Abstract
Corticotropin-releasing factor (CRF), originally characterized as the principal neuroregulator of the hypothalamus-pituitary-adrenal axis, has broad central and peripheral distribution and actions. We demonstrate the presence of CRF receptor type 1 (CRFR1) on primary β cells and show that activation of pancreatic CRFR1 promotes insulin secretion, thus contributing to the restoration of normoglycemic equilibrium. Stimulation of pancreatic CRFR1 initiates a cAMP response that promotes insulin secretion in vitro and in vivo and leads to the phosphorylation of cAMP response element binding and the induction of the expression of several immediate-early genes. Thus, the insulinotropic actions of pancreatic CRFR1 oppose the activation of CRFR1 on anterior pituitary corticotropes, leading to the release of glucocorticoids that functionally antagonize the actions of insulin. Stimulation of the MIN6 insulinoma line and primary rat islets with CRF also activates the MAPK signaling cascade leading to rapid phosphorylation of Erk1/2 in response to CRFR1-selective ligands, which induce proliferation in primary rat neonatal β cells. Importantly, CRFR1 stimulates insulin secretion only during conditions of intermediate to high ambient glucose, and the CRFR1-dependent phosphorylation of Erk1/2 is greater with elevated glucose concentrations. This response is reminiscent of the actions of the incretins, which potentiate insulin secretion only during elevated glucose conditions. The presence of CRFR1 on β cells adds another layer of complexity to the intricate network of paracrine and autocrine factors and their cognate receptors whose coordinated efforts can dictate islet hormone output and regulate β cell proliferation.
Keywords: diabetes, pancreas, corticotropin-releasing factor, hypothalamus-pituitary-adrenal axis , glucose homeostasis
Corticotropin-releasing factor receptor type 1 (CRFR1) and type 2 (CRFR2) are closely related class B G protein-coupled receptors (GCPRs) that relay signals from corticotropin-releasing factor (CRF) and its paralogues urocortin 1 (Ucn 1), Ucn 2, and Ucn 3 (1). CRF preferentially activates CRFR1, Ucn 1 can activate both CRF receptors, and Ucn 2 and Ucn 3 are selective CRFR2 agonists (2). CRFR1 is expressed in anterior pituitary corticotropes and induces the release of adrenocorticotropic hormone in response to hypothalamic CRF during challenge (3). This release leads to the downstream secretion of glucocorticoids from the adrenal cortex, which has widespread effects on most cells and tissues, including the stimulation of liver gluconeogenesis to increase circulating glucose levels (4–6). In addition to the indirect effects of CRFR1 on glucose homeostasis that are mediated through the hypothalamus-pituitary-adrenal (HPA) axis, there have been indications that CRF can affect the endocrine pancreas directly. Treatment of dissociated rat islet cells with CRF stimulates Ca2+ flux and intracellular Ca2+ content (7, 8), increasing insulin secretion in rodent islets (9, 10). We previously demonstrated that Ucn 3 is expressed in β cells and is released in a glucose-dependent manner (9). Inhibition of endogenous Ucn 3 suppresses glucose-stimulated insulin secretion (GSIS) (11), suggesting the presence of local CRFR2 receptors that may relay the CRF signal. Key questions remain regarding CRF-mediated effects on the endocrine pancreas, including the CRF receptors involved and identification of the pancreatic cell type in which they are expressed. In this study we present data demonstrating that β cells express CRFR1 and respond directly to CRF. CRFR1 activation potentiates GSIS in vitro and in vivo. In addition, CRF causes cAMP response element binding (CREB) phosphorylation and the glucose-dependent activation of MAPK, leading to rapid transcriptional responses and the proliferation of primary rat neonatal β cells.
Results
CRFR1 Is Expressed on β Cells of the Mouse Pancreas.
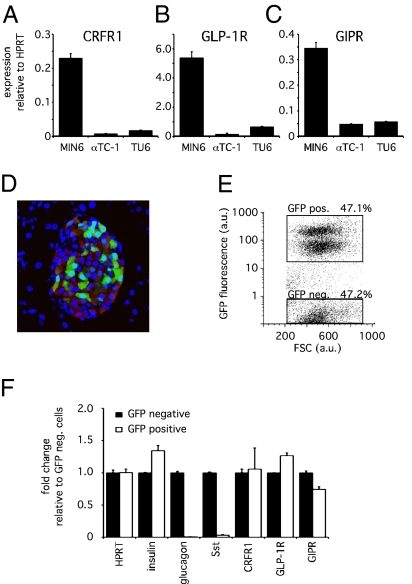
To identify the cell type within the islet that expresses CRFR1, we compared the expression profile of CRFR1 in MIN6 cells, αTC1 cells, and TU6 cells representing the β, α, and δ lineages, respectively. Although CRFR1 transcript was detectable in total RNA isolated from each of these cell lines, levels of CRFR1 transcript were over 10-fold more abundant in the MIN6 β cell line than in the α and δ cell lines (Fig. 1A). This expression pattern mimicked that of the incretin receptors glucagon-like peptide-1 receptor (GLP-1R) and glucose-dependent insulinotropic peptide receptor (GIPR), which are expressed on β cells and belong to the class B family of GPCRs along with CRFR1 (Fig. 1 B and C). To establish unequivocally the presence of CRFR1 in primary β cells, we employed a transgenic reporter mouse that expresses GFP under control of the insulin promoter (mIP-GFP) (12). The expression of the GFP reporter gene is restricted to β cells, although only ≈50% of all insulin-positive cells coexpress GFP (Fig. 1D) (13). The expression profile of the GFP-positive cells following FACS sorting (Fig. 1E) revealed the expression of CRFR1 transcript in the GFP-expressing population in addition to established β cell markers such as insulin, GLP-1R, and GIPR (Fig. 1F). Importantly, the expression of glucagon and somatostatin (Sst) is restricted to the GFP-negative population, indicating that the GFP-positive population does not contain significant amounts of α and δ cells and confirming that primary β cells are the source of CRFR1 transcript.
