Abstract
The development of vaccines and other immunotherapies has been complicated by heterogeneous antigen display and the use of incompletely defined immune adjuvants with complex mechanisms of action. We have observed strong antibody responses in mice without the coadministration of any additional adjuvant by noncovalently assembling a T and B cell epitope peptide into nanofibers using a short C-terminal peptide extension. Self-assembling peptides have been explored recently as scaffolds for tissue engineering and regenerative medicine, but our results indicate that these materials may also be useful as chemically defined adjuvants. In physiological conditions, these peptides self-assembled into long, unbranched fibrils that displayed the epitope on their surfaces. IgG1, IgG2a, and IgG3 were raised against epitope-bearing fibrils in levels similar to the epitope peptide delivered in complete Freund’s adjuvant (CFA), and IgM production was even greater for the self-assembled epitope. This response was dependent on self-assembly, and the self-assembling sequence was not immunogenic by itself, even when delivered in CFA. Undetectable levels of interferon-gamma, IL-2, and IL-4 in cultures of peptide-challenged splenocytes from immunized mice suggested that the antibody responses did not involve significant T cell help.
Keywords: biomaterial, epitope, nanofiber, self-assembly, vaccine
The development of vaccines and other immunotherapies has been challenged by imprecise antigen display and the use of heterogeneous immune adjuvants whose mechanisms of action are complex and incompletely understood. Synthetic peptides are useful as antigens because their precise chemical definition allows one to specify the exact epitopes against which an immune response is to be raised. However, peptides are poorly immunogenic by themselves and require coadministration with strong adjuvants. Although many adjuvants have been investigated for peptide immunotherapies to date, current strategies such as particulates (1, 2), oil emulsions (3), toll-like receptor (TLR) ligands (4), immunostimulating complexes (ISCOMs) (5), and other biologically sourced materials (6, 7) utilize chemically or structurally heterogeneous materials, making their characterization, mechanistic understanding, and regulatory approval challenging (1, 8, 9). This situation has motivated the pursuit of self-adjuvanting or adjuvant-free systems (10–12).
A broad goal in the field of biomaterials is to produce synthetic scaffolds capable of presenting multiple cell-interactive components in spatially resolved networks (13, 14). To accomplish this, supramolecular self-assembly is rapidly becoming a synthetic method of choice (15–17). One strategy that has received attention recently is based on fibrillized peptides, peptidomimetics, and peptide derivatives, which are being explored for a variety of biomedical and biotechnological applications, most notably as scaffolds for regenerative medicine (18, 19) and defined matrices for cell culture (20, 21). In these applications, self-assembled materials provide several advantages, including multifunctionality, multivalency, synthetic definition, molecular specificity, and control over the nanoscale positioning of ligands and other biomolecular features (17). In our laboratory, we have previously developed self-assembled, multicomponent matrices for cell culture using a short fibrillizing peptide, Q11 (Ac-QQKFQFQFEQQ-Am) (16, 17, 22, 23). This peptide, like other previously reported short fibrillizing peptides (21, 24–26), β-hairpins (27), peptide-amphiphiles (19, 28), and peptide derivatives (29), self-assembles in salt-containing aqueous environments to form networks of β-sheet-rich nanofibers. It is also capable of displaying functional amino acid sequences or chemical groups on the surface of its self-assembled fibers. For example, adding cell-binding amino acid sequences to the N terminus of Q11 leads to self-assembled fibrils that functionally present the cell-binding peptides on their surfaces (16). Q11 with an N-terminal cysteine and a C-terminal thioester can undergo native chemical ligation after assembly, which can be used to stiffen the fibrillar network (23). These peptides can also be mixed to display precise combinations of different ligands (16).
During initial development as scaffolds for regenerative medicine, Q11 and other self-assembling peptide-based materials have been found to be minimally immunogenic. In previous work, we found that Q11 and Q11 with N-terminal cell-binding RGDS sequences elicited little to no antibody responses in mice (16). Negligible tissue responses were also observed for β-sheet fibrillizing RAD16-II peptide assemblies injected within rat (30) or mouse (18) myocardium. Low antibody titers have also been reported in rabbits and goats for RAD16 peptides (24). Only one study to our knowledge has observed an inflammatory response to RAD16 peptides in rats, but the causes were not known (31). The minimal immunogenicity of these materials observed to date is clearly advantageous for applications in regenerative medicine, but previous work has focused largely on amino acid sequences that are already found in endogenous proteins, for example the RGDS cell-binding sequence from fibronectin (16, 32). Because of this, strong epitopes have effectively been avoided. In the present work, we sought to determine the extent to which the previously observed low immunogenicity also applied to peptide sequences containing stronger epitopes. Our results indicate a surprisingly robust antibody response generated against a self-assembled peptide containing antigenic determinants from ovalbumin known to be recognized by both T cells and B cells. This indicates that in certain circumstances, self-assembled peptides can serve as powerful chemically defined adjuvants.
Results
Peptide Design and Supramolecular Assembly.
