Abstract
Plant cell walls represent an abundant, renewable source of biofuel and other useful products. The major bottleneck for the industrial scale-up of their conversion to simple sugars (saccharification), to be subsequently converted by microorganisms into ethanol or other products, is their recalcitrance to enzymatic saccharification. We investigated whether the structure of pectin that embeds the cellulose-hemicellulose network affects the exposure of cellulose to enzymes and consequently the process of saccharification. Reduction of de-methyl-esterified homogalacturonan (HGA) in Arabidopsis plants through the expression of a fungal polygalacturonase (PG) or an inhibitor of pectin methylesterase (PMEI) increased the efficiency of enzymatic saccharification. The improved enzymatic saccharification efficiency observed in transformed plants could also reduce the need for acid pretreatment. Similar results were obtained in PG-expressing tobacco plants and in PMEI-expressing wheat plants, indicating that reduction of de-methyl-esterified HGA may be used in crop species to facilitate the process of biomass saccharification.
Keywords: biofuel, pectin, plant cell wall, pectin methylesterase inhibitor, polygalacturonase
Plant biomass has been a source of energy for most part of human history and, due to the increasing demand for renewable materials and industrial products, is reconsidered today as a possible strategic resource. Plant cell walls comprise a significant proportion of the lignocellulosic biomass (1) and are a potentially abundant substrate for bioconversion to ethanol and other industrial products (2). They are composed of a heterogeneous polysaccharidic matrix associated with components like lignin and proteins. Saccharification, a key process for the production of ethanol, is the degradation of the wall polysaccharides into fermentable sugars. Enzymatic hydrolysis is the most promising and environmentally friendly technology available for saccharification (3, 4), but the recalcitrance of cell walls to hydrolysis is the major bottleneck for the industrial scale-up of this process (2). Thermochemical pretreatments using high temperature, toxic acids, peroxides, and ammonia, often along with some form of mechanical disruption, are currently required to make biomass accessible to cell wall–degrading enzymes and represent up to 30% of the cost of biofuel production (2).
Modification of the cell wall structure may be useful for reducing pretreatments and improving the overall saccharification process. For example, it has been shown that reducing the lignin content in transgenic alfalfa plants improves saccharification efficiency, although it can reduce biomass yield (5). A cell wall component that, particularly in dicots, is critical for tissue integrity and accessibility to cell wall–degrading enzymes is the cohesive pectin matrix embedding the cellulose-hemicellulose network, which in turn contains the major strength-conferring elements. It is well known that intermolecular bonds of pectin, mediated by acidic homogalacturonan (HGA), influence wall plasticity (6) and cell adhesion (7). HGA is synthesized and secreted as a highly methyl-esterified polymer (8) and is de-methyl-esterified in muro by pectin methylesterases (PMEs). These enzymes produce long stretches of free carboxylic residues that are necessary for Ca2+-mediated crosslinks of HGA into rigid “egg-box” structures (9) and, in lignified tissues, could enhance the formation of benzyl-uronate crosslinks (10). Here we show that plants with a reduced content of de-methyl-esterified HGA can be obtained by expressing in planta a fungal polygalacturonase (PG) or by overexpressing plant inhibitors of endogenous PMEs. We also show that these modified plants exhibit an increased efficiency of enzymatic saccharification, thus reducing the need for thermochemical pretreatments.
Results and Discussion
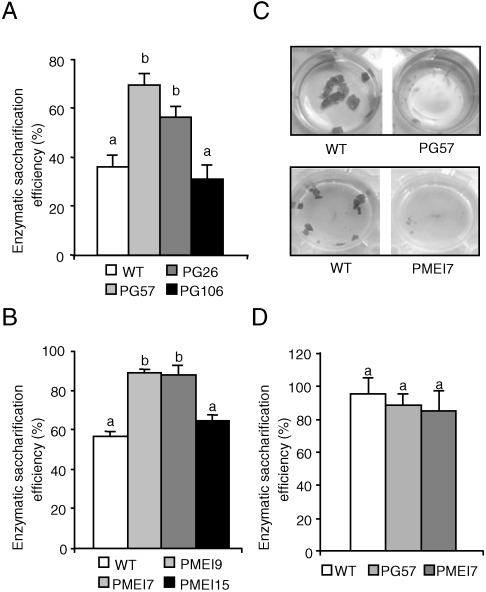
To test whether the content of acidic HGA and/or the methyl-esterification status of HGA affects the susceptibility of plant cell walls to enzymatic saccharification, we analyzed Arabidopsis plants expressing a mutated version of the Aspergillus niger pgaII gene encoding a PG with reduced specific activity (PG plants) (11, 12), and plants overexpressing AtPMEI-2, an endogenous inhibitor of PMEs (PMEI plants) (13, 14). Leaf material from untransformed [wild-type (WT)] plants, from two independent lines (PG26 and PG57) with high levels of PG expression, from one line (PG106) with low levels of PG expression (Fig. S1), from two independent lines (PMEI7 and PMEI9) expressing high levels of PMEI, and from one line (PMEI15) with low levels of PMEI (13) was treated with Celluclast 1.5 L, a commercial preparation which contains mostly cellulose-degrading activities. Large differences were observed in the enzymatic saccharification efficiency (reducing sugars released as a percentage of total sugars in the tissue) among the various lines. After 24 h of incubation, saccharification efficiency in the two independent lines with high levels of PG expression was up to 2-fold higher than in either WT or PG106 plants, whereas it was about 60% higher in highly expressing PMEI lines than in the respective control lines (Fig. 1A and B). Notably, enzymatic treatment of leaves from lines with higher saccharification efficiency also showed a remarkable tissue maceration (Fig. 1C). No significant tissue maceration or release of sugars were detected when leaf material from both transformed and control plants was incubated in the absence of enzymes, indicating that the expression of pgaII or AtPMEI-2 does not per se determine disassembly of the tissue and/or saccharification but rather promotes the ability of exogenous enzymes to hydrolyze the substrate in fresh tissues. No significant differences in the amount of total sugars, of alcohol insoluble solid (AIS), and of starch could be detected in leaf material of WT, PG, or PMEI plants with high levels of expression of the transgene (Table S1). Sugars present in the enzymatic digests of Arabidopsis leaf material treated with Celluclast comprised glucose and low amounts of xylose (Table S2). The increased level of glucose but not xylose in the digests of transgenic plants, as compared to WT plants, suggests that the expression of PG or PMEI mostly increases the accessibility of cellulose to hydrolytic enzymes. Note that no significant differences in enzymatic saccharification efficiency were observed when WT, PG, and PMEI leaves were pretreated with dilute acid (1.3% sulphuric acid) at 110 °C, a treatment that is known to remove mostly cell wall matrix components and to increase cellulose susceptibility to enzymes (15, 16) (Fig. 1D). We concluded that high expression of PGs or PMEIs in plants may reduce the need of acid pretreatments in biomass bioconversion.
