Abstract
We studied human cancer cell models in which we detected constitutive activation of ERK. A fraction of active ERK was found to be located in mitochondria in RWPE-2 cells, obtained by v-Ki-Ras transformation of the epithelial prostate RWPE-1 cell line; in metastatic prostate cancer DU145 cells; and in osteosarcoma SAOS-2 cells. All these tumor cells displayed marked resistance to death caused by apoptotic stimuli like arachidonic acid and the BH3 mimetic EM20-25, which cause cell death through the mitochondrial permeability transition pore (PTP). PTP desensitization and the ensuing resistance to cell death induced by arachidonic acid or EM20-25 could be ablated by inhibiting ERK with the drug PD98059 or with a selective ERK activation inhibitor peptide. ERK inhibition enhanced glycogen synthase kinase-3 (GSK-3)-dependent phosphorylation of the pore regulator cyclophilin D, whereas GSK-3 inhibition protected from PTP opening. Neither active ERK in mitochondria nor pore desensitization was observed in non-transformed RWPE-1 cells. Thus, in tumor cells mitochondrial ERK activation desensitizes the PTP through a signaling axis that involves GSK-3 and cyclophilin D, a finding that provides a mechanistic basis for increased resistance to apoptosis of neoplastic cells.
Keywords: glycogen synthase kinase-3, cyclophilin D, PTP, EM20-25, arachidonic acid
In cancer, disruption of the tightly regulated network that controls tissue homeostasis occurs as a result of perturbed signal transduction, leading to enhanced activation of biologic subroutines such as cell proliferation and survival. Dysregulation of the Ras/ERK transduction pathway is of particular relevance to neoplastic transformation. Many cancer-associated lesions lead to constitutive activation of ERK signaling (1, 2), and this activation is associated with unrestrained cell proliferation and poor prognosis (3). For instance, in prostate cancer, deregulation of growth factor pathways activates Ras/ERK signaling and correlates with cancer progression from a localized, androgen-dependent to a metastatic, hormone-refractory disease; conversely, Ras inhibition delivers a potent signal of growth arrest and death (4). The ultimate biological consequences of ERK1/2 activation depend on the specific targets of their kinase activity, and this is partly regulated by the subcellular localization of the enzyme (5). Recent work indicates that a fraction of cellular ERK1/2 is targeted to mitochondria, where it prevents the release of apoptogenic proteins (6), is involved in the response to oxidative insults (7, 8), regulates cholesterol transport (9), and takes part in the disposal of damaged organelles (10). Mitochondria are key players in apoptosis regulation. Opening of the mitochondrial permeability transition pore (PTP) constitutes a point of no return in cell commitment to death. The PTP is an inner membrane megachannel, whose stable opening results in mitochondrial depolarization, swelling, and rupture of the outer membrane with release of intermembrane proteins (11, 12). The lack of information on the molecular composition of the pore makes very difficult to comprehend the mechanisms controlling its open–closed transitions. It is established that the mitochondrial chaperone cyclophilin D (CyP-D) enhances PTP opening and is the molecular target of the PTP desensitizer drug cyclosporin A (CsA) (11, 12). It has been postulated that dynamic networks of kinase/phosphatase pathways might regulate the PTP, converging on the inhibition of glycogen synthase kinase-3β (GSK-3β) (13). A reduced sensitivity of mitochondrial PTP to diverse stress stimuli was described in in vitro and in vivo models of neoplastic transformation (11, 12), implying that dysregulation of pore opening might be a strategy used by tumor cells to escape death. Here we report that ERK is constitutively activated in mitochondria of several cancer cell types, where it inhibits GSK-3–dependent phosphorylation of CyP-D and renders these cells more refractory to pore opening and to the ensuing cell death.
Results
We first assessed cell sensitivity to PTP inducers in the human prostate epithelial cell lines RWPE-1 and -2, because they constitute a good model of in vitro transformation (14, 15). RWPE-1 cells are immortalized but lack any tumorigenic potential, whereas RWPE-2 cells are made tumorigenic by expression of v-Ki-Ras in RWPE-1 cells (14, 15). As expected, ERK is constitutively active only in RWPE-2 cells (Fig. S1A). We treated cells with arachidonic acid (AA), a fatty acid that acts as a potent PTP inducer (16, 17). AA caused marked cell death in RWPE-1 cells, whereas RWPE-2 cells were less sensitive (Fig. 1A and Fig. S1B). CsA, which desensitizes the PTP by inhibiting CyP-D, protected both cell types from AA. Because AA is a direct PTP inducer (17), v-Ki-Ras-mediated transformation might act on the pore itself. In this case, RWPE-2 cells should be similarly protected from the effects of a second PTP opener, unrelated to AA. We tested the BH3 mimetic EM20-25, which, unlike its parent compound HA14-1 (18), does not cause inhibition of respiration or uncoupling, and specifically kills cells by opening the PTP (19). RWPE-1 cells treated with EM20-25 underwent death to a significantly higher degree than RWPE-2, and cell death was inhibited by CsA (Fig. 1B and Fig. S1C). Both AA and EM20-25 also induced mitochondrial depolarization in RWPE-1 cells, whereas their effect was lower in RWPE-2 cells. In both cell types, CsA inhibited depolarization (Fig. S1D).
