Abstract
The intracellular signaling mechanisms regulating the generation and long-term persistence of memory T cells in vivo remain unclear. In this study, we used mouse models with conditional deletion of the key T cell receptor (TCR)-coupled adaptor molecule SH2-domain-containing phosphoprotein of 76 kDa (SLP-76), to analyze signaling mechanisms for memory CD4 T cell generation, maintenance, and homeostasis. We found that ablation of SLP-76 expression after T cell priming did not inhibit generation of phenotypic effector or memory CD4 T cells; however, the resultant SLP-76–deficient memory CD4 T cells could not produce recall cytokines in response to TCR-mediated stimulation and showed decreased persistence in vivo. In addition, SLP-76–deficient memory CD4 T cells exhibited reduced steady-state homeostasis and were impaired in their ability to homeostatically expand in vivo in response to the γc cytokine IL-7, despite intact proximal signaling through the IL-7R–coupled JAK3/STAT5 pathway. Direct in vivo deletion of SLP-76 in polyclonal memory CD4 T cells likewise led to impaired steady-state homeostasis as well as impaired homeostatic responses to IL-7. Our findings demonstrate a dominant role for SLP-76–dependent TCR signals in regulating turnover and perpetuation of memory CD4 T cells and their responses to homeostatic cytokines, with implications for the selective survival of memory CD4 T cells following pathogen exposure, vaccination, and aging.
Keywords: immune memory, signal transduction, linker-adapter
The enhanced functional and survival properties of memory T cells enable them to provide long-lasting secondary responses to recall antigens. Memory T cells are generated following antigen activation of naive T cells (1) and differ from naive T cells in their rapid production of effector cytokines following antigenic stimulation through the T cell receptor (TCR). The TCR-coupled signaling pathways for naive T cell activation have been well defined (2) and include an initial phosphorylation of TCR/CD3 components, leading to activation of the ZAP-70 proximal kinase, and the coupling of proximal phosphorylation events to distal signaling through linker-adapter molecules such as the SH2-containing phosphoprotein of 76 kDa (SLP-76) and linker for activated T cells (LAT) (3). The specific TCR-coupled signaling events important for promoting memory T cell development and persistence remain undefined.
Once generated, memory T cells have variable requirements for TCR engagement for their maintenance. Whereas memory CD8 T cells can persist and maintain recall function in the absence of MHC class I expression (4), memory CD4 T cells exhibit impaired functional responses when maintained in the absence of MHC class II expression (5, 6), although they can survive in MHC class II–deficient hosts (5, 7, 8). In addition, the presence of MHC class II or TCR signaling is associated with improved memory CD4 T cell survival and homeostasis (9–11), suggesting that memory CD4 T cells may depend on TCR signals during their long-term maintenance. Cytokines within the γc family, particularly IL-7, have also been shown to be required for long-term survival and homeostasis of memory CD4 T cells (12, 13), although whether TCR signals can compensate for and/or influence cytokine responses in memory T cells has not been demonstrated.
We have performed an extensive analysis of TCR-coupled signaling pathways in memory CD4 T cells to identify biochemical intermediates involved in their generation, function, and maintenance (14, 15). Importantly, we found differences in the expression and phosphorylation of SLP-76 in naive and memory CD4 T cells (15), suggesting that TCR signaling through SLP-76 may be critical in the pathway to memory T cell development and/or maintenance. SLP-76 is required for TCR-mediated IL-2 production, cytoskeletal reorganization, and calcium flux in primary T cells or T cell lines (3, 16, 17), although its role in memory T cell signaling and function remains undefined. However, SLP-76 deficiency in vivo results in a lack of peripheral T cells due to an early block in thymopoiesis (18, 19), precluding the use of fixed genetic knockouts to investigate the role of SLP-76 in memory development and persistence.
In this study, we used mice with conditional SLP-76 expression to dissect the role of SLP-76-dependent TCR signaling in memory CD4 T cell generation, maintenance, and homeostasis. We developed a unique system for deletion of SLP-76 expression after CD4 T cell priming by administration of a TAT-Cre recombinant fusion protein and also via drug-induced Cre activity and SLP-76 deletion. We found that SLP-76–deficient effector cells developed into phenotypic memory CD4 T cells in vivo, albeit at reduced persistence. SLP-76–deficient memory CD4 T cells generated by adoptive transfer or by direct drug-induced deletion were unable to produce recall cytokines in response to TCR engagement. Moreover, SLP-76–deficient memory CD4 T cells were significantly impaired in steady-state homeostasis and in vivo responses to IL-7, despite intact early cytokine signaling through the γc pathway. Our findings demonstrate a dominant role for SLP-76–dependent TCR signals in regulating turnover and perpetuation of memory CD4 T cells and in sustaining their homeostatic responses to cytokines.
Results
SLP-76 Deletion in Primed CD4 T Cells.