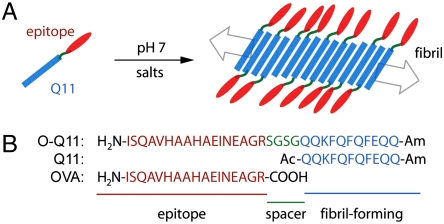
The peptide Q11 was previously designed as a self-assembling transglutaminase substrate (22) and was a variation on the DN1 peptide originally described by Aggeli and coworkers (25, 33). For the work reported here, we designed a peptide containing a Q11 self-assembling domain in tandem with OVA323-339, a 17-amino acid peptide from chicken egg ovalbumin (Fig. 1). OVA323-339 (ISQAVHAAHAEINEAGR, hereafter referred to as OVA) is an H-2b-restricted class II peptide containing multiple antigenic determinants, including known T and B cell epitopes (34). The self-assembling epitope peptide (O-Q11) was produced by solid phase synthesis and included a hydrophilic Ser-Gly-Ser-Gly spacer between the OVA and Q11 domains, with the OVA domain positioned at the N terminus (Fig. 1B).
Fig. 1.
Schematic (A) and sequences (B) of epitope-bearing self-assembling peptides. The Q11 domain (blue) assembles into fibrillar aggregates, displaying the epitope sequence (red) at the end of a flexible spacer (green).
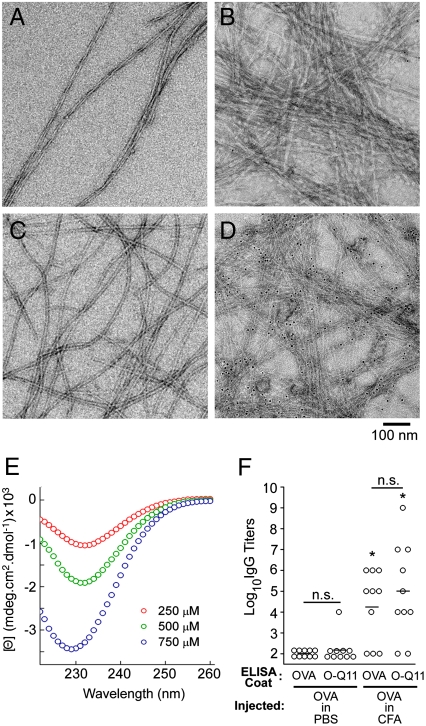
When dissolved in water at concentrations of 40 mM and lower, O-Q11 formed no visible precipitate. However, similar to Q11 and to other previously reported Q11 derivatives (16, 17, 22, 23), it formed networks of laterally entangled fibrils when it was first dissolved in water and then added to salt-containing buffers such as phosphate-buffered saline (PBS), (Fig. 1A, Fig. 2). By TEM, O-Q11 in PBS appeared as long, unbranched fibrils with widths of about 15 nm (Fig. 2C). Although the length of these fibrils was not directly measured, the relative scarcity of fibril ends in TEM images suggested that the fibril length was on the order of microns. Circular dichroism of O-Q11 showed a single concentration-dependent minimum at 229–232 nm (Fig. 2E). This spectrum is consistent with a high degree of β-sheet or β-turn structure, and it is similar to the spectra of Q11 and mixtures of Q11 with other previously reported ligand-bearing Q11 derivatives (16, 22).
Fig. 2.
Q11-based peptides self-assembled into β-sheet fibrils that displayed functional epitopes on their surfaces. Q11 (A) self-assembled into long, unbranched fibrils that did not bind streptavidin-gold (B). O-Q11 (C) also formed long, unbranched fibrils, and biotin-O-Q11 bound streptavidin-gold (D), indicating availability of the N terminus on the fibril surface. O-Q11 possessed a predominant β-sheet structure by CD (E). OVA antisera reacted similarly to ELISA plates coated with OVA or O-Q11 (F). Each point represents one mouse’s serum; bars represent the mean. *p < 0.01 compared to OVA coat/OVA in PBS injection, by ANOVA with Tukey HSD post hoc testing. n.s., not statistically different.
Epitopes Were Functionally Displayed on O-Q11 Fibrils.
The availability of the OVA epitope on the surface of O-Q11 nanofibers was confirmed by TEM and by ELISA. To label epitopes in TEM samples, an N-terminally biotinylated O-Q11 was synthesized with a single biotin tag directly adjacent to the OVA sequence. This biotinylated peptide produced fibrils that appeared morphologically similar to O-Q11 (Fig. 2D). Five-nanometer streptavidin-conjugated gold particles were then used to probe epitope availability on the fibril surface, with unmodified Q11 serving as a negative control. Biotin-O-Q11 fibrils stained strongly with streptavidin-gold, whereas Q11 samples bound negligible numbers of particles (Fig. 2B–D), demonstrating that a significant portion of the peptides’ N termini were available on the surface of O-Q11 fibrils. To confirm this finding and to quantify the availability of the entire epitope, ELISA was employed. Plates coated with OVA and O-Q11 peptides were probed with antisera from mice immunized with OVA, either with or without complete Freund’s adjuvant (CFA). Antisera from CFA-adjuvanted groups showed similar titers of OVA-reactive IgG, whether measured on OVA plates or on O-Q11 plates, and both showed low backgrounds for antisera from nonadjuvanted groups (Fig. 2F). This result indicated that the OVA epitope was functionally presented on the surface of the Q11 fibrils, and that plates coated with O-Q11 fibrils produced similar signal strengths to plates coated with the nonfibrillized OVA peptide, allowing the sera of mice immunized with different peptides to be compared. Slightly higher titers were observed for the O-Q11-coated plates in both cases, but it was not statistically significant.
High IgG Titers Were Elicited by O-Q11 Without Adjuvant in Mice.
