Abstract
We suggest that some of the most avian dromaeosaurs, such as Sinornithosaurus, were venomous, and propose an ecological model for that taxon based on its unusual dentition and other cranial features including grooved teeth, a possible pocket for venom glands, and a groove leading from that pocket to the exposed bases of the teeth. These features are all analogous to the venomous morphology of lizards. Sinornithosaurus and related dromaeosaurs probably fed on the abundant birds of the Jehol forests during the Early Cretaceous in northeastern China.
Keywords: dromaeosaur, Jehol, grooved fangs, venomous delivery system
One of the more bizarre innovations in organismic evolution is the ability to manufacture toxic substances. Venomous taxa occur in a variety of ecologic settings and include insects, lizards, snakes, and mammals (1–5). Clearly, venom has evolved numerous times in many different lineages employing various delivery apparatus. A combination of morphological and molecular research has recently shown that venomous taxa are far more widespread and primitive within tetrapod lineages than had previously been suspected (6).
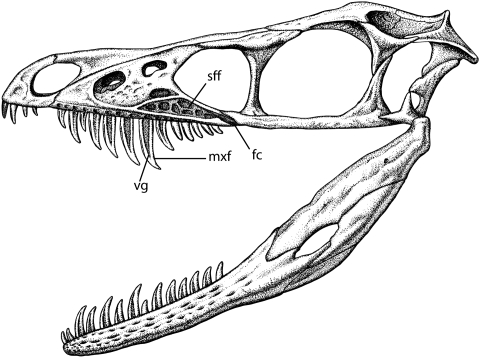
Sinornithosaurus is a dromaeosaurid closely related to the 4-winged glider Microraptor gui and therefore within the early avian radiation (7). It has unusually long maxillary teeth that are morphologically similar to those of “rear-fanged” snakes specialized to carry poison (Fig. 1). This type of fang discharges venom along a groove on the outer surface of the tooth that enters the wound of the bitten animal by capillary action (8, 9). Supporting this interpretation in Sinornithosaurus is an additional space on the lateral surface of the maxillary bone that we interpret on the basis of analogy with venomous squamates as having housed a venom gland. This previously undescribed fossa, herein termed the subfenestral fossa, could have housed an elongate, ascinar venom gland similar to that found in rear-fanged (i.e., opisthoglyphous) snakes (10, 11). We suggest that the venom traveled in ducts to the bases of the teeth and mixed with the saliva in a manner also similar to extant venomous squamates (6). The position of the venom collecting duct was probably along the oblique ventral surface of the maxilla, where there is a supradental groove (i.e., longitudinal depression running along the base of the tooth row). This groove bears small pits that seem to be related to tooth sites and may represent the location of small venom reservoirs. These depressions were illustrated and mentioned in the original description of Sinornithosaurus, but their purpose was not addressed. As in modern venomous taxa that employ grooved fangs, the ducts feed the venom to the base of the teeth. The mechanism for dispensing the venom may be similar to the system used by open-fanged snakes and lizards that discharge it under low pressure provided largely by force of the bite—a strategy for prey control rather than quick death (12). We believe Sinornithosaurus was a venomous predator that fed on birds by using its long fangs to penetrate through the plumage and into the skin, and the toxins would induce shock and permit the victim to be subdued rapidly.
Fig. 1.
Line drawing of reconstructed skull of Sinornithosaurus redrawn, in part, from the holotype IVPP V12811 described by Xu and Wu (14) and further modified using information from additional specimens in the collections of the Dalian Natural History Museum and Shandong Tianyu Museum of Natural History. Skull is approximately 75 mm long. vg, venom groove; mxf, maxillary fang; sff, subfenestral fossa; fc, fossa canal.
Results
Sinornithosaurus millenii (13) comprises a well preserved skull and most of the skeleton. Our recent inspection of the holotype and several additional specimens of Sinornithosaurus confirm the presence of lateral grooves on the tooth crowns and the presence of a subfenestral fossa in all specimens. The maxillary teeth are strongly heterodont, whereas on the lower jaws the height of the tooth crowns are similar along the tooth row.