To study the role of SLP-76 in memory T cell development and maintenance, we used a mouse model for conditional deletion of SLP-76 expression by the Cre recombinase protein (16). Mice containing a knock-in SLP-76 allele flanked by LoxP sites (“Floxed”) heterozygous with either a null allele (slp76F/null) or an intact WT allele (slp76F/+) of SLP-76 (16) were crossed to ROSA26yfp (R26Ryfp) Cre-reporter mice (20) with transgenic expression of the yellow fluorescent protein (YFP) gene preceded by a floxed STOP cassette. The resultant SLP-76F/nullR26Ryfp (F/null) mice and SLP-76F/+R26Ryfp (F/+) mice (Fig. S1A) both had wild-type levels of thymic and peripheral CD4 and CD8 T cells (16). Introduction of the Cre recombinase deletes the floxed alleles of SLP-76 and the R26R stop cassette, resulting in SLP-76Δ/nullYFP+ (conditional knockout, cKO) and SLP-76Δ/+YFP+ (conditional heterozygous control, cHET) phenotypes, respectively (Fig. S1A).
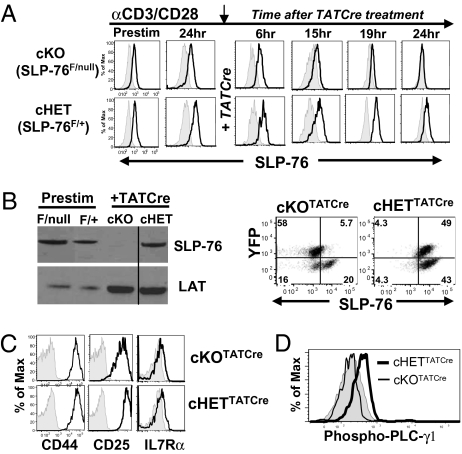
We determined the kinetics of YFP up-regulation and concomitant down-regulation of SLP-76 protein expression in activated CD4 T cells from F/null and F/+ mice, after administration of Cre recombinase in the form of a fusion protein with HIV Tat protein (TATCre) (21), enabling direct entry into cells. CD4 T cells from F/null and F/+ mice were activated in vitro for 24 h with anti-CD3 and anti-CD28 antibodies, treated with TATCre, and YFP and SLP-76 expression was monitored 6–24 h later. We found that YFP expression appeared within 15 h after TATCre administration and was maximal by 24 h in both cKO and cHET CD4 T cells (Fig. S1B). Coincident with YFP up-regulation, SLP-76 expression decreased to the level of isotype control 15–20 h post-TATCre in cKO YFP+CD4 T cells (Fig. 1A, Upper row), yet persisted at levels substantially above isotype control in cHET YFP+CD4 T cells (Fig. 1A, Lower row). By 48 h after TATCre treatment, both Western blot (Fig. 1B, Left) and flow cytometry (Fig. 1B, Right) analyses show that YFP+ cKOTATCre CD4 T cells were SLP-76 negative, whereas YFP+ cHETTATCre CD4 T cells were SLP-76+. These results demonstrate efficient ablation of SLP-76 expression in the YFP+ population of cKOTATCre CD4 T cells within 24 h post-TATCre treatment.
Fig. 1.
Downmodulation of SLP-76 expression after T cell activation using a conditional knockout mouse model. (A) Kinetics of SLP-76 knockdown. Splenic CD4+ T cells from SLP-76F/nullR26Ryfp (F/null) and SLP-76F/+R26Ryfp (F/+) mice were activated with anti-CD3/anti-CD28 antibodies and cultured with TATCre protein, and SLP-76 expression was determined by flow cytometry 6–24 h later. Histograms show intracellular SLP-76 expression gated on total CD4+ T cells or YFP+CD4+ T cells (for 19 and 24 h). Shaded histograms represent isotype controls. (B) SLP-76 deletion occurs in the YFP+ fraction of cKOTATCre cells. (Left) Western blot of SLP-76 and LAT expression in CD4 T cells from F/null and F/+ mice before stimulation (Prestim) and sorted, CD4+YFP+ cKO or cHET cells after TATCre. The line separates the noncontiguous lanes. (Right) Flow cytometric analysis of YFP versus SLP-76 expression in cKOTATCre and cHETTATCre CD4 T cells analyzed 48 h post-TATCre administration, gated on CD4+ T cells, representative of seven experiments. (C) Surface expression of CD44, CD25, and IL-7Rα on cKOTATCre (Upper) and cHETTATCre (Lower) primed cells gated on CD4+YFP+cells. Results are representative of six experiments. (D) Intracellular phospho-PLC-γ1 expression of cKOTATCre and cHETTATCre primed CD4 T cells gated on CD4+YFP+cells, with shaded histograms denoting control. Results are representative of three experiments.
We examined whether deletion of SLP-76 after priming would affect the survival and activation state of the resultant effector cells. Both cKOTATCre and cHETTATCre YFP+ CD4 T cells persisted in vitro in similar numbers 48 h after priming and TATCre administration as above (Fig. S2A) and exhibited surface phenotypes characteristic of activated cells, including up-regulation of CD25 and CD44 and down-regulation of IL-7R (22) (Fig. 1C). However, primed SLP-76-deficient cKOTATCre CD4 T cells exhibited reduced phosphorylation of the TCR signaling intermediate PLC-γ (Fig. 1D), known to be coupled to SLP-76 signaling in T cells (17, 23), and reduced IL-2 and IFN-γ production compared to control cHETTATCre effector CD4 T cells (Fig. S2B). These results indicate that deletion of SLP-76 following TCR stimulation does not alter the phenotype of primed CD4 T cells, but reduces TCR-mediated signaling and function.
