Abstract
The threat of smallpox as a bioweapon and the emerging threat of human monkeypox, among other poxviral diseases, highlight the need for effective poxvirus countermeasures. ST-246, which targets the F13L protein in vaccinia virus and its homologs in other orthopoxvirus species, provides full protection from lethal poxviral disease in numerous animal models and seems to be safe in humans. All previous evaluations of ST-246 efficacy have been in immunocompetent animals. However, the risk of severe poxviral disease is greater in immunodeficient hosts. Here we report on the efficacy of ST-246 in preventing or treating lethal poxviral disease in immunodeficient mice. After lethal challenge with the Western Reserve strain of vaccinia, Nude, SCID, and JH knockout mice additionally depleted of CD4+ and CD8+ T cells were not fully protected by ST-246, although survival was significantly extended. However, CD4+ T cell deficient, CD8+ T cell deficient, JH knockout, and JH knockout mice also deficient for CD4+ or CD8+ T cells survived lethal challenge when treated with ST-246 starting on the day of challenge. Delaying treatment until 72 h after infection reduced ST-246 efficacy in some models but provided full protection from lethal challenge in most. These findings suggest that ST-246 may be effective in controlling smallpox or other pathogenic orthopoxviruses in some immunodeficient human populations for whom the vaccine is contraindicated.
Keywords: antiviral, immunodeficiency, ST-246, smallpox, orthopoxvirus
Naturally occurring smallpox has been eradicated but is still considered a threat as a bioweapon (1,2–3). Smallpox virus (variola) stocks are known to exist in two laboratories in the United States and Russia, but there is a possibility the virus exists outside these high-security facilities. Additionally, there is a growing threat from zoonotic poxvirus outbreaks, particularly monkeypox (4, 5), cowpox (6,7–8), and vaccinia (9, 10), all close relatives of variola. Currently there is no postexposure therapy for orthopoxviral disease, although the smallpox vaccine may have limited effectiveness as postexposure prophylaxis and is clearly effective as a preexposure prophylactic (11, 12). Although effective, there are also a number of severe adverse events associated with the vaccine (13). Approximately 10% of target military personnel are excluded from receiving the vaccine during preemptive medical screening, and it is estimated that ≈25% of the civilian population would be excluded (14) owing to risk factors predisposing toward adverse events. Although adverse events may occur in apparently healthy individuals, risk factors include immunodeficiency, skin conditions such as eczema or atopic dermatitis, and heart disease. It is assumed that these same populations would be at higher risk of mortality if exposed to smallpox, monkeypox, or other pathogenic poxviral diseases as well. Therefore, military personnel most susceptible to smallpox and the vast majority of the civilian population are not protected in the event of a smallpox outbreak—either through intentional release as a bioweapon or through accidental escape from a biocontainment facility. Prophylactic and therapeutic measures are needed to ensure that these populations are protected. To address this need, new, safer vaccines are in development, which include subunit DNA or proteins and attenuated/replication deficient strains of vaccinia, such as LC16m8, MVA, NYVAC, or dVV-L (reviewed in ref. 15). Although the new vaccines are likely to improve on the safety of the current Food and Drug Administration–approved smallpox vaccine, ACAM2000, it is not a foregone conclusion that they will in fact provide adequate immune protection from smallpox, particularly in immunodeficient populations. An antiviral therapeutic to protect immunologically naïve (unvaccinated) or immunodeficient individuals, incapable of mounting protective immune responses, may be beneficial.
ST-246 is a small-molecule compound identified through a high-throughput screen of a library of compounds for the ability to inhibit the cytopathic effect of poxviruses in vitro (16). ST-246 treatment of infected cells inhibits plaque formation and reduces the formation of enveloped virus (EV) without affecting the production of intracellularly retained mature virus particles or EV-associated proteins. ST-246 is effective in vitro against a number of orthopoxvirus strains, including variola, vaccinia, cowpox, ectromelia, monkeypox, and camelpox viruses (16,17,18,19,20–21). The target of ST-246 was discovered to be p37, the product of the F13L gene in vaccinia. This protein is highly conserved among orthopoxviruses, particularly in the region of the protein targeted by ST-246, explaining the genus-wide susceptibility to the drug. Further testing demonstrated that it is orally bioavailable, potent, nontoxic, and a specific inhibitor of orthopoxvirus envelopment, thus reducing virus dissemination both in vitro and in vivo. ST-246 has been demonstrated to be very effective in numerous animal models for lethal poxviral disease, essentially providing full protection as a postexposure prophylactic and therapeutic (16,17,18–19). In two phase I clinical trials it seems to be safe in humans (22). All previous evaluations of ST-246 efficacy have been in immunocompetent animals. The present study was designed to determine whether ST-246 is effective against lethal poxvirus challenge in murine models for immunodeficiency.
Results
Virulence of the F13L-Deleted Vaccinia Virus in Nude and SCID Mice.