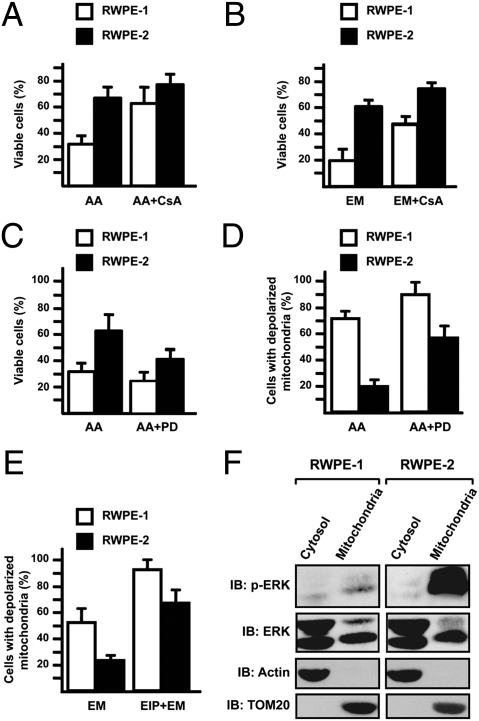
Fig. 1.
RWPE-2 cells are more resistant than RWPE-1 cells to PTP agonists and are sensitized by ERK inhibition. (A–C) Viable cells are identified as double negative for propidium iodide and annexin V–FITC in FACS analyses. (D and E) Cells with depolarized mitochondria are assessed by FACS analysis after TMRM staining. RWPE-1/2 cells were exposed for 1 h to 50 μM arachidonic acid (AA) (A, C, and D) or to 500 μM EM20-25 (EM; B and E). CsA (4 μM), PD98059 (PD, 40 μM), or ERK inhibitor peptide (EIP) (50 μM) were preincubated for 30 min. In all histograms, bars are mean values of percentages (±SD). (F) Subcellular distribution of phospho- and total ERK was assessed by Western immunoblot (IB) in the mitochondrial and cytosolic fractions of RWPE-1/2 cells. Blots were probed with an anti-TOM20 as a mitochondrial marker and with an anti-actin as a cytosolic marker.
We then investigated whether ERK activation contributed to the reduced sensitivity of RWPE-2 cells to PTP inducers. PD98059, a potent inhibitor of MEK (i.e., the kinase responsible for ERK activation) (Fig. S2A) increased both cell death and mitochondrial depolarization caused by pore inducers (Fig. 1 C and D). Similarly, a cell-permeable ERK activation inhibitor peptide (EIP), which corresponds to the N terminus of MEK and selectively binds ERK2 (Fig. S2B), strongly increased mitochondrial depolarization prompted by pore inducers (Fig. 1E and Fig. S2C). We also found that a fraction of the p42ERK2 isoform localizes to mitochondria both in RWPE-1 and RWPE-2 cells (Fig. 1F). Mitochondrial ERK was activated in RWPE-2 cells (Fig. 1F), and this activation did not involve relocation of K-Ras (Fig. S2D). We investigated PTP opening with the whole-cell Ca2+ retention capacity (CRC) assay (20). RWPE-1 cells had a lower PTP opening threshold than RWPE-2 cells, both in basal conditions and after treatment with AA or EM20-25 (Fig. 2A). Treatment with PD98059 markedly enhanced PTP opening (Fig. 2B).
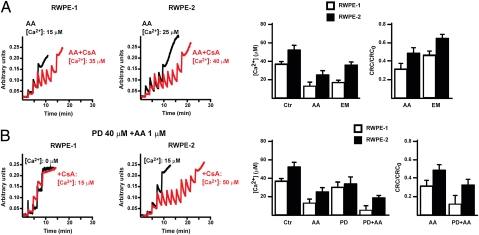
Fig. 2.
RWPE-2 cells are less sensitive than RWPE-1 cells to pore opening, which is enhanced by ERK inhibition. (A) PTP opening of RWPE-1/2 cells treated with arachidonic acid (AA) (1 μM) or with EM20-25 (EM, 50 μM) is measured with the whole-cell CRC assay. In B, cells were also pretreated with the ERK inhibitor PD98059 (PD, 40 μM). Calcium Green-5N fluorescence is reported as arbitrary units on the y axis. Because the probe does not permeate mitochondria, Ca2+ uptake into the organelles is displayed by a rapid decrease of the fluorescence spike after administration to the cells of subsequent Ca2+ pulses (5 μM each). CsA (red) increases the number of spikes before the permeability transition, recorded as a marked fluorescence increase, occurs. This establishes the PTP dependence of Ca2+ release. In the histograms, both the Ca2+ concentration required to open the pore (Left) and the ratio between the CRC detected in the presence (CRC) and absence (CRC0) of the agonist (Right) are reported.
