Abstract
Varicella-zoster virus (VZV) and herpes simplex virus (HSV) are prevalent neurotropic herpesviruses that cause various nervous system diseases. Similar to other enveloped viruses, membrane fusion is an essential process for viral entry. Therefore, identification of host molecules that mediate membrane fusion is important to understand the mechanism of viral infection. Here, we demonstrate that myelin-associated glycoprotein (MAG), mainly distributed in neural tissues, associates with VZV glycoprotein B (gB) and promotes cell-cell fusion when coexpressed with VZV gB and gH/gL. VZV preferentially infected MAG-transfected oligodendroglial cells. MAG also associated with HSV-1 gB and enhanced HSV-1 infection of promyelocytes. These findings suggested that MAG is involved in VZV and HSV infection of neural tissues.
Keywords: herpes simplex virus, neurotropism, membrane fusion, Varicella-zoster virus, virus entry
Varicella-zoster virus (VZV) mainly causes two human diseases, varicella (chickenpox) in children and zoster (shingles) in immune-compromised or elderly individuals (1); VZV also causes diseases of the nervous system, such as meningitis and encephalitis (2). Herpes simplex virus (HSV) also causes neurological disease. A unique characteristic of these viruses is establishment of latency in sensory ganglia (1, 3). Both VZV and HSV are enveloped viruses of the alphaherpesvirus family, whose interactions between its envelope proteins and cell-surface molecules are crucial events for the entry of enveloped viruses into cells (4).
Glycoproteins gB, gD, gH, and gL are essential envelope protein for membrane fusion during HSV infection. gD associates with several cell-surface proteins, such as herpesvirus entry mediator (HVEM) and nectin, and gB associates with paired Ig-like type-2 receptor α (PILRα) (5). These interactions can play important roles in HSV-1 infection, depending upon cell types (5). On the other hand, glycoproteins gB, gE, gH, and gL have been suggested to participate in membrane fusion during VZV infection (6). Because mannose 6-phosphate (M6P) inhibits cell-free VZV infection, a M6P receptor has been suggested to be involved in cell-free VZV infection by the interaction with VZV glycoproteins that contain M6P (7, 8). Indeed, Chen et al. showed that cation-independent M6P receptor (MPRci) is involved in cell-free VZV infection (9). MPRci is ubiquitously expressed on various tissues and mainly functions as a molecular chaperone that transports proteins modified with N-linked oligosaccharides from the trans-Golgi network to early endosomes (10, 11).
VZV-gE is an essential glycoprotein for VZV infection and has been suggested to be required, in concert with its heterodimer partner, gI, for viral replication and for virion assembly in the trans-Golgi network (12,13,14–15). In addition, gE also seems to be involved in membrane fusion between the viral envelope and cellular membrane, although gE alone does not induce membrane fusion (6, 16). Recently, insulin-degrading enzyme (IDE), ubiquitously expressed on various cell populations, has been shown to associate with gE and is involved in both cell-free and cell-associated VZV infection (17). However, VZV-expressing mutant gE that does not associate with IDE is still infectious (13, 18); therefore, the exact function of IDE in VZV infection has remained unclear.
gB, an envelope protein conserved among all herpesviruses, has been suggested to play an important role in membrane fusion by most herpesviruses (19). We have recently found that HSV-gB associates with PILRα and is involved in HSV-1 infection (20). gB is also essential for VZV infection (21), although cellular receptors for VZV-gB have not been identified. Here, we analyzed the molecules that associate with VZV-gB and found that VZV-gB associated with myelin-associated glycoprotein (MAG). Interestingly, cell-cell fusion was observed when cells expressing MAG, but not IDE or MPRci, were cocultured with cells expressing VZV glycoproteins. Furthermore, MAG-expressing cells were susceptible to VZV infection. MAG is a cell-surface molecule that is preferentially expressed in neural tissues, especially on myelin sheath, and plays an important role in the regulation of axonal growth (22,23,24–25). These results suggested that MAG might be involved in the membrane fusion step of VZV entry. Furthermore, MAG also associated with HSV-gB and enhanced HSV-1 infection. These data suggested that MAG can promote infection of neurotropic herpesviruses.