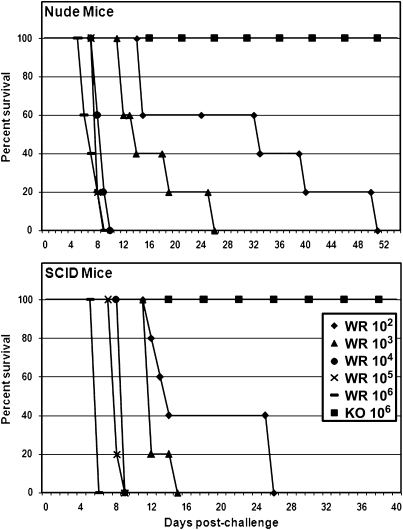
ST-246 targets the F13L protein in vaccinia. Therefore, to determine the significance of F13L as a virulence determinant in mice, Nude and SCID mice were challenged intranasally with the wild-type Western Reserve strain of vaccinia (VV-WR) or the F13L-deleted virus derived from VV-WR (F13L-KO), at doses ranging from sublethal (102 pfu) to more than 50 times the mean lethal dose (LD50) (106 pfu). The mice were then observed for 8 weeks for symptoms of disease, temperature, and weight changes. Nude and SCID mice challenged with VV-WR at doses ranging from 104–106 pfu (high-dose challenges) died of acute-onset disease, with a mean time to death from postchallenge day 6 to postchallenge day 9 (Fig. 1). Challenge with lower doses (102–103 pfu) resulted in a more prolonged disease course, with deaths occurring between days 12 and 26 in SCID mice and between days 12 and 51 in Nude mice. All of the SCID and Nude mice that were challenged with the F13L-KO virus survived regardless of the challenge dose. None of the animals showed any signs of disease and maintained or gained weight throughout the 8-week monitoring period following the challenge. Thus, the F13L protein is essential for virulence and is validated as a target for antiviral therapeutics to prevent or treat poxviral disease in immunodeficient hosts.
Fig. 1.
Virulence of F13L-KO in Nude and SCID mice. Nude and SCID mice (as indicated in each panel) were intranasally challenged (day 0) with the wild-type VV-WR (WR) or the F13L-KO virus (KO) at doses ranging from 102 pfu to 106 pfu, as indicated in the legend. Survival percentages are shown. Legend applies to both panels.
ST-246 Efficacy in Immunodeficient Mice.
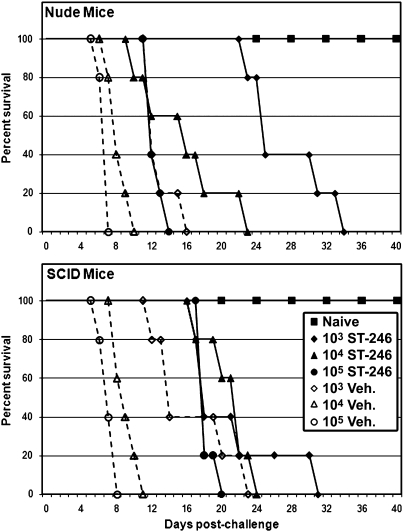
Initially, ST-246 efficacy was tested in Nude and SCID mice. Nude and SCID mice were challenged with VV-WR by the intranasal route using virus doses ranging from 103 pfu to 105 pfu. ST-246 treatment was initiated at the time of challenge and continued for 21 consecutive days. Similar to our first experiment, the vehicle-treated mice succumbed to disease in a dose-dependent fashion (Fig. 2). ST-246 treatment delayed disease progression initially, but then the mice developed increasingly severe disease even while receiving ST-246, and all eventually succumbed. In Nude mice, ST-246 significantly extended survival at all challenge doses (Table S1), essentially inhibiting acute-onset disease caused by the higher challenge doses, resulting in prolonged disease characteristic of the low challenge dose. Likewise, ST-246 inhibited acute-onset disease in SCID mice, significantly extending survival at the higher challenge doses, but did not significantly extend survival at the lowest challenge dose as was seen in Nude mice. Considering that the F13L-KO virus was avirulent in these mice, it is apparent that, in vivo, ST-246 efficacy in targeting the F13L protein is less than genetic knockout, although its effect is clearly evident.
Fig. 2.
Efficacy of ST-246 vs. lethal VV-WR challenge in Nude and SCID mice. Nude and SCID mice (as indicated in each panel) were intranasally challenged (day 0) with the wild-type VV-WR at doses ranging from 103 pfu to 105 pfu. At each challenge dose (as indicated in the legend), one group was treated with vehicle (Veh.) (dashed lines), and the other was treated with ST-246 (solid lines) for 21 consecutive days. Mice were monitored for evidence of disease and were killed if moribund. Survival percentages are shown. Legend applies to both panels.