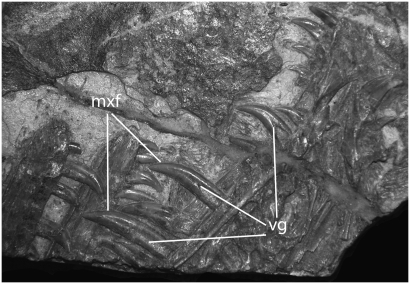
The anterior premaxillary teeth are procumbent and are not recurved or serrated (14). The first premaxillary teeth are rotated so the carinae are approximately 90° to the rest of the dentition. The premaxillary teeth are shorter than the maxillary teeth. The second premaxillary alveolus has the longest tooth, and this crown, along with the premaxillary tooth crowns for 3 and 4, have deep, narrow grooves on the lingual surface running behind the anterior carinae (Fig. 2). The lateral grooves on the other teeth tend to be larger and more centrally located.
Fig. 2.
Photograph of the holotype of S. millenii (IVPP V12811) showing dentition with venom grooves (vg). mxf, maxillary fang.
The anterior maxillary teeth are so long and fanglike (Figs. 1 and 2) that the animal appears to be saber-toothed. They are laterally compressed and fairly straight compared with other dromaeosaur teeth (15). Interestingly, much of the effective erupted length of the teeth is composed of the tooth root. The erupted portion of the largest maxillary tooth in the type specimen of S. millenii (IVPP V12811) measures 12 mm long and occupies the seventh alveolus. There is a distinct groove on the labial side running from the base of the root to the tip. The tooth crown is not really as elongated as it appears because of a hyper-erupted tooth root, and the tooth sockets are not especially deep. The posterior maxillary teeth are much shorter, straighter, and flattened. The morphology of the grooved maxillary fangs is similar to that in Uatichodon (1), in which there is a labial groove that is V-shaped and widest at the base of the exposed portion of the tooth. There is also a smaller, narrower labial groove along the anterior carinae of the crown. Both grooves follow the curvature of the tooth crown nearly to the tip and, although more prominent on the longest maxillary teeth, can be found throughout the dentition on the upper and lower jaws.
There are 13 dentary tooth positions, and most of the teeth are uniform in height with the exception of the second or third anterior tooth, which is slightly longer (Fig. 1). These longer teeth also appear to be hyper-erupted and expose a portion of the root. The first dentary tooth is short and procumbant. Serrations can be found on all of the dentary tooth crowns except for the anteriormost (14).
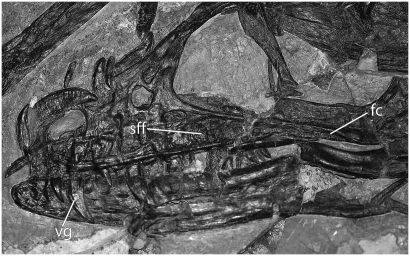
The lateral surface of the maxilla has a complicated architecture not evident in other archosaurs. On the posteroventral surface just anterior to the lacrimal, and separate from the antorbital fossa, is a distinct triangular area bound by ridges that nearly encompass a depression herein termed the subfenestral fossa. The surface of this fossa is covered by a system of large pits. Judging from the holotype of Sinornithosaurus haoiana (D 2140) (Fig. 3), this fossa is open posteriorly and confluent with the shallow maxillary (i.e., supradental) groove that runs labially above the teeth as reported by Xu and Wu (14) for the holotype. This groove exhibits a number of shallow pits.
Fig. 3.
Photograph of the skull of the holotype S. haoiana (D 2140) showing subfenestral fossa (sff) and venom grooves (vg) on the maxillary teeth. The length of the maxilla is approximately 63.6 mm (23). fc, fossa canal.
Hotton (16) proposed a venomous delivery system for a Late Permian synapsid based on the combination of a maxillary fossa and grooved teeth. Pocketing within the maxilla in conjunction with grooved fangs is considered well supported evidence for venom delivery systems in fossil taxa (17).