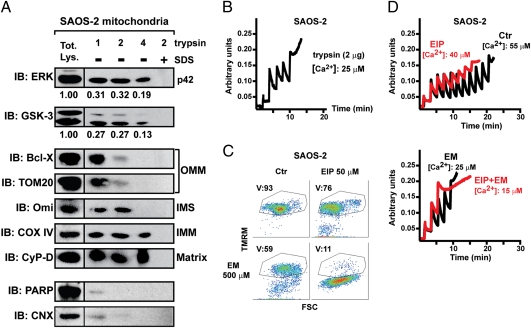
Similarly to RWPE-2 cells, both DU145 and SAOS-2 cells, which were obtained from a brain metastasis of prostate cancer and from an osteosarcoma, respectively, displayed constitutive ERK activation and mitochondrial localization of a fraction of active ERK (Fig. S3A). Using a trypsin digestion assay on isolated mitochondria, we found that the 42-kDa isoform of ERK (ERK2) was not digested under conditions that disrupted external mitochondrial markers (central lane of Fig. 3A) retaining mitochondrial ability to take up Ca2+ (Fig. 3B). Increasing trypsin concentrations fully digested intermembrane space proteins but not inner membrane or matrix components and only partially affected ERK2. ERK2 was eliminated only when mitochondrial membranes were disrupted with SDS before trypsin treatment (right lane of Fig. 3A). Therefore, the protease-resistant fraction of ERK resides in the matrix, whereas the portion digested by trypsin (see the densitometry of Fig. 3A) is in external mitochondrial compartments. Densitometric analysis on the trypsin-treated mitochondria vs. the cytosol showed that approximately 1.5% of total ERK is in the matrix. Both DU145 and SAOS-2 cells underwent death and pore opening after treatment with EM20-25, and this was markedly increased by ERK inhibition (Fig. 3 C and D and Fig. S3B).
Fig. 3.
A fraction of ERK and of GSK-3 is located in mitochondria, and ERK inhibition sensitizes SAOS-2 cells to mitochondrial depolarization and PTP opening. (A) Isolated mitochondria (70 μg per point) were treated for 1 h with the reported quantities of trypsin (in micrograms; trypsin concentration: 5, 10, or 20 μg/mL) and analyzed by Western immunoblot (IB). Where indicated, 0.1% SDS was added before trypsin. Blots were probed for ERK and GSK-3; and for a panel of mitochondrial markers of the outer and inner membrane (OMM and IMM, respectively), of the intermembrane space (IMS), and of the matrix. COX-IV is a cytochrome oxidase subunit. Poly(ADP-ribose)polymerase (PARP) was used as an example of a non-mitochondrial soluble protein; and calnexin (CNX) as an endoplasmic reticulum marker. Densitometry analyzes ERK or GSK-3 levels. (B) Ca2+ uptake in trypsin-treated mitochondria was assessed by Calcium Green-5N fluorescence. Because the probe does not permeate mitochondria, Ca2+ entering viable organelles is displayed by a rapid decrease of the fluorescence spike after administration to the cells of subsequent Ca2+ pulses (5 μM each). The final increase in fluorescence indicates pore opening. (C) FACS analysis [forward scatter (FSC) vs. TMRM] showing mitochondria depolarization in cells exposed to EM20-25 (EM) for 1 h with or without a 30-min preincubation with EIP. The percentage of viable cells (V, TMRM positive, in the quadrant) is reported. (D) PTP opening was measured as in B, but on whole cells. Treatment with ERK inhibitor peptide (EIP) (5 μM, in red) decreased the number of spikes before the permeability transition occurred, either in control conditions or when cells were incubated with EM20-25 (EM, 50 μM).