Fig. 1.
Saccharification of leaf material from Arabidopsis PG or PMEI plants. Leaf material from untransformed Arabidopsis plants (WT) and from transgenic PG (A) and PMEI (B) plants were treated with Celluclast, and saccharification efficiency was measured after 24 hours. (C) Tissue maceration of representative samples of WT (Left) and transgenic (Right) PG57 and PMEI7 leaf material after 24 h of enzymatic saccharification. (D) Enzymatic saccharification efficiency of leaf tissue from WT, PG, and PMEI plants after dilute acid pretreatment. Numbers indicate different independent transgenic lines. Bars represent average saccharification efficiency ± SEM (N≥6). Different letters indicate statistically significant differences, according to ANOVA followed by Tukey’s test (P < 0.05). These experiments were repeated at least twice with similar results.
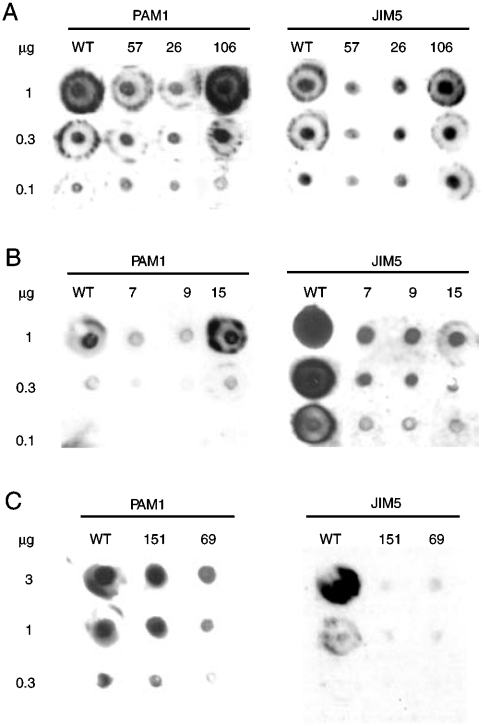
Taken together, the above results indicate that cell wall recalcitrance is reduced in plants expressing PG or PMEI, likely due to a modification of pectin composition and/or architecture. Levels of de-methyl-esterified HGA regions of Arabidopsis plants expressing PG and PMEI were therefore investigated in chelating agent-soluble solid (ChASS) fractions, which contain mainly pectin, by immunodot assay, using the monoclonal antibodies PAM1, which specifically recognizes large de-methyl-esterified blocks of HGA (at least 30 continuous GalA units) (17), and JIM5, which binds pectin with a low degree of methyl-esterification (18). This analysis revealed that Arabidopsis plants expressing high levels of PG or PMEI have lower levels of PAM1-binding epitopes, and therefore a reduced quantity of long stretches of unesterified HGA, than WT plants or plants with low transgene expression (Fig. 2A and B). JIM5-binding epitopes were also less abundant in plants highly expressing PG and PMEI as compared with WT plants or plants with low transgene expression, confirming a higher degree of HGA methyl-esterification in PG and PMEI plants (Fig. 2A and B).
Fig. 2.
Immunodot analysis of pectin in PG and PMEI plants. ChASS fractions were extracted from leaves of untransformed and transgenic Arabidopsis PG (A) and PMEI plants (B) and of untransformed and transgenic wheat AcPMEI plants (C). Numbers indicate different independent transgenic lines. The indicated amounts (in μg) of ChASS fraction were applied at a single point to a nitrocellulose membrane. Specific HGA epitopes were detected by using PAM1 and JIM5 monoclonal antibodies.