Results
Association of MAG with VZV and HSV gB.
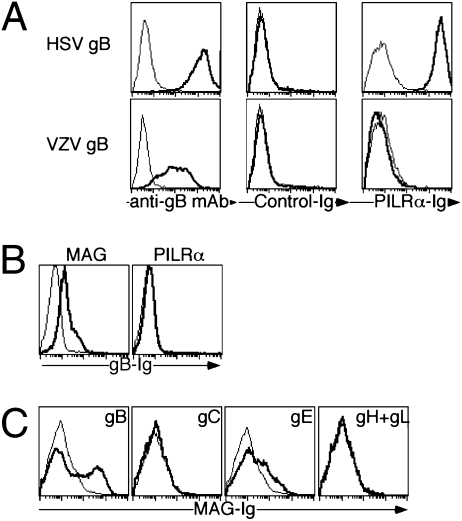
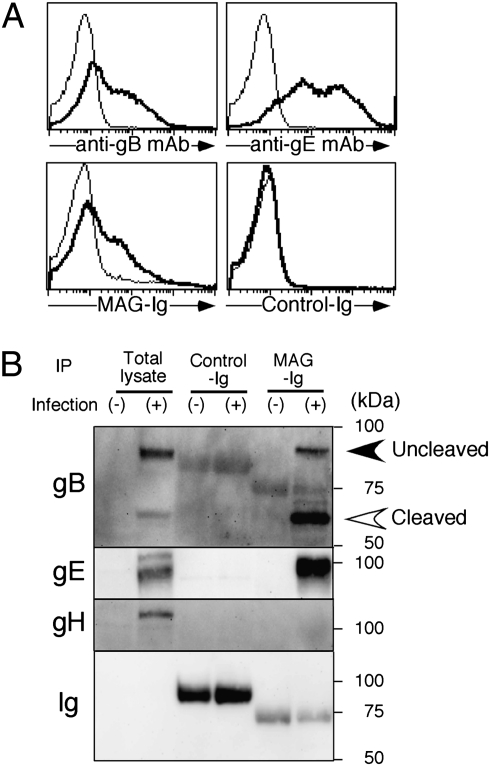
Because there is 48% homology between VZV-gB and HSV-gB, we first addressed whether VZV-gB also associated with PILRα using PILRα-Ig fusion protein (PILRα-Ig) (20). HSV-gB-transfectants were clearly stained with PILRα-Ig, whereas VZV-gB-transfectants were not stained with PILRα-Ig (Fig. 1A). We have previously proposed a hypothesis that paired inhibitory and activating receptors, like PILR, might be involved in host-pathogen interaction (26). We then focused on various paired receptors, and noticed that Sialic-acid-binding Ig-like lectin (Siglec) family molecules, which include paired receptors, have a 5 to 12% homology with PILRα (27). In particular, Siglec-1, -4, and -5 showed relatively high homology with human PILRα. We analyzed various Siglec molecules and found that Siglec-4 (also called MAG)-transfectants were stained with VZV-gB-Ig (Fig. 1B and Fig. S1). Unexpectedly, MAG also associated with VZV-gE as well as VZV-gB but not with other envelope proteins (Fig. 1C and Fig. S2). Although it has been reported that VZV-gE is involved in VZV infection by associating with IDE (17), the VZV-gE did not bind to IDE-transfectants, whereas VZV-gE, as well as VZV-gB, clearly bound to the cell surfaces of MAG-transfectants (Fig. S3). Interestingly, when we analyzed association of MAG with HSV-1 envelope proteins, MAG also associated with HSV-gB, which is essential for membrane fusion during HSV-1 infection (Fig. S4A). Next, We confirmed association of MAG-Ig with VZV- (Fig. 2A) or HSV-infected cells (Fig. S4B). When the lysate of VZV-infected or mock-infected cells was immuoprecipitated with MAG-Ig, association of gB and gE with MAG was detected using the lysate of VZV-infected cells (Fig. 2B).
Fig. 1.
