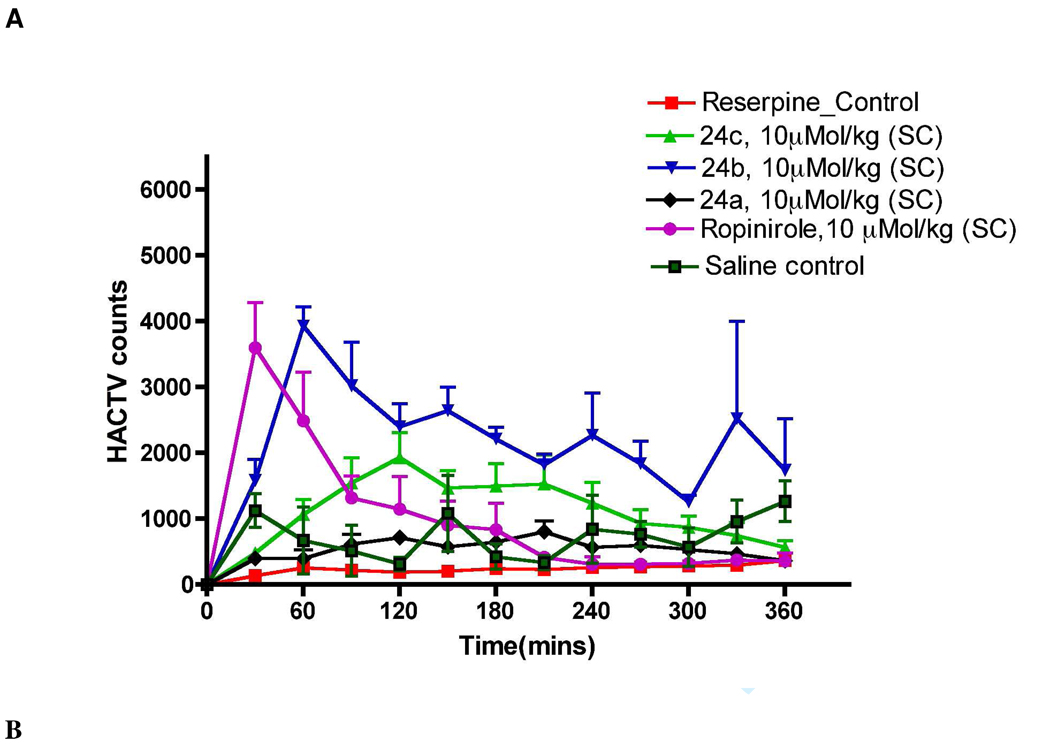
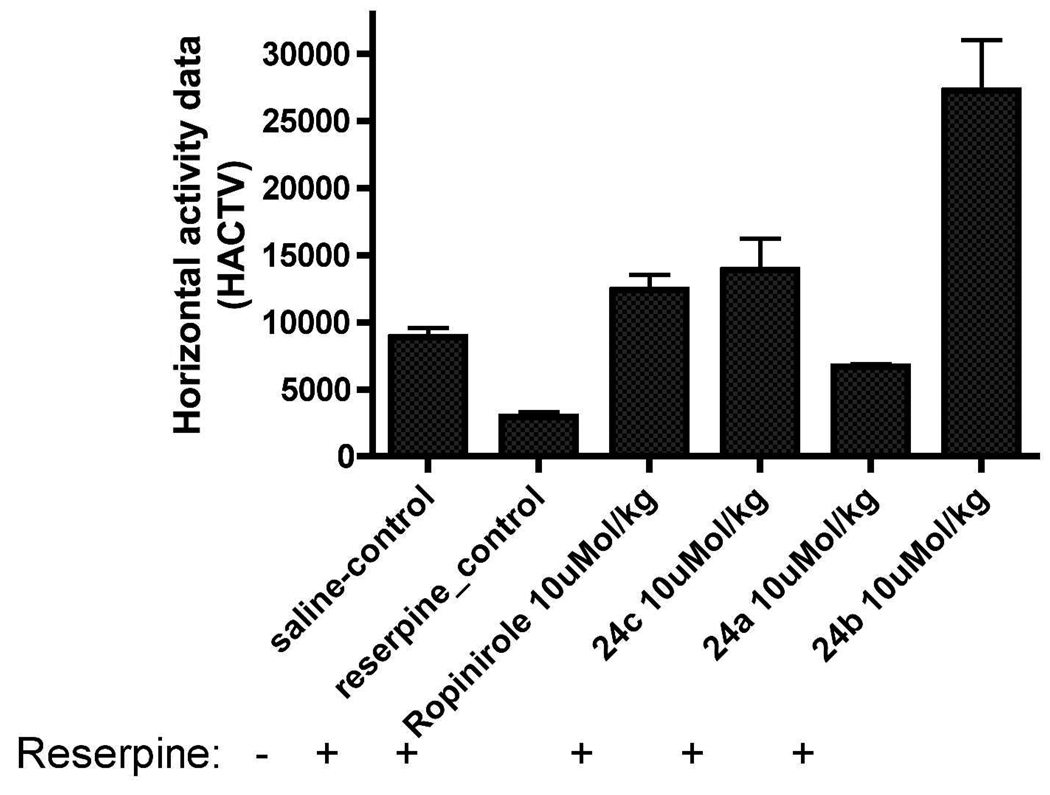
Reserpine induces depletion of catecholamines in nerve terminals resulting in a cataleptic condition in rats, which is a well established animal model for PD 55. Significant reduction of locomotion of the rats was observed 18 h after the administration of reserpine (5 mg/kg, s.c.) which indicated the development of akinesia in rats. Compounds 24c and 24b were highly efficacious in producing complete reversal of reserpine induced hypolocomotion. At a dose of 10 µmol/kg (6.5 mg/kg), s.c., 24b not only reversed the reserpine-induced hypokinetic condition to a level that was similar or higher than that in control (vehicle treated non-reserpinized rats) rats, but also maintained an increased level of locomotor activity of the reserpinized rats significantly throughout the 6-h period (Fig. 3A). Subcutaneous administration of 10 µMol/kg (6.3 mg/kg) 24c and 10 µMol/kg (2.96 mg/kg) ropinirole could restore the locomotion to the normal state. The reference drug ropinirole at a dose of 10 µMol/kg s.c. exhibited a much shorter duration of action compared to 24b; the peak of action of ropinirole was reached within 30 min and the pharmacological action ceased after 150 min. It is evident that out of the three compounds, 24b and 24c exhibited the highest CNS locomotor activity (Figure 3A). On the other hand, compound 24a (10 µMol/kg) was much less active compared to 24b and 24c. Cumulative horizontal activity data for 6 h period of observation for saline-control meaning animals receive saline instead of reserpine, reserpinized control, and 10 µMol/kg of ropinirole, 24a, 24b and 24c is presented as a bar graph in Figure 3B.
Figure 3.
Effects of different drugs (administered s.c.) upon reserpine (5.0 mg/Kg, s.c., 18 h pretreatment)-induced hypolocomotion in rats. Each point is the mean ± S.E.M for six rats. Horizontal activity was measured as described under materials and methods section. Panel A. Representation of horizontal locomotor activity at discrete 30-min intervals after the administration of 24a, 24b, 24c and ropinirole at the dose of 10 µMol/kg compared to control rats in 18 h reserpine post treatment. Panel B. Representation of cumulative horizontal activity data for 6 h period of observation for saline-control meaning animals receive saline instead of reserpine, reserpinized control, and 10 µMol/kg of ropinirole, 24a, 24b and 24c. One way ANOVA analysis demonstrates significant effect among treatments: Panel A, F (6,95) = 13.69 (P< 0.0001). Dunnett’s analysis following ANOVA showed that the effects of 24b (P< 0.01), 24c (P< 0.01) and ropinirole (P< 0.05) were significantly different statistically compared to reserpine control but for 24a was not statistically significant (P> 0.05).
Effect of 24b, 24c and ropinirole in 6-OHDA lesioned rats
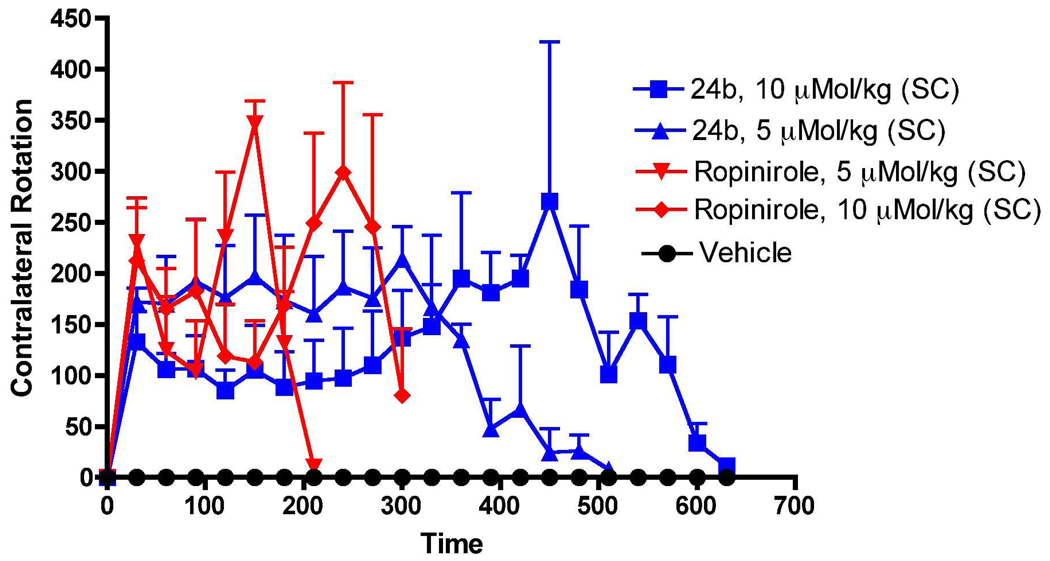
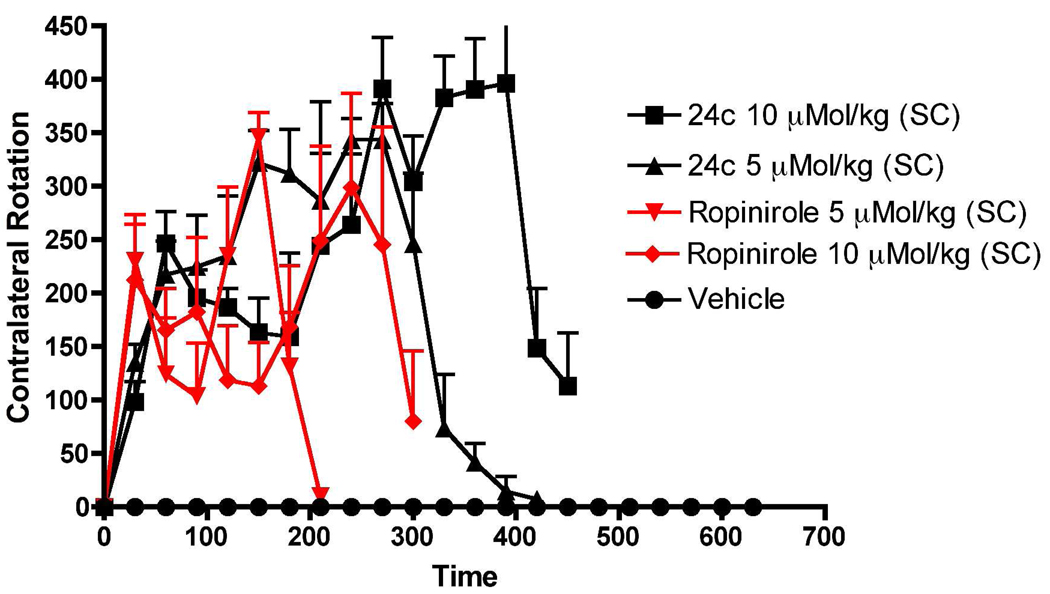
Based on the above locomotor activity results, compounds 24b and 24c were selected for in vivo evaluation in rats carrying a unilateral lesion in the medial forebrain bundle induced by application of the neurotoxin 6-hydroxydopamine (6-OHDA). This results in destruction of dopamine neurons and development of supersensitivity of dopamine receptors on the lesioned side. Such surgically modified rats when challenged with direct acting dopamine agonists, produce contralateral rotations away from the lesioned side. This rat model is considered to be one of the standard models for preclinical screening of drugs for possible antiparkinsonian property.56 Both compounds 24b and 24c produced potent rotational activity in a dose dependent manner when administered subcutaneously. Thus, 24b produced dose dependently contralateral rotations with the highest dose (10 µMol/kg, 6.5 mg/kg) exhibiting the longest duration of action (> 10 h). At a lower dose (5 µMol/kg, 3.28 mg/kg), 24b reached its peak effect at around 5 h and produced a number of total rotations of 2295 whereas at a higher dose (10 uMol/kg) the peak effect was reached at around 7.5 h with 2648 total rotations (Figure 4). It was clear that 24b was more efficacious in producing rotations at a higher dose. However, in the first 360 min, the lower dose of 24b produced significant higher rotations compared to higher dose, indicating possible involvement of stereotype at a higher dose. In comparison, compound 24c when tested at a dose of 10 µMol/kg (6.3 mg/kg), produced an even higher number of total rotations of 3688 with a long duration of action (>8 h), Figure 5. The peak activity for 24c was reached at about 7 h. At the lower dose of 5 µMol/kg (3.16 mg/kg), compound 24c produced a lower number of rotations (2805) which lasted for 7 h (Figure 5). It is evident that contrary to stimulating locomotor activity in reserpinized rats, 24c was more efficacious compared to 24b in regards to total number of rotations produced by respective doses. However, 24b exhibited relatively longer duration of action. The reference drug ropinirole at 5 and 10 µMol/kg exhibited shorter duration of action and was less active in producing rotations compared to 24b and 24c at the same respective doses.