Memory CD4 T Cell Generation in the Absence of SLP-76.
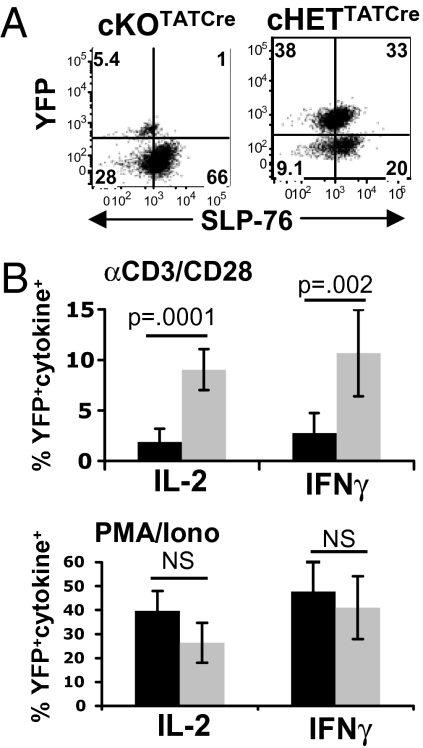
We next asked whether primed CD4 T cells deficient in SLP-76 expression could develop into memory CD4 T cells when transferred into lymphocyte-deficient or intact adoptive hosts in vivo. We transferred primed cKOTATCre and cHETTATCre CD4 T cells (Fig. 1B) initially into lymphocyte-deficient RAG2−/− hosts and found that cKOTATCre CD4 T cells maintained SLP-76 deficiency and were recovered at lower frequencies compared to SLP-76+ cHETTATCre CD4 T cells (Fig. 2A). Functionally, ex vivo stimulation with TCR/CD3 cross-linking resulted in significant frequencies of rapid IFN-γ and IL-2 producers from cHETTATCre CD4 T cells, with negligible cytokine production from cKOTATCre CD4 T cells (Fig. 2B and Fig. S3). By contrast, comparable frequencies of rapid IFN-γ and IL-2 producers resulted from stimulation of cKOTATCre and cHETTATCre CD4 T cells with phorbol 12-myristate 13-acetate (PMA)/ionomycin, which bypasses TCR-mediated SLP-76 signaling. These results indicate that persisting cKOTATCre and cHETTATCre CD4 T cells were similarly primed for Th1 cytokine production, but that ablation of SLP-76 expression following priming inhibited the ability of the TCR to signal for rapid production of IL-2 and IFN-γ.
Fig. 2.
In vivo transfer of primed SLP-76-deficient CD4 T cells. CD4 T cells from cKO and cHET mice were primed as in Fig.1, transferred into RAG2−/− adoptive hosts, and recovered 2 weeks posttransfer. (A) SLP-76 and YFP expression of persisting cKOTATCre and cHETTATCre CD4 T cells, representative of three independent experiments. (B) IL-2 and IFN-γ production by persisting cKOTATCre and cHETTATCre CD4 T cells stimulated for 6 h with anti-CD3/anti-CD28 antibodies (Upper) or PMA/ionomycin (Lower) as determined by intracellular cytokine staining. Results are expressed as mean percentage of cytokine+YFP+ CD4 T cells (n = 6), from two experiments.
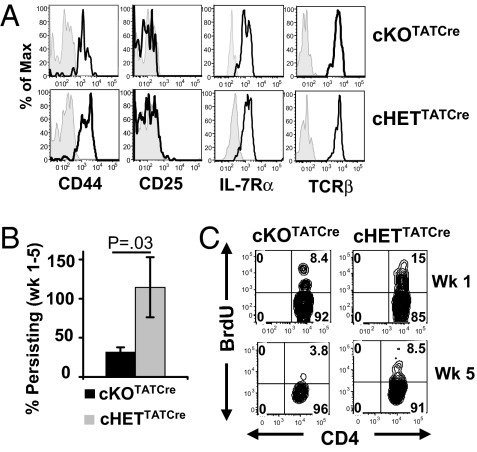
We also examined the ability of cKOTATCre and cHETTATCre memory CD4 T cells to persist in intact hosts. CD4 T cells from cKO and cHET mice were primed with anti-CD3/CD28 antibodies for 72 h, treated with TATCre in vitro, and directly transferred into congenic B6.CD45.1 hosts. After 4–6 weeks in vivo, persisting cKOTATCre YFP+ CD4 T cells remained SLP-76 deficient (Fig. S4A) and exhibited a resting memory phenotype (CD44hi/CD25lo/IL-7R+) and surface TCR expression comparable to that of cHETTATCre YFP+ CD4 T cells (Fig. 3A). Despite similar phenotypes, the number of persisting cKOTATCre YFP+ CD4 T cells was markedly diminished 5 weeks posttransfer compared with cHETTATCre YFP+ cells (Fig. 3B and Fig. S4B). We investigated whether the attrition of SLP-76–deficient memory CD4 T cells was caused by altered in vivo turnover and homeostasis by BrdU incorporation. At 1 week posttransfer, a lower proportion of cKOTATCre YFP+ CD4 T cells incorporated BrdU compared to cHETTATCre YFP+ CD4 T cells (Fig. 3C), and by 5 weeks posttransfer, cKOTATCre memory CD4 T cells displayed negligible BrdU incorporation, whereas a significant proportion of cHETTATCre memory CD4 T cells incorporated BrdU (Fig. 3C and Fig. S4C). The persisting memory CD4 T cells from both groups did not exhibit a difference in expression of the anti-apoptotic molecule Bcl-2 (Fig. S4D), suggesting that the diminished persistence of SLP-76–deficient memory CD4 T cells may be directly due to their reduced homeostatic turnover in vivo.