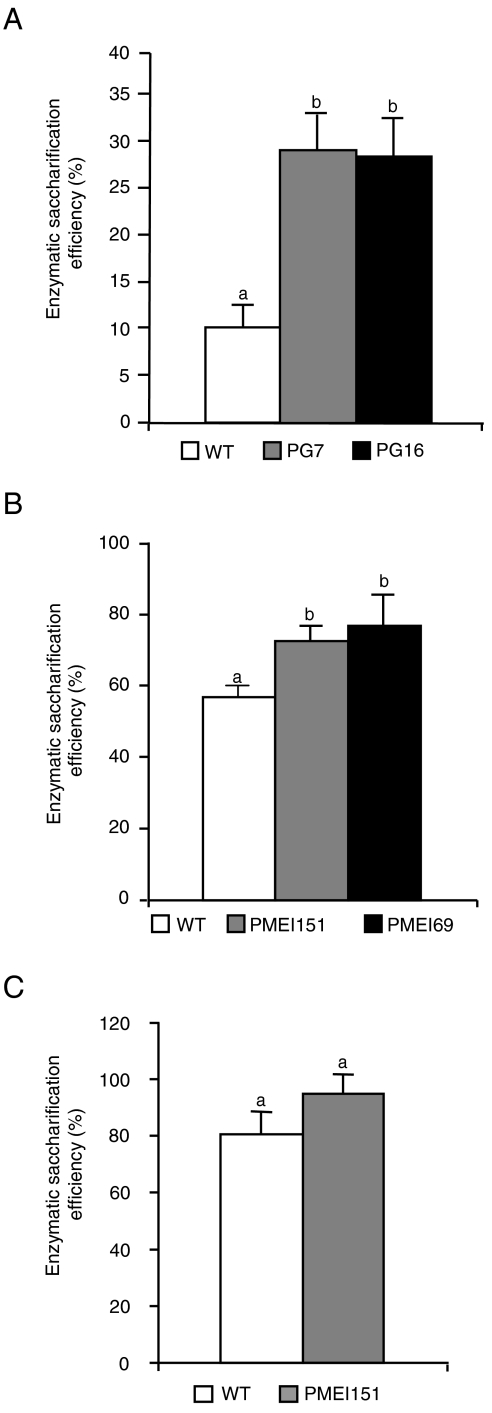
After proving the concept that Arabidopsis plants expressing PG or PMEI are more susceptible to enzymatic saccharification, we tested whether the expression of these proteins also increases saccharification efficiency in crop species more suitable for industrial bioconversion. Indeed, two tobacco lines expressing high levels of pgaII (PG7 and PG16), previously described as exhibiting a reduced content of de-methyl-esterified blocks of HGA (11), showed a three times higher saccharification efficiency than WT plants (Fig. 3A) and increased levels of glucose in the hydrolysate (Table S2), yet have a similar content of total sugars and AIS per leaf fresh weight (Table S1). Commelinid monocots, which include most energy crops currently used or under development, like switchgrass (Panicum virgatum), Miscanthus (Miscanthus×giganteus), sorghum (Sorghum bicolor), and maize (Zea mays), have a lower content of HGA as compared to dicots (19). Therefore, we tested whether transformation with a PMEI could also affect saccharification in wheat (Triticum durum L. cv. Svevo), a commelinid grass. We used a kiwifruit (Actinidia chinensis) PMEI isoform (AcPMEI) (20), which has stronger inhibitory activity on endogenous wheat PMEs as compared to Arabidopsis PMEIs: 15 ng of AcPMEI or AtPMEI-2 resulted in 100% or 75% inhibition of the PME activity present in 9 μg of wheat leaf total proteins, respectively. Two T2 independent wheat lines (AcPMEI151 and AcPMEI69) expressing high levels of AcPMEI and showing about 90% reduction of endogenous PME activity (Fig. S2) were selected. Consistently, PAM1 and JIM5-binding epitopes were much less abundant in the ChASS fractions of plants expressing AcPMEI compared to WT plants, indicating a lower level of de-esterified HGA in pectins (Fig. 2C). As in Arabidopsis PMEI plants, no significant differences in total sugar, AIS fraction, and starch per leaf fresh weight were observed between transformed and WT plants (Table S1). Treatment of wheat leaf tissues with Celluclast alone resulted in a low level of soluble sugars released and no significant differences in the saccharification efficiency between WT and transgenic plants; instead, when Celluclast was used in combination with Macerozyme, a commercial cocktail of cell wall–degrading enzymes reported to facilitate cellulase-mediated hydrolysis of cellulose in tissues of monocots such as corn cobs and stover (2, 21), a 35–40% increase in saccharification efficiency was observed in transgenic plants as compared with WT plants (Fig. 3B). Determination of glucose and xylose in the enzymatic hydrolysates indicated a greater release of both monosaccharides from PMEI expressing plants as compared with the control WT plants (Table S2), suggesting that both cellulose and hemicellulose are more easily digested in transformed plants. As in the case of Arabidopsis, a dilute acid pretreatement resulted in WT plants a degree of saccharification similar to that observed in PMEI plants without pretreatment (Fig. 3C).
Fig. 3.
Saccharification of leaf material from tobacco PG and wheat PMEI plants. (A) Leaf material from tobacco untransformed (WT) and PG-expressing plants was treated with Celluclast, and saccharification efficiency was measured after 24 h. (B and C) Leaf material from untransformed (WT) and transgenic wheat plants overexpressing AcPMEI was treated with a mixture of Macerozyme R-10 and Celluclast 1.5 L without pretreatment (B) or after dilute acid pretreatment (C), and saccharification efficiency was measured after 24 h. Numbers indicate different independent transgenic lines. Bars represent average saccharification efficiency ± SEM (N≥6). These experiments were repeated at least twice with similar results. Different letters indicate statistically significant differences, according to ANOVA followed by Tukey’s test (P < 0.01).