Figure 4.
Effect on turning behavior of (−)-24b and ropinirole in 6-OH-DA unilaterally lesioned rats studied over ten hours. Each point is the mean ± SEM for four rats. All drugs were administered s.c. immediately before counting rotational activity. One way ANOVA analysis demonstrates a significant effect among treatments: F (5,95) = 16.93 (P< 0.0001). Dunnett’s analysis shows that the effect of (−)-24b at two doses (5 and 10 µMol/kg) and ropinirole at two doses (5 and 10 µMol/kg) are significantly different compared to vehicle (P<0.01).
Figure 5.
Effect on turning behavior of (−)-24c and ropinirole in 6-OH-DA unilaterally lesioned rats studied over seven and half hours. Each point is the mean ± SEM for four rats. All drugs were administered s.c. immediately before counting rotational activity. One way ANOVA analysis demonstrates a significant effect among treatments: F (5,95) = 16.23 (P< 0.0001). Dunnett’s analysis shows that the effect of (−)-24c at two doses (5 and 10 µMol/kg) and ropinirole at two doses (5 and 10 µMol/kg) are significantly different compared to vehicle (P<0.01).
Conclusion
The present study describes the development of compounds with high affinity and selectivity for dopamine D3 receptors. SAR results have demonstrated that Isoquinoline derivatives have higher selectivity for D3 receptor. In both binding and functional assays compound isoquinoline derivative 24c exhibited the highest selectivity for the D3 over D2 receptor. Lead compounds 24b and 24c also exhibited potent free radical quenching property, possibly indicating antioxidant activity. The two lead compounds were tested in two PD animal models. Compound 24b was the most potent in the reserpinized animal model where it exhibited appreciable locomotor activity, while compound 24c was as active as ropinirole in this animal model. However, in the 6-OH-DA animal model studies, 24c produced the highest number of rotations and was the most active among the three compounds. Compound 24b produced a slightly longer duration of action. Both compounds were more efficacious than ropinirole. Relative contribution of D2 receptor vs. D3 receptor in reversing reserpine effect and to induce rotations by compounds 24b and 24c will be assessed in future. Compounds 24b and 24c will next be studied in the in vitro and in vivo neuroprotection experiment model to evaluate their potential as a neuroprotective treatment agent for PD.
Experimental Section
Reagents and solvents were purchased from commercial suppliers and used as received unless otherwise indicated. Dry solvent was obtained according to the standard procedure. All reactions were performed under inert atmosphere (N2) unless otherwise noted. Analytical silica gel 60 F254-coated TLC plates were obtained from EMD Chemicals, Inc. and were visualized with UV light or by treatment with phosphomolybdic acid (PMA), Dragendorff’s reagent or ninhydrin. Flash column chromatographic purifications were performed using Whatman Purasil® 60A silica gel 230–400 mesh. The proton nuclear magnetic resonance (1H NMR) spectra were measured on Varian 400 MHz FT NMR spectrometer using tetramethylsilane (TMS) as an internal standard. The NMR solvent used was CDCl3 as indicated. Optical rotations were recorded on Perkin-Elmer 241 polarimeter. Melting points were recorded using MEL-TEMP II (Laboratory Devices Inc., USA) capillary melting point apparatus and were uncorrected. Elemental analyses were performed by Atlantic Microlab, Inc. and were within ± 0.4% of the theoretical value.
Procedure A. Preparation of 1-(naphthalen-1-yl)piperazine (8a)
Into a solution of 1-bromo naphthalene (3 g, 14.5 mmol) and piperazine (9.98 g, 115.6 mmol) in 50 ml of diglyme was added Na-t-butoxide (4.89 g, 43.46 mmol). The reaction mixture was stirred for few minutes before the addition of palladium catalyst, dichlorobis(tri-o-tolylphosphine) palladium(II) (0.724 g, 0.724mmol) and refluxed at 170 °C for 48 h. The reaction mixture was cooled and the diglyme was evaporated under reduced pressure. The solid residue was partitioned between ethyl acetate and water. The aqueous layer was extracted with ethyl acetate (3×100 ml). The combined organic layer was dried (Na2SO4), evaporated under educed pressure to get the crude product which was purified by column chromatography (ethylacetate :MeOH 9:1) to yield 1.94 g of pure yellowish oily compound 8a (63 %). 1H NMR (400 MHz, CDCl3): δ ppm 7.68–7.74 (m, 2H), 7.54–7.56 (d, 1H, J = 8 Hz), 7.45–7.48 (t, 1H, J = 6 Hz), 7.38–7.42 (m, 1H), 7.26–7.30 (t, 1H, J = 8 Hz), 7.08–7.14 (dd, 1H, J = 8 Hz); 3.08–3.33 (m, 8H).
1-(naphthalen-2-yl)piperazine (8b)
Compound 8b was synthesized from 2-bromo naphthalene (1.8 g, 8.9 mmol) and piperazine (6.13 g, 71.17 mmol) according to the procedure A to afford 1.88 g of oily pure compound 8b (63.55 %). 1H NMR (400 MHz, CDCl3): δ ppm 7.72–7.73 (d, 1H, J = 4 Hz); 7.70–7.71 (d, 1H, J = 4 Hz); 7.68 (s, 1H); 7.37–7.41 (t, 1H, J = 8 Hz); 7.25–7.30 (m, 2H); 7.11–7.12 (d, 1H, J = 4 Hz); 3.09–3.33 (m, 8H).
Procedure B. Preparation of 2-(4-(naphthalen-1-yl)piperazin-1-yl)acetonitrile (9a)
A suspension of 1-(naphthalene-1-yl)-piperazine (8a) (1.94 g, 9.13 mmol), potassium carbonate (3.77 g, 27.39 mmol), and 2-chloroacetonitrile (1.16 mL, 18.26 mmol) in toluene was refluxed for 3 h. Toluene was removed under reduced pressure, and the residue was diluted with ethyl acetate, washed with water and brine, dried over sodium sulfate (Na2SO4), concentrated, and purified by column chromatography (ethyl acetate/hexane = 1:1) to afford the product 9a as a thick yellow solid (1.95 g, 85%): 1H NMR (400 MHz, CDCl3) δ ppm 7.68-7.64 (m,2H); 7.54–7.56 (d, 1H, J = 8 Hz); 7.45–7.48 (t, 1H, J = 6 Hz); 7.38–7.42 (m, 1H); 7.26–7.30 (t, 1H, J = 8 Hz); 7.08–7.14 (dd, 1H, J = 8 Hz); 3.60 (s, 2H); 3.33–3.36 (t, 4H, J = 6 Hz); 2.81–2.83 (t, 4H, J = 4 Hz).
2-(4-(naphthalen-2-yl)piperazin-1-yl)acetonitrile (9b)
Compound 9b was synthesized from 1-(naphthalene-2-yl)-piperazine (8b) (1.2 g, 5.6 mmol) and 2-chloropropionitrile (1.08 mL, 16.96 mmol) according to the procedure B to afford product 9b as a thick yellow solid (1.0 g, 83%): 1H NMR (400 MHz, CDCl3) δ ppm 7.72–7.73 (d, 1H, J = 4 Hz); 7.70–7.71 (d, 1H, J = 4 Hz); 7.68 (s, 1H); 7.37–7.41 (t, 1H, J = 8 Hz); 7.25–7.30 (m, 2H); 7.11–7.12 (d, 1H, J = 4 Hz); 3.33–3.36 (t, 4H, J = 6 Hz); 2.81–2.83 (t, 4H, J = 4 Hz).
Procedure C. Preparation of 2-(4-(naphthalen-1-yl)piperazin-1-yl)ethanamine (10a)
A solution of compound 9a in methanol (0.86 g, 3.42 mmol) was hydrogenated in a parr hydrogenator apparatus in the presence of raney nickel catalyst at a pressure of 60 psi for 8 h. The reaction mixture was passed through celite, dried over Na2SO4, evaporated, and purified over a silica gel column using the solvent system ethyl acetate/methanol/triethylamine (80:15:5) to afford compound 10a as transparent thick liquid (0.823 g, 94%): 1H NMR (400 MHz, CDCl3) δ ppm 7.68-7.64 (m,2H); 7.54–7.56 (d, 1H, J = 8 Hz); 7.45–7.48 (t, 1H, J = 6 Hz); 7.38–7.42 (m, 1H); 7.26–7.30 (t, 1H, J = 8 Hz); 7.08–7.14 (dd, 1H, J = 8 Hz); 3.31 (t, 4H, J = 4 Hz); 3.11–3.15 (m, 2H); 2.91–2.96 (m, 2H); 2.71–2.74 (t, 4H, J = 6 Hz).
2-(4-(naphthalen-2-yl)piperazin-1-yl)ethanamine (10b)
Compound 10b was synthesized from compound 9b (1 g, 3.98 mmol) according to the procedure C to afford 10b as transparent thick liquid (0.90 g, 88%): 1H NMR (400 MHz, CDCl3) δ ppm 7.72–7.73 (d, 1H, J = 4 Hz); 7.70–7.71 (d, 1H, J = 4 Hz); 7.68 (s, 1H); 7.37–7.41 (t, 1H, J = 8 Hz); 7.25–7.30 (m, 2H); 7.11–7.12 (d, 1H, J = 4 Hz); 3.30–3.34 (t, 4H, J = 6 Hz); 3.13–3.14 (bs, 2H); 2.90–2.92 (bs, 2H); 2.62–2.73 (m, 4H).
Procedure D. Preparation of 7-methoxy-N-(2-(4-(naphthalen-1-yl)piperazin-1-yl)ethyl)-1,2,3,4-tetrahydronaphthalen-2-amine (11a)
A mixture of compound 10a (0.823 g, 3.22 mmol), 7-methoxy-2-tetralone (0.624 g, 3.5 mmol), and glacial acetic acid (HOAc) (0.19 mL) in 1,2-dichloroethane (50 mL) was stirred at room temperature under N2 atmosphere for 20 min. Sodium cyanoborohydride (NaCNBH3) (0.81 g, 12.88 mmol) dissolved in a minimum volume of methanol was added to the reaction mixture. The reaction mixture was stirred at room temperature under nitrogen atmosphere for 12 h. The solvent was evaporated, and saturated NaHCO3/H2O (50 mL) was added to the mixture, which was then extracted with ethyl acetate (3 × 100 mL). The combined organic phase was dried over Na2SO4 and evaporated to afford the crude product, which was purified by flash chromatography (EtOAc/MeOH/Et3N = 95:4:1) to give the product 11a as brown wax (0.84 g, 56%): 1H NMR (400 MHz, CDCl3) δ ppm 7.68-7.64 (m,2H); 7.54–7.56 (d, 1H, J = 8 Hz); 7.45–7.48 (t, 1H, J = 6 Hz); 7.38–7.42 (m, 1H); 7.26–7.30 (t, 1H, J = 8 Hz); 7.08–7.14 (dd, 1H, J = 8 Hz); 6.69–7.01 (d, 1H, J = 8 Hz); 6.67–6.70 (d, 1H, J = 12 Hz); 6.62 (s, 1H); 3.76 (s, 3H); 3.27–3.33 (t, 4H, J = 6 Hz); 2.48–2.93 (m, 13H); 1.65–1.68 (m, 2H).
7-methoxy-N-(2-(4-(naphthalen-2-yl)piperazin-1-yl)ethyl)-1,2,3,4-tetrahydronaphthalen-2-amine (11b)
Compound 10b (0.90 g, 3.5 mmol) was reacted with 7-methoxy-2-tetralone (0.745 g, 4.23 mmol), NaCNBH3 (0.866 g, 14.99 mmol), and HOAc (0.2 mL) in 1,2-dichloroethane (50 mL) to yield 11b brown wax (0.855 g, 59%) (procedure D): 1H NMR (400 MHz, CDCl3) δ ppm 7.72–7.73 (d, 1H, J = 4 Hz); 7.70–7.71 (d, 1H, J = 4 Hz); 7.68 (s, 1H); 7.37–7.41 (t, 1H, J = 8 Hz); 7.25–7.30 (m, 2H); 7.11–7.12 (d, 1H, J = 4 Hz); 6.69–7.01 (d, 1H, J = 8 Hz); 6.67–6.70 (d, 1H, J = 12 Hz); 6.62 (s, 1H); 3.76 (s, 3H); 3.27–3.33 (t, 4H, J = 6 Hz); 2.48–2.93 (m, 13H); 1.65–1.68 (m, 2H).
Procedure E. Preparation of N-(7-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)-N-(2-(4-(naphthalen-1-yl)piperazin-1-yl)ethyl)propionamide (12a)
Propionyl chloride (0.22 mL, 2.5 mmol) was added into a solution of compound 11a (0.343 g, 0.83 mmol) and Et3N (1.0 mL) in anhydrous methylene chloride at 0 °C under N2 atmosphere and then stirred at room temperature for 4 h. The reaction was diluted with CH2Cl2 and washed with water and brine, and the organic layer was dried over Na2SO4, evaporated, and purified by flash chromatography (EtOAc/MeOH/Et3N = 95:4:1) to yield 12a as yellow oil (0.318 g, 82 %): 1H NMR (400 MHz, CDCl3) δ ppm 7.68-7.64 (m,2H); 7.54–7.56 (d, 1H, J = 8 Hz); 7.45–7.48 (t, 1H, J = 6 Hz); 7.38–7.42 (m, 1H); 7.26–7.30 (t, 1H, J = 8 Hz); 7.08–7.14 (dd, 1H, J = 8 Hz); 6.69–7.01 (d, 1H, J = 8 Hz); 6.67–6.70 (d, 1H, J = 12 Hz); 6.62 (s, 1H); 3.76 (s, 3H); 3.27–3.33 (t, 4H, J = 6 Hz); 2.48–2.93 (m, 13H); 1.95–1.99 (m, 2H); 1.65–1.68 (m, 2H); 1.15–1.19 (t, 3H, J = 8 Hz).