Fig. 3.
Reduced persistence and homeostasis of SLP-76–deficient memory CD4 T cells. CD4 T cells from cKO and cHET mice were primed in vitro for 72 h, TATCre treated, and transferred into B6.CD45.1 hosts. Persisting cKOTATCre and cHETTATCre memory CD4 T cells were harvested from spleen and lymph nodes 1–5 weeks posttransfer. (A) Surface expression of CD44, CD25, IL-7Rα, and TCRβ gated on CD45.2+CD4+YFP+ cells of cKOTATCre and cHETTATCre memory CD4 T cells 5 weeks posttransfer. Shaded histograms are controls. Results are representative of three experiments (n = 5 mice per group). (B) Persistence of cKOTATCre and cHETTATCre CD4+YFP+ memory CD4 T cells in adoptive hosts calculated by dividing the average number of YFP+ memory T cells at >5 weeks posttransfer by the average number of YFP+ cells at day 6 posttransfer, with n = 12–14 mice for cKOTATCre and n = 9–10 mice for cHETTATCre. (C) Flow cytometry plots of BrdU incorporation in cKOTATCre and cHETTATcre memory CD4 T cells gated on YFP+CD45.2+ CD4+ cells.
Impaired Homeostatic Turnover in SLP-76–Deficient Memory CD4 Cells to IL-7 in Vivo.
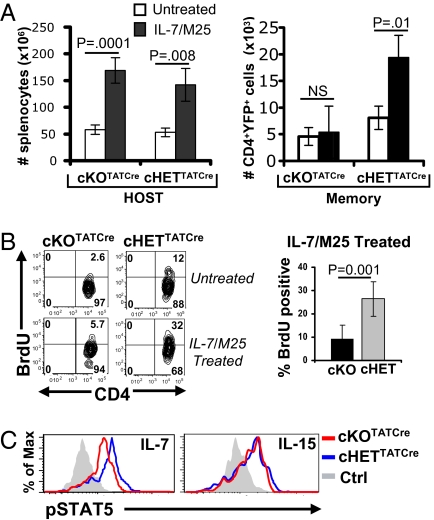
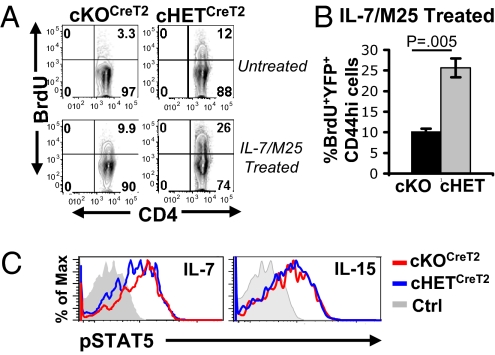
Previous studies have identified a dominant role for the γc cytokine IL-7 in the survival and homeostasis of memory CD4 T cells (7, 12). We therefore investigated whether homeostatic turnover of cKOTATCre memory cells could be restored by the addition of IL-7 in vivo. We administered IL-7/anti-IL-7(M25) antibody complexes, shown to promote robust T cell homeostasis in intact mice (24), to mouse hosts of cKOTATCre and cHETTATCre memory CD4 T cells and measured in vivo proliferation and cumulative expansion of CD4 T lymphocyte populations. Whereas the number of endogenous lymphocytes in IL-7/M25-treated compared to untreated host mice was markedly increased (Fig. 4A, Left), as were the numbers of cHETTATCre memory CD4 T cells (Fig. 4A, Right), the numbers of cKOTATCre memory CD4 T cells were unchanged or slightly decreased in IL-7/M25–treated compared to control hosts (Fig. 4A, Right). BrdU incorporation studies revealed that cHETTATCre memory CD4 T cells proliferated significantly more than cKOTATCre memory CD4 T cells in IL-7/M25–treated mice (Fig. 4B), indicating that SLP-76–deficient memory CD4 T cells were impaired in their ability to undergo homeostatic proliferation triggered by IL-7.
Fig. 4.