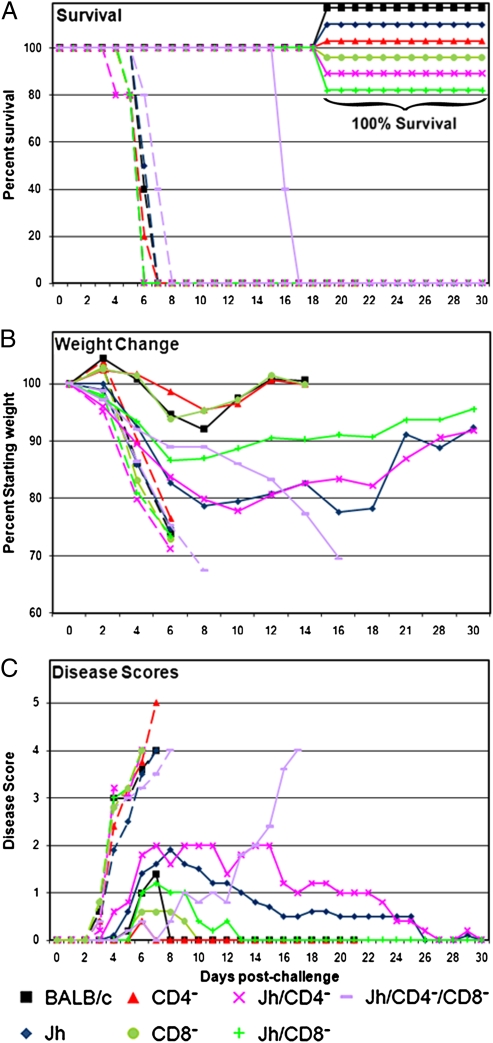
We then evaluated ST-246 in six additional models for immunodeficiency: BALB/c mice depleted of CD4+ or CD8+ T cells (mAb depletion), as well as JH mice (lacking mature B cells owing to genetic deficiency) with or without additional mAb depletion of T cells (CD4+ alone, CD8+ alone, or combined CD4+ and CD8+ T cell depletion), referred to in the text and figures as CD4−, CD8−, Jh, Jh/CD4−, Jh/CD8−, or Jh/CD4−/CD8−, respectively. Mice were challenged with a 10 × LD50 dose of VV-WR by the intranasal route. This dose of virus (2.5 × 105 pfu) and route of challenge results in 100% lethality in normal immunocompetent BALB/c mice. ST-246 treatment was initiated at the time of challenge (postexposure prophylaxis) or at 72 h after challenge (postexposure therapy) and continued for 14 consecutive daily doses. The 72-h delay is based on previous observations (23) that by 72 h after infection the virus has replicated in the lungs and is disseminating to the visceral organs; clinical disease becomes evident at this time. All vehicle-treated mice displayed evidence of disease beginning on day 3, continually lost weight, failed to maintain normal body temperature, and eventually succumbed to disease between 6 and 8 days after challenge (Fig. 3A). In marked contrast, ST-246 as postexposure prophylaxis resulted in 100% survival for BALB/c, CD4−, CD8−, Jh, Jh/CD4−, and Jh/CD8− mice. ST-246 treatment of Jh/CD4−/CD8− mice resulted in delayed disease onset relative to vehicle-treated controls, but the mice developed severe disease even while receiving ST-246 and eventually succumbed to disease between 15 and 17 days after challenge. Observations of morbidity in ST-246–treated mice (with the exception of the Jh/CD4−/CD8−) ranged from slight ruffling of the fur (disease score 1; see Fig. 3C) to pronounced ruffling of the fur (disease score 2), without additional clinical symptoms such as conjunctivitis, hunched posture, labored breathing, loss of appetite, and lack of socialization, as was commonly seen in the vehicle-treated mice before death. BALB/c, CD4−, and CD8− mice experienced relatively mild disease and lost less than 10% of their initial weight (Fig. 3B) between postchallenge days 4 and 8, then regained it by postchallenge day 12. Jh mice, with or without additional T cell depletions, were more susceptible to challenge (Jh/CD4−/CD8− > Jh ≥ Jh/CD4− > Jh/CD8−) than nondepleted BALB/c, CD4−, or CD8− mice. They began to lose weight more rapidly, lost more weight, received higher disease scores, and took ≈30 days to fully recover from disease by weight measurement and clinical evaluation.
Fig. 3.
Efficacy of ST-246 vs. lethal VV-WR challenge in B cell and/or T cell deficient mice. Immunodeficient mice (as indicated in the legend) and BALB/c mice as controls were intranasally challenged with a 10 × LD50 dose of the wild-type VV-WR on day 0 (n = 5 mice per group). Each immunodeficient mouse model was treated daily with vehicle (dashed lines) or with ST-246 (solid lines) for 14 consecutive days starting at the time of challenge. (A) Survival percentages after challenge. (B) Average weights reported as a percentage of the starting weight. Weight observations were discontinued when animals died (≈70% starting weight) or recovered weight approached 100%. (C) Average disease scores.
When ST-246 treatment was delayed for 72 h, morbidity increased in all groups relative to the prophylaxis treatment groups, although the drug still provided full protection from death in BALB/c, CD4−, CD8−, and Jh/CD8− mice (Fig. S1). Eighty percent of Jh/CD4− mice survived the challenge, whereas Jh/CD4−/CD8− and Jh mice all succumbed to disease between postchallenge days 13 and 17. Weight loss was evident in all groups by postchallenge day 3, reaching a nadir between postchallenge days 8 and 10 for survivors. From that point, BALB/c, CD4−, and CD8− mice regained weight more rapidly than the others, similar to the prophylaxis experiment, and attained their starting weight ≈3 weeks after challenge, whereas the Jh/CD8− and the surviving Jh/CD4− required 6 to 7 weeks to fully recover their starting weight.