N-(7-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)-N-(2-(4-(naphthalen-2-yl)piperazin-1-yl)ethyl)propionamide (12b)
Compound 11b (0.855 g, 2.07 mmol) was reacted with propionyl chloride (0.54 mL, 6.22 mmol) and Et3N (2.0 mL) in CH2Cl2 (20 mL) (procedure E). The crude product was purified by flash chromatography using solvent system EtOAc/MeOH = 90:10 to yield pure compound 12b as yellow oil (0.5 g, 51.5%): 1H NMR (400 MHz, CDCl3) δ ppm 7.72–7.73 (d, 1H, J = 4 Hz); 7.70–7.71 (d, 1H, J = 4 Hz); 7.68 (s, 1H); 7.37–7.41 (t, 1H, J = 8 Hz); 7.25–7.30 (m, 2H); 7.11–7.12 (d, 1H, J = 4 Hz); 6.69–7.01 (d, 1H, J = 8 Hz); 6.67–6.70 (d, 1H, J = 12 Hz); 6.62 (s, 1H); 3.76 (s, 3H); 3.27–3.33 (t, 4H, J = 6 Hz); 2.48–2.93 (m, 13H); 1.95–1.99 (m, 2H); 1.65–1.68 (m, 2H); 1.15–1.19 (t, 3H, J = 8 Hz).
Procedure F. Preparation of 7-methoxy-N-(2-(4-(naphthalen-1-yl)piperazin-1-yl)ethyl)-Npropyl-1,2,3,4-tetrahydronaphthalen-2-amine (13a)
Compound 12a (0.318 g, 0.68 mmol) in anhydrous THF (20 mL) was added dropwise into a suspension of lithium aluminum hydride (LiAlH4) (0.0.154 g, 4.07 mmol) in anhydrous THF (15 mL) at 0 °C under N2 atmosphere. The reaction mixture was refluxed for 4 h, cooled to room temperature, and then cooled further to 0 °C. Saturated NaOH/H2O (3 mL) was added dropwise to quench excess LiAlH4. The mixture was filtered, and the reaction mixture was dried over Na2SO4. The solvent was removed under vacuum to afford compound 13a as brown wax (0.246 g, 79%): 1H NMR (400 MHz, CDCl3) δ ppm 7.68-7.64 (m,2H); 7.54–7.56 (d, 1H, J = 8 Hz); 7.45–7.48 (t, 1H, J = 6 Hz); 7.38–7.42 (m, 1H); 7.26–7.30 (t, 1H, J = 8 Hz); 7.08–7.14 (dd, 1H, J = 8 Hz); 6.69–7.01 (d, 1H, J = 8 Hz); 6.67–6.70 (d, 1H, J = 12 Hz); 6.62 (s, 1H); 3.76 (s, 3H); 3.27–3.33 (t, 4H, J = 6 Hz); 2.48–2.93 (m, 15H); 1.95–1.99 (m, 2H); 1.65–1.68 (m, 2H); 0.89–0.92 (t, 3H, J = 6 Hz).
7-methoxy-N-(2-(4-(naphthalen-2-yl)piperazin-1-yl)ethyl)-N-propyl-1,2,3,4-tetrahydronaphthalen-2-amine (13b)
Compound 12b (0.50 g, 1.07 mmol) was reacted with LiAlH4 (0.243 g, 6.4 mmol) in THF (20 mL) by following the procedure F. The crude product was purified by flash chromatography using solvent system EtOAc/MeOH/Et3N = 95:4:1 to yield compound 13b as brown wax (0.393 g, 80.7%): 1H NMR (400 MHz, CDCl3) δ ppm 7.72–7.73 (d, 1H, J = 4 Hz); 7.70–7.71 (d, 1H, J = 4 Hz); 7.68 (s, 1H); 7.37–7.41 (t, 1H, J = 8 Hz); 7.25–7.30 (m, 2H); 7.11–7.12 (d, 1H, J = 4 Hz); 6.69–7.01 (d, 1H, J = 8 Hz); 6.67–6.70 (d, 1H, J = 12 Hz); 6.62 (s, 1H); 3.76 (s, 3H); 3.27–3.33 (t, 4H, J = 6 Hz); 2.48–2.93 (m, 15H); 1.95–1.99 (m, 2H); 1.65–1.68 (m, 2H); 0.89–0.92 (t, 3H, J = 6 Hz).
Procedure G. Preparation of 7-((2-(4-(naphthalen-1-yl)piperazin-1-yl)ethyl)(propyl)amino)-5,6,7,8-tetrahydronaphthalen-2-ol (14a, hydrochloride salt)
Boron tribromide (1 M solution in dichloromethane) (1.62 mL, 1.62 mmol) was added into a solution of 13a (0.246 g, 0.54 mmol) in anhydrous methylene chloride (CH2Cl2) (20 mL) at −40 °C under N2 atmosphere. The reaction mixture was stirred at −40 °C for 2 h and was continued overnight at room temperature. The reaction was quenched by the addition of saturated NaHCO3 solution, and the mixture was extracted with CH2Cl2. The combined organic layer was dried over Na2SO4 and evaporated under vacuum, and the crude product was purified by flash chromatography (EtOAc/MeOH = 95:5) to afford compound 14a as yellowish thick oily (0.075 g, 32%): 1H NMR (400 MHz, CDCl3) δ ppm 7.68-7.64 (m,2H); 7.54–7.56 (d, 1H, J = 8 Hz); 7.45–7.48 (t, 1H, J = 6 Hz); 7.38–7.42 (m, 1H); 7.26–7.30 (t, 1H, J = 8 Hz); 7.08–7.14 (dd, 1H, J = 8 Hz); 6.87–6.89 (d, 1H, J = 8 Hz); 6.55–6.58 (d, 1H, J = 12 Hz); 6.46 (s, 1H); 3.27–3.33 (t, 4H, J = 6 Hz); 2.48–2.93 (m, 15H); 1.95–1.99 (m, 2H); 1.65–1.68 (m, 2H); 0.89–0.92 (t, 3H, J = 6 Hz). The product was converted into the corresponding trihydrochloride salt as white solid, mp 202–205°C. Anal. Calcd for (C29H40N3Cl3O, 2H2O) C, H, N.
7-((2-(4-(naphthalen-2-yl)piperazin-1-yl)ethyl)(propyl)amino)-5,6,7,8-tetrahydronaphthalen-2-ol (14b, hydrochloride salt)
Compound 13b (0.393 g, 0.86 mmol) was reacted with 1 M BBr3/CH2Cl2 (2.6 mL, 2.6 mmol) in CH2Cl2 (20 mL) by following the procedure G to furnish 14b as yellow wax (0.227 g, 59.4%): 1H NMR (400 MHz, CDCl3) δ ppm 7.72–7.73 (d, 1H, J = 4 Hz); 7.70–7.71 (d, 1H, J = 4 Hz); 7.68 (s, 1H); 7.37–7.41 (t, 1H, J = 8 Hz); 7.25–7.30 (m, 2H); 7.11–7.12 (d, 1H, J = 4 Hz); 6.87–6.89 (d, 1H, J = 8 Hz); 6.55–6.58 (d, 1H, J = 12 Hz); 6.46 (s, 1H); 3.27–3.33 (t, 4H, J = 6 Hz); 2.48–2.93 (m, 15H); 1.95–1.99 (m, 2H); 1.65–1.68 (m, 2H); 0.89–0.92 (t, 3H, J = 6 Hz). The product was converted into the corresponding trihydrochloride salt as white solid, mp 208–211 °C. Anal. Calcd for (C29H40Cl3N3O) C, H, N.
4-chloroquinoline (5)57
This intermediate was made according to the reported procedure49. Commercially available 4-hydroxyquinoline (1.0 g, 6.9 mmol) was dissolved in POCl3 (3.2 ml, 34.4 mmol) under nitrogen atmosphere and refluxed for 2 h. It was then cooled and concentrated under vacuo. The solid concentrate was taken in a beaker with 100 ml water, neutralized with NaHCO3 powder and extracted with EtOAc. The solution was dried over Na2SO4, filtered, and concentrated. This crude product was then purified by flash chromatography (EtOAc/Hexan = 50:50) to afford compound 5 as off white solid (0.92 g, 81.6%). 1H NMR (400 MHz, CDCl3) δ ppm 8.74–8.75 (d, 1H, J = 4.8 Hz); 8.16–8.18 (d, 1H, J = 8 Hz); 8.10–8.12 (d, 1H, J = 8 Hz); 7.71–7.75 (t, 1H, J = 8 Hz); 7.57–7.61 (t, 1H, J = 8 Hz); 7.43–7.44 (d, 1H, J = 4 Hz).
Procedure H. Preparation of 4-(Piperazin-1-yl)quinoline (8c)
A mixture of 4-chloroquinoline 5 (0.92 g, 5.6 mmol) and piperazine (4.8 g, 56.2 mmol) in isopropanol (30 ml) was refluxed for 4 h. The solvent was evaporated in vacuo and the solid obtained was dissolved in dichloromethane (20 ml) and washed with sat. NaHCO3 solution followed by water (2×20 ml). The organic layer was dried (Na2SO4) and the solvent was evaporated in vacuo to get yellow oily liquid in quantitative yield. It was used without further purification in the next step. 1H NMR (400 MHz, CDCl3) δ ppm 8.73–8.74 (d, 1H, J = 4 Hz); 8.02–8.06 (t, 2H, J = 8 Hz); 7.63–7.67 (t, 1H, J = 8 Hz); 7.46–7.50 (t, 1H, J = 8 Hz); 6.83–6.84 (d, 1H, J = 4 Hz); 3.16–3.18 (m, 8H).
Quinolin-5-amine (6)
Into the solution of 5-nitroquinoline (1.5 g, 8.6 mmol) in absolute ethanol was added stannous chloride dihydrate (10.92 g, 43.06 mmol). The mixture was refluxed at 60 °C for 1 h. NaBH4 (0.022 g, 4.3 mmol) was added to it and refluxed for another 30 min. The reaction mixture was cooled, ethanol was evaporated and the concentrate was dissolved in water. The reaction mixture was made alkaline with 40% aq. NaOH and extracted with ethyl acetate (3×100 mL). The organic layer was evaporated under reduced pressure, dried (Na2SO4) to get a yellow liquid of almost quantitative yield. It was used in the next step without further purification. 1H NMR (400 MHz, CDCl3) δ ppm 8.89–8.90 (d, 1H, J = 4 Hz); 8.17–8.19 (d, 1H, J = 8 Hz); 7.57–7.59 (d, 1H, J = 8 Hz); 7.49–7.53 (t, 1H, J = 8 Hz); 7.34–7.37 (dd, 1H, J = 4 Hz); 6.82–6.84 (d, 1H, J = 8 Hz).
5-(Piperazin-1-yl)quinoline (8d)
5-Aminoquinoline 6 (2.2 g, 15.3 mmol) and bis(2-chloroethyl)amine HCl (3.27 g, 18.3 mmol, 1.2 equiv) were refluxed in diglyme for 2 days. The reaction mixture was cooled and diglyme was evaporated in vacuo. To the solid residue water and ethyl acetate were added and extracted with (3×100 ml) ethyl acetate. The combined organic layers were washed with water, dried (Na2SO4) and the solvent was evaporated to get the crude product which was purified by column chromatography (dichloromethane/MeOH = 4:1) to afford a resinous pure mass. Yield: 56 %. 1H NMR (400 MHz, CDCl3) δ ppm 8.85–8.86 (d, 1H, J = 4 Hz); 8.70–8.71 (d, 1H, J = 4 Hz); 7.80–7.82 (d, 1H, J = 8 Hz); 7.72–7.76 (t, 1H, J = 8 Hz); 7.57–7.60 (dd, 1H, J = 4 Hz), 7.34–7.36 (d, 1H, J = 8 Hz), 3.51–3.54 (t, 4H, J = 6 Hz), 3.30–3.34 (m, 4H).
1-(Piperazin-1-yl)isoquinoline (8e)
It was synthesized following the procedure H to furnish 8e using 1-chloroisoquinoline 7 (0.95 g, 5.8 mmol) and piperazine (2.5 g, 29 mmol) in isopropanol (50 ml) with quantitative yield of 8e as a yellow semisolid. It was used without further purification in the next step. 1H NMR (400 MHz, CDCl3) δ ppm 8.14–8.16 (d, 1H, J = 8 Hz); 8.08–8.10 (d, 1H, J = 8 Hz); 7.72–7.74 (d, 1H, J = 8 Hz); 7.57–7.61 (t, 1H, J = 8 Hz); 7.48–7.52 (t, 1H, J = 8 Hz); 7.23–7.24 (d, 1H, J = 4 Hz); 3.39–3.41 (t, 4H, J = 4 Hz); 3.15–3.18 (t, 4H, J = 6 Hz);), 2.72 (brs, 1H).