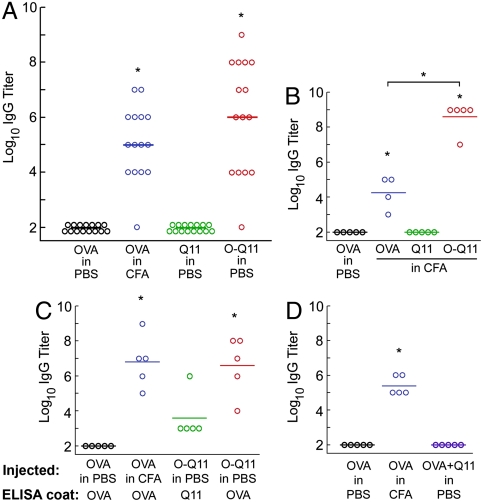
To investigate how fibrillization affected the immunogenicity of OVA, C57BL/6 mice were immunized subcutaneously with the different peptides and boosted with additional peptide at 28 d (see Methods). Serum was collected 7 d after the boost, and levels of various immunoglobulins were measured. It was found that fibrillized Q11 alone did not raise any detectable IgG, whether delivered with or without CFA (Fig. 3A and B, raw data in Fig. S1). This result reiterated our previous findings that Q11 was not immunogenic (16) and further indicated that Q11 continued to be nonimmunogenic even when delivered in CFA. Also, we previously showed that Q11 and functionalized Q11 peptides did not induce cell death in cultures of primary human endothelial cells, indicating that the basic Q11 sequence was noncytotoxic as well as nonimmunogenic (16, 23).
Fig. 3.
Fibrillization by the Q11 domain strongly adjuvanted IgG responses to OVA. Similar titers of total IgG were raised against O-Q11 delivered in PBS and OVA delivered in CFA, whereas Q11 or OVA delivered in PBS did not elicit a response (A). Q11 was nonimmunogenic even in CFA, whereas CFA increased IgG titers for O-Q11 (B). O-Q11 antisera were strongly cross-reactive to OVA-coated ELISA plates and showed a small amount of reactivity to Q11-coated plates that was not statistically significant (C). The adjuvant activity of Q11 was entirely dependent on covalent conjugation between the fibrillizing domain and epitope domain, as mixtures of Q11 and OVA did not raise any OVA-specific IgG (D). Each point represents one mouse; bars represent the mean. *p < 0.01 by ANOVA with Tukey HSD post hoc testing, compared with OVA in PBS, or between groups as indicated.
In surprising contrast to the immeasurably low immunogenicity of Q11, O-Q11 elicited high IgG titers without any added adjuvant (Fig. 3A, raw data in Fig. S1). Even higher titers were produced when it was delivered in CFA (Fig. 3B). Anti-peptide IgG titers were similar between mice injected with O-Q11 in PBS and OVA peptide in CFA, indicating that the Q11 sequence itself functioned as a strong adjuvant, presumably by assembling the OVA peptide into nanofibers. O-Q11 antisera also bound to OVA-coated plates, demonstrating that the epitope was conserved (Fig. 3C). O-Q11 antiserum reactivity to OVA-coated plates also excluded the possibility that the high antibody titers found in O-Q11 antisera were a measurement artifact arising from increased antigen density on fibril-coated ELISA plates. In addition, endotoxin levels were less than 0.3 EU/mL for all samples (Table S1), eliminating the possibility that inadvertent contamination caused the observed adjuvant effect. O-Q11 antisera also reacted with Q11, though at smaller, statistically insignificant levels (Fig. 3C), possibly indicating a small degree of epitope spreading to the Q11 domain.
We hypothesized that the multivalent surface display of the epitope on the fibrils was the source of Q11’s strong adjuvant properties, but alternate explanations were possible. For example, if Q11 functioned as an adjuvant by activating TLRs, similarly to LPS or unmethylated CpG motifs, or if it simply slowed the diffusion of the epitope from the injection site by surrounding it with fibrils (the so-called “depot effect”), then outright conjugation of the epitope and the fibril would not be required. Additionally, if Q11 fibrils functioned in a manner similar to particulate adjuvants such as aluminum salts, whereby the adsorption and entrapment of the antigen onto and within the particle is sufficient for adjuvancy, then conjugation would also not be required. We did not investigate TLR activation in this study, but to determine whether covalent conjugation between the epitope sequence and the self-assembling sequence was required for the strong antibody responses observed for O-Q11, we measured responses to unconjugated mixtures of Q11 peptide and OVA peptide. Notably, the strong antibody response generated by O-Q11 was completely abolished in the absence of covalent coupling between the Q11 fibrillizing domain and the epitope domain (Fig. 3D). No detectable IgG was observed for mixtures of OVA and Q11. This result indicated that Q11’s adjuvant properties were dependent on its covalent attachment to the epitope peptide.