It has been previously reported that after intranasal challenge, virus initially replicates in the nasal passages and lungs (days 0–3 after infection) and thereafter disseminates to visceral organs and brain, increasing in titer until death or clearance mediated by the immune response (23). Additionally, it has been demonstrated in immunocompetent BALB/c mice that ST-246 treatment of a lethal intranasal challenge results in slight decreases in viral load in the lungs but dramatically reduces dissemination to the spleen, liver, and brain (24). By postchallenge day 14, no live virus is detectable by plaque assay, although a low level of virus DNA is still detectable in the lungs by quantitative PCR. To address the possibility that ST-246 may keep the virus in check while immunodeficient mice are being treated, but that the virus may rebound after the drug is discontinued, resulting in persistent infections or death, we performed an experiment in which we evaluated viral load 24 h and 14 days after discontinuing drug treatment. We included BALB/c mice deficient for CD4+ and CD8+ T cells as an additional model in this experiment. Antibody depletion of T cells was maintained throughout the recovery period. Similar to previous results, we found that viral DNA was below the limit of detection in blood and visceral organs but was still detectable in the lungs of immunocompetent BALB/c mice at 14 days after infection, and by day 28 that too was below the limit of detection (Table S2). All immunodeficient mice still had detectable viral DNA in the lungs at day 14, although this varied significantly, but the majority did not have detectable virus in the blood or visceral organs. Exceptions include BALB/c/CD4−/CD8− and Jh/CD4−/CD8− mice, which had very high levels of viral DNA in the lungs as well as detectable virus in the spleen and brain. The Jh/CD4−/CD8− mice also had a high level of virus in the blood. They did not survive past postinfection day 17. The BALB/c/CD4−/CD8− mice developed progressively severe disease once ST-246 was discontinued and were killed on day 25 for humane reasons. At day 25 in these mice, the viral load had increased in the blood and all organs relative to day-14 values. It seems apparent that in this immunodeficient setting, ST-246 kept the virus in check so long as treatment continued, but after treatment cessation, in the absence of adequate immunity, the virus rebounded. In all other immunodeficient mice, the viral load decreased drastically between days 14 and 28, most to the level of detection or below. It is notable that the Jh and Jh/CD4− mice still had detectable viral DNA in the lungs at day 28. These mice also lost more weight and required a longer recovery period in the prophylaxis experiment and displayed increased mortality in the therapy experiment (Fig. 3 and Fig. S1).
Discussion
It is presumed that the immunodeficient host would be relatively more susceptible to smallpox and at greater risk of severe morbidity and mortality after infection. Although there is scarce evidence to directly support this claim, it is clear that antiviral immunity, either naturally acquired after infection with variola or after prophylactic vaccination with vaccinia, is largely responsible for recovery/protection from smallpox. Additionally, severe adverse events and death after vaccination with the related, but attenuated, vaccinia virus occur frequently as a result of immunodeficiency. Therapeutic options are needed for the treatment of poxvirus infections in immunodeficient individuals. ST-246 is currently being developed as a postexposure therapeutic for smallpox. Considering that ST-246 does not block virus replication but only inhibits its spread, it was not obvious to what extent ST-246 would be effective in immunodeficient hosts.
Initially we performed a proof-of-concept experiment using an F13L-deleted derivative of VV-WR (F13L-KO) to infect Nude and SCID mice, to evaluate the validity of targeting the F13L protein as a means of treating poxvirus infections in immunodeficient hosts. It has been previously reported that F13L-deleted viruses are “severely attenuated” (25, 26), although the details of those studies have not been presented. Nude and SCID mice were susceptible to intranasal VV-WR challenge even at doses as low as 100 pfu, but survived a F13L-KO challenge with 106 pfu and showed no evidence of disease during the 2-month monitoring period. This demonstrated to us that the F13L protein was essential for virulence and that in its absence infections are self-limiting even in severely immunodeficient mice. Thus, F13L is validated as a target for prevention of poxviral disease even in immunodeficient hosts.
Next, we challenged Nude and SCID mice with VV-WR and treated the mice with ST-246. ST-246 significantly extended survival relative to vehicle-treated controls, but eventually all mice succumbed to disease. Considering that drug treatment only extended survival, it seems apparent that drug efficacy in vivo is not equal to genetic knockout. This suggests a number of possibilities: (i) ST-246 may not efficiently target and reduce F13L function, (ii) the drug is rapidly metabolized or neutralized, (iii) drug-resistant mutants may emerge if the virus is not rapidly eliminated by the immune system, or (iv) drug exposure in infected tissues is not optimal. Inefficient inhibition of F13L function is unlikely to be a major factor: in vitro, ST-246 treatment inhibits EV formation and plaque-formation to the same extent as a genetic knockout of F13L (16). Pharmacokinetic analysis has demonstrated that ST-246 has good blood exposure and metabolizes slowly (in mice, nonhuman primates, and humans) (16, 22, 27). These studies suggest that the most likely factor contributing to the relative inefficacy of the drug compared with the genetic knockout may be inefficient penetration of infected tissues by the drug, although drug resistance remains a possibility. Prolonging drug treatment or increasing the dose likely would not solve this issue (in mice), considering that we have achieved maximal exposure at the doses given and that ST-246-treated mice that eventually succumbed to disease displayed severe disease symptoms while receiving the drug.