Procedure I. Preparation of (7-Methoxy-1,2,3,4-tetrahydro-naphthalen-2-yl)-propyl-amine (16a).45
7-Methoxy-2-tetralone (10g, 56.75 mmol) and acetic acid (13.5 ml, 226.9 mmol) were dissolved in dichloroethane (150 ml) and cooled to 0° C. Propyl amine (11.7 ml, 141.87 mmol) was added and the mixture was stirred under a N2 atmosphere for 30 min. NaCNBH3 (8.915 g, 141.87 mmol) in anhydrous MeOH (15 ml) was then added to the mixture and allowed to stir overnight at ambient temperature. The volatiles were then evaporated and saturated NaHCO3 solution was added into the mixture. The compound was then extracted into dichloromethane, dried over Na2SO4, filtered, and concentrated. The crude residue was dissolved in EtOAc and ethereal HCl was added. The crude salt was filtered and dried in a vacuum oven. The crude salt was then recrystalized from ethanol. The white salt was collected via filtration and dried to yield 9.5 g (65%) and used in the subsequent transformations. 1H NMR (free base) (400 MHz, CDCl3) 6.95–6.98 (d, 1H, J = 8.8 Hz), 6.65–6.78 (m, 1H), 6.60–6.61 (dd, 1H, J = 1.6 Hz), 3.81 (s, 3H), 2.97–3.04 (m, 1H), 2.88–2.92 (m, 2H), 2.67–2.71 (t, 3H, J = 7.6 Hz), 2.54–2.62 (m, 2H), 2.04–2.09 (m, 1H), 1.38 (bs, 1H), 1.48–1.60 (m, 3H), 0.91–0.95 (t, 3H, J = 7.6 Hz),
(5-Methoxy-1,2,3,4-tetrahydro-naphthalen-2-yl)-propyl-amine (16b)
Compound 16b was prepared by following the procedure I from 5-methoxy 2-tetralone (64%). 1H NMR (free base) (400 MHz, CDCl3) 7.07–7.11 (t, 1H, J = 7.2 Hz), 6.96-6.71 (d, 1H, J = 8 Hz), 6.65–6.67 (d, 1H, J = 8 Hz); 3.81 (s, 3H), 2.98–3.03 (m, 1H), 2.87–2.94 (m, 2H), 2.66–2.70 (t, 3H, J = 7.6 Hz), 2.53–2.62 (m, 2H), 2.05–2.10 (m, 1H), 1.49–1.61 (m, 3H), 1.39 (bs, 1H), 0.92–0.964 (t, 3H, J = 7.6 Hz).
Resolution of 5-Methoxy-N-propyl-1,2,3,4-tetrahydronaphthalen-2-amine (−)-16b
Racemic compound 16b was resolved into its (−) isomer by using the (+) isomer of a synthetic resolving agent 4-(2-chlorophenyl)-5,5-dimethyl-2-hydroxy-1,3,2-dioxaphosphorinane 2-oxide. The (+) and the (−) resolving agents were prepared according to the method of Ten Hoeve and Wynberg. 16b (free base 14.77 g, 67.36 mmol) and (+)-4-(2-chlorophenyl)-5,5-dimethyl-2-hydroxy-1,3,2-dioxaphosphorinane 2-oxide (20.5 g, 74.1 mmol) were dissolved by warming in 100 ml of ethanol. The solution was cooled to room temperature and then at 0°C. The precipitated crystals were filtered off, washed with cold ether to yield 17.4 g of the salt ([α] D= (−)1.2°, c =1 in methanol). Further recrystallization (2 times) from hot ethanol yielded the salt (12.9 g, [α] D= (−)14.1°, c =1 in methanol). Further crystallization of the salt from hot ethanol did not change the optical rotation to a significant extent. The salt was then neutralized in presence of 20% NaOH solution in water under stirred condition for 2 h at room temperature. The aqueous layer was extracted with dichloromethane (3×100 ml), dried over Na2SO4 and evaporated to dryness to yield (−)-16b as transparent liquid (5.8 g, [α] D of the white solid, HCl salt of (−)-16b = (−)71.5°, (c =1 in methanol) Yield. 78.5 %.
Procedure J. Preparation of 2-Chloro-N-(7-methoxy-1,2,3,4-tetrahydro-naphthalen-2-yl)-N-propyl-acetamide (17a)
Compound 16a (HCl salt, 3.117 g, 12.18 mmol) and Et3N (8.4 ml, 60.9 mmol) was stirred at 0° C in CH2Cl2 (75 ml) for 30 min. Chloroacetyl chloride (1.94 ml, 24.37 mmol) was added dropwise and the resulting solution was stirred at room temperature for 20 min, at which time the reaction mixture was poured onto a 1M solution of NaOH (50 ml) and the product was extracted with dichloromethane, dried (Na2SO4), filtered, and concentrated. The crude material was purified by column chromatography (Hex:EtOAc, 3:1) to give 3.42 g (95%) of 17a as a transparent liquid. 1H NMR (400 MHz, CDCl3)) 0.90–0.98 (m, 3H), 1.64–1.72 (m, 2H), 3.19–3.27 (m, 2H), 4.00 (s, 3H), 6.61–6.62 (dd, 1H, J = 1.6 Hz), 6.64–6.77 (m, 1H), 6.96–6.99 (d, 1H, J = 8.8 Hz).
2-Chloro-N-(5-methoxy-1,2,3,4-tetrahydro-naphthalen-2-yl)-N-propyl-acetamide (17b)
This compound was prepared from 16b by following the procedure J (93 %). 1H NMR (400 MHz, CDCl3) 6.96–7.04 (m, 1H), 6.61–6.68 (m, 2H), 4.00 (s, 3H), 3.19–3.27 (m, 2H), 1.64–1.72 (m, 2H), 0.90–0.98 (m, 3H).
(−)-2-Chloro-N-(5-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)-N-propylacetamide (−)-17b
Compound (−)-16b (1.20 g, 5.47 mmol) was reacted under similar conditions as reported above (procedure J) to afford the optically pure (−)-17b as transparent liquid (1.58 g, 97%) : 1H NMR (400 MHz, CDCl3) δ 7.07–7.15 (m, 1H), 6.65–6.71 (m, 2H), 4.08–4.12 (m, 2H), 3.95–4.03 (m, 1H), 3.80–3.82 (d, 3H, -OCH3), 3.15–3.26 (m, 2H), 3.00–3.10 (m, 2H), 2.84–2.89 (dd, 1H, J1 = 16.0 Hz, J2 = 4.8 Hz), 1.83–2.12 (m, 2H), 2.58−2.70 (m, 1H), 1.61–1.73 (m, 2H), 0.90–0.97 (m, 3H, CH2CH3),
Procedure K. Preparation of N-(7-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)-N-propyl-2-(4-(quinolin-4-yl)piperazin-1-yl)acetamide (18a)
Compound 17a (0.70 g, 2.4 mmol) and 8c (0.614 g, 2.84 mmol) were refluxed in CH3CN (100 ml) in presence of K2CO3 (1.635 g, 11.83 mmol) for 2 h. The solution was cooled, filtered, and concentrated. The crude material was then partitioned between EtOAc and H2O, and the organic layer was separated, dried (Na2SO4), and concentrated. The crude mixture was purified by column chromatography (EtOAc:MeOH; 4:1) to give 0.45 g (41 %) of 18a as yellow sticky mass. 1H NMR (400 MHz, CDCl3) δ ppm 8.73–8.74 (d, 1H, J = 4 Hz); 8.04–8.06 (d, 1H, J = 8 Hz); 7.99–8.02 (d, 1H, J = 12 Hz); 7.64–7.68 (t, 1H, J = 8 Hz); 7.46–7.50 (t, 1H, J = 8 Hz); 7.01–7.03 (d, 1H, J = 8 Hz); 6.83–6.84 (d, 1H, J = 4 Hz); 6.62–6.64 (d, 1H, J = 8 Hz); 6.66 (s, 1H); 3.76 (s, 3H); 3.49 (s, 2H); 2.82–3.30 (m, 13H); 2.55 (brs, 2H); 1.85–2.07 (m, 2H); 1.63 (brs, 2H); 0.88–0.91 (t, 3H, J = 6 Hz).
N-(7-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)-N-propyl-2-(4-(quinolin-5-yl)piperazin-1-yl)acetamide (18b)
Compound 17a (0.472 g, 1.6 mmol) was reacted with 8d (0.23 g, 1.1 mmol) in CH3CN (50 ml) by following the procedure K to furnish 18b as a yellow sticky mass (0.240 g, 23%). 1H NMR (400 MHz, CDCl3) δ ppm 8.89–8.90 (d, 1H, J = 4 Hz); 8.48–8.54 (t, 1H, J = 12 Hz); 7.80–7.82 (d, 1H, J = 8 Hz); 7.61–7.65 (t, 1H, J = 8 Hz); 7.37–7.40 (dd, 1H, J = 4 Hz), 7.11–7.13 (d, 1H, J = 8 Hz), 6.99–7.00 (dd, 1H, J = 4 Hz); 6.65–6.75 (m, 1H), 6.60 (s, 1H); 3.76 (s, 3H); 2.80–3.45 (m, 15H); 2.55 (brs, 2H); 1.85–2.07 (m, 2H); 1.63 (brs, 2H); 0.88–0.91 (t, 3H, J = 6 Hz).
2-(4-(isoquinolin-1-yl)piperazin-1-yl)-N-(7-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)-N-propylacetamide (18c)
Compound 17a (1.56 g, 5.27 mmol) was reacted with 8e (0.760 g, 3.5 mmol) in CH3CN (100 ml) by following the procedure K to furnish 18c as a yellow sticky mass (1.45 g, 87%). 1H NMR (400 MHz, CDCl3) δ ppm 8.14–8.16 (d, 1H, J = 8 Hz); 8.08–8.10 (d, 1H, J = 8 Hz); 7.72–7.74 (d, 1H, J = 8 Hz); 7.57–7.61 (t, 1H, J = 8 Hz); 7.48–7.52 (t, 1H, J = 8 Hz); 7.23–7.24 (d, 1H, J = 4 Hz); 6.98–7.02 (t, 1H, 8 Hz); 6.64–6.77 (m, 1H); 6.63 (s, 1H); 3.78 (s, 3H); 2.78–3.84 (m, 16H); 1.63–2.04(m, 5H); 0.90–0.98 (m, 3H).
N-(5-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)-N-propyl-2-(4-(quinolin-4-yl)piperazin-1-yl)acetamide (18d)
Compound 17b (1.1 g, 3.7 mmol) was reacted with 8c (0.855 g, 4.1 mmol) in CH3CN (100 ml) by following the procedure K to furnish 18d as a yellow sticky mass (1.63 g, 92.7%). 1H NMR (400 MHz, CDCl3) δ ppm 8.73–8.74 (d, 1H, J = 4 Hz); 7.99–8.06 (m, 2H Hz); 7.63–7.67 (t, 1H, J = 8 Hz); 7.46–7.50 (t, 1H, J = 8 Hz); 7.07–7.16 (m, 1H); 6.83–6.87 (m, 1H); 6.65–6.73 (m, 2H), 3.83 (s, 3H); 2.80–3.45 (m, 15H), 2.63 (brs, 1H), 2.05 (m, 2H); 1.65–1.71 (m, 2H); 1.24–1.28 (t, 1H, J = 4 Hz); 0.90–0.98 (m, 3H).
(S)-N-(5-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)-N-propyl-2-(4-(quinolin-4-yl)piperazin-1-yl)acetamide (−)-18d
Compound (−)-17b (0.67 g, 2.25 mmol) was reacted with 8c (0.539 g, 2.5 mmol) in CH3CN (100 ml) by following procedure K to furnish (−)-18d as a yellow sticky mass (0.854 g, 79.8%). 1H NMR (400 MHz, CDCl3) δ ppm 8.73–8.74 (d, 1H, J = 4 Hz); 7.99–8.06 (m, 2H Hz); 7.63–7.67 (t, 1H, J = 8 Hz); 7.46–7.50 (t, 1H, J = 8 Hz); 7.07–7.16 (m, 1H); 6.83–6.87 (m, 1H); 6.65–6.73 (m, 2H), 3.83 (s, 3H); 2.80–3.45 (m, 15H), 2.63 (brs, 1H), 2.05 (m, 2H); 1.65–1.71 (m, 2H); 1.24–1.28 (t, 1H, J = 4 Hz); 0.90–0.98 (m, 3H).
7-methoxy-N-propyl-N-(2-(4-(quinolin-4-yl)piperazin-1-yl)ethyl)-1,2,3,4-tetrahydronaphthalen-2-amine (19a)
Compound 18a (0.45 g, 0.95 mmol) was reacted with LiAlH4 (0.18 g, 4.76 mmol) in THF (20 mL) by following procedure F to furnish 19a as a yellow thick liquid (0.14 g, 35%). 1H NMR (400 MHz, CDCl3) δ ppm 8.72–8.73 (d, 1H, J = 4 Hz); 8.04–8.06 (d, 1H, J = 8 Hz); 8.01–8.03 (d, 1H, J = 8 Hz); 7.63–7.67 (t, 1H, J = 8 Hz); 7.45–7.49 (t, 1H, J = 8 Hz); 6.98–7.00 (d, 1H, J = 8 Hz); 6.83–6.84 (d, 1H, J = 4 Hz); 6.67–6.70 (d, 1H, J = 12 Hz); 6.64 (s, 1H); 3.77 (s, 3H); 3.26 (brs, 4H); 2.99–3.02 (m, 1H); 2.73–2.88 (m, 10H); 2.54–2.64 (m, 4H); 2.05 (brs, 1H); 1.49–1.66 (m, 3H); 0.90–0.94 (t, 3H, J = 8 Hz).