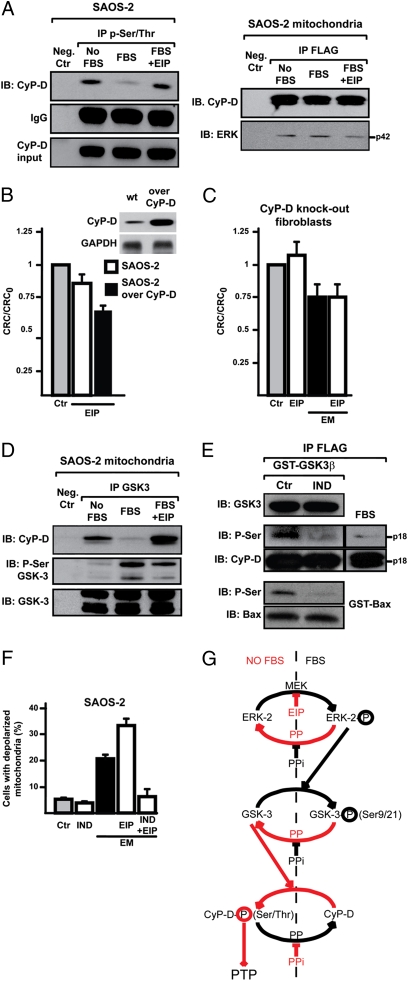
We then evaluated whether ERK could modulate the PTP regulator CyP-D. CyP-D was phosphorylated on Ser or Thr residues (Fig. 4A, Left), this phosphorylation was abolished by ERK activation (Fig. 4A, Left), and ERK directly interacted with CyP-D (Fig. 4A, Right). The slight PTP induction caused by ERK inhibition was enhanced after CyP-D overexpression in SAOS-2 cells (Fig. 4B), whereas EIP could not modulate the pore in fibroblasts derived from CyP-D knockout cells (20; Fig. 4C). Thus, ERK activity could impinge upon pore opening through the negative regulation of CyP-D phosphorylation, possibly by inhibiting an intermediate kinase. Inhibition of the kinase GSK-3 by Ser-phosphorylation was reported to desensitize PTP opening (13). We found that a fraction of GSK-3 is located in the mitochondrial matrix (Fig. 3A), corresponding to approximately 1% of the total cell enzyme. GSK-3 associated with CyP-D, and it was Ser-phosphorylated in an ERK-dependent fashion, favoring dissociation of the CyP-D/GSK-3 complex (Fig. 4D). An in silico analysis was performed with Scansite and NetPhosK databases, and residues S38, S39, and S123 were identified as possible GSK-3 targets on human CyP-D. A purified GSK-3 fusion protein was able to phosphorylate CyP-D, and this was inhibited by the GSK-3 inhibitor indirubin-3′-oxime (Fig. 4E), which also abolished both mitochondrial depolarization induced by EM20-25 per se, and the enhancing effect of EIP (Fig. 4F).
Fig. 4.
ERK activity modulates mitochondrial depolarization and CyP-D phosphorylation through GSK-3. (A) Immunoprecipitations (IP) on lysates of SAOS-2 cells starved overnight (No FBS) and then stimulated for 15 min with serum with or without a 30 min preincubation with EIP (50 μM; lanes FBS and FBS+EIP). Left: P-Ser/Thr were immunoprecipitated from total cell lysates; coimmunoprecipitation of CyP-D is shown, and IgG and input of CyP-D are reported as loading control. Right: the FLAG peptide was immunoprecipitated from mitochondria of SAOS-2 cells expressing FLAG-CyP-D. Immunoprecipitation of CyP-D and coimmunoprecipitation of ERK2 (p42) are shown. The negative control was a SAOS-2 lysate incubated with the nonconjugated resin (Left) or a lysate of SAOS-2 wild-type mitochondria incubated with the FLAG-conjugated resin (Right). (B and C) Ratio between CRC detected in the presence (CRC) and absence (CRC0) of EIP are reported for wild-type or CyP-D-overexpressing SAOS-2 cells (B), or for CyP-D knockout fibroblasts (C), which were also treated with EM20-25 (EM). Numbers are mean values of four experiments (±SD). (D) GSK-3 was immunoprecipitated from SAOS-2 mitochondria. Coimmunoprecipitation of CyP-D and Ser-phosphorylation of GSK-3 are shown. The negative control was as in A (Left). (E) In vitro CyP-D phosphorylation by recombinant GST-GSK-3β, without or with the GSK-3 inhibitor indirubin-3′-oxime (IND, 3 μM). The FLAG peptide was immunoprecipitated from lysates of SAOS-2 cells expressing FLAG-CyP-D. GST-Bax was used as a positive control for enzyme activity. Right: Ser phosphorylation was analyzed on the same quantity of FLAG-CyP-D immunoprecipitated from cells kept with serum. (F) Mitochondria depolarization of SAOS-2 cells exposed to 500 μM EM20-25 (EM) for 1 h with or without a 3-h preincubation with IND (3 μM) and/or a 30-min preincubation with EIP (50 μM). Numbers are mean values of percentages (±SD) of four FACS analyses. (G) Model of CyP-D regulation by ERK2/GSK-3. Red denotes the final sensitizing effect on the PTP (i.e., an increased probability of pore opening). PP, protein phosphatase; PPi, protein phosphatase inhibitor.