Fig. 1.
CRFR1 is expressed on insulinoma cells and on primary mouse β cells. CRFR1 expression levels are relatively abundant in the mouse insulinoma cell line MIN6 compared with the α cell line αTC-1 or the δ cell line TU6 (A). Similarly, GLP-1R (B) and GIPR (C) are expressed abundantly in the MIN6 insulinoma cells. Using dissociated primary islets from an mIP-GFP transgenic reporter mouse (D), we obtained primary β cells by FACS separation of GFP-positive and GFP-negative cells (E). The expression profile of the GFP-positive population demonstrates the retention of expression of β cell markers such as insulin, GLP-1R, and GIPR, whereas glucagon and Sst are not expressed (F). CRFR1 expression in GFP-positive cells demonstrates β cell localization of CRFR1. GFP and insulin are stained green and red, respectively, in (D); DAPI (blue) is applied as a nuclear counterstain.
Activation of CRFR1 Promotes Intracellular cAMP and Potentiates GSIS.
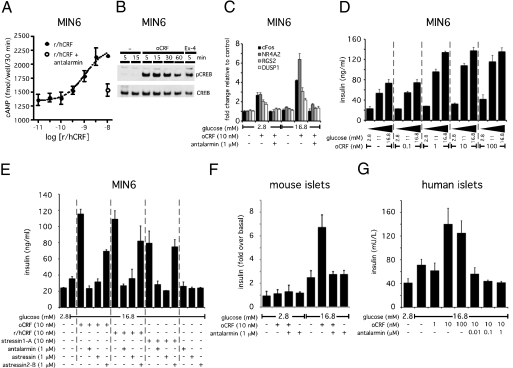
Stimulation of the mouse insulinoma line MIN6 with increasing doses of the CRFR1 agonist rat/human CRF (r/hCRF) reveals a dose-dependent accumulation of intracellular cAMP (Fig. 2A). Coadministration of the CRFR1-selective antagonist antalarmin abrogated the r/hCRF-induced accumulation of cAMP, indicating that this response is mediated by CRFR1. The elevation of cAMP by the selective CRFR1 agonist ovine CRF (oCRF) in β cells leads to the phosphorylation of CREB (Fig. 2B), enabling its nuclear translocation to initiate downstream transcriptional responses (Fig. 2C). Because the generation of cAMP in β cells can potentiate Ca2+-mediated GSIS, we tested whether the CRFR1-dependent induction of cAMP would enhance insulin secretion. Stimulation with 1 nM oCRF maximized insulin release, provided ambient glucose levels were intermediate (11 mM) to high (16.8 mM) (Fig. 2D). The potentiating effect of CRF on GSIS is prevented by coadministration of the CRFR1-selective antagonist antalarmin or the general CRFR antagonist astressin, whereas the CRFR2-selective antagonist astressin2-B only partially suppressed the actions of oCRF (Fig. 2E). Similarly, the insulin release by MIN6 cells induced by r/hCRF or the synthetic CRFR1-selective peptide agonist stressin1-A is blocked by coadministration of antalarmin or astressin but not by astressin2-B. None of these antagonists alone affected GSIS. Stimulation of primary mouse islets with oCRF also potentiated glucose-induced, but not basal, insulin secretion (Fig. 2F), whereas 10 nM oCRF augmented GSIS from human islets (Fig. 2G). Furthermore, the additive effect of oCRF on GSIS from mouse (Fig. 2F) and human (Fig. 2G) islets was abrogated by coadministration of antalarmin, corroborating that the effects of oCRF on insulin secretion from mouse and human islets are mediated via CRFR1.
Fig. 2.
CRFR1 activation induces a cAMP response that augments GSIS in MIN6 insulinoma cells and primary rodent and human islets. CRF dose-dependently increases intracellular cAMP levels in MIN6 cells; this increase is blocked by the simultaneous administration of the CRFR1-selective antagonist antalarmin (A). Stimulation of MIN6 cells with the CRFR1-selective agonist oCRF rapidly induces robust phosphorylation of CREB (B) and leads to the stimulation of the expression of immediate-early genes including cFos, nuclear receptor subfamily 4 group A member 2 (NR4A2), regulator of G protein signaling 2 (RGS2), and dual-specificity phosphatase 1 (DUSP1) (C). These transcriptional changes are enhanced by high ambient glucose concentrations and can be blocked by the CRFR1 antagonist antalarmin. Stimulation of MIN6 cells with a relatively low dose of oCRF (1 nM) suffices to augment insulin secretion in the presence of intermediate (11 mM) or high (16.8 mM) glucose concentrations (D). The augmentation of GSIS induced by selective or preferential CRFR1 agonists (oCRF, r/hCRF, stressin1-A) is completely inhibited by general (astressin) or CRFR1-selective (antalarmin) antagonists but not by the CRFR2-selective antagonist astressin2-B (E). Stimulation with oCRF potentiates GSIS in mouse (F) and human (G) primary islets in a CRFR1-dependent manner, because coadministration of antalarmin complete blocks the effects of oCRF.