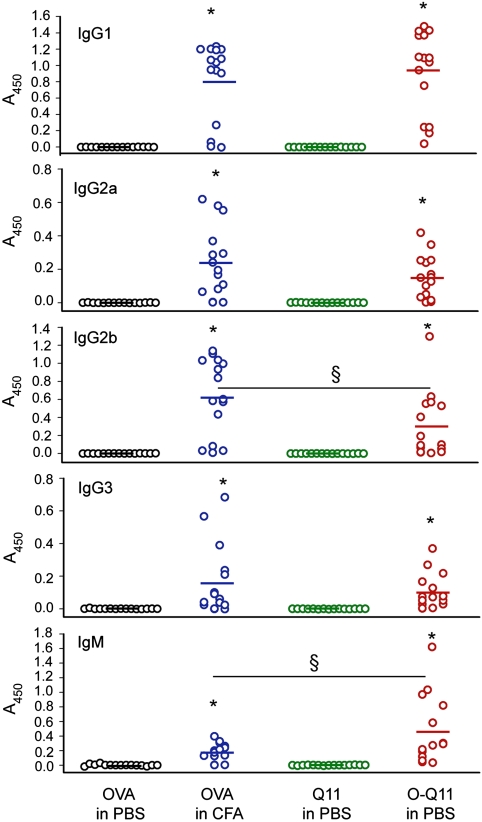
In order to determine the nature of the immune response to Q11-adjuvanted peptides, the isotypes of the responding antibodies were evaluated. For both OVA in CFA and fibrillized O-Q11, the dominant antibody isotype was IgG1, with smaller amounts of IgG2a, IgG2b, IgG3, and IgM being produced for both (Fig. 4). Comparing CFA-adjuvanted responses with Q11-adjuvanted responses, IgG1, IgG2a, and IgG3 were produced in statistically similar quantities, but IgG2b production was greater in the CFA-adjuvanted group, and IgM production was greater in the Q11-adjuvanted group.
Fig. 4.
Antibody isotypes in sera from mice immunized with peptides. Each point represents one mouse; bars represent the mean. *p < 0.01 compared with OVA delivered in PBS by ANOVA with Tukey HSD post hoc test. §p < 0.05 as indicated.
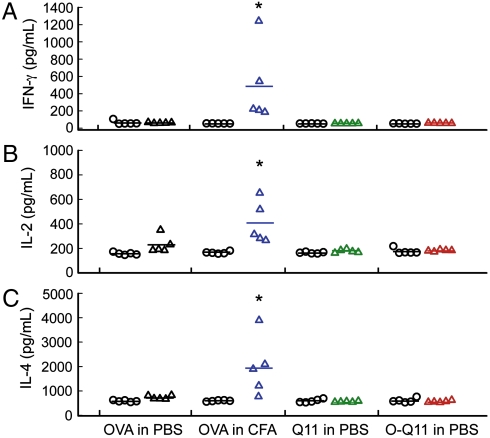
To investigate the involvement of T cell help in the immune responses to O-Q11, splenocytes from immunized mice were challenged in vitro with the peptides, and the production of interferon-γ (IFN-γ), interleukin-2 (IL-2), and interleukin-4 (IL-4) was measured. These three cytokines were selected to provide measures of either a Th1 response (IFN-γ and IL-2) or a Th2 response (IL-4). However, somewhat surprisingly, when splenocytes from immunized mice were challenged in vitro with the immunizing peptide, they did not produce significant levels of any of these three cytokines compared with positive controls (Fig. 5). This lack of a strong cytokine response, robust antibody production, and an elevated IgM response in the groups adjuvanted with Q11 is suggestive of a mechanism that does not depend strongly on T cell help.
Fig. 5.
IFN-γ, IL-2, and IL-4 production in peptide-challenged (triangles) and unchallenged (circles) splenocyte cultures. Each point represents one mouse; bars represent the mean. ∗p < 0.01 compared with corresponding unchallenged control, by ANOVA with Tukey HSD post hoc test.
Discussion
Simply attaching a short self-assembling amino acid sequence to a peptide epitope’s C terminus can dramatically enhance the peptide’s immunogenicity. Our results indicate that fibrillizing peptide domains may be useful as simple adjuvant systems for peptide-based immunotherapies, and these results also provide guidance for the development of nonimmunogenic peptide biomaterials within applications such as regenerative medicine. As a means to specifically enhance peptide immunogenicity, the unique Q11-based approach reported here has a number of advantages. First, a self-assembling peptide domain can be easily added to any known epitope using conventional solid phase peptide synthesis. Also, by utilizing self-assembly, highly multivalent nanoscale objects can be directly produced from only one molecule, which enables precision in the production, purity, and study of the material. In contrast, many adjuvants currently employed or under development are composed of multiple molecular constituents or heterogeneous mixtures, making their definition, formulation, purification, and characterization challenging. For example, immunotherapies based on attenuated live viruses or heat-killed organisms contain intrinsic adjuvants such as lipopolysaccharide or unmethylated CpG motifs that are critical to their efficacy (35). Adjuvants based on natural products such as saponins or squalene (7) are by nature associated with some degree of molecular heterogeneity, and particulate adjuvants such as aluminum salts depend on antigen adsorption or entrapment, processes that are complexly dependent on multiple chemical and physical factors during formulation (1, 35). Accordingly, specific molecular features of current adjuvants are not easily adjusted independently within a given vaccine formulation, making it difficult both to optimize an immunotherapy as well as understand its mechanism of action. Such efforts will be greatly enhanced with the availability of chemically defined adjuvants.