7-Methoxy-N-propyl-N-(2-(4-(quinolin-5-yl)piperazin-1-yl)ethyl)-1,2,3,4-tetrahydronaphthalen-2-amine (19b)
Compound 18b (0.24 g, 0.51 mmol) was reacted with LiAlH4 (0.097, 2.5 mmol) in THF (20 mL) by following procedure F to furnish 19b as a yellow thick liquid (0.200 g, 85.9%). 1H NMR (400 MHz, CDCl3) δ ppm 8.87–8.89 (d, 1H, J = 8 Hz); 8.50–8.52 (d, 1H, J = 8 Hz); 7.79–7.81 (d, 1H, J = 8 Hz); 7.60–7.64 (t, 1H, J = 8 Hz); 7.36–7.39 (dd, 1H, J = 4 Hz), 7.12–7.14 (d, 1H, J = 8 Hz), 6.97–7.00 (dd, 1H, J = 4Hz); 6.65–6.75 (m, 1H), 6.60 (s, 1H); 3.76 (s, 3H); 2.80–3.45 (m, 15H); 2.04 (brs, 2H); 1.47-1.1.68 (m, 4H); 1.63 (brs, 2H); 0.88–0.91 (t, 3H, J = 6 Hz).
N-(2-(4-(Isoquinolin-1-yl)piperazin-1-yl)ethyl)-7-methoxy-N-propyl-1,2,3,4-tetrahydronaphthalen-2-amine (19c)
Compound 18c (1.42 g, 3.0 mmol) was reacted with LiAlH4 (0.570 g, 15.02 mmol) in THF (30 mL) by following procedure F to furnish 19c as a yellow thick liquid (1.2 g, 87%). 1H NMR (400 MHz, CDCl3) δ ppm 8.14–8.16 (d, 1H, J = 8 Hz); 8.08–8.10 (d, 1H, J = 8 Hz); 7.72–7.74 (d, 1H, J = 8 Hz); 7.57–7.61 (t, 1H, J = 8 Hz); 7.48–7.52 (t, 1H, J = 8 Hz); 7.23–7.24 (d, 1H, J = 4 Hz); 6.98–7.02 (t, 1H, 8 Hz); 6.64–6.77 (m, 1H); 6.63 (s, 1H); 3.77 (s, 3H); 3.45 (brs, 4H); 2.78–3.00 (m, 11H); 2.57 (m, 5H); 2.05 (S, 1H); 1.51 (m, 2H); 0.90–0.93 (t, 3H, 6Hz).
5-Methoxy-N-propyl-N-(2-(4-(quinolin-4-yl)piperazin-1-yl)ethyl)-1,2,3,4-tetrahydronaphthalen-2-amine (19d)
Compound 18d (1.63 g, 3.45 mmol) was reacted with LiAlH4 (0.654 g, 17.24 mmol) in THF (30 mL) by following procedure F to furnish 19d as a yellow thick liquid (0.325 g, 21%). 1H NMR (400 MHz, CDCl3) δ ppm 8.73–8.74 (d, 1H, J = 4 Hz); 7.99–8.06 (m, 2H); 7.63–7.67 (t, 1H, J = 8 Hz); 7.46–7.50 (t, 1H, J = 8 Hz); 7.08–7.12 (m, 2H); 6.83–6.84 (d, 1H, J = 4 Hz); 6.71–6.73 (d, 1H, J = 8 Hz), 3.83 (s, 3H); 2.80–3.45 (m, 17H), 2.63 (brs, 1H), 2.05 (m, 2H); 1.65–1.71 (m, 2H); 1.24–1.28 (t, 1H, J = 4 Hz); 0.90–0.98 (m, 3H).
(S)-5-Methoxy-N-propyl-N-(2-(4-(quinolin-4-yl)piperazin-1-yl)ethyl)-1,2,3,4-tetrahydronaphthalen-2-amine (−)-19d
Compound (−)-18d (0.83 g, 1.76 mmol) was reacted with LiAlH4 (0.333 g, 8.8 mmol) in THF (20 mL) by following procedure F to furnish (−)-19d as a yellow thick liquid (0.20 g, 24%). 1H NMR (400 MHz, CDCl3) δ ppm 8.73–8.74 (d, 1H, J = 4 Hz); 7.99–8.06 (m, 2H); 7.63–7.67 (t, 1H, J = 8 Hz); 7.46–7.50 (t, 1H, J = 8 Hz); 7.08–7.12 (m, 2H); 6.83–6.84 (d, 1H, J = 4 Hz); 6.71–6.73 (d, 1H, J = 8 Hz), 3.83 (s, 3H); 2.80–3.45 (m, 17H), 2.63 (brs, 1H), 2.05 (m, 2H); 1.65–1.71 (m, 2H); 1.24–1.28 (t, 1H, J = 4 Hz); 0.90–0.98 (m, 3H).
7-(Propyl(2-(4-(quinolin-4-yl)piperazin-1-yl)ethyl)amino)-5,6,7,8-tetrahydronaphthalen-2-ol (20a, hydrochloride salt)
Compound 19a (0.14 g, 0.31 mmol) was reacted with 1 M BBr3/CH2Cl2 (1.22 mL, 1.22 mmol) in CH2Cl2 (20 mL) by following procedure G to furnish 20a as a white wax (0.030 g, 31%). 1H NMR (400 MHz, CDCl3) δ ppm 8.72–8.73 (d, 1H, J = 4 Hz); 8.04–8.06 (d, 1H, J = 8 Hz); 8.01–8.03 (d, 1H, J = 8 Hz); 7.63–7.67 (t, 1H, J = 8 Hz); 7.45–7.49 (t, 1H, J = 8 Hz); 6.89–6.91 (d, 1H, J = 4 Hz); 6.84–6.85 (d, 1H, J = 4 Hz); 6.63–6.65 (d, 1H, J = 8 Hz); 6.58 (s, 1H); 3.26 (brs, 4H); 2.99–3.02 (m, 1H); 2.73–2.88 (m, 10H); 2.54–2.64 (m, 4H); 2.05 (brs, 1H); 1.49–1.66 (m, 3H); 0.90–0.94 (t, 3H, J = 8 Hz).
The product was converted into the corresponding hydrochloride salt as white solid, mp 225–228 °C. Anal. Calcd for (C28H40Cl4N4O, 2.6H2O) C, H, N
7-(Propyl(2-(4-(quinolin-5-yl)piperazin-1-yl)ethyl)amino)-5,6,7,8-tetrahydronaphthalen-2-ol (20b, oxalate salt)
Compound 19b (0.20 g, 0.44 mmol) was reacted with 1 M BBr3/CH2Cl2 (1.74 mL, 1.74 mmol) in CH2Cl2 (20 mL) by following procedure G to furnish 20b as a white wax (0.123 g, 63.4%). 1H NMR (400 MHz, CDCl3) δ ppm 8.87–8.89 (d, 1H, J = 8 Hz); 8.50–8.52 (d, 1H, J = 8 Hz); 7.79–7.81 (d, 1H, J = 8 Hz); 7.60–7.64 (t, 1H, J = 8 Hz); 7.36–7.39 (dd, 1H, J = 4 Hz), 7.12–7.14 (d, 1H, J = 8 Hz), 6.88–6.90 (d, 1H, J = 8Hz); 6.62–6.64 (m, 1H), 6.55 (s, 1H); 2.80–3.45 (m, 15H); 2.04 (brs, 2H); 1.47–1.1.68 (m, 4H); 1.63 (brs, 2H); 0.88–0.91 (t, 3H, J = 6 Hz).
The product was converted into the corresponding oxalate salt as a light yellowish solid, mp 155–158 °C. Anal. Calcd for (C34H42N4O13, 1.5H2O) C, H, N
7-((2-(4-(Isoquinolin-1-yl)piperazin-1-yl)ethyl)(propyl)amino)-5,6,7,8-tetrahydronaphthalen-2-ol (20c, hydrochloride salt)
Compound 19c (1.0 g, 2.18 mmol) was reacted with 1 M BBr3/CH2Cl2 (8.7 mL, 8.7 mmol) in CH2Cl2 (50 mL) by following procedure G to furnish 20c as white wax (0.600 g, 62%). 1H NMR (400 MHz, CDCl3) δ ppm 8.10–8.12 (d, 1H, J = 8 Hz); 8.05–8.07 (d, 1H, J = 8 Hz); 7.72–7.74 (d, 1H, J = 8 Hz); 7.59–7.63 (t, 1H, J = 8 Hz); 7.49–7.53 (t, 1H, J = 8 Hz); 7.24–7.26 (d, 1H, J = 8 Hz); 6.84–6.86 (d, 1H, 8 Hz); 6.64–6.66 (d, 1H, J = 8 Hz); 6.57 (s, 1H); 3.47 (brs, 4H); 3.28 (brs, 1H); 3.04-2.66 (m, 14H); 2.21 (m, 1H); 2.04 (s, 1H); 1.69 (brs, 2H); 0.90–0.93 (t, 3H, 6Hz).
The product was converted into the corresponding trihydrochloride salt as white solid, mp 140–142 °C. Anal. Calcd for (C28H39Cl3N4O, 1.4H2O) C, H, N
6-(Propyl(2-(4-(quinolin-4-yl)piperazin-1-yl)ethyl)amino)-5,6,7,8-tetrahydronaphthalen-1-ol (20d, hydrochloride salt)
Compound 19d (0.30 g, 0.65 mmol) was reacted with 1 M BBr3/CH2Cl2 (3.3 mL, 3.3 mmol) in CH2Cl2 (20 mL) by following procedure G to furnish 20d as white wax (0.175 g, 45%). 1H NMR (400 MHz, CDCl3) δ ppm 8.70–8.71 (d, 1H, J = 4 Hz); 8.05–8.07 (d, 1H, J = 8 Hz); 7.99–8.01(d, 1H, J = 8 Hz); 7.63–7.67 (t, 1H, J = 8 Hz); 7.46–7.50 (t, 2H, J = 8 Hz); 6.93–6.97 (t, 1H, J = 8 Hz); 6.83–6.84 (d, 1H, J = 4 Hz), 6.70–6.72 (m, 1H); 6.59–6.60 (d, 1H, J = 4 Hz); 2.80–3.45 (m, 17H), 2.63 (brs, 1H), 2.05 (m, 2H); 1.65–1.71 (m, 2H); 1.24–1.28 (t, 1H, J = 4 Hz); 0.94–0.97 (t, 3H, J = 6 Hz).
The product was converted into the corresponding hydrochloride salt as white solid, mp 223–225 °C. Anal. Calcd for (C28H40Cl4N4O, C2H5OH) C, H, N.
(S)-6-(Propyl(2-(4-(quinolin-4-yl)piperazin-1-yl)ethyl)amino)-5,6,7,8-tetrahydronaphthalen-1-ol. [(−)-20d (hydrochloride salt)]
Compound (−)-19d (0.193 g, 0.42 mmol) was reacted with 1 M BBr3/CH2Cl2 (2.1 mL, 2.1 mmol) in CH2Cl2 (20 mL) by following procedure G to furnish (−)-20d as white wax (0.83 g, 45%). 1H NMR (400 MHz, CDCl3) δ ppm 8.70–8.71 (d, 1H, J = 4 Hz); 8.05–8.07 (d, 1H, J = 8 Hz); 7.99–8.01(d, 1H, J = 8 Hz); 7.63–7.67 (t, 1H, J = 8 Hz); 7.46–7.50 (t, 2H, J = 8 Hz); 6.93–6.97 (t, 1H, J = 8 Hz); 6.83–6.84 (d, 1H, J = 4 Hz), 6.70–6.72 (m, 1H); 6.59–6.60 (d, 1H, J = 4 Hz); 2.80–3.45 (m, 17H), 2.63 (brs, 1H), 2.05 (m, 2H); 1.65–1.71 (m, 2H); 1.24–1.28 (t, 1H, J = 4 Hz); 0.94–0.97 (t, 3H, J = 6 Hz). [α]25 D= −36° (c=1, CH3OH). The product was converted into the corresponding hydrochloride salt as white solid, mp 219–222 °C. Anal. Calcd for C28H40Cl4N4O, C2H5OH) C, H, N.
4-(4-(Tert-Butoxycarbonyl)piperazin-1-yl)phenylboronic acid (7b)
1-(4-Iodophenyl)-piperazine HCl (0.5 g, 1.54 mmol) was suspended in dichloromethane (15 ml) when Et3N (1 equiv) was added drop-wise. The clear solution was washed with water, dried (Na2SO4) and the solvent evaporated to obtain pale brown semisolid which was dissolved in absolute EtOH. Di-tert-butyl dicarbonate (0.34 g, 1.54 mmol) was added to the above ethanolic solution and the reaction mixture stirred for 30 min. The solvent was evaporated and the residue taken in dichloromethane, washed with water, dried (Na2SO4) with subsequent removal of the solvent to yield tert-butyl 4-(4-iodophenyl)piperazine-1-carboxylate (0.56 g, 93 %) which was used without further purification in the next step.