Association of MAG with VZV-gB. (A) HSV-gB- (Upper, bold lines) and VZV-gB- (Lower, bold lines) transfected 293T cells were stained with control-Ig and PILRα-Ig, as well as anti-HSV-gB or anti-VZV-gB mAb. Mock-transfectants were used as control (thin lines). (B) 293T cells were transfected with MAG and PILRα (bold line), and these cells, as well as control-transfectants (thin line), were stained with VZV-gB-Ig. (C) 293T cells transfected with VZV-glycoproteins (bold line) and control-transfectants (thin line) were stained with MAG-Ig.
Fig. 2.
Association of MAG with gB and gE expressed in VZV-infected cells. (A) VZV-infected MeWo (bold line) or mock-infected MeWo cells (thin line) were stained with anti-VZV-gB mAb, anti-VZV-gE mAb, MAG-Ig, or control-Ig, respectively. (B) Association of MAG with gB and gE expressed in VZV-infected cells. Cell lysates of VZV-infected MeWo or mock-infected MeWo cells were immunoprecipitated with control-Ig or MAG-Ig and the precipitants were analyzed by Western blotting using anti-VZV-gB, anti-VZV-gE, or anti-VZV-gH mAbs. Uncleaved and cleaved forms of gB were indicated in the figure. Data are representative of three independent experiments.
Cell-Cell Fusion Mediated by MAG and VZV or HSV Glycoproteins.
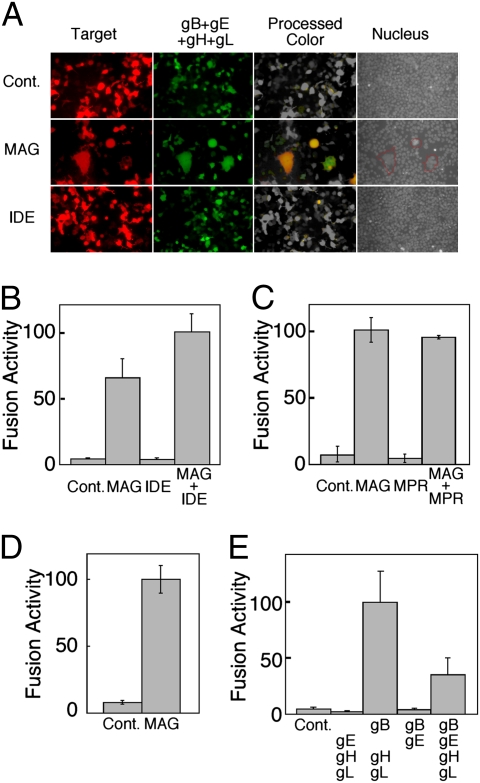
MAG is a cell-surface molecule that is preferentially expressed in neural tissues, and plays an important role in the regulation of axonal growth by associating with host ligands, such as Nogo-66 receptor and paired Ig-like receptor B (22,23,24–25). Because enveloped viruses including VZV and HSV require membrane fusion between their envelope and host cell membrane, we examined the role of MAG in membrane fusion by VZV and HSV envelope proteins using cell-cell fusion assay, which defines a minimal set of viral fusion proteins. When MAG and DsRed cotransfected 293T cells and VZV-gB, gE, gH, gL, and GFP-cotransfected 293T cells were cocultured, many fused polykaryons were observed. Fused polykaryons were not observed when transfectants of VZV envelope proteins were cocultured with mock- or IDE-alone transfectants (Fig. 3A). We then quantitatively evaluated the fusion efficiency using a luciferase reporter system (28). Similar to microscopic analyses, MAG-transfectants but not IDE-transfectants showed cell fusion with transfectants of viral envelope proteins (Fig. 3B). The efficiency of MAG-mediated cell-cell fusion was slightly increased in the presence of IDE, although there was no statistical difference. On the other hand, MPRci has been suggested to be involved in VZV infection (9). However, the expression of MPRci did not induce cell-cell fusion and also did not augment cell-cell fusion induced by MAG (Fig. 3C and Fig. S5). These results suggest that VZV-envelope glycoproteins mediate cell-cell fusion by associating with MAG. Furthermore, MAG-mediated cell-cell fusion was also observed using CHO cells transfected with HSV-1 envelope proteins (Fig. 3D).