Molecular definition is becoming more favored in the development of immunotherapies, as reflected by the recent interest in microparticle- and nanoparticle-based approaches (2, 36, 37), and on multivalent polymeric ligands (38). Other multivalent synthetic epitopes, such as the polymers generated by ring-opening metathesis polymerization recently investigated by Kiessling and coworkers, also elicit significant antibody production (38). These polymers were investigated in vivo using CFA, however, so it is not clear whether they also induce strong responses without adjuvant. Another recent approach employing noncovalent aggregation was based on DNA vaccination and used 103 tandem glutamine repeats to oligomerize protein antigens (39); however, with DNA vaccination it may be challenging to tightly control the ultimate protein dose or the stoichiometries of multiple epitopes. In contrast, the molecular definition inherent in a synthetic self-assembling peptide enables precise dosing and should enable the predictable cofibrillization of multiple different epitope-displaying Q11 derivatives, as has been previously accomplished with cell-binding ligands (16). This may be a useful way to clarify the mechanism of Q11’s adjuvant properties and to optimize its use toward a range of therapeutic targets. Although definitive long-term safety studies have yet to be performed, it is encouraging that Q11 and other fibrillizing peptide biomaterials have been found to be noncytotoxic in cell culture (16, 23). At this time, however, it is not entirely clear what the molecular determinants of β-sheet fibril toxicity are, or why some β-sheet fibrils appear to be nontoxic, while others are toxic (40). This aspect requires clarification as fibrillar biomaterials are developed (41). In addition, the lack of a robust cytokine response in the present system may indicate that this approach may not provide for lasting B cell or T cell memory, necessary for vaccinations that would provide long-term immunity. Immunological memory was not investigated in this study, but if it is found in the future that peptide fibrils are unable to provide memory, it may be possible to combine the fibril-forming peptides with other chemically defined adjuvants or other epitopes via coassembly. Regardless of whether the present system is able to produce lasting memory, its ability to produce high antibody titers would be immediately useful in other applications such as bioreagent antibody production against specific peptide epitopes in animals.
Owing to the significant production of IgG1, IgG2a, IgG2b, IgG3, and IgM and the relative lack of IFN-γ, IL-2, and IL-4 in cultures of challenged splenocytes, it appears that this fibrillar adjuvant system induces an immune response that does not significantly involve T cell help. Although the molecular definition of the fibrils may allow a clearer understanding of this mechanism of action in the future, at present several explanations are possible, as is the case with other current adjuvants as well (1, 35). It is known that fibrillized peptides tend to be significantly stabilized against proteolytic attack, which would extend the residence time of the peptide at the injection site, the so-called “depot effect”. At the same time, it is possible that epitope fibrillization facilitates phagocytosis and activation of antigen-presenting cells. Also, while unlikely, it cannot be ruled out that some degree of TLR activation occurs with fibrillized antigens, because this aspect was not investigated directly. However, the nonimmunogenicity of Q11 codelivered with soluble OVA suggests against this possible mechanism. Although interaction between antigen-specific B cells and T helper cells is usually required for high-affinity antibody production and class switching from IgM to IgG, T cell-independent (TI) mechanisms of B cell activation and Ig class switching have been known for some time (42–44), and are the most likely explanation for the response observed with Q11. Previous studies with haptenated polymers have shown that highly multivalent synthetic antigens can activate B cells in the absence of T cell help, and O-Q11’s mechanism of action may be similar to these previously investigated TI antigens (45, 46). Studies in T cell-deficient mice could be used to clarify this mechanism.
Do the adjuvant properties of β-sheet fibrillized peptides mean that such biomaterials are globally at risk of being inappropriately immunogenic in biomedical applications such as regenerative medicine? We do not believe so, and it is more likely that a confluence of attributes is necessary for producing the strong immune responses we observed. First, there is mounting evidence that most self-assembled peptide biomaterials are well tolerated in vivo (16, 18, 19, 24, 30). In addition, antibodies could not be detected against the unmodified Q11 peptide, even when it was delivered in CFA or when soluble OVA was codelivered with it. Only when OVA was directly conjugated to the Q11 peptide were measurable antibody titers generated. In light of these results, it appears that the immunogenicity of fibrillized peptide biomaterials is dominated by the epitope present on the nanofibers to a much greater extent than the self-assembling nature of the material itself, and immunogenicity can be largely avoided simply by avoiding strong epitopes. Taken further, much of current biomaterials research deals with the biofunctionalization of solid materials, be they self-assembled or covalently constructed, particulate or macroscale, synthetic or biological. In all of these cases, underlying adjuvant activity of the biomaterials themselves is increasingly being shown to modulate the tissue response (47, 48), so the field as a whole will need to establish which modes of surface presentation of biomolecular features lead to nonimmunogenicity, tolerance, or immunogenicity. It is expected that molecularly defined systems such as the one we describe here will directly enable such future studies.
Conclusions
Peptide epitopes that were assembled into nanofibers by a short synthetic fibrillization domain elicited high antibody titers in the absence of any adjuvant. This response was dependent on covalent conjugation between the epitope and fibrillizing domain, and Q11 by itself was nonimmunogenic, even in CFA. Titers of IgG1, IgG2a, and IgG3 were similar between CFA-adjuvanted and Q11-adjuvanted groups, but IgG2b production was greater for CFA-adjuvanted responses, and IgM was greater for Q11-adjuvanted responses. Interferon-γ, IL-2, and IL-4 were not produced in measurable quantities for Q11-adjuvanted responses, which along with the enhanced IgM response suggests that this response is not strongly dependent on T cell help. This strategy represents a simple, chemically defined way of dramatically enhancing antibody responses to peptide epitopes.
Materials and Methods
Peptide Synthesis and Purification.