Nude and SCID mice are models for severe immunodeficiencies. To expand our analysis to include immunodeficient populations occurring with higher frequencies in humans, we also tested ST-246 efficacy in mice with B cell deficiency, CD4+ or CD8+ T cell deficiency, and combined B and T cell immunodeficiencies. If ST-246 treatment was initiated immediately after infection (postexposure prophylaxis), then all immunodeficient animals survived challenge, with the exception of the Jh/CD4−/CD8− mice. However, the time to death was significantly extended in this group. Normal BALB/c, CD4−, and CD8− mice experienced mild morbidity and recovered more quickly than other groups (Fig. 3 A–C). Jh mice with or without additional cellular depletions experienced moderate levels of morbidity and required more than 30 days to fully recover from disease. This result is similar to observations made by Chaudhri et al. (28) after infection of B cell deficient mice with ectromelia virus, whereby T cells responded acutely to poxvirus infection and played a major role in “natural resistance” to poxviruses but, notably, the humoral response was necessary to fully clear persisting virus. In the absence of humoral immunity, but with “normal” CD8+ T cell responses, B cell deficient mice had a prolonged disease course and eventually succumbed. Considering our results in all eight immunodeficient models, it seems that ST-246 treatment, when initiated immediately after infection, is effective so long as a single T cell component is intact, whether it is CD4+ or CD8+ T cells, while ST-246, by acting to inhibit virus spread, is, in effect, augmenting or replacing the role of antibody. This is supported by our previous observations (24) that ST-246 did not significantly decrease viral load at early times after infection but that dissemination via the blood to visceral organs was drastically reduced.
In the event of a smallpox outbreak, it is unlikely that infected persons will be treated immediately after exposure. Clinical manifestations of smallpox are not evident until 12–17 days after exposure. In the early days after an outbreak, before widespread knowledge of it, most patients will not receive treatment until the disease is evident. To model this scenario (postexposure therapy), we delayed treatment of infected mice until 72 h after infection. In addition to the Jh/CD4−/CD8− group, Jh mice, and to a lesser extent Jh/CD4− mice, were now susceptible when ST-246 treatment was delayed 72 h. Although the severe immunodeficiency in Jh/CD4−/CD8− mice provides a strong rationale as to why these mice are susceptible to lethal infection, it is not clear why the Jh/CD8− mice are protected by delayed ST-246 treatment, whereas Jh mice (with CD4+ and CD8+ T cells intact) and to a lesser extent Jh/CD4− mice are not. Although this may be a model-specific phenomenon, requiring confirmation in other animal models for poxvirus disease, it is possible that in the absence of B cells, immunopathology, possibly mediated by T cells, contributes to increased morbidity and mortality. In addition to the well-accepted role of B cells as positive regulators of the immune response (antibody formation, antigen presentation, and costimulation of T cells), a subset of B cells has been described as having negative regulatory functions as well, particularly in inflammation, cancer, autoimmunity, and infection (29). Although the vast majority of data in the literature is supportive of a positive regulatory/stimulatory role for B cells in resisting or clearing poxvirus infections (reviewed in ref. 30), our survival data from the therapy experiment (Fig. S1) are suggestive of an additional negative regulatory role for B cells as well. T cell responses are severely blunted in Jh mice (31, 32). Therefore, if T cell–mediated immunopathologies result in the absence of regulation by B cells, the mechanism by which this may occur is not clear from our data.
Previous reports show that immunodeficient mice may be safely vaccinated with replication deficient MVA (33, 34) and that, after vaccination within the context of partial immunodeficiency (humoral or cellular), animals are protected from subsequent lethal vaccinia challenge. Although the correlates of protective antipoxvirus immunity are still debated (reviewed in ref. 35), our results, along with others, suggest that, in response to a vaccinia infection, partial immunodeficiencies may be compensated for by any arm of the immune system. Immunodeficiencies may result in persistent infections, however, and we considered the possibility that the virus was not fully cleared after ST-246 treatment. Quantitative PCR analysis of viral DNA in the lungs, spleen, brain, and blood demonstrate that ST-246 effectively prevented dissemination from the lungs while the mice were being treated, except in situations in which CD4+ and CD8+ T cells were absent. Mice deficient for CD4+ and CD8+ T cells did not survive long after drug treatment was discontinued. In support of the concept that limited partial immunity contributes to virus clearance in the presence of ST-246, survivors of the initial challenge (data in Fig. 3) also resisted rechallenge at a higher virus dose without ST-246 and displayed no evidence of disease (Fig. S2). It is possible although that prolonged infections result in extended activation of innate immunity: it may be that innate immune components participate in viral clearance after challenge, and if the virus is not rapidly cleared they may remain active at the time of rechallenge, giving the illusion of protective adaptive immunity.
The US population retains very little protective immunity to smallpox because the vaccine was discontinued for general use many years ago. Although there is a potential threat from smallpox (or other pathogenic orthopoxviruses) owing to possible use as a bioterror/biowarfare agent, it is deemed unlikely. Thus, the risks associated with vaccinating even a limited number of people outweigh the benefit. Therefore, the population is increasingly vulnerable. In the event of a smallpox outbreak, previously existing contraindications to receiving the vaccine would be disregarded in many instances, and essentially all suspected of exposure would be vaccinated. Immunodeficient persons unaware of their condition may inadvertently be vaccinated and, unfortunately, they may suffer severe adverse events, including death. New vaccines that are safe in immunodeficient persons are in development. ST-246 is a promising candidate as a therapeutic option not only for those exposed to smallpox (or other pathogenic orthopoxviruses) but also for those suffering from unintended, severe adverse events arising from vaccination, particularly those with immunodeficiencies.