Discussion
Chronic activation of the Ras/ERK signaling pathway is frequently induced in neoplastic transformation (2, 3), where it correlates with worsening of tumor stage and grade and favors metastasis formation (1, 3, 4). In the present report we have provided evidence that a functional circuit connects the Ras/ERK transduction pathway and the mitochondrial PTP; and that in cancer cell models mitochondrial activation of ERK results in desensitization of pore opening and increased resistance to death stimuli, a finding that has major implications for tumorigenesis. We found that ERK is constitutively activated in mitochondria of cancer cells but not in immortalized, non-transformed prostate cells. Mitochondria-specific ERK activation might provide a key advantage to tumor cells during the oncogenic process, by placing the death/survival mitochondrial rheostat in an anti-apoptotic mode. Mitochondrial ERK inhibition was reported to cause ATP depletion and apoptosis (6). In other studies, although the subcellular localization of ERK was not assessed, its inhibition prompted block of ATP synthase and mitochondrial depolarization (21, 22). In this context, an important finding of this study is that an essential component of the mitochondrial death machinery, the PTP, is a downstream target of ERK. This is a further mechanistic clue into ERK survival signaling.
The PTP is a voltage- and Ca2+-dependent channel of unknown molecular nature, whose openings lead to mitochondrial depolarization, release of apoptogenic proteins, and cell death (11). Several observations link the PTP to tumor progression. The pore is desensitized by the product of the oncogene BCL-2 (23), by arylamines in the early phases of liver tumor promotion (24), in models of resistance to chemotherapeutics, in tumor-associated hypoxia, and in anoikis (11). Signal transduction through kinase/phosphatase pathways has been proposed to impinge upon PTP regulation. The stress-activated kinase JNK promotes inner membrane depolarization and release of apoptogenic proteins (25–27) and induces permeability transition in acetaminophen-induced liver injury (28). A protein phosphatase 2C family member inhibits PTP opening and is essential for cell survival, embryonic development, and cardiac function (29). ERK, together with the survival kinase Akt, is responsible for the cardioprotective phenomena of ischemic preconditioning, possibly leading to the eventual inhibition of the PTP through abrogation of GSK-3β activity (13, 30, 31). Release of hexokinase II from mitochondria, where it is involved in the aggressive phenotype of highly glycolytic tumors, is inhibited by the survival kinase Akt and favored by GSK-3β (32), and prompts a rapid permeability transition (20).
Our data are in line with these observations and provide a mechanistic connection between ERK activation and PTP regulation through GSK-3. Indeed, we demonstrate that in diverse tumor cell models resilience to undergo cell death is caused by ERK-dependent desensitization of the mitochondrial PTP. We postulate a model in which ERK activation inhibits GSK-3 activity, its association to CyP-D, and CyP-D phosphorylation, leading to PTP desensitization, whereas the Ser/Thr phosphorylation of CyP-D favors pore opening (Fig. 4G). Our trypsin-digestion experiments suggest that a small fraction of both ERK and GSK-3 is constitutively found in a protease-protected mitochondrial compartment, which we assume to be the matrix space or a domain associated to the inner membrane. ERK and GSK-3 do not possess recognizable mitochondria targeting sequences, and the amount of the enzymes located in internal mitochondrial compartments is a minor fraction of the total enzyme content in the cell. How this fraction of ERK and GSK-3 enters mitochondria is an open question that has not been addressed here or in previous reports (6–10, 13). An alternative model that would also be consistent with our data is that a pool of ERK and GSK-3 associates to the outer mitochondrial membrane and regulates PTP opening through one or more as yet unknown intermediate step(s), including the phosphorylation of CyP-D by another kinase. Further work is needed to clarify this issue, even if our data favor the first model because the mitochondrial pool of the kinases is resistant to concentrations of trypsin that digest outer-membrane proteins. A matrix location is also corroborated by the finding that CyP-D, which is a matrix protein, co-immunoprecipitates with both ERK2 and GSK-3. Notably, CyP-D is Ser/Thr phosphorylated, and this phosphorylation correlates with CyP-D binding to GSK-3. In accord with a pivotal role played by the GSK-3/CyP-D complex in PTP regulation downstream to ERK, in silico analysis reveals putative GSK-3 phosphorylation sites on CyP-D. More importantly, GSK-3 can phosphorylate CyP-D in vitro, and GSK-3 pharmacologic inhibition abolishes pore opening, even after ERK inhibition. Furthermore, ERK blockade prompts a higher mitochondrial depolarization in cells overexpressing CyP-D than in their wild-type counterparts, whereas it is ineffective in CyP-D knockout cells. ERK-mediated inhibition of GSK-3 activity is a well-characterized event (33), also in tumor models (34). ERK acts as a priming kinase on GSK-3, therefore requiring the activity of a second kinase for complete GSK-3 inhibition; and GSK-3 itself needs a priming kinase to phosphorylate its target (35). Moreover, these reactions must be finely tuned by the action of phosphatases. Thus, a network of phosphorylation events could control CyP-D activity and the ensuing pore regulation.