Peptides were synthesized using standard Fmoc chemistry as previously reported (16, 23). For TEM studies, O-Q11 was N-terminally biotinylated on-resin using biotin o-nitrophenyl ester. Peptides were purified using a Varian ProStar HPLC system, Grace-Vydac C18 reverse phase columns, and water-acetonitrile gradients. Peptide identity and purity were confirmed by MALDI-MS and HPLC, respectively (Table S1). Endotoxin levels of all immunizations were < 0.3 EU/mL by LAL chromogenic endpoint assay (Lonza), well within acceptable limits (Table S1). Endotoxin measurements were conducted on samples containing exactly the same or similar concentrations of peptides as the immunizations (see SI Text for detailed methods).
Circular Dichroism.
An AVIV 202 CD spectrometer (Aviv Biomedical, NJ), 0.1 cm path length quartz cells, and initial disaggregation in TFA were employed as previously reported (16). Working concentrations of 250 μM, 500 μM, and 750 μM were prepared in degassed water using Phe absorbance at 257 nm. Owing to peptide fibrillization and the resultant low CD signal strength, spectra below 220 nm could not be measured accurately and so are not reported.
Transmission Electron Microscopy.
Peptides were dissolved in deionized water and mixed 6∶1 with PBS to produce working peptide concentrations of 330 μM. After fibrillizing for 4 h, peptides were applied to 400 mesh gold grids with carbon support films, negative-stained with 1% uranyl acetate, and imaged on a Tecnai F30 TEM. For gold staining, prior to negative-staining grids were placed upside-down on a droplet of blocking solution (0.2% acetylated BSA, 0.1% gelatin from cold water fish skin in PBS) for 5 min, then for 2 h on a droplet of 5 nm colloidal gold conjugated to streptavidin (Sigma). Grids were washed once with blocking solution, twice with PBS, and stained with 1% uranyl acetate.
Immunizations.
Peptides were dissolved in sterile water (8 mM) and allowed to fibrillize overnight at 4 °C. Stock solutions were then diluted in sterile, endotoxin-free PBS to working concentrations. Female C57BL/6 mice (6–8 weeks old, Taconic Farms, IN) were each given two 50 μL subcutaneous injections near the shoulder blades, each injection containing 100 nmol of peptide. CFA-adjuvanted groups received the same volume and total peptide dose, prepared by emulsifying peptide/PBS solutions 1∶1 in CFA. Mice were boosted at 28 d with two additional 25 μL injections, each containing 50 nmol of peptide. CFA groups were boosted in incomplete Freund’s adjuvant (IFA). Mice receiving mixtures of Q11 and OVA received 100 nmol of each peptide in the first injection and 50 nmol of each in the second. Seven days after the boost, the mice were sacrificed, and sera and spleens were harvested. In all animal work, institutional guidelines for the care and use of laboratory animals were strictly followed under a protocol approved by the University of Chicago’s Institutional Animal Care and Use Committee.
Determination of Antibody Titers.
High-binding ELISA plates (eBioscience) were coated with either 20 μg/mL peptide in PBS or PBS alone (for uncoated background subtraction) overnight at 4 °C. Wells were blocked with 1% BSA/0.5% Tween 20 in PBS, and serial dilutions of serum between 1∶102 and 1∶109 were applied, followed by peroxidase-conjugated goat antimouse IgG (H+L) (Jackson Immuno Research). Washing steps were performed with 0.5% Tween 20 in PBS, plates were developed using TMB substrate (eBioscience), and absorbance values were read at 450 nm (absorbance values shown in Fig. S1). To determine titers for each antiserum, background absorbance values from uncoated wells were subtracted from coated wells, and net absorbances were compared to cutoff values. The cutoff consisted of the mean plus three times the standard deviation of the negative control group (mice receiving OVA without adjuvant) for each corresponding dilution. Any sample dilutions having absorbances above this cutoff value were considered positive readings. The titer was considered as the highest dilution for which it and all lower dilutions had positive readings. If no positive dilutions were present the titer was considered to be 102. Negative control mice (OVA without adjuvant) did not raise detectable IgG, and no single mouse in the negative control groups had absorbance values greater than three standard deviations above the group’s mean for a given dilution; therefore all negative control mice are reported as having titers of 102, which is the baseline level of detection for this study. Antibody isotypes were analyzed similarly using a mouse monoclonal antibody kit containing goat antimouse IgG1, IgG2a, IgG2b, IgG3, and IgM (Sigma). See SI Text for detailed methods.
Splenocyte Isolation and Challenge.
Spleens of the immunized mice were pressed through 70 μm cell strainers, and isolated splenocytes were washed in RPMI medium containing 10% FBS. Red blood cells were lysed using ACK buffer (150 mM NH4Cl, 10 mM KHCO3/0.1 mM EDTA) and washed twice. 1 × 106 cells/well (96 well plate) were plated in complete T cell medium (S-MEM supplemented with 3.75 mM dextrose, 0.9% L-glutamine, 0.6% essential amino acids, 1.26% nonessential amino acids, 0.9% sodium pyruvate, 9 mM sodium bicarbonate, 95 μM gentamycin, 140 μM penicillin-G, 60 μM streptomycin sulfate, 44 μM 2-mercaptoethanol, and 10% fetal bovine serum) containing 5 μg/ml challenging peptide or no peptide. After 24 h, interferon-γ (IFN-γ), IL-2, and IL-4 concentrations were measured in the culture medium using a sandwich ELISA format, capture antibodies for IFN-γ, IL-2, and IL-4, biotin-conjugated detection antibodies, and avidin-horseradish peroxidase (see SI for detailed methods). Antibodies and reagents were purchased from eBioscience, and cytokine concentrations were calculated from standard curves.