To a solution of tert-butyl 4-(4-iodophenyl)piperazine-1-carboxylate (0.5 g, 2.601 mmol) in dry THF was added dropwise 2.5 M nBuLi in hexane solution (1.36 ml, 3.381 mmol, 1.3 equiv) at −78 °C and the mixture stirred at the same temperature for 30 min. Trimethyl borate (0.38 ml, 3.381 mmol, 1.3 equiv) was added dropwise over 15 min period. The mixture was stirred at −78 °C for 1 hr and then gradually warmed to RT when it was quenched with 1.2 M HCl solution (pH 5–7) and then extracted with diethyl ether (3×20 ml). The combined organic layers were washed with brine, dried (Na2SO4) and the solvent evaporated to get the boronic acid 7b (0.23 g, 58 %). 1H NMR(400 MHz, CDCl3) δ ppm 8.11–8.13 (d, 2H, J = 8 Hz), 6.98–7.0 (d, 2H, J = 8 Hz), 3.61–3.63 (t, 4H, J = 4 Hz), 3.31–3.33 (t, 4H, J = 4 Hz), 1.50 (s, 9H).
1-(4-(Pyridin-4-yl)phenyl)piperazine (8f)
A mixture of Pd(PPh3)4 (31.5 mg, 5 mol %) and 4-bromopyridine HCl (0.106 g, 0.544 mmol) in DME was stirred at RT for 30 min. The suspension of boronic acid 7b (0.2 g, 0.653 mmol, 1.2 equiv) in DME was added to the above solution followed by addition of 2 M Na2CO3 solution (0.55 ml, 2 equiv). The reaction mixture was refluxed overnight, cooled to RT and the solids filtered. To the filtrate was added sat. aq. NH4Cl solution with subsequent extraction by dichloromethane (3×20 ml). The combined organic layers were dried (Na2SO4) and the solvent evaporated in vacuo to get tert-butyl 4-(4-(pyridin-4-yl)phenyl)piperazine-1-carboxylate (0.15 g, 68 %).
The deprotection of the N-Boc group was done by stirring tert-butyl 4-(4-(pyridin-4-yl)phenyl)-piperazine-1-carboxylate (0.15 g) in trifluoroacetic acid (2 ml) and chloroform (2 ml). The trifluoro-acetate salt (obtained after the removal of the solvent in vacuo) was converted to the free base using 1 M NaOH aq. solution to yield 8f (0.1 g) as an oil. 1H NMR(400 MHz, CDCl3) δ ppm 8.51–8.52 (d, 2H, J = 4 Hz), 7.50–7.53 (d, 2H, J = 12 Hz), 7.39–7.40 (d, 2H, J = 4 Hz), 6.92–6.94 (d, 2H, J = 8 Hz), 3.16–3.18 (t, 4H, J = 4 Hz), 2.98–3.0 (t, 4H, J = 4 Hz), 1.89 (brs, 1H).
General Procedure for the Preparation of 2-(4-Arylpiperazin-1-yl)ethanols 21a–21d
Into a stirring suspension of 1-arylpiperazine 8c-f and anhydrous K2CO3 (2 equiv) in acetonitrile, bromoethanol (1.2 equiv) and KI (catalytic amt) were added. The mixture was refluxed overnight and cooled to RT. The solvent was evaporated in vacuo and the residue was partitioned between ethyl acetate and water. The aqueous layer was further extracted with ethyl acetate (2×20 ml). The combined organic layers were dried (MgSO4) and the solvent evaporated in vacuo to yield oily liquid which was purified by column chromatography (dichloromethane:MeOH 95:5) to obtain pure 21a-d.
2-(4-(Quinolin-4-yl)piperazin-1-yl)ethanol (21a)
Yield: 71 %, yellow oil, starting with 0.7 g (3.28 mmol) of 4-(piperazin-1-yl)quinoline 8c. 1H NMR(400 MHz, CDCl3) δ ppm 8.73–8.74 (d, 1H, J = 4 Hz), 8.06–8.08 (d, 1H, J = 8 Hz), 8.01–8.03 (d, 1H, J = 8 Hz), 7.65–7.69 (m, 1H), 7.47–7.51 (m, 1H), 6.85–6.87 (d, 1H, J = 8 Hz), 3.70–3.73 (t, 2H, J = 6 Hz), 3.30 (brs, 4H), 3.01 (brs, 1H), 2.84 (brs, 2H), 2.70–2.73 (t, 2H, J = 6 Hz).
2-(4-(Quinolin-5-yl)piperazin-1-yl)ethanol (21b)
Yield: 67 %, starting with 1.0 g (4.69 mmol) of 5-(piperazin-1-yl)quinoline 8d. 1H NMR(400 MHz, CDCl3) δ ppm 8.92–8.96 (m, 1H), 8.53–8.59 (m, 1H), 7.87–7.92 (t, 1H, J = 10 Hz), 7.64–7.71 (m, 1H), 7.40–7.47 (m, 1H), 7.12–7.19 (m, 1H), 4.09 (s, 1H), 3.80–3.84 (t, 2H, J = 8 Hz), 3.13 (brs, 4H), 2.83 (brs, 4H), 2.72–2.78 (t, 2H, J = 12 Hz).
2-(4-(Isoquinolin-1-yl)piperazin-1-yl)ethanol (21c)
Yield: 64 %, oil, starting with 1.3 g (6.1 mmol) of 1-(piperazin-1-yl)isoquinoline 8e. 1H NMR(400 MHz, CDCl3) ppm 8.13–8.14 (d, 1H, J = 4 Hz), 8.07–8.09 (d, 1H, J = 8 Hz), 7.73–7.75 (d, 1H, J = 8 Hz), 7.58–7.62 (t, 1H, J = 8 Hz), 7.48–7.52 (t, 1H, J = 8 Hz), 7.24–7.25 (d, 1H, J = 4 Hz), 3.68–3.7 (t, 2H, J = 4 Hz), 3.46 (brs, 4H), 2.80–2.82 (t, 4H, J = 4 Hz), 2.67–2.70 (t, 2H, J = 6 Hz), 2.56 (brs, 1H).
2-(4-(4-(Pyridin-4-yl)phenyl)piperazin-1-yl)ethanol (21d)
Yield: 64 %, sticky oil, starting with 0.1 g (0.43 mmol) of 1-(4-(pyridin-4-yl)phenyl)piperazine 8f. 1H NMR(400 MHz, CDCl3) δ ppm 8.51–8.52 (d, 2H, J = 4 Hz), 7.50–7.52 (d, 2H, J = 8 Hz), 7.39–7.40 (d, 2H, J = 4 Hz), 6.91–6.93 (d, 2H, J = 8 Hz), 3.61–3.64 (t, 2H, J = 6 Hz), 3.23–3.25 (t, 4H, J = 4 Hz), 3.0 (brs, 1H), 2.63–2.66 (t, 4H, J = 6 Hz), 2.56–2.58 (t, 2H, J = 4 Hz).
General Procedure for the Preparation of 2-(4-Arylpiperazin-1-yl)acetaldehydes 22a–22d
To a solution of oxalyl chloride (2 equiv) in dichloromethane (20 ml) at −78 °C was added dimethyl sulfoxide (4 equiv). The mixture was stirred and a solution of 2-(4-arylpiperazin-1-yl)ethanol 21 in dichloromethane (10 ml) was added dropwise within 5 min. The stirring was continued for 15–20 min at −78 °C. After the addition of Et3N (6 equiv), the mixture was allowed to reach to RT and then poured into water (25 ml). The aqueous phase was further extracted with dichloromethane (2×20 ml). The combined organic phases were concentrated in vacuo and the residue obtained was stirred with ether (20 ml). The ether solution was dried (Na2SO4) and concentrated in vacuo to yield 2-(4-arylpiperazin-1-yl)acetaldehyde which was used without further purification in the next step.
2-(4-(Quinolin-4-yl)piperazin-1-yl)acetaldehyde (22a)
Yield: 73 %, starting with 0.6 g (2.33 mmol) of 2-(4-(quinolin-4-yl)piperazin-1-yl)ethanol 21a. This compound was found highly unstable on exposure to air and was used in the next step without further purification.
2-(4-(Quinolin-5-yl)piperazin-1-yl)acetaldehyde (22b)
Yield: 65 %, starting with 0.8 g (3.1 mmol) of 2-(4-(quinolin-5-yl)piperazin-1-yl)ethanol 21b. 1H NMR(400 MHz, CDCl3) δ ppm 9.69 (s, 1H), 8.80–8.81 (d, 1H, J = 4 Hz), 8.40–8.42 (d, 1H, J = 8 Hz), 7.74–7.76 (d, 1H, J = 8 Hz), 7.53–7.57 (t, 1H, J = 6 Hz), 7.28–7.31 (dd, 1H, J1= 4 Hz, J2 = 8 Hz), 7.05–7.07 (d, 1H, J = 8 Hz), 3.24 (s, 2H), 3.0–3.1 (t, 4H, J = 20 Hz), 2.74–2.77 (t, 4H, J = 6 Hz).
2-(4-(Isoquinolin-1-yl)piperazin-1-yl)acetaldehyde (22c)
Yield: 52 %, starting with 0.8 g (3.1 mmol) of 2-(4-(isoquinolin-1-yl)piperazin-1-yl)ethanol 21c. 1H NMR(400 MHz, CDCl3) δ ppm 9.72 (s, 1H), 8.07–8.09 (d, 1H, J = 8 Hz), 7.99–8.01 (d, 1H, J = 4 Hz), 7.67–7.69 (d, 1H, J = 8 Hz), 7.52–7.54 (t, 1H, J = 4 Hz), 7.43–7.46 (t, 1H, J = 6 Hz), 7.18–7.20 (d, 1H, J = 8 Hz), 3.38–3.44 (t, 4H, J = 12 Hz), 3.23 (s, 2H), 2.75–2.77 (t, 4H, J = 4 Hz).
2-(4-(4-(Pyridin-4-yl)phenyl)piperazin-1-yl)acetaldehyde (22d)
Yield: 70 %, starting with 0.6 g (2.12 mmol) of 2-(4-(4-(pyridin-4-yl)phenyl)piperazin-1-yl)ethanol 21d. 1H NMR(400 MHz, CDCl3) δ ppm 9.67 (s, 1H), 8.50–8.52 (d, 2H, J = 8 Hz), 7.50–7.52 (d, 2H, J = 8 Hz), 7.38–7.40 (d, 2H, J = 8 Hz), 6.91–6.93 (d, 2H, J = 8 Hz), 3.25–3.28 (t, 4H, J = 6 Hz), 3.19 (s, 2H), 2.62–2.65 (t, 4H, J = 6 Hz).
General Procedure for the Preparation of (S)-N6-Propyl-N6-(2-(4-arylpiperazin-1-yl)ethyl)-4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diamine 24a–24d
To a solution of 2-(4-arylpiperazin-1-yl)acetaldehyde 22 (1.5 equiv) in dichloroethane, (S)-(−)-pramipexole 23 {[α]D = −94° (c = 0.5, CH3OH)} was added followed by acetic acid (1 equiv). (S)-(−) pramipexole was made according o the reported procedure44. The mixture was stirred for 1 hr at RT. NaCNBH3 (3 equiv) was added to the reaction mixture under vigorous stirring. The contents of the flask were stirred overnight. Saturated NaHCO3 was added and the layers separated. Aqueous layer was extracted with dichloromethane (2×20 ml). The combined organic layers were dried (Na2SO4) and the solvent evaporated in vacuo to yield the crude product which was purified by column chromatography.
(S)-N6-Propyl-N6-(2-(4-(quinolin-4-yl)piperazin-1-yl)ethyl)-4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diamine (24a)
Yield: 35 mg, starting with 100 mg of (S)-(−)-pramipexole 23. Mobile phase used for column chromatographic purification was dichloromethane:MeOH (95:5). 1H NMR(400 MHz, CDCl3) δ ppm 8.65–8.66 (d, 1H, J = 4 Hz), 7.94–7.99 (m, 2H), 7.57–7.60 (t, 1H, J = 6 Hz), 7.39–7.43 (t, 1H, J = 8 Hz), 6.77–6.78 (d, 1H, J = 4 Hz), 4.89 (brs, 2H), 3.20 (brs, 4H), 3.01 (brs, 1H), 2.62–2.73 (m, 9H), 2.40–2.55 (m, 5H), 1.94–1.96 (brm, 1H), 1.65–1.71 (m, 1H), 1.39–1.46 (m, 2H), 0.82–0.86 (t, 3H, J = 8 Hz). [α]25D= −26.0° (c=1.0, CHCl3). The free base was converted to HCl salt as white solid, mp=212–214 °C. Analysis calculated for (C25H39Cl5N6S.0.5C2H5OC2H5) C, H, N.
(S)-N6-Propyl-N6-(2-(4-(quinolin-5-yl)piperazin-1-yl)ethyl)-4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diamine (24b)
Yield: 24 mg, starting with 50 mg of (S)-(−)-pramipexole 23. Mobile phase used for column chromatographic purification was dichloromethane:MeOH (95:5). 1H NMR(400 MHz, CDCl3) δ ppm 8.87–8.89 (dd, 1H, J1 = 1.6 Hz, J2 = 4 Hz), 8.48–8.51 (d, 1H, J = 12 Hz), 7.79–7.81 (d, 1H, J = 8 Hz), 7.60–7.64 (t, 1H, J = 8 Hz), 7.36–7.39 (dd, 1H, J1 = 4 Hz, J2 = 8.4 Hz), 7.12–7.14 (d, 1H, J = 8 Hz), 4.84 (brs, 2H), 3.14 (brs, 5H), 2.59–2.81 (m, 14H), 2.04–2.07 (brm, 1H), 1.73–1.82 (m, 1H), 1.54–1.56 (m, 2H), 0.9–0.94 (t, 3H, J = 8 Hz). [α]25D= −23.85° (c=0.9, CHCl3). The free base was converted to HCl salt as yellow solid, mp = 112–114 °C. Analysis calculated for (C25H38Cl4N6S) C, H, N.