Stimulation of CRFR1 Phosphorylates ERK1/2 in Synergism with Glucose.
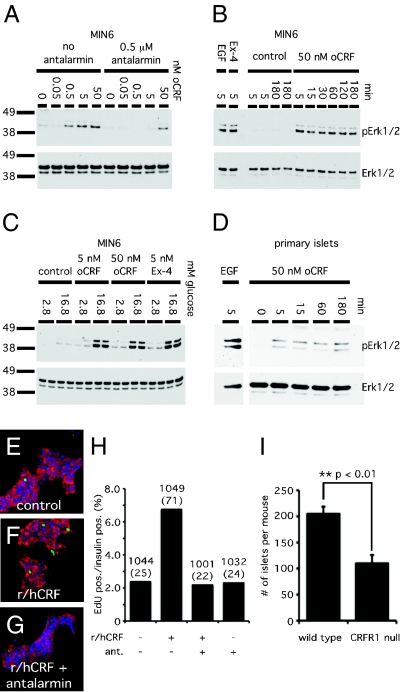
Because the MAPK signaling pathway can be activated downstream of CRFRs (14) and is implicated in β cell proliferation (15), we tested whether activation of CRFR1 on β cells leads to MAPK signaling. Stimulation of MIN6 cells with the CRFR1-selective agonist oCRF resulted in a dose-dependent phosphorylation of Erk1/2, which was inhibited by pretreatment with a 100-fold molar excess of antalarmin (Fig. 3A). The oCRF-induced phosphorylation of Erk1/2 in MIN6 cells persists for at least 3 h poststimulation at levels comparable to the Erk1/2 phosphorylation induced by exendin-4 or epidermal growth factor (EGF) (Fig. 3B). The oCRF- or exendin-4–induced phosphorylation of Erk1/2 is greater at 16.8 mM glucose, indicating that the activation of MAPK downstream of class B GPCRs is dependent on high ambient glucose levels (Fig. 3C). Finally, stimulation of intact primary rat islets in vitro with oCRF induces Erk1/2 phosphorylation that persists for several hours, suggesting that primary β cells respond to CRFR1-selective agonists with lasting activation of the MAPK pathway (Fig. 3D).
Fig. 3.
Activation of CRFR1 activates the MAPK signaling cascade and induces proliferation in rat neonatal β cells. Stimulation of CRFR1 dose-dependently increases phosphorylation of Erk1/2, an increase that is inhibited by coadministration of antalarmin (A). The induction of pErk1/2 is rapid (5 min) and persists for at least 3 h (B). oCRF and exendin-4 synergize with glucose (C). Isolated primary rat islets respond to the CRFR1-selective agonist oCRF with increased levels of pErk1/2 (D). Stimulation of rat neonatal islet cells with r/hCRF increased the nuclear incorporation of EdU into insulin-positive cells; this incorporation was blocked by antalarmin (E–G). Proliferation was quantified as the fraction of EdU insulin-positive cells (H). Numbers indicate the total number of insulin-positive cells; the number of insulin-positive EdU cells is given in parentheses. The total number of islets isolated from CRFR1-null pancreata was markedly lower than in control animals (I).
Activation of CRFR1 Promotes Proliferation of Primary Rat Neonatal β Cells.
To test whether the activation of CRFR1 induces proliferation of primary β cells, we measured the incorporation of 5-ethynyl-2′-deoxyuridine (EdU) in dissociated rat neonatal β cells in vitro. Stimulation of rat neonatal islet cells with CRF increased the fraction of insulin-containing EdU-positive cells to 6.7%, compared with less than 2.5% of control cells (Fig. 3 E–H). The proliferative actions of CRF are blocked by coadministration of antalarmin, confirming CRFR1 dependence. In keeping with the proliferative actions of CRF in vitro, the total number of islets isolated from mice null for CRFR1 (16) is approximately half that isolated from littermate controls (Fig. 3I), whereas overall islet architecture and expression profile remain largely unaltered, with the exception of a possible modest increase in α cell number and glucagon expression (Fig. S1).
Stimulation of Pancreatic CRFR1 Leads to Enhanced Insulin Secretion and Improved Glucose Tolerance in Vivo.