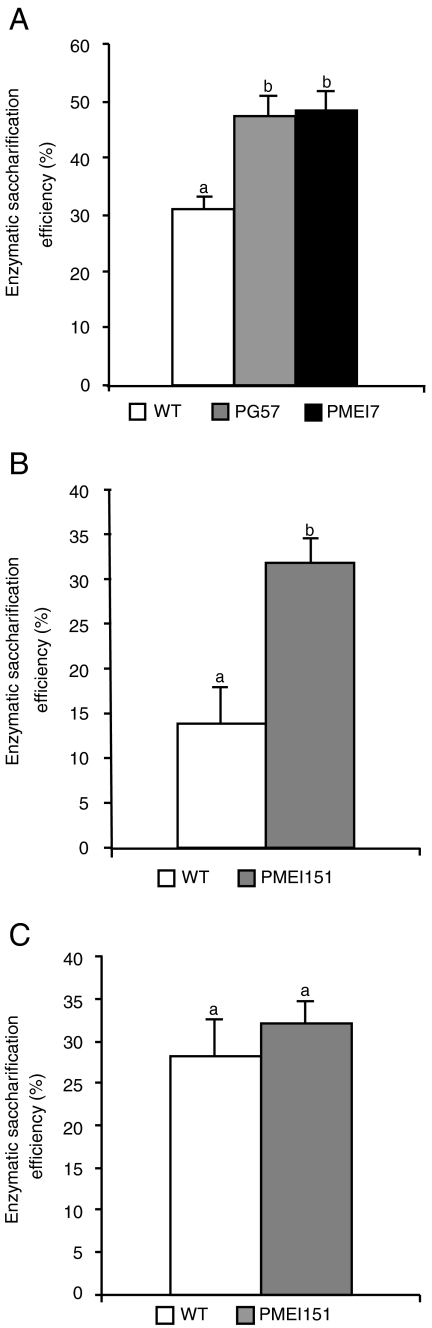
We then investigated if the increased degree of saccharification of PG and PMEI plants could also be observed in stems, which are more lignified tissues with secondary walls, since stem tissues are largely utilized for biofuel production (15). As expected, the saccharification efficiency of stem material was lower than that observed in leaves; however, Arabidopsis PG and PMEI stems treated with Celluclast showed a 50% increase of saccharification, compared to WT plants (Fig. 4A). Similarly, saccharification efficiency of wheat PMEI stems treated with Celluclast and Macerozyme was about 2.5 times higher than in WT stems (Fig. 4B). In addition, dilute acid pretreatment of wheat stems did not result in significantly different release of total reducing sugars after enzymatic saccharification of the residual PMEI and WT biomass (Fig. 4C).
Fig. 4.
Saccharification of stem material from PG and PMEI plants. (A) Stem segments from Arabidopsis untransformed (WT), PG57, and PMEI7 plants were treated with Celluclast, and saccharification efficiency was measured after 24 h. (B and C) Stem segments from wheat untransformed (WT) and PMEI151 plants were treated with a mixture of Macerozyme and Celluclast without pretreatment (B) or after dilute acid pretreatment (C), and saccharification efficiency was measured after 24 h. Bars represent average saccharification efficiency ± SEM (N≥6). These experiments were repeated at least twice with similar results. Different letters indicate statistically significant differences, according to ANOVA followed by Tukey’s test (P < 0.01). Total sugars in stem material were the following (mg g-1fresh weight ± SEM↦): Arabidopsis WT, 19.7 ± 0.5; PG57, 18.5 ± 0.04; PMEI7, 22.5 ± 1.6; wheat WT, 57.0 ± 4.8; PMEI151, 53.2 ± 4; pretreated wheat WT, 64.1 ± 3.3; and pretreated PMEI151, 64.8 ± 2.4. Differences in sugar content between transgenic and parental lines were not statistically different, according to Student’s t-test (P > 0.05).
Our results show that the recalcitrance of cell walls to enzymatic saccharification is reduced in leaves and stems of both dicot and monocot plants expressing PGs and PMEIs. It is likely that a reduced content of egg-box structures or other crosslinks mediated by de-methyl-esterified HGA is responsible for the accessibility of cellulases and hemicellulases to their substrates, resulting in increased saccharification efficiency. However, in the case of PG plants, high expression of the transgene also causes a reduced biomass yield both in tobacco (11) and in Arabidopsis (Table 1). It is conceivable that the level to which the content of de-methyl-esterified HGA may be reduced without affecting plant performance and tissue integrity is critical to develop crops with improved saccharification. Work is in progress to establish a system to control the ectopic PG activity and to minimize the effect of its activity on biomass production. This may involve the expression of PG under inducible or developmentally regulated promoters at the end of the plant life cycle, or coexpression with specific inhibitors. Instead, Arabidopsis plants overexpressing PMEI show enhanced growth (Fig. 5A) and a higher vegetative biomass yield, with an increase of about 80 and 68% in rosette leaf fresh weight and dry matter, respectively, compared to WT plants (Table 1). The increase in biomass yield of PMEI plants may be due to an enhanced cell expansion, as suggested by previous results showing an increased root cell elongation (13) and by comparison of cell size of transverse sections of hypocotyls of etiolated WT and PMEI seedlings (Fig. 5B). It has been suggested that higher levels of HGA methyl-esterification without a reduction of total HGA promote cell expansion and positively affect growth of dicot plants (13, 22). In contrast to Arabidopsis, no significant difference in biomass yield was observed between control and wheat plants expressing PMEI (Table 1), suggesting that HGA methyl-esterification has a minor impact on the biomass of monocots.