Fig. 3.
Cell-cell fusion induced by MAG (A) MAG but not IDE mediates cell-cell fusion by associating with VZV-glycoproteins. 293T cells transfected with VZV-gB, gE, gH, gL, and GFP and 293T cells transfected with MAG and IDE, as well as DsRed (Target), were cocultured for 18 h and were analyzed by fluorescence microscopy. Green and red fluorescence from nonfused cells were converted to gray color, and yellow colors of fused cells were left unchanged (processed color). Cell nuclei were stained with Hoechst 33258 fluorescence dye, and blue fluorescence from nuclei is shown (gray). Fused cells are delineated by red lines. Mock-transfectants were used as control (Cont.). When mock- or IDE-transfectants were cocultured, number of obvious polykaryons in each field of microscopy was 0.5 ± 0.6 or 0.5 ± 0.7 (mean ± SD of 20 fields), respectively. On the other hand, when MAG transfectants were cocultured, the number of obvious polykaryons was 5.7 ± 1.5. (B) Quantification of efficiency of MAG-mediated membrane fusion. 293T cells transfected with VZV-gB, gE, gH, gL, and T7 polymerase and 293T cells transfected with MAG and IDE, as well as firefly luciferase and Renilla luciferase, were cocultured for 18 h. (C) MPRci does not mediate membrane fusion. 293T cells transfected with VZV-gB, gE, gH, gL, and T7 polymerase and 293T cells transfected with MAG and MPRci, as well as with firefly luciferase and Renilla luciferase, were cocultured for 18 h. (D) MAG mediates membrane fusion by associating with HSV-1 glycoproteins. CHO cells transfected with HSV-gB, gD, gH, gL, and T7 polymerase and CHO cells transfected with MAG and firefly luciferase and Renilla luciferase were cocultured for 18 h. (E) Requirement of VZV-gB, gH, and gL for MAG-mediated membrane fusion. 293T cells cotransfected with various combinations of VZV-gB, gE, gH, or gL, as well as T7 polymerase and Renilla luciferase, and 293T cells transfected with MAG, IDE, and firefly luciferase and were cocultured for 18 h. Combinations of glycoproteins used for effector cells were shown. Mock-transfectants were used as control (Cont.). Relative firefly luciferase activities are presented as means ± SD of at least triplicates. Statistical significance (P-value) was shown in the figure. Data are representative of at least three independent experiments.
Because MAG associates with both VZV-gB and VZV-gE (Figs. 1C and 2B), the roles of VZV-gB and VZV-gE in MAG-mediated cell-cell fusion were further addressed. Although 293T cells cotransfected with VZV-gB, gE, gH, and gL showed cell-cell fusion with MAG-transfected 293T cells (Fig. 3E), cell-cell fusion was not observed in the absence of VZV-gB or VZV-gH. The efficiency of cell-cell fusion was augmented in the absence of VZV-gE. There was no significant difference in the efficiency of cell-cell fusion in the presence of gI that associates with gE (Fig. S6) (14). VZV-gE did not affect the cell-surface expression of VZV-gB and VZV-gH (Fig. S7). These results suggest that the association of MAG with VZV-gB is involved in membrane fusion in collaboration with VZV-gH and VZV-gL. Furthermore, VZV-gE seemed not to be directly involved in membrane fusion.
VZV Infection of MAG-Expressing Cells.