Discussion
The maxillary teeth of Sinornithosaurus are long enough to reduce bite force (18) and restrict the size of prey that will fit in its gape. Sinornithosaurus had a strikingly heterodont dentition that placed restrictions on its use as a simple “grab and gulp” mechanism. These changes reflect specialized uses not required by more ordinary predators, which may result from problems inherent to predation on feathered birds. The long maxillary teeth do not appear to have been deeply inserted into the prey, and if they had been, they would have been difficult to extract without damage. Their length may result from the need to penetrate a thick layer of feathers. Once through the feathers, the grooved fang would penetrate 4 to 6 mm into the skin. This would be sufficient to cut into the subdermal tissue and allow poison to enter the bloodstream but would be too shallow to cause death or immobilization through trauma alone (19). The poison of Sinornithosaurus may have been similar in properties to rear-fanged snakes and helodermid lizards in that it did not kill the envenomated animal quickly but rather placed it into a rapid state of shock (11). If the venom was indeed similar to these animals, then a “bite and hold” method of venom delivery was probably used.
Many predators on birds pluck the feathers off their victims (20), and the procumbant teeth at the tip of the snout with their unusual rotation might have been used for this function. Other types of prey probably included small-sized dinosaurs, lizards, pterosaurs, and mammals. Dismemberment of the prey, if it occurred at all, was probably accomplished by posterior maxillary teeth that were shorter, broader, and more suitable for cutting.
The use of venom is consistent with the overall morphology of the skull in Sinornithosaurus. It has a narrow snout; the cranium has a tall lateral profile and a large gape. These features suggest only a moderate bite force that may have had difficulty in subduing larger prey. An embedded tooth from a small dromaeosaur on the limb bone of a pterosaur (21) suggests that the teeth had difficulty penetrating even the thin bone of the flying reptile.
The presence of a venom delivery system in a wide variety of squamates (12, 22) suggests that this adaptation can be expected in other diapsids, including the dromaeosaurs. Venom glands in lizards tend to be mandibular whereas those in snakes are maxillary; the basal condition is to have both (6). Sinornithosaurus probably had the primitive system. Osteological evidence such as the grooved mandibular and maxillary teeth, subfenestral fossa, and supradental channel with pits found in multiple specimens of Sinornithosaurus deserves some explanation, and venom seems to be the most parsimonious conclusion.
Materials and Methods
We were especially interested in inspecting the dentition of Sinornithosaurus for taphonomic artifacts and were careful to consider the affects of crushing on the morphology of the teeth. We directly examined the skull of the holotype of S. millenii at the Institute of Vertebrate Paleontology and Paleoanthropology with binocular microscopes and photographed the dentition using a Nikon D80 with the AF-S Micro Nikkor 105 mm 1:2.8 GED lens. The holotype of S. haoiana (D 2140) was examined at the Dalian Natural History Museum and was photographed with the same equipment. Additional specimens were examined and documented at the Tianyu Museum of Natural History but were not photographed.