Table 1.
Effects of the expression of PG and PMEI on plant biomass
| FW (mg) |
DW (mg) |
DW/FW |
||
| Arabidopsis | WT | 138 ± 10 | 15.7 ± 0.2 | 0.11 ± 0.02 |
| PG57 | 82 ± 3* | 8.1 ± 0.1* | 0.10 ± 0.02 | |
| PMEI7 | 253 ± 18* | 27.4 ± 1.8* | 0.11 ± 0.02 | |
| Tobacco | WT | 40,550 ± 50 | 1,465 ± 15 | 0.04 ± 0.00 |
| PG16 | 6,100 ± 500* | 240 ± 15* | 0.04 ± 0.00 | |
| Wheat | WT | 2,785 ± 569 | 875 ± 186 | 0.31 ± 0.12 |
| PMEI151 | 3,107 ± 119 | 1,080 ± 46 | 0.35 ± 0.03 |
Fresh weight (FW) and dry weight (DW) of aerial vegetative parts of Arabidopsis, tobacco and wheat untransformed (WT), and transgenic plants. Data represent the average ± SEM of at least six plants.
*Statistically significant differences between transgenic and WT parental lines, according to Student’s t-test (P < 0.001).
Fig. 5.
Morphological analysis of Arabidopsis PMEI plants. (A) Photographs of representative Arabidopsis untransformed (Left) and PMEI7 (Right) plants grown for 30 days. (B) Transverse sections of hypocotyls of etiolated untransformed (Left) and PMEI7 (Right) seedlings were photographed in light microscopy. (Scale bar, 40 μm.)
In conclusion, we suggest that PGs and, more in general, other HGA-degrading enzymes such as pectate lyases may be used to reduce the acidic HGA content in crop plants as well as in energy plants and make them a better substrate for the production of biofuel and other commercial products. The technology described in this paper is in agreement with our vision of the cell wall architecture and with the concept that HGA-mediated cross links act as a glue for other cell wall polysaccharides. Our results are also in agreement with our knowledge of the mode of action of soft rot pathogenic microorganisms and, more generally, of those microorganisms that, by growing on plant tissues, need to saccharify the cell wall polysaccharides to use them as nutrients. These microorganisms produce pectic enzymes before other cell wall–degrading enzymes, and their action is a prerequisite for the exposure of the plant polysaccharides to the hydrolytic machinery of the pathogens. However, the use of microbial pectic enzymes has to be further developed and implemented, because their expression in planta without any kind of control is detrimental for plant growth and tissue integrity (11). On the other hand, inhibitors of PME may be used to the same purpose since their expression may reduce the stretches of acidic HGA in crop and energy plants. This technology, unlike that involving PGs, is readily available for application purposes, because plants overexpressing PMEI have a normal growth and, in the case of Arabidopsis, even exhibit an increased biomass. Furthermore, since plants overexpressing PMEI display a higher resistance to microbial pathogens (13, 23), crops with increased HGA methyl-esterification may also possess this additional desirable trait. Independently of the kind of genes that are used, we have shown that a reduction of the acidic HGA content ultimately determines an increased cell wall susceptibility to hydrolytic degradation. As an alternative to genetic transformation, it is conceivable that either natural variability or variability induced by mutagenesis can be exploited to isolate genotypes with low levels of unesterified HGA. For example, we have shown that the antibody PAM1 can be used as a tool to detect mutants with a low content of unesterified HGA. In addition, it may be useful to develop a high-throughput screening of plants with lower PME/higher PMEI levels or with higher levels of HGA-degrading enzymes.
Materials and Methods
Generation of Transgenic Lines and Plant Growth.
Generation of tobacco (Nicotiana tabacum) Petit Havana-SR1 plants expressing PG and of Arabidopsis (Arabidopsis thaliana) Columbia-0 (Col-0) plants expressing AtPMEI-2 was previously described (11, 13). Arabidopsis Col-0 plants expressing PG were obtained using the pCAMBIA3300 vector (CAMBIA, Australia) harboring the same 35S-PG-NOS cassette previously described (11). Wheat (Triticum durum cv. Svevo) transgenic plants expressing a full-length open reading frame of AcPMEI (20) under the control of the maize Ubi-1 promoter (24) were obtained as previously reported (25). Selection of wheat lines expressing high levels of the transgene was performed by RT-PCR and radial gel diffusion assay (Fig. S2). Total RNA was extracted by using RNeasy Plant Mini kit (Qiagen SpA, Milano, Italy) following the manufacturer’s procedure. Contaminating DNA was removed by using the DNA-free™ mix (Ambion Ltd., Huntingdon, UK), and RNA concentration was determined spectrophotometrically. RT-PCR experiments were performed by using the QuantyTect® SYBR® Green RT-PCR (Qiagen) in a MyCycler™ thermal cycler (Bio-Rad Life Science, Segrate, Italy) using oligonucleotides specific for AcPMEI (5′-CTTGTATCTTTGAGAACTGCAC-3′ and 5′-CCAAGAGAATCAATAGCATCAGC-3′). Amplification of a wheat actin gene (accession number AB181991) was used as reference for transcript amplification by using the following primers: 5′-AAGAGTCGGTGAAGGGGACT and 5′-TTCATACAGCAGGCAAGCAC.