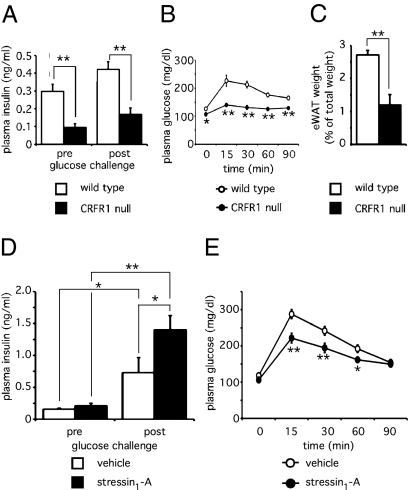
To address the potential of CRFR1 to augment GSIS in vivo, we examined CRFR1-null mice that were maintained on a replacement dose of glucocorticoids to mitigate the adrenal phenotype of this line (16). Fasting plasma insulin levels in CRFR1-null mice are significantly lower than in age- and gender-matched wild-type animals, a difference that persists 5 min following an i.p. glucose challenge (Fig. 4A). Despite lower fasting- and glucose-induced plasma insulin concentrations, CRFR1-null mice have slightly but significantly lower fasting plasma glucose levels and demonstrate markedly higher glucose tolerance when compared with age-matched controls in a glucose tolerance test (Fig. 4B). Furthermore, CRFR1-null animals display reduced adiposity as measured by the reduced fraction of body weight composed of epidydimal white adipose tissue (eWAT) (Fig. 4C). The paradoxically high glucose tolerance and reduced adiposity in the CRFR1-null mice likely result from the lack of a functional HPA axis and present significant confounds that limit the utility of CRFR1-null mice as a suitable model to study the role of pancreatic CRFR1 (see Discussion). Using a different approach, we administered the CRFR1-selective peptide agonist stressin1-A or vehicle to adrenalectomized mice (preventing acute activation of the HPA axis) to test whether activation of pancreatic CRFR1 would enhance GSIS. Stressin1-A had no effect on fasting insulin levels, and both groups of animals demonstrated significant increases in plasma insulin in response to glucose challenge. Importantly, the group treated with stressin1-A displayed significantly higher GSIS in vivo than the vehicle-treated controls (Fig. 4D). Furthermore, adrenalectomized animals that received a single dose of stressin1-A demonstrated improved glucose tolerance compared with vehicle-treated controls (Fig. 4E). This effect probably is secondary to CRFR1-dependent augmentation of GSIS, which expedites clearance of plasma glucose by insulin-sensitive tissues such as liver and skeletal muscle.
Fig. 4.
Acute stimulation of CRFR1 in vivo augments GSIS leading to enhanced glucose tolerance. Fasted plasma insulin levels in CRFR1-null males receiving a replacement dose of glucocorticoids to mitigate HPA deficiency were significantly lower than in age-matched wild-type males (A). Despite reduced fasting and glucose-induced insulin levels, glucose tolerance paradoxically was superior in CRFR1-null mice than in wild-type controls (B). CRFR1-null mice have reduced relative eWAT weight (C). Circulating insulin levels in fasted adrenalectomized wild-type animals were unaffected by prior stressin1-A administration (0.5 mg/kg i.p.) as compared with controls (D). Although glucose challenge (2 g/kg i.p.) significantly elevated circulating insulin in both groups, preadministration of stressin1-A significantly elevated GSIS (D). Stressin1-A (2.5 mg/kg, i.p.) administered 15 min before a glucose tolerance test significantly improved glucose tolerance as compared with vehicle-treated controls (E). *, P < 0.05; **, P < 0.01 vs. wild-type or vehicle-treated controls.
Discussion
Several studies have documented actions of CRF on primary mouse islets or dissociated islet cells, suggesting the presence of local CRF receptors (7–10). However, the islet cell type that responds directly to CRF and the source of the endogenous ligand acting on CRFR1 are unclear. CRF immunoreactivity has been reported in α cells of the murine islet (17), although it has not been confirmed whether this staining represents chemically authentic CRF. Alternatively, the endogenous ligand for CRFR1 may reach the mouse islet via the circulation or through innervation, as has been reported for other neuropeptides including vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating peptide (18). It is possible that the actions of CRF are mediated by CRFR2, which CRF activates with lower potency (19). The presence of CRFR2 in islets is inferred from the insulinotropic actions of the CRFR2-selective peptide Ucn 3. This peptide is secreted from β cells and is required for full glucose- and exendin 4–induced insulin secretion (9, 11). We detected CRFR1 transcript in primary mouse β cells and demonstrated that CRF-induced insulin secretion, phosphorylation of MAPK and CREB, transcriptional changes, and β cell proliferation are fully blocked by coadministration of the CRFR1-selective antagonist antalarmin, corroborating independence from CRFR2.
CRF-induced insulin secretion is evident only at high ambient glucose concentrations that mimic postprandial levels and amplify the CRF-induced phosphorylation of Erk1/2. This response is reminiscent of the glucose-dependent actions of the incretins glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP). Defining characteristics of incretins are the potentiation of GSIS and a relative inability to induce insulin secretion under low-glucose conditions. In vivo pretreatment of fasted mice with stressin1-A has no effect on basal insulin levels but potentiates insulin secretion following a subsequent glucose challenge. Of note, the incretin receptors GLP-1R and GIPR belong to the class B subfamily of GPCRs that includes CRFR1 and activate common downstream signaling cascades. The effects of CRFR1 and incretin receptors are not restricted to the release of cAMP, which is responsible for the insulinotropic effects that follow the activation of class B GPCRs expressed on islet cells, but extend to the activation of additional signaling cascades including MAPK that can promote β cell mass by proliferation and islet neogenesis and reduce apoptosis of β cells (20–22). CRFR1-mediated Erk activity resembles the GLP-1R–mediated phosphorylation of Erk by exendin-4, in that both can persist for at least several hours following the application of the peptide (23). This observation, along with the demonstration that CRFR1 activation stimulates proliferation of primary rat neonatal β cells, raises the possibility that CRFR1 could be a therapeutic target to promote the intrinsic capability of mature β cells to proliferate slowly (24, 25) and regenerate β cell mass under conditions characterized by progressive β cell loss such as type 1 diabetes. The insulinotropic actions of CRF mimic similar effects by GLP-1 and exendin-4, which have led to the development of successful therapeutic treatments to improve insulin secretion and alleviate insulin resistance in type 2 diabetic patients. The utility of CRFR1 agonists for therapeutic intervention in either type 1 or type 2 diabetes requires the development of strategies to activate pancreatic CRFR1 receptors selectively without activating CRFR1 on anterior pituitary corticotropes, which would result in release of glucocorticoids. Activation of CRFR1 could have opposing effects on plasma glucose concentrations depending on the site of activation: CRFR1 in the anterior pituitary releases glucocorticoids, which act as functional antagonists to insulin by elevating plasma glucose levels. In contrast, activation of pancreatic CRFR1 would, under high glucose levels, potentiate insulin secretion, resulting in the lowering of plasma glucose.