ERK plays a major role in complex survival responses leading to carcinogenesis by orchestrating transient signals and transcription modulation in different subcellular locations. Our data indicate that ERK-dependent desensitization of the PTP in mitochondria requires modulation of GSK-3 and possibly of other molecules. This seems to be an important regulatory hotspot in survival signaling networks, offering a selective target for antitumor agents that may restore the death threshold in tumor cells.
Experimental Procedures
Chemicals and Reagents.
FITC-conjugated annexin-V was from Boehringer Mannheim; Calcium Green-5N, MitoTracker Red, and tetramethylrhodamine methyl ester (TMRM) were from Molecular Probes; EM20-25 (Bcl-2 inhibitor III), PD98059, indirubin-3′-oxime, and the ERK activation inhibitor peptide I (Ste-MPKKKPTPIQLNP-NH2) were from Calbiochem; all other chemicals were from Sigma. Arachidonic acid was from Alexis Biochemicals. The mouse monoclonal anti-actin antibody was from Sigma; the mouse monoclonal anti-GAPDH was from Millipore; the mouse monoclonal anti-K-Ras and anti–GSK-3α/β, the goat polyclonal anti-calnexin, and the rabbit polyclonal anti-ERK, anti–poly(ADP-ribose)polymerase, and anti-TOM20 antibodies were from Santa Cruz Biotechnology; the rabbit monoclonal anti–Bcl-XL and the rabbit polyclonal anti–phospho-ERK and anti–phospho Ser21/9 GSK-3 α/β antibodies were from Cell Signaling; the mouse monoclonal anti–phospho-Ser/Thr antibody was from Qiagen; the mouse monoclonal anti-CyP-D was from Calbiochem; the mouse monoclonal anti–cytochrome oxidase subunit IV antibody was from MitoSciences. FITC-conjugated secondary antibodies were from Sigma. The full-length human recombinant GST-GSK-3β was from Enzo Life Sciences; GST-Bax was a generous gift of Mario Zoratti (University of Padua, Padua, Italy). Cell death inducers were added to exponentially growing cells in HBSS buffer (Sigma) supplemented with calcium chloride, magnesium sulfate, and 10 mM Hepes. Each experiment was repeated at least four times. The CyP-D cDNA was cloned in a pcDNA3 vector (Invitrogen) with a FLAG tag added at its 3′ end and used to stably transfect SAOS-2 cells.
Cell Lysis, Fractionation, Kinase Assay, and Western Immunoblot Analysis.
Total cell extracts were prepared at 4°C in 140 mM NaCl, 20 mM Tris∣HCl (pH 7.4), 5 mM EDTA, 10% glycerol, and 1% Triton X-100 in the presence of phosphatase and protease inhibitors (Sigma). To prepare mitochondrial extracts, cells were placed in an isolation buffer (250 mM sucrose, 10 mM Tris·HCl, 10 mM EGTA-Tris, pH 7.4) and homogenized at 4°C. Mitochondria were then isolated by differential centrifugation (3 times, first at 700 × g and twice at 7,000 × g, all at 4°C, 10 min) in mitochondrial isolation buffer. For the trypsin assay, mitochondria were treated with trypsin at the reported concentrations at 4°C for 1h. Where indicated, 0.1% SDS was added before trypsin. After inactivating trypsin, mitochondria were spun (18,000 × g, 10 min, 4°C) and loaded on an SDS/PAGE or used for CRC assays. Immunoprecipitations were performed according to standard procedures on 3 mg of extracted proteins per reaction. Anti-FLAG immunoprecipitations were made using an anti-FLAG M2 Affinity Gel (Sigma). The kinase assay was performed by incubating FLAG-CyP-D obtained from 107 cells with GST-GSK-3β (200 ng) for 30 min at 30°C in a buffer containing 25 mM Tris·HCl (pH 7.5), 5 mM β-glycerophosphate, 2 mM DTT, 0.1 mM Na3VO4, and 10 mM MgCl2, supplemented with 50 μM ATP. Indirubin-3′-oxime was preincubated 30 min with the enzyme.
Western immunoblots were carried out under standard conditions, and proteins were visualized by enhanced chemiluminescence (Millipore). Densitometric analysis was performed with Quantity One software (Bio-Rad Laboratories).
Flow Cytometry Analysis of Apoptosis Induction.
Flow cytometry recordings of apoptotic changes were performed as described previously (36, 37) to detect mitochondrial depolarization (reduced TMRM staining), phosphatidylserine exposure on the cell surface (increased FITC-conjugated annexin-V staining), and loss of plasma membrane integrity (propidium iodide staining). Samples were analyzed on a FACSCalibur flow cytometer (Becton Dickinson). Data acquisition was performed using CellQuest software (Becton Dickinson) and data analysis with WinMDI free software.