Statistical Analysis.
Statistical analysis was performed by ANOVA with Tukey’s HSD post hoc comparisons. The data reported in Figs. 3A and 4 represent three separate experiments, each containing five mice per group. Positive and negative controls were not statistically different between the three trials, and so the data were pooled into the groups of 15 shown.
Supplementary Material
Acknowledgments.
We thank Karl Matlin, Anita Chong, Roger Sciammas, Jose Guevara-Patino, James McCracken, Audrea Troutman, and David Hildeman for helpful discussions. This research was supported in part by the National Institutes of Health (NIDCR, grant number R21DE017703 and NIBIB grant numbers 7R21EB007335 and 1R01EB009701). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. TEM and CD were performed at the University of Chicago Electron Microscopy Facility and Biophysics Core Facility, respectively.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912124107/DCSupplemental.
References
- 1.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminum. Nat Rev Immunol. 2009;9:287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wendorf J, et al. A practical approach to the use of nanoparticles for vaccine delivery. J Pharm Sci. 2006;95:2738–2750. doi: 10.1002/jps.20728. [DOI] [PubMed] [Google Scholar]
- 3.Daftarian P, et al. Eradication of established HPV 16-expressing tumors by a single administration of a vaccine composed of a liposome-encapsulated CTL-T helper fusion peptide in a water-in-oil emulsion. Vaccine. 2006;24:5235–5244. doi: 10.1016/j.vaccine.2006.03.079. [DOI] [PubMed] [Google Scholar]
- 4.Ishii KJ, Akira S. Toll or toll-free adjuvant path toward the optimal vaccine development. J Clin Immunol. 2007;27:363–371. doi: 10.1007/s10875-007-9087-x. [DOI] [PubMed] [Google Scholar]
- 5.Maraskovsky E, et al. Development of prophylactic and therapeutic vaccines using the ISCOMATRIX adjuvant. Immunol Cell Biol. 2009;87:371–376. doi: 10.1038/icb.2009.21. [DOI] [PubMed] [Google Scholar]
- 6.McSorley SJ, Ehst BD, Yu Y, Gewirtz AT. Bacterial flagellin is an effective adjuvant for CD4+ T cells in vivo. J Immunol. 2002;169:3914–3919. doi: 10.4049/jimmunol.169.7.3914. [DOI] [PubMed] [Google Scholar]
- 7.Sun HX, Xie Y, Ye YP. Advances in saponin-based adjuvants. Vaccine. 2009;27:1787–1796. doi: 10.1016/j.vaccine.2009.01.091. [DOI] [PubMed] [Google Scholar]
- 8.Lambrecht BN, Kool M, Willart MA, Hammad H. Mechanism of action of clinically approved adjuvants. Curr Opin Immunol. 2009;21:23–29. doi: 10.1016/j.coi.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Purcell AW, McCluskey J, Rossjohn J. More than one reason to rethink the use of peptides in vaccine design. Nat Rev Drug Discov. 2007;6:404–414. doi: 10.1038/nrd2224. [DOI] [PubMed] [Google Scholar]
- 10.Bettahi I, et al. Antitumor activity of a self-adjuvanting glyco-lipopeptide vaccine bearing B cell, CD4+ and CD8+ T cell epitopes. Cancer Immunol Immunother. 2009;58:187–200. doi: 10.1007/s00262-008-0537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao CH, Lin XY, Wahi MM, Jackson EA, Potter H. Successful adjuvant-free vaccination of BALB/c mice with mutated amyloid beta peptides. BMC Neurosci. 2008;9:25. doi: 10.1186/1471-2202-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toth I, Simerska P, Fujita Y. Recent advances in design and synthesis of self-adjuvanting lipopeptide vaccines. Int J Pept Res Ther. 2008;14:333–340. [Google Scholar]
- 13.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 14.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8:457–470. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 15.Silva GA, et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303:1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 16.Jung JP, et al. Co-assembling peptides as defined matrices for endothelial cells. Biomaterials. 2009;30:2400–2410. doi: 10.1016/j.biomaterials.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collier JH. Modular self-assembling biomaterials for directing cellular responses. Soft Matter. 2008;4:2310–2315. doi: 10.1039/b805563g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis ME, et al. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation. 2005;111:442–450. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tysseling-Mattiace VM, et al. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J Neurosci. 2008;28:3814–3823. doi: 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Genove E, Shen C, Zhang S, Semino CE. The effect of functionalized self-assembling peptide scaffolds on human aortic endothelial cell function. Biomaterials. 2005;26:3341–3351. doi: 10.1016/j.biomaterials.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Horii A, Wang X, Gelain F, Zhang S. Biological designer self-assembling peptide nanofiber scaffolds significantly enhance osteoblast proliferation, differentiation and 3-D migration. PLoS ONE. 2007;2:e190. doi: 10.1371/journal.pone.0000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collier JH, Messersmith PB. Enzymatic modification of self-assembled peptide structures with tissue transglutaminase. Bioconjugate Chem. 2003;14:748–755. doi: 10.1021/bc034017t. [DOI] [PubMed] [Google Scholar]