Impaired homeostasis of SLP-76–deficient memory CD4 T cells to IL-7/anti-IL-7 complexes. (A) (Left) Absolute numbers of host splenocytes in untreated and IL-7/M25-treated hosts of cKOTATCre and cHETTATCre memory cells. (Right) Absolute numbers of CD45.2+ YFP+CD4+ cKOTATCre and cHETTATCre memory T cells. (B) BrdU incorporation by cKOTATCre and cHETTATCre memory CD4 T cells in untreated and IL-7/M25-treated hosts. (Left) BrdU incorporation of lymph node cKOTATCre and cHETTATCre memory CD4 T cells gated on CD45.2+YFP+SLP-76lo or CD45.2+YFP+, respectively, with the percentage of BrdU incorporation indicated in the upper right quadrant. (Right) Average BrdU incorporation of splenic cKOTATCre (n = 6) and cHETTATCre (n = 5) memory CD4 T cells compiled from two experiments. (C) cKOTATCre and cHETTATCre memory CD4 T cells were incubated for 30 min with media alone (Ctrl), 0.1 ng/mL recombinant IL-7, or 10 ng/mL IL-15 and phosphorylation of STAT5 (pSTAT5) was analyzed by intracellular staining. Histograms show pSTAT5 expression gated on CD45.2+YFP+CD4+ T cells (n = 3–4).
We hypothesized that the reduced in vivo proliferative responses of SLP-76–deficient memory CD4 T cells to IL-7/M25 complexes were caused by impairments in the IL-7R–coupled JAK3/STAT5 signaling pathway (25). We therefore analyzed the ability of cKOTATCre and cHETTATCre memory CD4 T cells to phosphorylate the STAT5 transcription factor in response to IL-7 ex vivo. Treatment of cHET and cKO memory CD4 T cells with IL-7 resulted in significant STAT5 phosphorylation, which was only slightly reduced in cKO memory CD4 T cells (Fig. 4C). Both cHET and cKO memory CD4 T cells also exhibited comparable STAT5 phosphorylation in response to the related γc cytokine IL-15 (Fig. 4C), which also regulates memory CD4 T cell homeostasis (7). These results demonstrate that homeostatic defects in SLP-76–deficient memory CD4 T cells are independent of proximal γc cytokine signaling.
In Vivo Deletion of SLP-76 Impairs Memory CD4 T Cell Homeostasis.
To rule out specific effects of the adoptive transfer system, we also analyzed homeostasis and cytokine responses in polyclonal CD44hi endogenous memory CD4 T cells where SLP-76 was directly deleted in vivo using a mouse model with drug-induced Cre recombinase activity. We crossed SLP-76F/nullR26Ryfp and SLP-76F/+R26Ryfp mice to CreT2 mice transgenic for a tamoxifen-regulated Cre recombinase (26), resulting in cKOCreT2 and cHETCreT2 mouse strains, respectively (Fig. S5A). Treatment of cKOCreT2 and cHETCreT2 mice with tamoxifen resulted in ablation of SLP-76 expression specifically in the YFP+ fraction of cKOCreT2 CD4 T cells, but not in cHETCreT2 mice (Fig. S5B). CD44hi memory CD4 T cells from cKOCreT2 mice also exhibited comparable IL-7Rα expression (Fig. S5C) and IFN-γ and IL-2 production in response to PMA/ionomycin as in cHETCreT2 mice, yet had impaired TCR-coupled cytokine responses (Fig. S6).
We examined the capacity of cKOCreT2 and cHETCreT2 CD44hi memory CD4 T cells to undergo in vivo homeostasis in untreated and IL-7/M25-treated mice. In untreated mice, cKOCreT2 CD44hi memory CD4 T cells exhibited significantly reduced BrdU incorporation compared to cHETCreT2 CD44hi memory cells (Fig. 5A). In IL-7/M25–treated mice, a greater proportion of cKOCreT2 memory CD4 T cells incorporated BrdU compared to untreated mice; however, the extent of BrdU incorporation was still threefold less than that of IL-7/M25–treated cHETCreT2 memory CD4 T cells (Fig. 5A). Thus, similar to memory CD4 T cells generated by adoptive transfer, in vivo SLP-76 deficiency impairs steady-state and IL-7-driven homeostasis of memory CD4 T cells. We also examined γc signaling in cKOCreT2 and cHETCreT2 memory CD4 T cells and did not find significant differences in pSTAT5 induction following stimulation of cHET or cKO memory CD4 T cells with IL-7, IL-15 (Fig. 5B), or IL-2 (Fig. S7), further establishing that SLP-76 deficiency in memory CD4 T cells does not appreciably affect early γc cytokine signaling through the JAK/STAT pathway.
Fig. 5.
Homeostasis and cytokine responses in SLP-76-deficient memory CD4 T cells generated by drug-induced deletion. (A) Tamoxifen-treated mice (Fig. S1) were administered IL-7/M25 complexes or left untreated and in vivo proliferation was assessed by BrdU incorporation. Representative plots show CD4 versus BrdU incorporation gated on CD44hiYFP+SLP-76lo or CD44hiYFP+ cells, for cKOCreT2 and cHETCreT2 groups, respectively. (B) Compiled BrdU incorporation (mean ± SD) of cKOCreT2 and cHETCreT2 groups after IL-7/M25 treatment. (n = 3). (C) cKOCreT2 and cHETCreT2 CD4 T cells were incubated for 30 min with media alone (Ctrl), 0.1 ng/mL recombinant IL-7, or 10 ng/mL IL-15 and phosphorylation of STAT5 (pSTAT5) was analyzed by intracellular staining. Histograms show pSTAT5 expression gated on YFP+CD44hi CD4 T cells (n = 3–4 mice per group).