Measurement of Mitochondrial Ca2+ Retention Capacity.
The CRC assay was used as described to assess PTP opening in whole cells after trains of Ca2+ pulses (20, 38). Briefly, cells were washed in an isotonic buffer (130 mM KCl, 1 mM Pi-Tris, 10 mM Tris/Mops, and 0.1 mM EGTA/Tris, pH 7.4), and then permeabilized with 150 μM digitonin (15 min, 4°C), increasing EGTA to 1 mM. Digitonin was then eliminated and the number of cells carefully assessed before starting each experiment. Permeabilized cells were placed in low (10 μM) EGTA in the presence of 2 μM rotenone/5 mM succinate, 10 μM cytochrome c, and of the Ca2+ indicator Calcium Green-5N, which does not permeate mitochondria. Cells were then exposed to Ca2+ spikes, and fluorescence drops were used to assess mitochondrial Ca2+ uptake. PTP opening was detected as a fluorescence increase.
Supplementary Material
Acknowledgments
We thank Davide Bartoli and Mattia Renzani for technical assistance; and our system operators Otello Piovan and Cristiano Cebba for inexhaustible support. This work was made possible by grants from Progetti di Ricerca di Interesse Nazionale del Ministero dell'Università e della Ricerca (to P.B.), from Associazione Italiana Ricerca sul Cancro (P.B.), and from Progetti di Ateneo dell'Università di Padova (A.R.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912742107/DCSupplemental.
References
- 1.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 2.Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer. 2007;7:295–308. doi: 10.1038/nrc2109. [DOI] [PubMed] [Google Scholar]
- 3.McCubrey JA, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gioeli D. Signal transduction in prostate cancer progression. Clin Sci (Lond) 2005;108:293–308. doi: 10.1042/CS20040329. [DOI] [PubMed] [Google Scholar]
- 5.Dhanasekaran DN, Johnson GL. MAPKs: Function, regulation, role in cancer and therapeutic targeting. Oncogene. 2007;26:3097–3099. doi: 10.1038/sj.onc.1210395. [DOI] [PubMed] [Google Scholar]
- 6.Monick MM, et al. Constitutive ERK MAPK activity regulates macrophage ATP production and mitochondrial integrity. J Immunol. 2008;180:7485–7496. doi: 10.4049/jimmunol.180.11.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alonso M, et al. Mitochondrial extracellular signal-regulated kinases 1/2 (ERK1/2) are modulated during brain development. J Neurochem. 2004;89:248–256. doi: 10.1111/j.1471-4159.2004.02323.x. [DOI] [PubMed] [Google Scholar]
- 8.Chu CT, Levinthal DJ, Kulich SM, Chalovich EM, DeFranco DB. Oxidative neuronal injury. The dark side of ERK1/2. Eur J Biochem. 2004;271:2060–2066. doi: 10.1111/j.1432-1033.2004.04132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poderoso C, et al. A mitochondrial kinase complex is essential to mediate an ERK1/2-dependent phosphorylation of a key regulatory protein in steroid biosynthesis. PLoS One. 2008;3:e1443. doi: 10.1371/journal.pone.0001443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu JH, Guo F, Shelburne J, Watkins S, Chu CT. Localization of phosphorylated ERK/MAP kinases to mitochondria and autophagosomes in Lewy body diseases. Brain Pathol. 2003;13:473–481. doi: 10.1111/j.1750-3639.2003.tb00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasola A, Bernardi P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis. 2007;12:815–833. doi: 10.1007/s10495-007-0723-y. [DOI] [PubMed] [Google Scholar]
- 12.Bernardi P, et al. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006;273:2077–2099. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- 13.Juhaszova M, et al. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bello D, Webber MM, Kleinman HK, Wartinger DD, Rhim JS. Androgen responsive adult human prostatic epithelial cell lines immortalized by human papillomavirus 18. Carcinogenesis. 1997;18:1215–1223. doi: 10.1093/carcin/18.6.1215. [DOI] [PubMed] [Google Scholar]
- 15.Webber MM, Bello D, Kleinman HK, Hoffman MP. Acinar differentiation by non-malignant immortalized human prostatic epithelial cells and its loss by malignant cells. Carcinogenesis. 1997;18:1225–1231. doi: 10.1093/carcin/18.6.1225. [DOI] [PubMed] [Google Scholar]
- 16.Penzo D, et al. Arachidonic acid released by phospholipase A(2) activation triggers Ca(2+)-dependent apoptosis through the mitochondrial pathway. J Biol Chem. 2004;279:25219–25225. doi: 10.1074/jbc.M310381200. [DOI] [PubMed] [Google Scholar]
- 17.Petronilli V, Penzo D, Scorrano L, Bernardi P, Di Lisa F. The mitochondrial permeability transition, release of cytochrome c and cell death. Correlation with the duration of pore openings in situ. J Biol Chem. 2001;276:12030–12034. doi: 10.1074/jbc.M010604200. [DOI] [PubMed] [Google Scholar]