(S)-N6-(2-(4-(Isoquinolin-1-yl)piperazin-1-yl)ethyl)-N6-propyl-4,5,6,7-tetrahydrobenzo[d]-thiazole-2,6-diamine (24c)
Yield: 48 mg, starting with 50 mg of (S)-(−)-pramipexole 23. Mobile phase used for column chromatographic purification was dichloromethane:MeOH (95:5). 1H NMR(400 MHz, CDCl3) δ ppm 8.06–8.07 (d, 1H, J = 4 Hz), 8.0–8.02 (d, 1H, J = 8 Hz), 7.66–7.68 (d, 1H, J = 8 Hz), 7.51–7.55 (t, 1H, J = 8 Hz), 7.41–7.45 (t, 1H, J = 8 Hz), 7.16–7.18 (d, 1H, J = 8 Hz), 4.79 (brs, 2H), 3.40 (brs, 4H), 3.04 (brm, 1H), 2.62–2.74 (m, 9H), 2.43–2.58 (m, 5H), 1.95–1.97 (brm, 1H), 1.64–1.73 (m, 1H), 1.40–1.50 (m, 2H), 0.82–0.86 (t, 3H, J = 8 Hz). [α]25D= −30.56° (c=0.82, CHCl3). The free base was converted to oxalate salt as white solid, mp=100–102 °C. Analysis calculated for (C35H44N6O20S.H2O) C, H, N.
(S)-N6-Propyl-N6-(2-(4-(4-(pyridin-4-yl)phenyl)piperazin-1-yl)ethyl)-4,5,6,7-tetrahydrobenzo[d]-thiazole-2,6-diamine (24d)
Yield: 20 mg, starting with 35 mg of (S)-(−)-pramipexole 23. Mobile phase used for column chromatographic purification was dichloromethane:MeOH (95:5). 1H NMR(400 MHz, CDCl3) δ ppm 8.51–8.52 (d, 2H, J = 8 Hz), 7.50–7.52 (d, 2H, J = 8 Hz), 7.39–7.40 (d, 2H, J = 4 Hz), 6.91–6.93 (d, 2H, J = 8 Hz), 4.65 (brs, 2H), 3.21–3.23 (t, 4H, J = 4 Hz), 3.0 (brs, 1H), 2.44–2.66 (m, 14H), 1.92–1.99 (brm, 1H), 1.61–1.72 (m, 1H), 1.39–1.45 (m, 2H), 0.81–0.85 (t, 3H, J = 8 Hz). [a]25D= −22.08° (c=0.56, CHCl3). The free base was converted to HCl salt, off white solid, mp = 206–210 °C. Analysis calculated for (C27H41Cl5N6S.0.8H2O.0.5C2H5OC2H5) C, H, N.
Evaluation of potency in binding to and activating dopamine D2 and D3 receptors
Binding potency was monitored by inhibition of [3H]spiperone (15.0 Ci/mmole, Perkin-Elmer) binding to dopamine rD2 and rD3 receptors expressed in HEK-293 cells, in a buffer containing 0.9% NaCl as described by us previously 44. Functional activity of test compounds in activating dopamine hD2 and hD3 receptors expressed in CHO and AtT cells, respectively, was measured by stimulation of [35S]GTPγS (1250 Ci/mmole, Perkin-Elmer) binding in comparison to stimulation by the full agonist dopamine as described by us previously 44.
Evaluation of antioxidant activity
DPPH Radical Scavenging Assay
To a 96 well plate 40 µL of different concentrations of methanolic drug solutions ranging from 20 µM to 250 µM were added. 200 µL of 100 µM methanolic solution of DPPH (1,1-Diphenyl-2-picryl-hydrazyl) was added, shaken vigorously at 30 °C for 20 min. Control wells received 40 µL methanol and 200 µL methanolic DPPH solution. Wells containing only 240 µL methanol served as back ground for base line correction. The changes in the absorbance of all the samples and standard (ascorbic acid) were measured at 517 nm. the radical scavenging activity was expressed as the inhibition percentage and was calculated using the following formula:- % scavenging activity = [(absorbance of control- absorbance of sample)/absorbance of control] × 100.54
Animal Experiment
Drugs and chemicals
The following commercially available drugs were used in the experiment: reserpine hydrochloride (Alfa Aesar), Ropinirole (Sigma Aldrich), The hydrochloride salts of compounds 24a, 24b, 24c and ropinirole were dissolved in water for both locomotor and 6-OH-DA rotational experiments. Reserpine was dissolved in 10–25 µL of glacial acetic acid and further diluted with 5.5% glucose solution. All compounds for this study were administered s.c. in a volume of 0.1 to 0.2 mL into each rat.
Animals
In rodent studies, animals were male Sprague-Dawley rats from Harlan (Indianapolis, IN) weighing 220–225 g unless otherwise specified. The lesioned rats (290–320 g) were purchased from Taconic Biotechnology (Rensselaer, NY) and their unilateral lesion was checked twice by apomorphine challenge following the surgery. Animals were maintained in sawdust-lined cages in a temperature and humidity controlled environment at 22 ± 1°C and 60 ± 5 % respectively, with a 12-h light/dark cycle, with lights on from 6:00 AM to 6:00 PM. They were group housed with unrestricted access to food and water. All experiment was performed during the light component. All animal use procedures were in compliance with the Wayne State University Animal Investigation Committee consistent with AALAC guidelines.
Reversal of Reserpine-Induced Hypolocomotion in Rats
Administration of reserpine induces catalepsy in rodents primarily by blocking the vesicular monoamine trasporter (VMAT) which helps in the internalization of monoamines into vesicles, resulting in metabolism of unprotected monoamines in the cytosol that ultimately causes depletion of monoamines in the synapse of the peripheral sympathetic nerve terminals 55, 58. The ability of the compounds (24a, 24b and 24c) to reverse the reserpine induced hypolocomotion was investigated 59. Ropinirole was used as standard reference compound in this study. Reserpine (5.0 mg/kg, s.c.) or saline (s.c.) were administered 18h before the injection of drug or vehicle (s.c.). The rats were placed individually in chambers for 1 h for acclimatization purpose before the administration of the test drug, standard drug or vehicle. Immediately after administration of drug or vehicle, animals were individually placed in versamax animal activity monitor chamber (45×30×20 cm) (AccuScan Instruments, Inc. Columbus, OH) to start measuring locomotor activity. Locomotion was monitored for 6 h. Consecutive interruption of two infrared beams situated 24 cm apart and 4 cm above the cage floor in the monitor chamber recorded movement. The data were presented as horizontal counts (HACTV). The effect of the individual doses of drugs on locomotor activity was compared with respect to saline treated controls (mean ± S.E.M.). The data were analyzed by one way analysis of variance (ANOVA) followed by Dunnett’s post-hoc test. The effect was considered significant if the difference from control group was observed at p<0.05.
In vivo Rotational experiment with 6-OH-DA lesioned rats
The first 14 days post-lesion challenge with apomorphine was done with lesioned animals to observe a complete rotation session post administration. In the second challenge with apomorphine (0.05 mg/kg) 21 days post lesion, contralateral rotations were recorded for 30 min; apomorphine produced rotations in all four rats (average rotation > 250) indicating successful unilateral lesion. In these rats, lesion was performed on the left side of the medial forebrain bundle in the brain and the coordinates used from Bregma are: AP-4.3, ML +1.2, DV −8.3. The rotations produced upon agonist challenge occurring clockwise. In this study, apomorphine was also used as a reference compound. The test drugs including ropinirole were dissolved in saline and were administered SC. The rats were brought to the test room 1 h before the administration of the test drug, standard drug or vehicle for acclimatization purpose. The rotations were measured over 7–10 hours. For control, vehicle was administered alone. Rotations were measured in the Rotomax Rotometry System (AccuScan Instruments, Inc. Columbus, Ohio) equipped with Rotomax Analyser, high resolution sensor and animal chambers with harnesses. Data were analyzed with Rotomax Window software program. Test drugs 24c (5 and 10 µMol/kg), 24b (5 and 10 µMol/kg) and ropinirole (5 and 10 µMol/kg) dissolved in saline were administered sc. The rotations were measured in a rotational chamber immediately after administration of drugs. The data were collected at every 30 min. Data were analyzed by Graph Pad (Version 4, San Diego) program. All drugs produced contralateral rotations in all lesioned rats which lasted over 3–10 hours.
Supplementary Material
Figure 1.
Molecular structure of dopamine D3 receptor preferring agonists and antagonists.
Table 1.
Inhibition constants for competing for [3H]spiperone binding to cloned rat D2L and D3 receptors expressed in HEK cells. Results are means ± SEM for 3–5 independent experiments each performed in triplicate.
| Compound | Ki, (nM), D2L [3H]Spiperone |
Ki, (nM), D3 [3H]Spiperone |
D2L/D3 |
|---|---|---|---|
| 7-OH-DPAT | 202 ± 34 | 2.35 ± 0.29 | 86 |
| (−)-5-OH-DPAT | 58.8 ± 11.0 | 1.36 ± 0.28 | 43 |
| 4a | 26.0 ± 7.5 | 0.825 ± 0.136 | 31.5 |
| 2 b | 264 ± 40 | 0.92 ± 0.23 | 253 |
| 1 c | 40.6 ± 3.6 | 1.77 ± 0.42 | 22.9 |
| 3 c | 47.5 ± 6.2 | 0.570 ± 0.094 | 83 |
| 14a | 13.5 ± 3.0 | 0.435 ± 0.13 | 31.0 |
| 14b | 8.85 ± 1.75 | 0.685 ± 0.217 | 12.9 |
| 20a | 7.08 ± 0.34 | 2.65 ± 0.65 | 2.67 |
| 20b | 5.22 ± 0.71 | 1.27 ± 0.52 | 4.11 |
| 20c | 21.7 ± 4.1 | 0.441 ± 0.101 | 49.2 |
| 20d | 4.89 ± 0.20 | 0.40 ± 0.09 | 12.23 |
| (−)-20d | 3.74 ± 0.70 | 0.19 ± 0.03 | 19.68 |
| (−)-24a | 109 ± 14 | 2.61 ± 0.18 | 41.8 |
| (−)-24b | 57.7 ± 3.3 | 1.21 ± 0.16 | 47.7 |
| (−)-24c | 269 ± 16 | 2.23 ± 0.60 | 121 |
| (−)-24d | 270 ± 28 | 4.78 ± 0.89 | 56.5 |
Acknowledgements
This work is supported by National Institute of Neurological Disorders and Stroke/ National Institute of Health (NS047198, AKD). We are grateful to Dr. K. Neve, Oregon Health and Science University, Portland, USA, for D2L and D3 expressing HEK cells. We are also grateful to Dr. J. Shine, Garvan Institute for Medical Research, Sydney, Australia, for D2L expressing CHO cells and Dr. Eldo Kuzhikandathil of UMDNJ-New Jersey Medical School, Newark for AtT-D3 cells.