The complex interactions between the HPA axis and the endocrine pancreas are exemplified by the phenotype of the CRFR1-null mice. Based on the insulinotropic actions of CRFR1 agonists in vitro, we expected reduced glucose tolerance secondary to impaired insulin secretion in the absence of pancreatic CRFR1. Instead, CRFR1-null mice maintain slightly but significantly lower fasting plasma glucose levels and demonstrate markedly higher glucose tolerance as compared with age-matched controls. The paradoxically high glucose tolerance could be explained by the lack of a functional HPA axis because of the absence of pituitary CRFR1. These animals have low circulating glucocorticoid levels, which correlate with low plasma insulin concentrations (26–28). We hypothesize that in the absence of glucocorticoids, well known to antagonize the actions of insulin, CRFR1-null mice maintain higher insulin sensitivity, enabling enhanced glucose tolerance despite lower fasting and glucose-stimulated insulin levels. Additionally, the reduced eWAT weight in CRFR1-null animals is in agreement with studies correlating reduced glucocorticoids with diminished adiposity. Conversely, persistently elevated concentrations of glucocorticoids, such as those associated with Cushing’s disease, chronic stress, or metabolic syndrome, are associated with increases in adipose reservoirs (4, 29–31). The lack of a functional HPA axis with normal circadian pulsatility has created an animal in which insulin sensitivity, glucose clearance, and peripheral energy reservoirs are profoundly and permanently perturbed. Consequently, the altered overall metabolic landscape of the CRFR1-null mice prohibits their use in the study of specific contributions of pancreatic CRFR1 in vivo.
In summary, we demonstrated that CRFR1 is expressed on pancreatic β cells and potentiates GSIS in vitro and in vivo. Activation of CRFR1 initiates MAPK signaling and leads to proliferation of rat neonatal β cells. CRFR1-null mice have lower total islet numbers, consistent with a role for CRFR1 in the regulation of β cell mass. The consequences of β cell CRFR1 activity resemble the effects of incretin receptor activity. These similarities include the requirement for high ambient glucose levels to potentiate insulin secretion and maximize the phosphorylation of Erk1/2 and CREB. Harnessing beneficial effects following activation of CRFR1 to improve β cell mass and augment insulin secretion in type 1 and type 2 diabetic states, respectively, will depend on the development of strategies to activate pancreatic CRFR1 selectively without the undesirable side effects of the activation of CRFR1 on the anterior pituitary. The presence of CRFR1 on β cells adds another layer of complexity to the intricate network of paracrine and autocrine factors and their cognate receptors whose coordinated efforts can dictate islet hormone output and maintain β cell mass.
Materials and Methods
Animals and Procedures.
Male CRFR1-null mice (16) and wild-type littermates were studied at 8–12 weeks of age. CRFR1-null mice received a replacement dose of 25 mg/L corticosterone (Sigma) in the drinking water after weaning. For adrenalectomy experiments, we used male C57/Bl6 mice (Harlan Laboratories) at 3 months of age. Animals received physiological saline (0.9% NaCl) supplemented with corticosterone (25 mg/mL) and were allowed to recover for 10 days postsurgery. Adrenalectomized animals did not respond to handling with elevated postchallenge plasma corticosterone levels, indicating that adrenalectomies were successful. The mIP-GFP mice (12) were obtained from the Jackson Laboratory. All animals were maintained on a 12-h light (0600–1800)/12-h dark (1800–0600) cycle with free access to water and standard rodent chow. All animal protocols were approved by the Salk Institute for Biological Studies Institutional Animal Care and Use Committee.
Glucose Tolerance Test and Insulin Release.
Animals were fasted overnight and weighed in the morning. For the glucose tolerance test, animals received a single 2-g/kg i.p. injection of glucose (Sigma) at time 0, and plasma glucose levels were determined using tail vein blood by glucometer (Novamax; Nova Biomedical). For insulin release, animals received a single 2-g/kg i.p. injection of glucose at time 0. Blood was collected by retro-orbital bleed. Plasma insulin levels were determined using an RIA kit (Millipore) according to the manufacturer’s instructions. When applicable, stressin1-A was injected i.p 15 min before glucose challenge at 0.5 mg/kg (insulin release) or 2.5 mg/kg (glucose tolerance test).
Islet isolation.
Islets were isolated by injecting collagenase P (1.5 mg/mL in HBSS; Roche Diagnostics) (Invitrogen) via the common bile duct while the ampulla of Vater was clamped. The entire pancreas was collected following the injection of 2 mL (mouse) or 10 mL (rat) collagenase solution and was incubated at 37 °C for 20 min while being shaken regularly. Pancreata were washed three times with cold HBSS containing 10% FBS. The digested suspension was passed through a nylon mesh (pore size 425 μm; Small Parts Inc.), and islets were isolated by density gradient centrifugation on a Histopaque gradient (1.077 g/mL density) (Sigma) for 20 min at 1400 × g without brake. Islets were collected from the interface, washed once with ice-cold HBSS containing 10% FBS, and hand picked several times under a dissecting microscope before being flash frozen in lysis buffer for expression analysis or transfer to a cell-culture dish containing RPMI 1640 with 10% FBS and penicillin/streptomycin for overnight culture before experimental procedures.