- 18.Wang JL, et al. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci USA. 2000;97:7124–7129. doi: 10.1073/pnas.97.13.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milanesi E, et al. The mitochondrial effects of small organic ligands of BCL-2: Sensitization of BCL-2-overexpressing cells to apoptosis by a pyrimidine-2,4,6-trione derivative. J Biol Chem. 2006;281:10066–10072. doi: 10.1074/jbc.M513708200. [DOI] [PubMed] [Google Scholar]
- 20.Chiara F, et al. Hexokinase II detachment from mitochondria triggers apoptosis through the permeability transition pore independent of voltage-dependent anion channels. PLoS ONE. 2008;3:e1852. doi: 10.1371/journal.pone.0001852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HJ, et al. Mitogen-activated protein kinase/extracellular signal-regulated kinase attenuates 3-hydroxykynurenine-induced neuronal cell death. J Neurochem. 2004;88:647–656. doi: 10.1111/j.1471-4159.2004.02191.x. [DOI] [PubMed] [Google Scholar]
- 22.Yung HW, Wyttenbach A, Tolkovsky AM. Aggravation of necrotic death of glucose-deprived cells by the MEK1 inhibitors U0126 and PD184161 through depletion of ATP. Biochem Pharmacol. 2004;68:351–360. doi: 10.1016/j.bcp.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 23.Susin SA, et al. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J Exp Med. 1996;184:1331–1341. doi: 10.1084/jem.184.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klöhn PC, et al. Early resistance to cell death and to onset of the mitochondrial permeability transition during hepatocarcinogenesis with 2-acetylaminofluorene. Proc Natl Acad Sci USA. 2003;100:10014–10019. doi: 10.1073/pnas.1633614100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aoki H, et al. Direct activation of mitochondrial apoptosis machinery by c-Jun N-terminal kinase in adult cardiac myocytes. J Biol Chem. 2002;277:10244–10250. doi: 10.1074/jbc.M112355200. [DOI] [PubMed] [Google Scholar]
- 26.Chauhan D, et al. JNK-dependent release of mitochondrial protein, Smac, during apoptosis in multiple myeloma (MM) cells. J Biol Chem. 2003;278:17593–17596. doi: 10.1074/jbc.C300076200. [DOI] [PubMed] [Google Scholar]
- 27.Kharbanda S, et al. Translocation of SAPK/JNK to mitochondria and interaction with Bcl-x(L) in response to DNA damage. J Biol Chem. 2000;275:322–327. doi: 10.1074/jbc.275.1.322. [DOI] [PubMed] [Google Scholar]
- 28.Hanawa N, et al. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu G, et al. A novel mitochondrial matrix serine/threonine protein phosphatase regulates the mitochondria permeability transition pore and is essential for cellular survival and development. Genes Dev. 2007;21:784–796. doi: 10.1101/gad.1499107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishihara M, et al. Modulation of the mitochondrial permeability transition pore complex in GSK-3beta-mediated myocardial protection. J Mol Cell Cardiol. 2007;43:564–570. doi: 10.1016/j.yjmcc.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Hausenloy DJ, Tsang A, Yellon DM. The reperfusion injury salvage kinase pathway: A common target for both ischemic preconditioning and postconditioning. Trends Cardiovasc Med. 2005;15:69–75. doi: 10.1016/j.tcm.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Robey RB, Hay N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene. 2006;25:4683–4696. doi: 10.1038/sj.onc.1209595. [DOI] [PubMed] [Google Scholar]
- 33.Ding Q, et al. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol Cell. 2005;19:159–170. doi: 10.1016/j.molcel.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Bergami P, et al. Rewired ERK-JNK signaling pathways in melanoma. Cancer Cell. 2007;11:447–460. doi: 10.1016/j.ccr.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Rasola A, Geuna M. A flow cytometry assay simultaneously detects independent apoptotic parameters. Cytometry. 2001;45:151–157. doi: 10.1002/1097-0320(20011001)45:2<151::aid-cyto1157>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 37.Gramaglia D, et al. Apoptosis to necrosis switching downstream of apoptosome formation requires inhibition of both glycolysis and oxidative phosphorylation in a BCL-X(L)- and PKB/AKT-independent fashion. Cell Death Differ. 2004;11:342–353. doi: 10.1038/sj.cdd.4401326. [DOI] [PubMed] [Google Scholar]
- 38.Fontaine E, Eriksson O, Ichas F, Bernardi P. Regulation of the permeability transition pore in skeletal muscle mitochondria. Modulation by electron flow through the respiratory chain complex J. Biol Chem. 1998;273:12662–12668. doi: 10.1074/jbc.273.20.12662. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.