Abbreviations
- GTPγS
Guanosine 5’-[g-thio]triphosphate
- 5-OH-DPAT
5-Hydroxy-2-(dipropylamino)tetralin
- 6-OHDA
6-Hydroxy dopamine
- CHO
Chinese Hamster Ovary
- HEK
Human embryonic kidney
- L-DOPA
(S)-(3,4-Dihydroxyphenyl) Alanine
- DPPH
1,1-Diphenyl-2-picryl-hydrazyl
- PD
Parkinson’s Disease
Footnotes
Supporting Information Available: Elemental analysis data for all final targets is available. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Mouradian MM. Recent advances in the genetics and pathogenesis of Parkinson disease. Neurology. 2002;58:179–185. doi: 10.1212/wnl.58.2.179. [DOI] [PubMed] [Google Scholar]
- 2.Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 3.Paulus W, Jellinger K. The neuropathologic basis of different clinical subgroups of Parkinson's disease. J Neuropathol Exp Neurol. 1991;50:743–755. doi: 10.1097/00005072-199111000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Sherer TB, Betarbet R, Greenamyre JT. Pathogenesis of Parkinson's disease. Curr Opin Investig Drugs. 2001;2:657–662. [PubMed] [Google Scholar]
- 5.Wooten GF. Movement Disorders. Neurologic Principles and Practice. New York, NY, USA: McGraw-Hill; 1997. pp. 153–160. [Google Scholar]
- 6.Forno LS. Neuropathology of Parkinson's disease. J Neuropathol Exp Neurol. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 8.Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 2003;53 Suppl 3:S26–S36. doi: 10.1002/ana.10483. discussion S36–38. [DOI] [PubMed] [Google Scholar]
- 9.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 10.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 11.Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson's disease. Annu Rev Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- 12.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 13.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 14.Marsden CD, Parkes JD. "On-off" effects in patients with Parkinson's disease on chronic levodopa therapy. Lancet. 1976;1:292–296. doi: 10.1016/s0140-6736(76)91416-1. [DOI] [PubMed] [Google Scholar]
- 15.Foley P, Gerlach M, Double KL, Riederer P. Dopamine receptor agonists in the therapy of Parkinson's disease. J Neural Transm. 2004;111:1375–1446. doi: 10.1007/s00702-003-0059-x. [DOI] [PubMed] [Google Scholar]
- 16.Schapira AH, Olanow CW. Rationale for the use of dopamine agonists as neuroprotective agents in Parkinson's disease. Ann Neurol. 2003;53 Suppl 3:S149–S157. doi: 10.1002/ana.10514. discussion S157–159. [DOI] [PubMed] [Google Scholar]
- 17.Clarke CE, Guttman M. Dopamine agonist monotherapy in Parkinson's disease. Lancet. 2002;360:1767–1769. doi: 10.1016/s0140-6736(02)11668-0. [DOI] [PubMed] [Google Scholar]
- 18.Lledo A. Dopamine agonists: the treatment for Parkinson's disease in the XXI century? Parkinson and Related Disorders. 2001;7:51–58. doi: 10.1016/s1353-8020(00)00038-9. [DOI] [PubMed] [Google Scholar]
- 19.Dutta AKLW. Existing Dopaminergic Therapies for Parkinson's Disease. Expert Opin. Ther. Patents. 2006;16:1613–1625. [Google Scholar]
- 20.Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature. 1979;277:93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- 21.Giros B, Martres MP, Sokoloff P, Schwartz JC. [Gene cloning of human dopaminergic D3 receptor and identification of its chromosome] C R Acad Sci III. 1990;311:501–508. [PubMed] [Google Scholar]
- 22.Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- 23.Van Tol HH, Bunzow JR, Guan HC, Sunahara RK, Seeman P, Niznik HB, Civelli O. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature. 1991;350:610–614. doi: 10.1038/350610a0. [DOI] [PubMed] [Google Scholar]
- 24.Sunahara RK, Guan HC, O'Dowd BF, Seeman P, Laurier LG, Ng G, George SR, Torchia J, Van Tol HH, Niznik HB. Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature. 1991;350:614–619. doi: 10.1038/350614a0. [DOI] [PubMed] [Google Scholar]
- 25.Strange PG. New insights into dopamine receptors in the central nervous system. Neurochem Int. 1993;22:223–236. doi: 10.1016/0197-0186(93)90050-f. [DOI] [PubMed] [Google Scholar]
- 26.Civelli O, Bunzow JR, Grandy DK. Molecular diversity of the dopamine receptors. Annu Rev Pharmacol Toxicol. 1993;33:281–307. doi: 10.1146/annurev.pa.33.040193.001433. [DOI] [PubMed] [Google Scholar]
- 27.Emilien G, Maloteaux JM, Geurts M, Hoogenberg K, Cragg S. Dopamine receptors--physiological understanding to therapeutic intervention potential. Pharmacol Ther. 1999;84:133–156. doi: 10.1016/s0163-7258(99)00029-7. [DOI] [PubMed] [Google Scholar]
- 28.Boeckler F, Gmeiner P. The structural evolution of dopamine D3 receptor ligands: structure-activity relationships and selected neuropharmacological aspects. Pharmacology & therapeutics. 2006;112:281–333. doi: 10.1016/j.pharmthera.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Park BH, Fishburn CS, Carmon S, Accili D, Fuchs S. Structural organization of the murine D3 dopamine receptor gene. J Neurochem. 1995;64:482–486. doi: 10.1046/j.1471-4159.1995.64020482.x. [DOI] [PubMed] [Google Scholar]
- 30.Chen S, Zhang X, Yang D, Du Y, Li L, Li X, Ming M, Le W. D2/D3 receptor agonist ropinirole protects dopaminergic cell line against rotenone-induced apoptosis through inhibition of caspase- and JNK-dependent pathways. FEBS Lett. 2008;582:603–610. doi: 10.1016/j.febslet.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 31.Bellucci A, Collo G, Sarnico I, Battistin L, Missale C, Spano P. Alpha-synuclein aggregation and cell death triggered by energy deprivation and dopamine overload are counteracted by D2/D3 receptor activation. J Neurochem. 2008;106:560–577. doi: 10.1111/j.1471-4159.2008.05406.x. [DOI] [PubMed] [Google Scholar]
- 32.Joyce JN, Millan MJ. Dopamine D3 receptor agonists for protection and repair in Parkinson's disease. Curr Opin Pharmacol. 2007;7:100–105. doi: 10.1016/j.coph.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Ramirez AD, Wong SK, Menniti FS. Pramipexole inhibits MPTP toxicity in mice by dopamine D3 receptor dependent and independent mechanisms. Eur J Pharmacol. 2003;475:29–35. doi: 10.1016/s0014-2999(03)02087-9. [DOI] [PubMed] [Google Scholar]
- 34.Gallagher DA, Schrag A. Impact of newer pharmacological treatments on quality of life in patients with Parkinson's disease. CNS Drugs. 2008;22:563–586. doi: 10.2165/00023210-200822070-00003. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto M, Schapira AH. Dopamine agonists in Parkinson's disease. Expert Rev Neurother. 2008;8:671–677. doi: 10.1586/14737175.8.4.671. [DOI] [PubMed] [Google Scholar]
- 36.Gu M, Iravani MM, Cooper JM, King D, Jenner P, Schapira AH. Pramipexole protects against apoptotic cell death by non-dopaminergic mechanisms. J Neurochem. 2004;91:1075–1081. doi: 10.1111/j.1471-4159.2004.02804.x. [DOI] [PubMed] [Google Scholar]
- 37.Joyce JN, Presgraves S, Renish L, Borwege S, Osredkar T, Hagner D, Replogle M, PazSoldan M, Millan MJ. Neuroprotective effects of the novel D3/D2 receptor agonist and antiparkinson agent, S32504, in vitro against 1-methyl-4-phenylpyridinium (MPP+) and in vivo against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a comparison to ropinirole. Exp Neurol. 2003;184:393–407. doi: 10.1016/s0014-4886(03)00353-4. [DOI] [PubMed] [Google Scholar]
- 38.Willner P. The mesolimbic dopamine system as a target for rapid antidepressant action. Int Clin Psychopharmacol. 1997;12 Suppl 3:S7–S14. doi: 10.1097/00004850-199707003-00002. [DOI] [PubMed] [Google Scholar]
- 39.Corrigan MH, Denahan AQ, Wright CE, Ragual RJ, Evans DL. Comparison of pramipexole, fluoxetine, and placebo in patients with major depression. Depress Anxiety. 2000;11:58–65. doi: 10.1002/(sici)1520-6394(2000)11:2<58::aid-da2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 40.Newman AH, Grundt P, Cyriac G, Deschamps JR, Taylor M, Kumar R, Ho D, Luedtke RR. N-(4-(4-(2,3-dichloro- or 2-methoxyphenyl)piperazin-1-yl)butyl)heterobiarylcarboxamides with functionalized linking chains as high affinity and enantioselective D3 receptor antagonists. J Med Chem. 2009;52:2559–2570. doi: 10.1021/jm900095y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cavalli A, Bolognesi ML, Minarini A, Rosini M, Tumiatti V, Recanatini M, Melchiorre C. Multi-target-directed ligands to combat neurodegenerative diseases. J Med Chem. 2008;51:347–372. doi: 10.1021/jm7009364. [DOI] [PubMed] [Google Scholar]
- 42.Dutta AK, Fei XS, Reith ME. A novel series of hybrid compounds derived by combining 2-aminotetralin and piperazine fragments: binding activity at D2 and D3 receptors. Bioorg Med Chem Lett. 2002;12:619–622. doi: 10.1016/s0960-894x(01)00820-4. [DOI] [PubMed] [Google Scholar]
- 43.Dutta AK, Venkataraman SK, Fei XS, Kolhatkar R, Zhang S, Reith ME. Synthesis and biological characterization of novel hybrid 7-[[2-(4-phenyl-piperazin-1-yl)-ethyl]-propyl-amino]-5,6,7,8-tetrahydro-na phthalen-2-ol and their heterocyclic bioisosteric analogues for dopamine D2 and D3 receptors. Bioorg Med Chem. 2004;12:4361–4373. doi: 10.1016/j.bmc.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 44.Biswas S, Hazeldine S, Ghosh B, Parrington I, Kuzhikandathil E, Reith ME, Dutta AK. Bioisosteric heterocyclic versions of 7-{[2-(4-phenyl-piperazin-1-yl)ethyl]propylamino}-5,6,7,8-tetrahydronaphth alen-2-ol: identification of highly potent and selective agonists for dopamine D3 receptor with potent in vivo activity. J Med Chem. 2008;51:3005–3019. doi: 10.1021/jm701524h. [DOI] [PubMed] [Google Scholar]
- 45.Biswas S, Zhang S, Fernandez F, Ghosh B, Zhen J, Kuzhikandathil E, Reith ME, Dutta AK. Further structure-activity relationships study of hybrid 7-{[2-(4-phenylpiperazin-1-yl)ethyl]propylamino}-5,6,7,8-tetrahydronaphtha len-2-ol analogues: identification of a high-affinity D3-preferring agonist with potent in vivo activity with long duration of action. J Med Chem. 2008;51:101–117. doi: 10.1021/jm070860r. [DOI] [PubMed] [Google Scholar]
- 46.Ling ZD, Robie HC, Tong CW, Carvey PM. Both the antioxidant and D3 agonist actions of pramipexole mediate its neuroprotective actions in mesencephalic cultures. J Pharmacol Exp Ther. 1999;289:202–210. [PubMed] [Google Scholar]
- 47.Zou L, Xu J, Jankovic J, He Y, Appel SH, Le W. Pramipexole inhibits lipid peroxidation and reduces injury in the substantia nigra induced by the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in C57BL/6 mice. Neurosci Lett. 2000;281:167–170. doi: 10.1016/s0304-3940(00)00853-3. [DOI] [PubMed] [Google Scholar]
- 48.Wang T, Magnin DR, Hamann LG. Palladium-catalyzed microwave-assisted amination of 1-bromonaphthalenes and 5- and 8-bromoquinolines. Org Lett. 2003;5:897–900. doi: 10.1021/ol034072h. [DOI] [PubMed] [Google Scholar]
- 49.Madrid PB, Sherrill J, Liou AP, Weisman JL, Derisi JL, Guy RK. Synthesis of ring-substituted 4-aminoquinolines and evaluation of their antimalarial activities. Bioorg Med Chem Lett. 2005;15:10151–10158. doi: 10.1016/j.bmcl.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 50.Dionne G, Humber LG, Asselin A, McQuillan J, Treasurywala AM. Ligand-receptor interactions via hydrogen-bond formation. Synthesis and pharmacological evaluation of pyrrolo and pyrido analogues of the cardiotonic agent 7-hydroxycyclindole. J Med Chem. 1986;29:1452–1457. doi: 10.1021/jm00158a022. [DOI] [PubMed] [Google Scholar]
- 51.Orus L, Perez-Silanes S, Oficialdegui AM, Martinez-Esparza J, Del Castillo JC, Mourelle M, Langer T, Guccione S, Donzella G, Krovat EM, Poptodorov K, Lasheras B, Ballaz S, Hervias I, Tordera R, Del Rio J, Monge A. Synthesis and molecular modeling of new 1-aryl-3-[4-arylpiperazin-1-yl]-1-propane derivatives with high affinity at the serotonin transporter and at 5-HT(1A) receptors. J Med Chem. 2002;45:4128–4139. doi: 10.1021/jm0111200. [DOI] [PubMed] [Google Scholar]
- 52.Ten Hoeve W, Wynberg H. The design of resolving agents. Chiral cyclic phosphoric acids. The Journal of Organic Chemistry. 2002;50:4508–4514. [Google Scholar]
- 53.Brown DA, Mishra M, Zhang S, Biswas S, Parrington I, Antonio T, Reith ME, Dutta AK. Investigation of various N-heterocyclic substituted piperazine versions of 5/7-{[2-(4-aryl-piperazin-1-yl)-ethyl]-propyl-amino}-5,6,7,8-tetrahydro-na phthalen-2-ol: effect on affinity and selectivity for dopamine D3 receptor. Bioorg Med Chem. 2009;17:3923–3933. doi: 10.1016/j.bmc.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Girennavar B, Jayaprakasha GK, Jadegoud Y, Nagana Gowda GA, Patil BS. Radical scavenging and cytochrome P450 3A4 inhibitory activity of bergaptol and geranylcoumarin from grapefruit. Bioorg Med Chem. 2007;15:3684–3691. doi: 10.1016/j.bmc.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 55.Carlsson A, Lindqvist M, Magnusson T. 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature. 1957;180:1200. doi: 10.1038/1801200a0. [DOI] [PubMed] [Google Scholar]
- 56.Ungerstedt U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. European journal of pharmacology. 1968;5:107–110. doi: 10.1016/0014-2999(68)90164-7. [DOI] [PubMed] [Google Scholar]
- 57.Schonafinger KY, Christine M, Vollman Anne, Ong Helen H. Synthesis of 7,8,9,10-tetrahydropyrido[3',4':4,5]pyrrolo[3,2-c]quinolines. Journal of Heterocyclic Chemistry. 1988;25:535–537. [Google Scholar]
- 58.Millan MJ, Di Cara B, Hill M, Jackson M, Joyce JN, Brotchie J, McGuire S, Crossman A, Smith L, Jenner P, Gobert A, Peglion JL, Brocco M. S32504, a novel naphtoxazine agonist at dopamine D3/D2 receptors: II. Actions in rodent, primate, and cellular models of antiparkinsonian activity in comparison to ropinirole. J Pharmacol Exp Ther. 2004;309:921–935. doi: 10.1124/jpet.103.062414. [DOI] [PubMed] [Google Scholar]
- 59.McCall RB, Lookingland KJ, Bedard PJ, Huff RM. Sumanirole, a highly dopamine D2-selective receptor agonist: in vitro and in vivo pharmacological characterization and efficacy in animal models of Parkinson's disease. J Pharmacol Exp Ther. 2005;314:1248–1256. doi: 10.1124/jpet.105.084202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.