FACS.
Isolated primary islets were dissociated in 0.25% Trypsin-EDTA solution (Invitrogen) for 5 min at 37 °C aided by gentle mechanical dissociation by pipetting. Dissociated primary islet cells were washed in HBSS with 10% FBS without phenol red and sorted on an FACS Vantage SE DiVa (Becton-Dickinson, Franklin Lakes, NJ) using a 488-nM argon excitation line. Cells were collected in lysis buffer for subsequent RNA isolation.
Cell Culture.
MIN6 insulinoma cells, obtained at passage 18 from Ulupi Jhala (UCSD, La Jolla, CA), were cultured in DMEM (Invitrogen) containing 11 mM glucose, 10% FBS, Glutamax (Invitrogen), and 10 μM β-mercaptoethanol and were used between passages number 21 and 35. The αTC-1 cell line was obtained from ATCC and was cultured in RPMI 1640 (Invitrogen) containing 10% FBS and Glutamax. TU6 cells were obtained from Marc Montminy (The Salk Institute, La Jolla, CA) and were cultured in DMEM containing Glutamax and 10% FBS.
Expression Analysis.
Cell lines were seeded at 100,000 cells/well in 24-well plates. The medium was aspirated 36 h later, and total RNA was isolated using the GenElute Mammalian Total RNA miniprep kit (Sigma) according to the manufacturer’s instructions. Sorted cells were collected directly in lysis buffer. RNA was converted into cDNA with the high-capacity cDNA archive kit (Applied Biosystems). Gene expression was assessed by qPCR using SYBR chemistry on a Lightcycler 480 platform (Roche Diagnostics). Primers are listed in Table S1.
cAMP Assay.
MIN6 cells were plated into 48-well plates at 50,000 cells/well. The next day cells were washed three times with DMEM containing 5.5 mM glucose, 2% FBS, Glutamax, and 10 μM β-mercaptoethanol. After 24 h, the cells were washed once and preincubated with 0.1 mM 3-isobutyl-1-methylxanthine (AG Scientific Inc.) for 20 min. The cells then were treated for 30 min as indicated. Intracellular cAMP was measured from triplicate wells using an RIA kit (Biomedical Technologies) as previously described (32).
Insulin Secretion Assay.
Following isolation, islets were handpicked twice under a dissecting microscope into RPMI 1640 containing 2.8 mM glucose and 0.1% BSA (ImmunO Grade; MP Biomedicals) without phenol red. Islets were incubated in the second wash for 1 h before handpicking to 24-well plates containing 0.4 mL RMPI with 2.8 mM glucose and 0.1% BSA at seven islets/well. After 60 min, a small aliquot of medium was collected for the determination of basal insulin release; then glucose and peptides were added as indicated. After 60 min, another aliquot of medium was collected for the determination of stimulated insulin secretion. Samples were stored at −20 °C until determination of insulin concentrations using an RIA kit (Millipore). Human islets were handpicked twice and cultured overnight in CMRL-1066 medium (Invitrogen) supplemented with 10% FBS and penicillin/streptomycin at 37 °C, 5% CO2. Islets were starved in RPMI 1640 medium supplemented with 1.4 mM glucose and 0.1% BSA for 60 min and then were transferred into 1.2 mL of medium (RPMI 1640 supplemented with 2.8 mM glucose, 0.1% BSA) in 24-well plates at 100 islets/well. After 60 min, 600 μL of medium was collected for the determination of basal insulin release, and then islets were stimulated for 60 min with glucose and peptide as indicated in RPMI 1640 containing 0.1% BSA. Samples were diluted 20 times in RPMI 1640 with 0.1% BSA and were stored at −20 °C until determination of insulin concentrations with an insulin ELISA kit (Mercodia AB).
Western Immunoblotting.
MIN6 cells were grown to 60% confluency and were starved for 1 h in Krebs Ringer solution supplemented with 0.1% BSA and 2.8 mM D-glucose. Antalarmin was added for the last 20 min of starvation. Subsequently, peptides were added for 5 min, unless otherwise indicated. Cells were lysed in PLC-lysis buffer [50 mM Hepes pH 7.5, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EGTA, 100 mM NaF, 10 mM NaPPi, 500 μM NaVO4, supplemented with protease inhibitors (Roche Diagnostics) and 1mM DTT]. Lysates were cleared by centrifugation and mixed with 4× sample buffer (Invitrogen) with DTT (50 mM). After separation by SDS/PAGE, transfer to nitrocellulose membranes (Whattman), and immunoblotting, reactive proteins were visualized using chemiluminescent substrate (Thermo Scientific). Rabbit anti-phospho-ERK and total ERK antibodies were from Cell Signaling Technology. Rabbit anti-phospho-CREB antibody (#5322) and total CREB antibody (#244) were produced in our laboratory. The secondary antibody was donkey anti rabbit/HRP (GE Healthcare U.K. Ltd.).
Rat Neonatal β Cell Proliferation.