- 23.Jung JP, Jones JL, Cronier SA, Collier JH. Modulating the mechanical properties of self-assembled peptide hydrogels via native chemical ligation. Biomaterials. 2008;29:2143–2151. doi: 10.1016/j.biomaterials.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holmes TC, et al. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc Natl Acad Sci USA. 2000;97:6728–6733. doi: 10.1073/pnas.97.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aggeli A, et al. Responsive gels formed by the spontaneous self-assembly of peptides into polymeric beta-sheet tapes. Nature. 1997;386:259–262. doi: 10.1038/386259a0. [DOI] [PubMed] [Google Scholar]
- 26.Gras SL, et al. Functionalised amyloid fibrils for roles in cell adhesion. Biomaterials. 2008;29:1553–1562. doi: 10.1016/j.biomaterials.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 27.Schneider JP, et al. Responsive hydrogels from the intramolecular folding and self-assembly of a designed peptide. J Am Chem Soc. 2002;124:15030–15037. doi: 10.1021/ja027993g. [DOI] [PubMed] [Google Scholar]
- 28.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294:1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 29.Zhou M, et al. Self-assembled peptide-based hydrogels as scaffolds for anchorage-dependent cells. Biomaterials. 2009;30:2523–2530. doi: 10.1016/j.biomaterials.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Hsieh PCH, Davis ME, Gannon J, MacGillivray C, Lee RT. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J Clin Invest. 2006;116:237–248. doi: 10.1172/JCI25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubois G, et al. Self-assembling peptide nanofibers and skeletal myoblast transplantation in infarcted myocardium. J Biomed Mater Res B Appl Biomater. 2008;87:222–228. doi: 10.1002/jbm.b.31099. [DOI] [PubMed] [Google Scholar]
- 32.Guler MO, et al. Presentation of RGDS epitopes on self-assembled nanofibers of branched peptide amphiphiles. Biomacromolecules. 2006;7:1855–1863. doi: 10.1021/bm060161g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riley JM, Aggeli A, Koopmans RJ, McPherson MJ. Bioproduction and characterization of a pH responsive self-assembling peptide. Biotechnol Bioeng. 2009;103:241–251. doi: 10.1002/bit.22274. [DOI] [PubMed] [Google Scholar]
- 34.Yang M, Mine Y. Novel T-cell epitopes of ovalbumin in BALB/c mouse: Potential for peptide-immunotherapy. Biochem Biophys Res Commun. 2009;378:203–208. doi: 10.1016/j.bbrc.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 35.McKee AS, Munks MW, Marrack P. How do adjuvants work? Important considerations for new generation adjuvants. Immunity. 2007;27:687–690. doi: 10.1016/j.immuni.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Carcaboso AM, et al. Enhancing immunogenicity and reducing dose of microparticulated synthetic vaccines: Single intradermal administration. Pharm Res. 2004;21:121–126. doi: 10.1023/b:pham.0000012159.20895.5b. [DOI] [PubMed] [Google Scholar]
- 37.Reddy ST, et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 38.Puffer EB, Pontrello JK, Hollenbeck JJ, Kink JA, Kiessling LL. Activating B cell signaling with defined multivalent ligands. ACS Chem Biol. 2007;2:252–262. doi: 10.1021/cb600489g. [DOI] [PubMed] [Google Scholar]
- 39.Ilyinskii PO, Thoidis G, Sherman MY, Shneider A. Adjuvant potential of aggregate-forming polyglutamine domains. Vaccine. 2008;26:3223–3226. doi: 10.1016/j.vaccine.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 40.Fowler DM, Koulov AV, Balch WE, Kelly JW. Functional amyloid—from bacteria to humans. Trends Biochem Sci. 2007;32:217–224. doi: 10.1016/j.tibs.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Jung JP, Gasiorowski JZ, Collier JH. Fibrillar peptide gels in biotechnology and biomedicine. Biopolymers. 2010 doi: 10.1002/bip.21326. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 43.Wen L, et al. Immunoglobulin-synthesis and generalized autoimmunity in mice congenitally deficient in alpha-beta(+)T cells. Nature. 1994;369:654–658. doi: 10.1038/369654a0. [DOI] [PubMed] [Google Scholar]
- 44.Test ST, Mitsuyoshi J, Connolly CC, Lucas AH. Increased immunogenicity and induction of class switching by conjugation of complement C3d to pneumococcal serotype 14 capsular polysaccharide. Infect Immun. 2001;69:3031–3040. doi: 10.1128/IAI.69.5.3031-3040.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dintzis HM, Dintzis RZ, Vogelstein B. Molecular determinants of immunogenicity—immunon model of immune-response. Proc Natl Acad Sci USA. 1976;73:3671–3675. doi: 10.1073/pnas.73.10.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vos Q, Lees A, Wu ZQ, Snapper CM, Mond JJ. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol Rev. 2000;176:154–170. doi: 10.1034/j.1600-065x.2000.00607.x. [DOI] [PubMed] [Google Scholar]
- 47.Jones KS. Biomaterials as vaccine adjuvants. Biotechnol Prog. 2008;24:807–814. doi: 10.1002/btpr.10. [DOI] [PubMed] [Google Scholar]
- 48.Sefton MV, Babensee JE, Woodhouse KA. Innate and adaptive immune responses in tissue engineering. Semin Immunol. 2008;20:83–85. doi: 10.1016/j.smim.2007.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.