Materials and Methods
Mice.
All animal protocols were designed and carried out according to the Guide for the Care and Use of Laboratory Animals. All protocols were approved by the Oregon State University Institutional Animal Care and Use Committee. Female BALB/c and JH-knockout mice were purchased from Taconic, and Nude and SCID mice were purchased from Charles River. BALB/c, Nude, and SCID mice were 7 weeks of age at the start of each experiment, whereas JH-knockout mice (Jh) were 5–7 weeks of age.
Viruses and Cells.
All tissue culture media and reagents were purchased from Invitrogen except where otherwise indicated. Cell lines and vaccinia virus strain Western Reserve (VV-WR; VR-1354) were purchased from the American Type Culture Collection (ATCC). BSC40 (CRL-2761) cell monolayers were maintained in a humidified atmosphere at 37°C and 5% CO2 in DMEM supplemented with 10% FBS, 2 mM L-glutamine, and 10 μg/mL gentamicin sulfate. Stocks of VV-WR and IHD-J (cultured from a stock originally obtained from S. Dales) were cultured in BSC40 cells and purified by sucrose gradient centrifugation (36). Viral titers were determined by plaque assay in monolayers of BSC40 cells. The F13L-deleted derivative of VV-WR (F13L-KO) was constructed as follows: an ≈3,000-bp pair segment of vaccinia DNA, comprising the VV-WR F13L gene and ≈1,000 bp of flanking sequence from each end of the gene, was PCR amplified from genomic DNA and cloned into a pCR2.1 plasmid vector. Essentially, the entire F13L coding sequence was removed by restriction enzyme digest and replaced with the EGFP gene—in frame with the ATG start codon and 18 nt remaining from the original F13L gene. The plasmid DNA and purified viral genomic DNA were cotransfected into BGMK cells infected with Shope fibroma virus. Recombinant viruses were identified by EGFP expression and the inability to form normal-sized plaques, because this is a characteristic of F13L-deleted viruses. After multiple rounds of clonal purification, F13L deletion was confirmed by diagnostic PCR of genomic DNA. The virus was amplified and titered in BSC40 cells as described above for VV-WR and IHD-J, although 7 days of culture were required to observe plaques.
ST-246.
ST-246 was synthesized by Pharmacore and delivered as a lyophilized powder. The drug was prepared for oral dosing of mice by suspension in an aqueous solution containing 0.75% methylcellulose (Sigma-Aldrich) and 1% Tween-80 (Sigma-Aldrich) at a concentration of 20 mg/mL Two milligrams per mouse (≈100 mg/kg) of ST-246 was delivered in a volume of 100 μL by oral gavage. Animals were dosed daily at 24-h intervals for 21 days (Nude and SCID animals in Fig. 2) or 14 consecutive days (all other experiments) beginning on the day of challenge with VV-WR or after a 72-h delay.
mAb-Mediated Depletion of CD4+ and/or CD8+ T Cells.
Rat hybridoma clones GK1.5 (TIB-207) and 2.43 (TIB-210) were purchased from ATCC and maintained in ATCC-formulated DMEM (catalog no. 30–2002) containing 20% FBS. IgG antibodies were purified from culture supernatants using Protein G Sepharose affinity columns and diluted in PBS before use in mice. Purified mAbs were tested by Limulus Amebocyte Lysate Chromo-LAL assay (Associates of Cape Cod) and verified to be very low in endotoxin (LPS) contamination. Mice were initially depleted of CD4+ and/or CD8+ T cells by i.p. injection of 200 μg GK1.5 and/or 500 μg 2.43 mAbs (in a total of 500 μL) for 3 consecutive days (days −5, −4, and −3 relative to the start of each experiment on day 0). Cellular depletions were then maintained for the duration of each experiment by once-weekly mAb injections (days +4, +11, etc.), similar to previous reports (34). The mice were confirmed to be depleted of the appropriate T cell subsets by flow cytometric analysis of blood cells obtained via retroorbital sampling. Cellular depletions were consistently >99% at all time points tested.
Vaccinia Challenge and Rechallenge.
The LD50 of the preparation of VV-WR used in all experiments, when delivered intranasally to BALB/c mice, was determined to be 2.5 × 104 pfu per mouse in 7-week-old mice and 7.9 × 104 pfu per mouse in 10-week-old mice, according to the method of Reed and Muench (37). Mice were anesthetized by inhalation of 3% isoflurane (IsoSol; Vedco), and the challenge virus was delivered to the nares in a total of 10 μL PBS. In the first set of experiments (Fig. 1 and Table S1), 7-week-old Nude and SCID mice were challenged with VV-WR or F13L-KO (doses ranging from 102 pfu to 106 pfu) on day 0. Mice were then monitored for survival, signs of disease, weight loss, and temperature maintenance, daily for up to 60 days. Daily observations included “disease scores” recorded as 0 (normal), 1 (slightly ruffled fur), 2 (significantly ruffled fur), 3 (hunched posture and/or conjunctivitis in addition to significant fur ruffling), 4 (score of 3 combined with labored breathing or lack of mobility; evidently moribund), and 5 (death). Mice were preemptively killed for humane reasons when exhibiting severe disease symptoms (such as >30% weight loss, labored breathing, hunched posture, and lack of mobility and socialization) and/or were otherwise moribund (disease score of 4). In the follow-up experiment, Nude and SCID mice were challenged with various doses of VV-WR as described above and then treated with ST-246 once daily for 21 consecutive days. In other lethal challenge experiments, mice were challenged with a 10 × LD50 dose of VV-WR by the intranasal route. In postexposure prophylaxis experiments, ST-246 treatment was started at the time of challenge, whereas in the postexposure therapy experiments, ST-246 treatment was delayed until 72 h after challenge. For the rechallenge, mice that were treated prophylactically with ST-246 and survived the initial challenge described in Fig. 3 A–C were challenged again 30 days later with a 10 × LD50 dose of VV-WR. These mice were not treated with ST-246 after rechallenge.