Proliferation of rat neonatal β cells was assessed as previously described (33). Briefly, islets were isolated from neonatal rats, and the islets were dispersed into single cells after 5 days of culture. Islets were seeded in culture flasks at 150,000 cells/flask and cultured for an additional 5 days in the presence of the indicated agents. EdU was added for the final 24 h of culture. Cells were fixed and stained for insulin, EdU, and DAPI. For each condition, more than 1,000 insulin-positive cells were counted, and the proportion of cells with EdU-positive nuclei was determined.
Immunohistochemistry.
Pancreata were immersion fixed in 4% paraformaldehyde in potassium phosphate-buffered saline (KPBS) for 4–6 h at 4 °C, followed by cryoprotection in 30% sucrose in KPBS overnight. Pancreata were embedded in Tissue-Tek (Sakura Finetek USA, Inc.), frozen on dry ice, and sectioned in 14-mm sections on a cryostat and stored at −20 °C until use. After thawing, slides were washed three times for 5 min in KPBS followed by an overnight incubation with primary antibody in KPBS containing 2% normal donkey serum and 0.4% Triton ×100. Slides were washed three times for 5 min in KPBS, incubated for 45 min with secondary antibody, followed by three more 5-min washings in KPBS before being embedded in Vectashield containing DAPI (Vector Laboratories Inc.). Primary antibodies were guinea pig anti-insulin (Millipore) at 1:500, guinea pig anti-glucagon (Millipore) at 1:7,000, sheep anti-Sst (American Research Products Inc.) at 1:1,000, guinea pig anti- polypeptide Y (Millipore) at 1:100. Secondary antibodies were donkey anti-guinea pig Cy3, donkey anti-sheep FITC, (all Jackson ImmunoResearch Laboratories Inc.), at 1:600. Slides were imaged using a Leica TCS SP2 AOBS confocal microscope (Leica Microsystems Inc.).
Supplementary Material
Acknowledgments
Human islets were obtained courtesy of the Islet Cell Resource Basic Science Islet Distribution Program. We thank Jean Rivier and Judit Erchegyi (The Salk Institute, La Jolla, CA) for providing the peptides used in this study. We thank Soon Lee (The Salk Institute, La Jolla, CA) for expert advice on surgical procedures and for supplying CRFR1-null mice. We thank David Chambers and Jonna Barrie of the Salk Center for Cytometry and Molecular Imaging for expert assistance and Peter Gray for constructive comments on an earlier version of this paper. The following have been licensed by The Salk Institute for Biological Studies and/or The Clayton Foundation: CRF to Ferring Pharmaceuticals, CRFR1 and Ucn 2 to Neurocrine Biosciences, and Ucn 3 to Johnson & Johnson. This work was supported by Grant P01DK026741-30 from the National Institute of Diabetes and Digestive and Kidney Diseases. We gratefully acknowledge support by the Juvenile Diabetes Research Foundation and the Adler Foundation. This work also was supported in part by the Clayton Medical Research Foundation, Inc.. W.W.V. is a Senior Clayton Medical Research Foundation Investigator. M.O.H. holds a postdoctoral fellowship from the Leona M. & Harry B. Helmsley Charitable Trust.
Footnotes
W.W.V. is a co-founder, member of the Board of Directors, and a shareholder of Neurocrine Biosciences, a company that is developing small molecule antagonists of corticotropin releasing factor and has licensed Urocortin 2 as a potential treatment for acute congestive heart failure. However, the work described in this manuscript is supported by the National Institutes of Health and private sources and is completely independent of Neurocrine Biosciences.
This article contains supporting information online at www.pnas.org/cgi/content/full/0913610107/DCSupplemental.
References
- 1.Kuperman Y, Chen A. Urocortins: Emerging metabolic and energy homeostasis perspectives. Trends Endocrinol Metab. 2008;19:122–129. doi: 10.1016/j.tem.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Fekete EM, Zorrilla EP. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: Ancient CRF paralogs. Front Neuroendocrinol. 2007;28:1–27. doi: 10.1016/j.yfrne.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 4.Chrousos GP. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: Neuro-endocrine and target tissue-related causes. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S50–S55. doi: 10.1038/sj.ijo.0801278. [DOI] [PubMed] [Google Scholar]
- 5.Strack AM, Sebastian RJ, Schwartz MW, Dallman MF. Glucocorticoids and insulin: Reciprocal signals for energy balance. Am J Physiol. 1995;268:R142–R149. doi: 10.1152/ajpregu.1995.268.1.R142. [DOI] [PubMed] [Google Scholar]
- 6.Barthel A, Schmoll D. Novel concepts in insulin regulation of hepatic gluconeogenesis. Am J Physiol Endocrinol Metab. 2003;285:E685–E692. doi: 10.1152/ajpendo.00253.2003. [DOI] [PubMed] [Google Scholar]
- 7.Kageyama K, et al. Modulation of Ca2+ influx by corticotropin-releasing factor (CRF) family of peptides via CRF receptors in rat pancreatic beta-cells. Peptides. 2006;27:1814–1819. doi: 10.1016/j.peptides.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Kanno T, Suga S, Nakano K, Kamimura N, Wakui M. Corticotropin-releasing factor modulation of Ca2+ influx in rat pancreatic beta-cells. Diabetes. 1999;48:1741–1746. doi: 10.2337/diabetes.48.9.1741. [DOI] [PubMed] [Google Scholar]