Discussion
We examined here signaling requirements at different stages of memory CD4 T cell development and persistence, using unique mouse models with conditional ablation of the TCR-coupled SLP-76 linker/adapter molecule. We found that SLP-76–deficient primed CD4 T cells developed into resting memory cells in vivo, yet exhibited reduced persistence and homeostasis. Importantly, SLP-76–deficient memory CD4 T cells did not undergo steady-state homeostatic turnover and were impaired in their ability to mediate cytokine- and lymphopenia-driven homeostasis, despite intact γc cytokine signaling. These results reveal a dominant requirement for SLP-76–dependent TCR signals in memory CD4 T cell maintenance and in sustaining homeostatic turnover.
We found that after initial priming, SLP-76–dependent TCR-mediated signals are not required for the generation of resting memory CD4 T cells from activated T cells. However, memory CD4 T cells require SLP-76 to signal for TCR-mediated cytokine production, establishing that SLP-76 is a central regulator of TCR signaling in memory T cells, as it is in naive T cells (16). Our results further reveal more profound requirements for TCR signals in memory CD4 T cell survival and homeostasis than was previously concluded from studies in MHC class II–deficient hosts (5, 8) or in mouse models with ablation of the TCR or the TCR-associated p56lck tyrosine kinase (10, 27). In our model, we follow the persistence of a specific population of primed CD4 T cells, rather than the polyclonal fraction of CD44hi cells examined in the previous studies. The decreased persistence of SLP-76-deficient memory CD4 T cells in vivo was associated with diminished homeostatic proliferation, suggesting that memory CD4 T cell persistence requires turnover (28). When taken together, our findings suggest that TCR-mediated signaling in memory CD4 T cells is the predominant regulator of their long-term persistence through steady-state turnover.
Previous studies have suggested that memory CD4 T cells can survive and undergo homeostasis via cytokine-mediated signals (11, 12, 29). We therefore investigated whether supraphysiological levels of IL-7 adminstered as IL-7/anti-IL-7 complexes could stimulate homeostatic turnover of SLP-76–deficient memory CD4 T cells. We found increased in vivo proliferation of SLP-76–deficient memory CD4 T cells in response to IL-7/anti-IL-7, albeit at a greatly reduced level compared to the high level of IL-7–induced proliferation of control memory T cells. This result prompted us to examine whether cytokine signals were impaired in the absence of SLP-76; however, we did not detect any defects in proximal JAK/STAT signaling in response to γc cytokines. Our findings suggest that TCR signals are required to sustain homeostatic turnover of memory CD4 T cells, rather than TCR and IL-7–mediated signals playing complementary roles in memory CD4 T cell homeostasis as previously suggested (11).
Our identification of a predominant TCR requirement for memory CD4 T cell maintenance predicts the selective survival of memory populations with the greatest capacity for TCR engagement. Indeed, the memory CD4 T cell compartment in aging is characterized by narrowing TCR repertoires and expanded clonal populations (30), suggesting that homeostatically expanding memory T cells may compose an increased proportion of the T cell repertoire. Conversely, certain populations of virus-specific memory CD4 T cells have been shown to decline over time (31). The requirement for continuous TCR signaling in long-term memory maintenance explains the eventual loss and selective long-term persistence of specific memory populations.
In conclusion, our results present unique evidence for a predominant role for the TCR signaling pathway in sustaining homeostatic turnover of memory CD4 T cells for their maintenance, with important implications for understanding memory CD4 T cell persistence following pathogen exposure, in vaccines, and during aging.
Materials and Methods
Mice.
C57BL/6 and B6.CD45.1(B6-Ly5.2) mice (8–16 weeks) were purchased from the National Cancer Institute Biological Testing Branch and RAG2−/− mice were from Taconic Farms. Mouse strains containing a floxed (F) allele of SLP-76(16) were crossed to R26RYFP mice (20) to generate SLP-76F/nullR26Ryfp(cKO) mice and SLP-76F/+R26Ryfp(cHET) mice (Fig. S1) and to CreT2 transgenic mice expressing a tamoxifen-regulated Cre recombinase protein (32) to generate SLP-76F/nullR26RyfpCreT2(cKOCreT2) and SLP-76F/+R26RyfpCreT2(cHETCreT2) strains (Fig. S1). All mice were maintained at the animal facilities at the University of Maryland, Baltimore, MD, and the University of Pennsylvania, Philadelphia, PA, under specific pathogen-free conditions, and animal procedures were approved by the Institutional Animal Care and Use Committee of each institution.
Antibodies and Reagents.