Tissue Collection, Processing, and Viral Load Quantitation.
On days 14 and 28 after VV-WR challenge, three mice per group (all ST-246 treated) were killed with CO2, and blood, spleen, lungs, and brain were collected into preweighed 2.0-mL tubes containing 1.0 mm silica spheres and one ¼-inch ceramic sphere (MP Biomedicals) and 500 μL PBS. The tubes were weighed again to determine blood volume or tissue weight and then frozen at −80°C. On the day of processing, the tissues were thawed and homogenized at a speed of 4.0 m/sec for 60 sec in a FASTPREP-24 (MP Biomedicals) tissue homogenizer. Qiagen’s DNeasy Blood and Tissue Kit was then used to purify total DNA from 75 μL of blood, 20 mg of lung and brain, or 8 mg of spleen homogenates as recommended by the manufacturer (Qiagen). Quantitation of VV genome copies was performed by real-time PCR on a 7500 Real Time PCR System (Applied Biosystems) using ribonucleotide reductase (I4L)-specific primers according to a protocol kindly provided by Dr. Eva-Jasmin Freyschmidt (Harvard Medical School) and as previously described (24). A standard curve generated using DNA extracted from known pfu of sucrose gradient purified VV-WR was used to quantitate virus genome copies in tissue samples.
Statistical Analysis.
Survival curves were plotted using GraphPad Prism (GraphPad Software) and compared using the log–rank (Mantel-Cox) test. In all tests, P values <0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported by Small Business Innovation Research Grant R43AI075747 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Conflict of interest statement: All authors are employed by SIGA Technologies.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912134107/DCSupplemental.
References
- 1.Henderson DA. The looming threat of bioterrorism. Science. 1999;283:1279–1282. doi: 10.1126/science.283.5406.1279. [DOI] [PubMed] [Google Scholar]
- 2.Henderson DA, et al. Working Group on Civilian Biodefense. Smallpox as a biological weapon: Medical and public health management. JAMA. 1999;281:2127–2137. doi: 10.1001/jama.281.22.2127. [DOI] [PubMed] [Google Scholar]
- 3.Fleck F. Conference warns of danger of re-emergence of smallpox as weapon of bioterror. Bull World Health Organ. 2003;81:917–918. [PMC free article] [PubMed] [Google Scholar]
- 4.Hutin YJ, et al. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg Infect Dis. 2001;7:434–438. doi: 10.3201/eid0703.010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parker S, Nuara A, Buller RM, Schultz DA. Human monkeypox: An emerging zoonotic disease. Future Microbiol. 2007;2:17–34. doi: 10.2217/17460913.2.1.17. [DOI] [PubMed] [Google Scholar]
- 6.Vorou RM, Papavassiliou VG, Pierroutsakos IN. Cowpox virus infection: An emerging health threat. Curr Opin Infect Dis. 2008;21:153–156. doi: 10.1097/QCO.0b013e3282f44c74. [DOI] [PubMed] [Google Scholar]
- 7.Lewis-Jones S. Zoonotic poxvirus infections in humans. Curr Opin Infect Dis. 2004;17:81–89. doi: 10.1097/00001432-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Baxby D, Bennett M, Getty B. Human cowpox 1969-93: A review based on 54 cases. Br J Dermatol. 1994;131:598–607. doi: 10.1111/j.1365-2133.1994.tb04969.x. [DOI] [PubMed] [Google Scholar]
- 9.Trindade GS, et al. Zoonotic vaccinia virus: Clinical and immunological characteristics in a naturally infected patient. Clin Infect Dis. 2009;48:e37–e40. doi: 10.1086/595856. [DOI] [PubMed] [Google Scholar]
- 10.de Souza Trindade G, et al. Zoonotic vaccinia virus infection in Brazil: Clinical description and implications for health professionals. J Clin Microbiol. 2007;45:1370–1372. doi: 10.1128/JCM.00920-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenner F, Henderson D, Arita I, Jezek Z, Ladnyi I. Smallpox and Its Eradication. Geneva: World Health Organization; 1988. pp. 1–68. [Google Scholar]
- 12.Mortimer PP. Can postexposure vaccination against smallpox succeed? Clin Infect Dis. 2003;36:622–629. doi: 10.1086/374054. [DOI] [PubMed] [Google Scholar]
- 13.Lane JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination, 1968: Results of ten statewide surveys. J Infect Dis. 1970;122:303–309. doi: 10.1093/infdis/122.4.303. [DOI] [PubMed] [Google Scholar]
- 14.Kemper AR, Davis MM, Freed GL. Expected adverse events in a mass smallpox vaccination campaign. Eff Clin Pract. 2002;5:84–90. [PubMed] [Google Scholar]
- 15.Artenstein AW. New generation smallpox vaccines: A review of preclinical and clinical data. Rev Med Virol. 2008;18:217–231. doi: 10.1002/rmv.571. [DOI] [PubMed] [Google Scholar]