Arabidopsis plants were grown in a growth chamber at 22° C, 70% relative humidity, with a 16 h light/8 h dark photoperiod (100 μmol m-2 s-1 of fluorescent light). For measurements of rosette fresh and dry weight, plants were grown for 15 days at 16 h light/8 h dark photoperiod and then for 15 days at 12 h light/12 h dark photoperiod (100 μmol m-2 s-1 of fluorescent light). Tobacco plants were grown in a growth chamber at 23 °C and 60% relative humidity, with a 16 h light/8 h dark photoperiod (130 μmol m-2 s-1). Wheat plants were grown in peat pots in growth chamber 14 h light/10 h dark photoperiod (300 μmol m-2 s-1) at 24 °C to stage 13, according to Zadoks system (26), vernalized for 3 weeks at 4 °C and grown at 18 °C for 10 days and then at 24 °C with 50–60% relative humidity until maturation. Before saccharification experiments, all plants were maintained for 24 h in darkness to minimize starch accumulation. For fresh and dry weight determination, the aerial vegetative portion of plants at the same stage of development of those used for saccharification experiments were weighed before and after treatment at 80 °C for 3 (Arabidopsis), 6 (tobacco), and 48 h (wheat).
For histological analysis, hypocotyls from 7-day-old etiolated Arabidopsis seedlings were fixed, dehydrated and embedded in hardgrade LR white resin (London Resin Company, Berkshire, UK). Semithin transverse sections (250 nm) were cut at the middle region of etiolated hypocotyls with a Reichert-Jung Ultracut E ultramicrotome and mounted on glass microslides previously coated with Vectabond reagent (Vector Laboratories). Sections were examined by light microscopy on an Axioscop microscope (Carl Zeiss), and photomicrographs were captured with a Canon Powershot G3 photocamera.
Enzymatic Saccharification and Acid Pretreatment.
Leaves were collected from 30-day-old Arabidopsis, 60-day-old tobacco, or wheat plants at stage 69–71, according to Zadoks system (26), corresponding to the formation of kernels. For stem saccharification, entire floral stems were collected from 6-week-old Arabidopsis plants, whereas the middle section of internodes 1, 2, and 3 was used for wheat. Leaves or stems were sterilized in a 1% sodium hypochlorite solution for 5 min and washed twice with sterile water; leaves were cut into 0.25-cm2 square pieces, and stems were cut in 0.5-cm-long segments. Plant material (100 mg of fresh weight) was incubated at 37 °C up to 24 h in a filter-sterilized solution containing 50 mM sodium acetate buffer, pH 5.5, and enzymes. The following enzymatic preparations were used: for Arabidopsis and tobacco, 0.5% (vol/vol) or 1% (vol/vol) Celluclast 1.5 L (cellulase from Trichoderema reseei ATCC 26921, 973 Endo-Glucanese Units/mL; Sigma, St. Louis, MO; product number C2730, lot number 077K0737), respectively; for wheat, a mixture containing 0.5% (vol/vol) Celluclast 1.5 L and 0.25% (wt/vol) Macerozyme R-10 (pectinase and hemicellulase activity derived from Rhizopus sp., Yakult Pharmaceutical Industry, Tokyo, Japan; lot number 202039). For acid pretreatments, plant material (100 mg fresh weight) was incubated in 1.3% sulphuric acid (vol/vol) at 110 °C for 20 min, the hydrolysates were removed, and the residual plant material was rinsed twice with distilled water before enzymatic saccharification.
Enzymatic saccharification efficiency was determined as percentage of reducing sugars released on the total sugars measured in untreated plant material or pretreated residual biomass.
Sugar Determination.