- 9.Li C, et al. Urocortin III is expressed in pancreatic beta-cells and stimulates insulin and glucagon secretion. Endocrinology. 2003;144:3216–3224. doi: 10.1210/en.2002-0087. [DOI] [PubMed] [Google Scholar]
- 10.O’Carroll AM, Howell GM, Roberts EM, Lolait SJ. Vasopressin potentiates corticotropin-releasing hormone-induced insulin release from mouse pancreatic beta-cells. J Endocrinol. 2008;197:231–239. doi: 10.1677/JOE-07-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li C, Chen P, Vaughan J, Lee KF, Vale W. Urocortin 3 regulates glucose-stimulated insulin secretion and energy homeostasis. Proc Natl Acad Sci USA. 2007;104:4206–4211. doi: 10.1073/pnas.0611641104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hara M, et al. Transgenic mice with green fluorescent protein-labeled pancreatic beta -cells. Am J Physiol Endocrinol Metab. 2003;284:E177–E183. doi: 10.1152/ajpendo.00321.2002. [DOI] [PubMed] [Google Scholar]
- 13.Rieck S, et al. The transcriptional response of the islet to pregnancy in mice. Mol Endocrinol. 2009;23:1702–1712. doi: 10.1210/me.2009-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: Implications for physiology and pathophysiology. Endocr Rev. 2006;27:260–286. doi: 10.1210/er.2005-0034. [DOI] [PubMed] [Google Scholar]
- 15.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 16.Smith GW, et al. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- 17.Petrusz P, Merchenthaler I, Maderdrut JL, Vigh S, Schally AV. Corticotropin-releasing factor (CRF)-like immunoreactivity in the vertebrate endocrine pancreas. Proc Natl Acad Sci USA. 1983;80:1721–1725. doi: 10.1073/pnas.80.6.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahrén B. Autonomic regulation of islet hormone secretion—implications for health and disease. Diabetologia. 2000;43:393–410. doi: 10.1007/s001250051322. [DOI] [PubMed] [Google Scholar]
- 19.Lovenberg TW, et al. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci USA. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trümper A, et al. Glucose-dependent insulinotropic polypeptide is a growth factor for beta (INS-1) cells by pleiotropic signaling. Mol Endocrinol. 2001;15:1559–1570. doi: 10.1210/mend.15.9.0688. [DOI] [PubMed] [Google Scholar]
- 21.Ahrén B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov. 2009;8:369–385. doi: 10.1038/nrd2782. [DOI] [PubMed] [Google Scholar]
- 22.Drucker DJ. Glucagon-like peptide-1 and the islet beta-cell: Augmentation of cell proliferation and inhibition of apoptosis. Endocrinology. 2003;144:5145–5148. doi: 10.1210/en.2003-1147. [DOI] [PubMed] [Google Scholar]
- 23.Kim MJ, et al. Exendin-4 induction of cyclin D1 expression in INS-1 beta-cells: Involvement of cAMP-responsive element. J Endocrinol. 2006;188:623–633. doi: 10.1677/joe.1.06480. [DOI] [PubMed] [Google Scholar]
- 24.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 25.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell. 2007;12:817–826. doi: 10.1016/j.devcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 26.la Fleur SE, Akana SF, Manalo SL, Dallman MF. Interaction between corticosterone and insulin in obesity: Regulation of lard intake and fat stores. Endocrinology. 2004;145:2174–2185. doi: 10.1210/en.2003-1359. [DOI] [PubMed] [Google Scholar]
- 27.Warne JP, et al. The gastroduodenal branch of the common hepatic vagus regulates voluntary lard intake, fat deposition, and plasma metabolites in streptozotocin-diabetic rats. Am J Physiol Endocrinol Metab. 2008;294:E190–E200. doi: 10.1152/ajpendo.00336.2007. [DOI] [PubMed] [Google Scholar]
- 28.Dallman MF, Warne JP, Foster MT, Pecoraro NC. Glucocorticoids and insulin both modulate caloric intake through actions on the brain. J Physiol. 2007;583:431–436. doi: 10.1113/jphysiol.2007.136051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell ME, et al. Voluntary sucrose ingestion, like corticosterone replacement, prevents the metabolic deficits of adrenalectomy. J Neuroendocrinol. 2000;12:461–470. doi: 10.1046/j.1365-2826.2000.00488.x. [DOI] [PubMed] [Google Scholar]
- 30.Stenzel-Poore MP, Cameron VA, Vaughan J, Sawchenko PE, Vale W. Development of Cushing’s syndrome in corticotropin-releasing factor transgenic mice. Endocrinology. 1992;130:3378–3386. doi: 10.1210/endo.130.6.1597149. [DOI] [PubMed] [Google Scholar]
- 31.Husebye E, Løvås K. Pathogenesis of primary adrenal insufficiency. Best Pract Res Clin Endocrinol Metab. 2009;23:147–157. doi: 10.1016/j.beem.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Kageyama K, Gaudriault GE, Suda T, Vale WW. Regulation of corticotropin-releasing factor receptor type 2beta mRNA via cyclic AMP pathway in A7r5 aortic smooth muscle cells. Cell Signal. 2003;15:17–25. doi: 10.1016/s0898-6568(02)00048-7. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen JH, Linde S, Welinder BS, Billestrup N, Madsen OD. Growth hormone is a growth factor for the differentiated pancreatic beta-cell. Mol Endocrinol. 1989;3:165–173. doi: 10.1210/mend-3-1-165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.