Acknowledgments
We thank Zhonghe Zhou, Xu Xing, and the staff at the Institute of Vertebrate Paleontology and Paleoanthropology for kind assistance, access to specimens, and allowing us to photograph IVPP V12811; Gao Chunling and staff at the Dalian Natural History Museum for kind assistance and allowing photographs of D 2140; Zheng Xiaoting and the staff at the Shandong Tianyu Museum of Natural History for their kind assistance in examining specimens including 9-25-1; we also thank E. Ebert, who illustrated the skull; and Rafe Brown, John Ruben, and Desui Miao, who read the manuscript and provided valuable comments.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Sues H-D. Venom-conducting teeth in a Triassic reptile. Nature. 1991;351:141–143. [Google Scholar]
- 2.Sues H-D. A reptilian tooth with apparent venom canals from the Chinle Group (Upper Triassic) of Arizona. J Vertebrate Paleontol. 1996;16:571–572. [Google Scholar]
- 3.Kita M, et al. Blarina toxin, a mammalian lethal venom from the short-tailed shrew Blarina brevicauda: Isolation and characterization. Proc Natl Acad Sci USA. 2004;101:7542–7547. doi: 10.1073/pnas.0402517101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox RC, Scott CS. First evidence of a venom delivery apparatus in extinct mammals. Nature. 2005;435:1091–1093. doi: 10.1038/nature03646. [DOI] [PubMed] [Google Scholar]
- 5.Reynosa V-H. Possible evidence of a venom apparatus in a Middle Jurassic sphenodontian from the Huizachal Red Beds of Tamaulipas, Mexico. J Vertebrate Paleontol. 2005;25:646–654. [Google Scholar]
- 6.Fry BG, et al. Early evolution of the venom system in lizards and snakes. Nature. 2006;439:584–588. doi: 10.1038/nature04328. [DOI] [PubMed] [Google Scholar]
- 7.Martin LD. Origin of avian flight - a new perspective. Oryctos. 2008;7:45–54. [Google Scholar]
- 8.Heatwole H. Sea Snakes. Sydney: New South Wales Univ Press; 1987. [Google Scholar]
- 9.Loeb L. The venom of Heloderma: Carnegie Institution of Washington Pub no. 177. Washington: Gibson Brothers; 1913. [Google Scholar]
- 10.Gans C, Parsons TS. Biology of the Reptilia. New York: Academic Press; 1973. [Google Scholar]
- 11.Weinstein SA, Smith TL, Kardong KV. In: Handbook of Venoms and Toxins of Reptiles. Mackessy SP, editor. New York: CRC Press; 2009. pp. 65–91. [Google Scholar]
- 12.Kardong KV, Lavin-Murcio PA. Venom delivery of snakes as high pressure and low-pressure systems. Copeia. 1993:644–650. [Google Scholar]
- 13.Xu X, Wang X-L, Wu X-C. A dromaeosaurid dinosaur with filamentous integument from the Yixian Formation of China. Nature. 1999;401:262–266. [Google Scholar]
- 14.Xu X, Wu X-C. Cranial morphology of Sinornithosaurus millenii Xu et al. 1999 (Dinosauria: Theropoda: Dromaeosauridea) from the Yixian Formation of Liaoning, China. Can J Earth Sci. 2001;38:1739–1752. [Google Scholar]
- 15.Currie PJ. Dinosaur Systematics Approaches and Perspectives. New York: Cambridge Univ Press; 1990. [Google Scholar]
- 16.Hotton NC., III . In: Origins of Higher Groups of Tetrapods. Schultze H-P, Trueb L, editors. New York: Cornell Univ Press; 1991. pp. 598–634. [Google Scholar]
- 17.Folinsbee KE, Müller J, Reisz RR. Canine grooves: morphology, function, and relevance to venom. J Vertebrate Paleontol. 2007;27:547–551. [Google Scholar]
- 18.Erickson GM, Lappin AK, Vliet KA. The ontogeny of bite-force per-formance in American alligator (Alligator mississippiensis) J Zool (Lond) 2003;260:317–327. [Google Scholar]
- 19.Kardong KV. Snake toxins and venoms: an evolutionary perspective. Herpetologica. 1996;52:36–46. [Google Scholar]
- 20.Zimmerman DA. Roadrunner predation on passerine birds. Condor. 1970;72:475–476. [Google Scholar]
- 21.Currie PJ, Jacobsen AR. An azhdarchid pterosaur eaten by a velociraptorine theropod. Can J Earth Sci. 1995;32:922–925. [Google Scholar]
- 22.Fry BG, et al. A central role for venom in predation by Varanus komodoensis (Komodo Dragon) and the extinct giant Varanus (Megalania) priscus. Proc Natl Acad Sci USA. 2009;106:8969–8974. doi: 10.1073/pnas.0810883106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Ji S, Tang F, Gao C. A new species of dromaeosaurids [sic] from the Yixian Formation of western Liaoning. Geo Bull China. 2004;23:778–783. [Google Scholar]