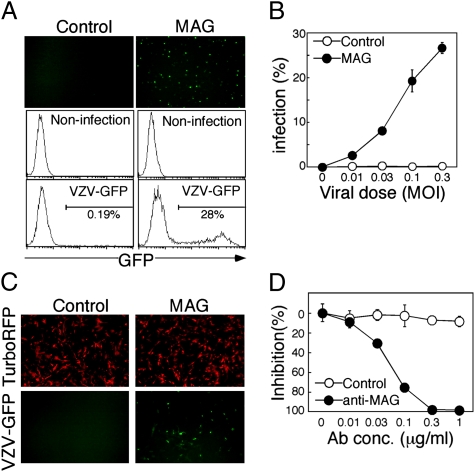
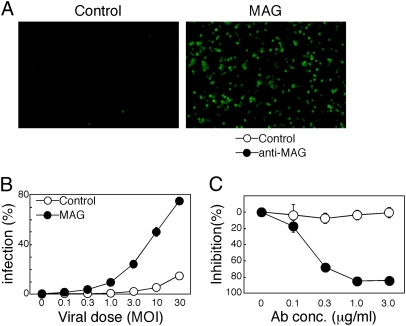
We then examined whether expression of MAG affects VZV and HSV-1 infection using recombinant viruses that possess a GFP reporter gene (VZV-GFP and HSV-GFP) (20, 29). Although MAG is specifically expressed on glial cells, such as oligodendroglial cells and Schwann cells in vivo (30), we could not find any human cell lines expressing endogenous MAG. Schwann cells obtained from primary culture also did not express MAG (Fig. S8A). Therefore, a MAG-negative human oligodendroglial cell line, OL, was stably transfected with MAG or mock, and these transfectants were used for VZV infection. The proportions of cells expressing GFP were increased in a dose-dependent manner when MAG-transfected OL cells but not mock-transfected OL cells were infected with VZV-GFP (Fig. 4 A and B). Similar results were obtained using nonrecombinant VZV (Fig. S9A). The MRC-5 cell line is a fibroblast-like cell line that is commonly used to amplify attenuated VZV vaccine. Interestingly, MAG-transfected MRC-5 cells were infected with VZV even at a dose at which mock-transfected MRC-5 cells or parental MRC-5 cells were hardly infected (Fig. 4C and Fig. S9B). This indicated that MRC-5 cells are not fully susceptible to VZV infection and MAG dramatically enhances susceptibility of MRC-5 cells to VZV infection. Reduced quantities of VZV gB receptors might restrict entry of cell-free VZV into cultured cells (31). Furthermore, VZV infection of MAG-expressing cells was completely blocked by anti-MAG mAb (Fig. 4D). On the other hand, VZV did not infect IDE-alone-transfected cells although expression of IDE was significantly augmented by transfection (Fig. S9 C and D). These results demonstrate that MAG enhances VZV infection.
Fig. 4.
MAG enhances VZV infection. (A) VZV infection of MAG-expressing cells. MAG- or mock- (Control) transfected OL cells were infected with VZV-GFP at 0.3 MOI. Expressions of GFP in VZV-infected cells were shown (Upper). Proportions of GFP-expressing cells in mock-infected cells and VZV-GFP-infected cells (VZV-GFP) were analyzed by flow cytometry (Middle and Lower). (B) MAG- (closed circle) or mock- (control, open circle) transfected OL cells were infected with VZV-GFP at the indicated MOI and the proportions of infected cells were calculated. (C) MAG-mediated VZV infection of MRC-5 cells. MAG and TurboRFP were transfected into MRC-5 cells using pMxs-IRES-TurboRFP expression vector, and the transfectants were infected with VZV-GFP. Expressions of TurboRFP (Upper) and GFP (Lower) were analyzed by fluorescence microscopy. (D) Inhibition of VZV infection of MAG-expressing cells by anti-MAG mAb. MAG-expressing OL cells were infected with VZV-GFP at 0.1 MOI in the presence of anti-MAG mAb (closed circle) or isotype-matched control mAb (open circle). Proportions of GFP-positive cells were determined by flow cytometry and the percentage inhibition of VZV infection by mAbs was calculated. All data are presented as means ± SD of duplicates. Data are representative of at least three independent experiments.
HSV-1 Infection of MAG-Expressing Cells.
HSV-1 gB also associates with MAG and can promote cell-cell fusion. We then addressed the role of MAG in HSV-1 infection. HL-60 is a promyelocytic cell line that expresses HVEM and nectin-1 (Fig. S8B). We stably transfected MAG into HL-60 and analyzed HSV-1 infection. Interestingly, the MAG-transfected HL-60 was efficiently infected with HSV (Fig. 5 A and B). The efficiency of infection was reduced in the presence of anti-MAG mAb (Fig. 5C). This finding suggests that MAG can promote HSV-1 entry into promyelocytes.