To determine the amount of sugars released after enzymatic saccharification, the incubation medium was collected, centrifuged at 11,000 × g for 10 min, and total reducing sugars in the supernatant were determined as described (27). The glucose and xylose content of enzymatic hydrolysates was determined by hifh-performance anion-exchange chromatography with pulsed amperometric detection (Ion Chromatography System ICS3000, Dionex, CA, USA) with CarboPac PA20 column 3 × 150 mm (Dionex, CA, USA). Before injection of each sample, the column was washed with 200 mM NaOH for 5 min, then equilibrated with 10 mM NaOH for 10 min. Samples were subjected to an isocratic elution with 10 mM NaOH at a flow rate of 0.4 mL min-1 for 20 min. Monosaccharides were detected by using a pulsed gold amperometric detector set on waveform A, according to the manufacturer’s instructions. Peaks were identified and quantified by comparison to a standard mixture of glucose and xylose (Sigma, St. Louis, MO, USA). The determination of total carbohydrates in untreated and pretreated plant material was performed according to the Laboratory Analytical Procedure of the National Renewable Energy Laboratory (http://www.nrel.gov/biomass/analytical_procedures.html). Plant material (100 mg) or pretreated residue derived from 100 mg of plant material were first hydrolysed in 72% (vol/vol) sulphuric acid at 30 °C for 1 h and then in 4% (vol/vol) sulphuric acid at 120 °C for 1 h. Total sugars were estimated spectrophotometrically by using the phenol-sulphuric acid assay (28). AIS was obtained as previously described (13), with minor modifications; leaves were frozen in liquid nitrogen and homogenized by using a Retschmill machine (model MM301; Retsch) at 25 Hz for 1 min. Ground tissue was washed twice in 70% ethanol, vortexed and pelleted by centrifugation at 10,000 × g for 10 min. The pellet was washed twice with chloroform:methanol (1∶1, vol/vol), vortexed, and centrifuged at 10,000 × g for 10 min. The pellet was washed twice with 80% acetone and, after centrifugation at 10,000 × g for 10 min, was air-dried and weighed. ChASS fractions were obtained by homogenizing AIS material (100 mg for Arabidopsis and 150 mg for wheat) in a buffer containing 50 mM Tris-HCl and 50 mM trans-1,2-cyclohexanediaminetetraacetic acid (CDTA), pH 7.2, at 80 °C. After centrifugation at 10,000 × g for 10 min, samples were dialysed and freeze-dried. To determine starch content in the AIS, the dried pellet was washed twice with 20 mM potassium phosphate buffer, pH 6.9, at 70 °C for 1 h, resuspended in 20 mM potassium phosphate buffer, pH 6.9, containing 7 mM NaCl, 0.01% (wt/vol) NaN2 and porcine Type VII-A α-amylase (100 units g-1 AIS; SIGMA, St. Louis, MO; product number A2643), and incubated 48 h at 30 °C. After inactivation of the enzyme at 70 °C for 10 min, the suspension was cooled, centrifuged at 8,000 × g for 20 min, and total sugars in the supernatant were determined with the phenol-sulphuric acid assay as described above. Sugar content in AIS and untreated and pretreated material and ChASS analysis were performed on tissues of the same plants used for saccharification experiments.
Immunodot Assays.
ChASS fractions (1–3 mg mL-1 from Arabidopsis and wheat, respectively) were applied as 1-μL aliquots to a nitrocellulose membrane (Amersham, UK) in a 3-fold dilution series and allowed to air dry. Membranes were blocked with 3% membrane blocking reagent powder (Amersham, UK) in phosphate-buffered saline (PBS; Bio-Rad) for 1 h prior to incubation for 1.5 h with primary antibodies. Specific epitopes were detected by using PAM1ScFv (17) and JIM5 (29) monoclonal antibodies. After extensive washes in PBS, membranes were incubated with anti-rat secondary antibody conjugated to horseradish peroxidase (Amersham, UK) for JIM5, and with anti-His conjugated to horseradish peroxidase (Sigma) for PAM1. Membranes were washed as described above prior to detection with ECL detection reagent (Amersham, UK).
Protein Analysis.
Extraction of leaf total proteins and immunoblot analysis were performed as previously described (11). PG activity and PMEI activity in total protein extracts were determined by radial gel diffusion assay, as previously described (11, 13).
Supplementary Material
Acknowledgments.
We thank D. Pontiggia for technical support to HPLC analysis and J. P. Knox for providing antibodies. This work was supported by the European Research Council [ERC Advanced Grant 233083 (F. C.)], the Institute Pasteur-Fondazione Cenci Bolognetti, the Italian Ministry of University and Research [PRIN 2007 Grant (G. D. L., F.C. and R.D) and AgroGen Grant (R.D.)] and the European Union COST Action 928 (D.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907549107/DCSupplemental.
References
- 1.Poorter H, Villar R. In: Plant Resource Allocation. Bazzaz FA, Grace J, editors. San Diego, CA: Academic; 1997. pp. 39–72. [Google Scholar]
- 2.Himmel ME, et al. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science. 2007;315:804–807. doi: 10.1126/science.1137016. [DOI] [PubMed] [Google Scholar]
- 3.Ogier JC, et al. Ethanol production from lignocellulosic biomass. Oil Gas Sci Technol. 1999;54:67–94. [Google Scholar]
- 4.Yu Z, Zhang H. Ethanol fermentation of acid-hydrolyzed cellulosic pyrolysate with Saccharomyces cerevisiae. Bioresour Technol. 2004;93:199–204. doi: 10.1016/j.biortech.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Chen F, Dixon RA. Lignin modification improves fermentable sugar yields for biofuel production. Nat Biotechnol. 2007;25:759–761. doi: 10.1038/nbt1316. [DOI] [PubMed] [Google Scholar]