Purified antibodies specific for CD8 (TIB 105), NK (PK136), and I-Ad (212.A1) were purchased from Bio X Cell. Fluorochrome-conjugated antibodies specific for CD62L, IL-7Rα, CD25, anti-TCRβ, anti-IL-2, anti-IFN-γ, anti-PLCγ1 (pY783), and CD4 were purchased from BD Pharmingen; antibodies specific for CD44, CD45.2, phospho-STAT5(Y694), and BrdU and recombinant mouse IL-7, IL-15 and IL-2 came from eBioscience. Anti-SLP-76 antibody came from Cell Signaling Technology and PE-conjugated donkey anti-Rabbit F(ab′)2 came from Jackson ImmunoResearch Laboratories. The TAT-fused Cre-recombinase protein (21) (TATCre) was expressed and purified as described (21). Anti-IL-7 antibody M25 (33) and human IL-7 were generously provided by Charles Surh (Scripps Research Institute, San Diego, CA).
In Vitro Priming, TATCre Treatment, and Adoptive Transfer.
CD4 T cells from cHET and cKO mice were purified as described (15) and primed in vitro by activation with plate-bound anti-CD3 (5 μg/mL) and anti-CD28 (5 μg/mL) antibodies in Complete Click’s Medium (15) at 37°C. Cells were cultured with recombinant TATCre protein (100 μg in HBSS per 107 cells) for 40 min at 37°C, washed with media, and recultured in vitro with plate-bound anti-CD3/anti-CD28 antibodies for 6–48 h. For generation of cKOTATCre and cHETTATCre memory T cells, CD4 T cells from cHET and cKO mice were activated for 72 h in vitro, treated with TATCre, transferred i.p. into B6.CD45.1 mouse hosts (3 × 106 cells/mouse), and harvested 1–5 weeks posttransfer. B6.CD45.1 recipients were administered anti-CD8 (TIB105, 100 μg/mouse) and anti-NK (PK136, 50 μg/mouse) antibodies i.p. at days −1 and 0 and every 3–5 days thereafter as described (7).
In Vivo Treatment with BrdU and Cytokine/Anti-Cytokine Complexes.
Adoptive mouse hosts were treated i.p. with BrdU diluted in PBS (1 mg/mouse), daily for 3 days before harvest. Intracellular staining for BrdU was done using the BrdU staining kit (BD Pharmingen). For cytokine administration in vivo, mice were treated with IL-7/anti-IL-7 complexes (24), using 3 μg recombinant IL-7 mixed with 15 μg of anti-IL-7 i.p. on days 0, 2, and 4. Cells were harvested 7 days after the first treatment.
Western Blotting and Flow Cytometry.
CD4 T cells from cHET and cKO mice and YFP+ CD4 T cells FACS sorted after activation and TATCre treatment above were lysed in SDS sample buffer with protease/phosphatase inhibitors as described (15). Lysates were resolved by 4–12% gradient SDS/PAGE and anti-SLP-76 and anti-LAT immunoblots were performed as described (15). Surface and intracellular staining was performed as previously described (14), and cells were analyzed using an LSRII flow cytometer (BD) and FlowJo software (Tree Star).
Tamoxifen-Induced SLP-76 Deletion.
SLP-76F/nullR26RyfpCreT2 and SLP-76F/+R26RyfpCreT2 mice (8–12 weeks) were administered Tamoxifen (Sigma) daily by oral gavage for 5 days (200 μg·g−1·day−1), and cells were harvested 5–20 days after the last tamoxifen dose.
Supplementary Material
Acknowledgments
The authors thank Gary Koretzky for reviewing this manuscript and Eric Brown, Frank Costantini, and Frank Edenhofer for mice and reagents. This project was supported by National Institutes of Health Grant AI042092 (to D.L.F.); N.D.B. was supported by National Institutes of Health Grant T32 AI007540. J.S.M. is an American Society of Nephrology-American Society of Transplantation John Merrill Transplant Research Scholar.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908126107/DCSupplemental.