Fig. 5.
MAG enhances HSV-1 infection. (A) HSV-1 infection of MAG-expressing cells. MAG- or control-transfected HL-60 cells were infected with HSV-GFP at 10 MOI. Expressions of GFP in HSV-1-infected cells were shown (Upper). (B) MAG- (closed circle) or mock- (open circle) transfectants were infected with HSV-GFP at the indicated MOI and the proportions of infected cells were calculated. Data are presented as means ± SD of triplicates. (C) Inhibition of HSV-1 infection of MAG-expressing cells by anti-MAG mAb. MAG-expressing cells were infected with HSV-GFP at 10 MOI in the presence of anti-MAG mAb (closed circle) or isotype-matched control mAb (open circle). The percentage inhibition of HSV-1 infection by mAbs was calculated. Data are presented as means ± SD of triplicates. Data are representative of at least three independent experiments.
Discussion
In the present study, we showed that VZV and HSV-1 gB associate with MAG, and both VZV and HSV-1 efficiently infect MAG-expressing cells. Furthermore, cell-cell fusion was observed when MAG-expressing cells were cocultured with cells expressing VZV or HSV-1 envelope glycoproteins. Although association of MAG with VZV-gE was also observed, cell-cell fusion was not induced in the presence of gE, gH, and gL or gE and gB. On the other hand, gB appeared to mediate cell-cell fusion by associating with MAG in the presence of gH and gL. Furthermore, MAG-mediated cell-cell fusion was decreased in the presence of VZV-gE. These results suggested that VZV-gB, gH, and gL were the minimal requirement for cell-cell fusion by VZV glycoproteins and that VZV-gE was not necessary for cell-cell fusion.
Previously, Maresova et al., using a cell fusion assay, have reported that VZV-gH alone or gE plus gB mediates cell-cell fusion (16). However, they used recombinant vaccinia virus to express envelope glycoproteins. Therefore, there is a possibility that certain cell-surface molecules derived from vaccinia virus affected the results of their cell fusion assay because many viral proteins derived from vaccinia virus, including envelope proteins of vaccinia virus, are also expressed on the cell surface of vaccinia virus-infected cells. In the present study, a virus-free transfection system was employed to express VZV glycoproteins on the cell surface and, therefore, it is unlikely that other molecules have affected the required composition of glycoproteins for cell-cell fusion.
MPRci has been suggested to play an important role in cell-free VZV infection (9); however, when the role of MPRci was analyzed using a cell-cell fusion assay, MPRci did not induce cell-cell fusion, although MPRci was highly expressed on the cell surface of MPRci-transfected cells. Therefore, MPRci seems not to be directly involved in membrane fusion. On the other hand, several molecules are known to associate with MPRci expressed on the cell surface (10). Therefore, it is possible that certain VZV glycoproteins associate with MPRci and this association is involved in VZV infection by enhancing the binding of virions to the cell surface.
In the case of HSV infection, gB, gD, gH, and gL are essential for membrane fusion and viral infection (19). Cellular receptors for HSV-gB can play an important role in HSV-1 infection. Indeed, MAG and PILRα enhanced HSV-1 entry into promyelocytes and monocytes, respectively. However, interaction between HSV-gB and PILRα alone does not mediate membrane fusion in the absence of gD (20). gD has been suggested to induce conformational changes in HSV-gB and gH by associating with gD receptors, such as nectin and HVEM, and the conformational changes of gB and gH appear to be essential for membrane fusion by HSV-1-envelope proteins (32). Because HL-60 cells express HVEM and nectin-1, MAG expressed on HL-60 promyelocytes might enhance HSV infection in collaboration with interaction between gD and its receptors. On the other hand, because VZV does not possess gD, VZV-gE has been considered to be a substitute for HSV-gD and, indeed, several studies have shown that VZV-gE is indispensable for VZV replication using gE mutant viruses (3). Recently, it has been reported that IDE associates with VZV-gE, and IDE-transfected cells are susceptible for VZV infection (17). However, gE did not associate with IDE on cell surfaces when the association was analyzed by flow cytometry using a gE-Ig fusion protein. In contrast, significant association of VZV-gE with MAG was detected on the cell surface by flow cytometry. Because interaction between gE and MAG did not mediate membrane fusion, gE was not necessary for cell-cell fusion involving MAG-expressing cells. There was also evidence that gE/gI was not required for fusion of these cells, and IDE was not required. Therefore, in these assays of cell-cell fusion, gB and gH/gL and MAG were sufficient, whereas IDE and gE were not important. Interaction between IDE and gE seems to play important, but undefined, roles in VZV infection. Because cell-cell fusion was observed in the presence of VZV-gB, gH and gL, mechanism of membrane fusion by VZV-glycoproteins seems to be different from that by HSV-glycoproteins.