- 6.Ezaki N, Kido N, Takahashi K, Katou K. The role of wall Ca2+ in the regulation of wall extensibility during the acid-induced extension of soybean hypocotyl cell walls. Plant Cell Physiol. 2005;46:1831–1838. doi: 10.1093/pcp/pci199. [DOI] [PubMed] [Google Scholar]
- 7.Willats WG, et al. Modulation of the degree and pattern of methyl esterification of pectic homogalacturonan in plant cell walls: Implications for pectin methyl esterase action, matrix properties and cell adhesion. J Biol Chem. 2001;276:19404–19413. doi: 10.1074/jbc.M011242200. [DOI] [PubMed] [Google Scholar]
- 8.Zhang GF, Staehelin LA. Functional compartmentation of the golgi-apparatus of plant-cells—Immunocytochemical analysis of high-pressure frozen-substituted and freeze-substituted sycamore maple suspension-culture cells. Plant Physiol. 1992;99:1070–1083. doi: 10.1104/pp.99.3.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ridley BL, O’Neill MA, Mohnen D. Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry. 2001;57:929–967. doi: 10.1016/s0031-9422(01)00113-3. [DOI] [PubMed] [Google Scholar]
- 10.Grabber JH, Ralph J, Hatfield RD. Cross-linking of maize walls by ferulate dimerization and incorporation into lignin. J Agric Food Chem. 2000;48:6106–6113. doi: 10.1021/jf0006978. [DOI] [PubMed] [Google Scholar]
- 11.Capodicasa C, et al. Targeted modification of homogalacturonan by transgenic expression of a fungal polygalacturonase alters plant growth. Plant Physiol. 2004;135:1294–1304. doi: 10.1104/pp.104.042788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrari S, et al. Transgenic expression of a fungal endo-polygalacturonase increases plant resistance to pathogens and reduces auxin sensitivity. Plant Physiol. 2008;146:669–681. doi: 10.1104/pp.107.109686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lionetti V, et al. Overexpression of pectin methylesterase inhibitors in Arabidopsis restricts fungal infection by Botrytis cinerea. Plant Physiol. 2007;143:1871–1880. doi: 10.1104/pp.106.090803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raiola A, et al. Two Arabidopsis thaliana genes encode functional pectin methylesterase inhibitors. FEBS Lett. 2004;557:199–203. doi: 10.1016/s0014-5793(03)01491-1. [DOI] [PubMed] [Google Scholar]
- 15.Gomez LD, Steele-King CG, McQueen-Mason SJ. Sustainable liquid biofuels from biomass: The writing’s on the walls. New Phytol. 2008;178:473–485. doi: 10.1111/j.1469-8137.2008.02422.x. [DOI] [PubMed] [Google Scholar]
- 16.Wyman CE, et al. Comparative sugar recovery data from laboratory scale application of leading pretreatment technologies to corn stover. Bioresour Technol. 2005;96:2026–2032. doi: 10.1016/j.biortech.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Willats WG, Gilmartin PM, Mikkelsen JD, Knox JP. Cell wall antibodies without immunization: Generation and use of de- esterified homogalacturonan block-specific antibodies from a naive phage display library. Plant J. 1999;18:57–65. doi: 10.1046/j.1365-313x.1999.00427.x. [DOI] [PubMed] [Google Scholar]
- 18.Willats WG, et al. Analysis of pectic epitopes recognised by hybridoma and phage display monoclonal antibodies using defined oligosaccharides, polysaccharides, and enzymatic degradation. Carbohydr Res. 2000;327:309–320. doi: 10.1016/s0008-6215(00)00039-2. [DOI] [PubMed] [Google Scholar]
- 19.Carpita NC, McCann MC. Maize and sorghum: Genetic resources for bioenergy grasses. Trends Plant Sci. 2008;13:415–420. doi: 10.1016/j.tplants.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Di Matteo A, et al. Structural basis for the interaction between pectin methylesterase and a specific inhibitor protein. Plant Cell. 2005;17:849–858. doi: 10.1105/tpc.104.028886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang M, Su R, Qi W, He Z. Enhanced enzymatic hydrolysis of lignocellulose by optimizing enzyme complexes. Appl Biochem Biotechnol. 2009 doi: 10.1007/s12010-009-8602-3. in press. [DOI] [PubMed] [Google Scholar]
- 22.Derbyshire P, McCann MC, Roberts K. Restricted cell elongation in Arabidopsis hypocotyls is associated with a reduced average pectin esterification level. BMC Plant Biol. 2007;7:31. doi: 10.1186/1471-2229-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.An SH, et al. Pepper pectin methylesterase inhibitor protein CaPMEI1 is required for antifungal activity, basal disease resistance and abiotic stress tolerance. Planta. 2008;228:61–78. doi: 10.1007/s00425-008-0719-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christensen AH, Quail PH. Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res. 1996;5:213–218. doi: 10.1007/BF01969712. [DOI] [PubMed] [Google Scholar]
- 25.Janni M, et al. The expression of a bean polygalacturonase-inhibiting proteins in transgenic wheat confers increased resistance to the fungal pathogen Bipolaris sorokiniana. Mol Plant-Microbe Interact. 2008;21:171–177. doi: 10.1094/MPMI-21-2-0171. [DOI] [PubMed] [Google Scholar]
- 26.Chang TT, Konzak CF, Zadoks JC. A Decimal Code for the Growth Stages of Cereals. Weed Res. 1974;14:415–421. [Google Scholar]
- 27.Lever M. A new reaction for colorimetric determination of carbohydrates. Anal Biochem. 1972;47:273–279. doi: 10.1016/0003-2697(72)90301-6. [DOI] [PubMed] [Google Scholar]
- 28.Dubois M, et al. Colorimetric methods for determination of sugars and related substances. Anal Chem. 1956;28:350–358. [Google Scholar]
- 29.Willats WG, et al. Analysis of pectic epitopes recognised by hybridoma and phage display monoclonal antibodies using defined oligosaccharides, polysaccharides, and enzymatic degradation. Carbohydr Res. 2000;327:309–320. doi: 10.1016/s0008-6215(00)00039-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.