References
- 1.Reiner SL, Sallusto F, Lanzavecchia A. Division of labor with a workforce of one: Challenges in specifying effector and memory T cell fate. Science. 2007;317:622–625. doi: 10.1126/science.1143775. [DOI] [PubMed] [Google Scholar]
- 2.Abraham RT, Weiss A. Jurkat T cells and development of the T-cell receptor signalling paradigm. Nat Rev Immunol. 2004;4:301–308. doi: 10.1038/nri1330. [DOI] [PubMed] [Google Scholar]
- 3.Jordan MS, Singer AL, Koretzky GA. Adaptors as central mediators of signal transduction in immune cells. Nat Immunol. 2003;4:110–116. doi: 10.1038/ni0203-110. [DOI] [PubMed] [Google Scholar]
- 4.Murali-Krishna K, et al. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 1999;286:1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- 5.Kassiotis G, Garcia S, Simpson E, Stockinger B. Impairment of immunological memory in the absence of MHC despite survival of memory T cells. Nat Immunol. 2002;3:244–250. doi: 10.1038/ni766. [DOI] [PubMed] [Google Scholar]
- 6.De Riva A, Bourgeois C, Kassiotis G, Stockinger B. Noncognate interaction with MHC class II molecules is essential for maintenance of T cell metabolism to establish optimal memory CD4 T cell function. J Immunol. 2007;178:5488–5495. doi: 10.4049/jimmunol.178.9.5488. [DOI] [PubMed] [Google Scholar]
- 7.Purton JF, et al. Antiviral CD4+ memory T cells are IL-15 dependent. J Exp Med. 2007;204:951–961. doi: 10.1084/jem.20061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swain SL, Hu H, Huston G. Class II-independent generation of CD4 memory T cells from effectors. Science. 1999;286:1381–1383. doi: 10.1126/science.286.5443.1381. [DOI] [PubMed] [Google Scholar]
- 9.Kassiotis G, Zamoyska R, Stockinger B. Involvement of avidity for major histocompatibility complex in homeostasis of naive and memory T cells. J Exp Med. 2003;197:1007–1016. doi: 10.1084/jem.20021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polic B, Kunkel D, Scheffold A, Rajewsky K. How alpha beta T cells deal with induced TCR alpha ablation. Proc Natl Acad Sci USA. 2001;98:8744–8749. doi: 10.1073/pnas.141218898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol. 2003;4:680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 12.Kondrack RM, et al. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797–1806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan JT, et al. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandok MR, Okoye FI, Ndejembi MP, Farber DL. A biochemical signature for rapid recall of memory CD4 T cells. J Immunol. 2007;179:3689–3698. doi: 10.4049/jimmunol.179.6.3689. [DOI] [PubMed] [Google Scholar]
- 15.Hussain SF, Anderson CF, Farber DL. Differential SLP-76 expression and TCR-mediated signaling in effector and memory CD4 T cells. J Immunol. 2002;168:1557–1565. doi: 10.4049/jimmunol.168.4.1557. [DOI] [PubMed] [Google Scholar]
- 16.Maltzman JS, Kovoor L, Clements JL, Koretzky GA. Conditional deletion reveals a cell-autonomous requirement of SLP-76 for thymocyte selection. J Exp Med. 2005;202:893–900. doi: 10.1084/jem.20051128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yablonski D, Kuhne MR, Kadlecek T, Weiss A. Uncoupling of nonreceptor tyrosine kinases from PLC-gamma1 in an SLP-76-deficient T cell. Science. 1998;281:413–416. doi: 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- 18.Clements JL, et al. Requirement for the leukocyte-specific adapter protein SLP-76 for normal T cell development. Science. 1998;281:416–419. doi: 10.1126/science.281.5375.416. [DOI] [PubMed] [Google Scholar]
- 19.Pivniouk V, et al. Impaired viability and profound block in thymocyte development in mice lacking the adaptor protein SLP-76. Cell. 1998;94:229–238. doi: 10.1016/s0092-8674(00)81422-1. [DOI] [PubMed] [Google Scholar]
- 20.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peitz M, Pfannkuche K, Rajewsky K, Edenhofer F. Ability of the hydrophobic FGF and basic TAT peptides to promote cellular uptake of recombinant Cre recombinase: A tool for efficient genetic engineering of mammalian genomes. Proc Natl Acad Sci USA. 2002;99:4489–4494. doi: 10.1073/pnas.032068699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moulton VR, Bushar ND, Leeser DB, Patke DS, Farber DL. Divergent generation of heterogeneous memory CD4 T cells. J Immunol. 2006;177:869–876. doi: 10.4049/jimmunol.177.2.869. [DOI] [PubMed] [Google Scholar]
- 23.Beach D, Gonen R, Bogin Y, Reischl IG, Yablonski D. Dual role of SLP-76 in mediating T cell receptor-induced activation of phospholipase C-gamma1. J Biol Chem. 2007;282:2937–2946. doi: 10.1074/jbc.M606697200. [DOI] [PubMed] [Google Scholar]
- 24.Boyman O, Ramsey C, Kim DM, Sprent J, Surh CD. IL-7/anti-IL-7 mAb complexes restore T cell development and induce homeostatic T cell expansion without lymphopenia. J Immunol. 2008;180:7265–7275. doi: 10.4049/jimmunol.180.11.7265. [DOI] [PubMed] [Google Scholar]
- 25.Moriggl R, Sexl V, Piekorz R, Topham D, Ihle JN. Stat5 activation is uniquely associated with cytokine signaling in peripheral T cells. Immunity. 1999;11:225–230. doi: 10.1016/s1074-7613(00)80097-7. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: A tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 27.Leignadier J, Hardy M-P, Cloutier M, Rooney J, Labrecque N. Memory T-lymphocyte survival does not require T-cell receptor expression. Proc Natl Acad Sci USA. 2008;105:20440–20445. doi: 10.1073/pnas.0806289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellier B, Thomas-Vaslin V, Saron MF, Klatzmann D. Turning immunological memory into amnesia by depletion of dividing T cells. Proc Natl Acad Sci USA. 2003;100:15017–15022. doi: 10.1073/pnas.1936194100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J Exp Med. 2003;198:1807–1815. doi: 10.1084/jem.20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naylor K, et al. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 31.Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- 32.Ruzankina Y, et al. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1:113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grabstein KH, et al. Inhibition of murine B and T lymphopoiesis in vivo by an anti-interleukin 7 monoclonal antibody. J Exp Med. 1993;178:257–264. doi: 10.1084/jem.178.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.