- 16.Yang G, et al. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus challenge. J Virol. 2005;79:13139–13149. doi: 10.1128/JVI.79.20.13139-13149.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quenelle DC, et al. Efficacy of delayed treatment of ST-246 given orally against systemic orthopoxvirus infections in mice. Antimicrob Agents Chemother. 2007;51:689–695. doi: 10.1128/AAC.00879-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sbrana E, et al. Efficacy of the antipoxvirus compound ST-246 for treatment of severe orthopoxvirus infection. Am J Trop Med Hyg. 2007;76:768–773. [PubMed] [Google Scholar]
- 19.Nalca A, et al. Evaluation of orally delivered ST-246 as postexposure prophylactic and antiviral therapeutic in an aerosolized rabbitpox rabbit model. Antiviral Res. 2008;79:121–127. doi: 10.1016/j.antiviral.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Duraffour S, et al. Activity of the anti-orthopoxvirus compound ST-246 against vaccinia, cowpox and camelpox viruses in cell monolayers and organotypic raft cultures. Antivir Ther. 2007;12:1205–1216. [PubMed] [Google Scholar]
- 21.Quenelle DC, et al. Synergistic efficacy of the combination of ST-246 with CMX001 against orthopoxviruses. Antimicrob Agents Chemother. 2007;51:4118–4124. doi: 10.1128/AAC.00762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jordan R, et al. Single-dose safety and pharmacokinetics of ST-246, a novel orthopoxvirus egress inhibitor. Antimicrob Agents Chemother. 2008;52:1721–1727. doi: 10.1128/AAC.01303-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smee DF, Bailey KW, Wong MH, Sidwell RW. Effects of cidofovir on the pathogenesis of a lethal vaccinia virus respiratory infection in mice. Antiviral Res. 2001;52:55–62. doi: 10.1016/s0166-3542(01)00159-0. [DOI] [PubMed] [Google Scholar]
- 24.Berhanu A, et al. ST-246(R) inhibits in vivo poxvirus dissemination, virus shedding, and systemic disease manifestation. Antimicrob Agents Chemother. 2009;53:4999–5009. doi: 10.1128/AAC.00678-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith GL. Vaccinia virus glycoproteins and immune evasion. The sixteenth Fleming Lecture. J Gen Virol. 1993;74:1725–1740. doi: 10.1099/0022-1317-74-9-1725. [DOI] [PubMed] [Google Scholar]
- 26.Borrego B, Lorenzo MM, Blasco R. Complementation of P37 (F13L gene) knock-out in vaccinia virus by a cell line expressing the gene constitutively. J Gen Virol. 1999;80:425–432. doi: 10.1099/0022-1317-80-2-425. [DOI] [PubMed] [Google Scholar]
- 27.Jordan R, et al. ST-246 antiviral efficacy in a nonhuman primate monkeypox model: Determination of the minimal effective dose and human dose justification. Antimicrob Agents Chemother. 2009;53:1817–1822. doi: 10.1128/AAC.01596-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaudhri G, Panchanathan V, Bluethmann H, Karupiah G. Obligatory requirement for antibody in recovery from a primary poxvirus infection. J Virol. 2006;80:6339–6344. doi: 10.1128/JVI.00116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev. 2008;224:201–214. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 30.Amanna IJ, Slifka MK, Crotty S. Immunity and immunological memory following smallpox vaccination. Immunol Rev. 2006;211:320–337. doi: 10.1111/j.0105-2896.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- 31.João C, Ogle BM, Geyer S. Immunoglobulin promotes the diversity and the function of T cells. Eur J Immunol. 2006;36:1718–1728. doi: 10.1002/eji.200635908. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, et al. Gene-targeted B-deficient mice reveal a critical role for B cells in the CD4 T cell response. Int Immunol. 1995;7:1353–1362. doi: 10.1093/intimm/7.8.1353. [DOI] [PubMed] [Google Scholar]
- 33.Wyatt LS, Earl PL, Eller LA, Moss B. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc Natl Acad Sci USA. 2004;101:4590–4595. doi: 10.1073/pnas.0401165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belyakov IM, et al. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc Natl Acad Sci USA. 2003;100:9458–9463. doi: 10.1073/pnas.1233578100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lane JM. Immunity to smallpox and vaccinia: The future of smallpox vaccines. Expert Rev Clin Immunol. 2006;2:325–327. doi: 10.1586/1744666X.2.3.325. [DOI] [PubMed] [Google Scholar]
- 36.Earl PL, Cooper N, Wyatt S, Moss B, Carroll MW. In: Current Protocols In Molecular Biology. Ausubel FM, et al., editors. Vol. 2. New York: John Wiley and Sons; 1998. pp. 16.16.11–16.16.13. [Google Scholar]
- 37.Reed LJ, Muench LH. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.