MAG is predominantly expressed on the cell surface of glial cells, and the association of MAG with Nogo-66 receptor or paired Ig-like receptor B regulates axonal growth of neurons (22,23,24–25). Because both VZV and HSV-1 infect neurons and epithelial cells that do not express MAG, there might exist certain molecules other than MAG that associate with gB on these cells. However, VZV and HSV-1 also infect glial cells in the acute phase of infection (33, 34). Therefore, MAG might be involved in certain aspects of neural disorders induced by VZV and HSV-1 infection. Because cells expressing intrinsic MAG were not available, we could not further analyze the role of intrinsic MAG in VZV and HSV-1 infection. In addition, because there are cell lines that do not express MAG and PILRα but are still susceptible to HSV or VZV infection, there might be other cell-surface molecules that associate with HSV-gB and VZV-gB and mediate infection. Further structural and functional analyses of the interactions between gB and MAG may provide important insights into the mechanism of VZV and HSV-1 infection.
Materials and Methods
Antibodies.
Anti-VZV-gE (SG1-1), -gB (SG2), -gH (SG3), -gI (SG4), -human IDE (9B12.225), and -human Sialoadhesin (Siglec-1) (HSn 7D2) mouse monoclonal antibodies (mAbs) were purchased from Genetex. Anti-MAG (513) and anti-immediate early gene 62 (IE62) (MAB8616) mouse mAb were purchased from Chemicon. Anti-HSV-gB (H1817), -gD (DL6), and –gH (53-S) mouse mAb were purchased from EastCoast Bio, Santa Cruz, and ATCC, respectively. Anti-FLAG (M2), and anti-human Siglec-5 (194128) mAbs were purchased from Sigma-Aldrich and R&D Systems, respectively. Rabbit anti-VZV gM Ab and anti-human PILRα mAb have been described previously (20, 35). Anti-MPRci (MEM-238), anti-α-tubulin (TU-01), anti-human Siglec-2 (CD22) (HIB22), and Siglec-3 (CD33) mAb (HIM3-4) were purchased from Biolegend, EXBIO, BD Bioscience, and eBioscience, respectively. Anti-HVEM (122) and anti-Nectin-1(CK8) were purchased from MBL and Zymed, respectively.
Cell Lines and Viruses.
Human melanoma MeWo cells, 293T cells, and human oligodendroglial OL cells (kindly provided by K. Ikuta, Osaka University) were cultured in DMEM (Nacalai Tesque). Human fetal lung fibroblasts (MRC-5) (kindly provided by Y. Gomi, The Research Foundation for Microbial Diseases of Osaka University) were cultured in MEM (Nissui) containing 1.55 g/l NaHCO3. Human promyelocytic leukemia HL-60 cells and CHO cells were cultured in RPMI-1640 (Nacalai Tesque) and F-12 Ham's medium (Sigma-Aldrich), respectively. Human Schwann cells derived from primary culture were purchased from ScienCell Research Laboratory and were cultured with manufacture's SCM medium. All cells were cultured at 37°C in 5% CO2 in medium supplemented with 10% FCS (Sigma-Aldrich), 100 U/mL penicillin, 100 μg/mL streptomycin, and 50 μM 2-mercaptoethanol.
VZV Oka strain and recombinant Oka strain carrying GFP reporter gene (VZV-GFP, kindly provided by A. M. Arvin, Stanford University) were used for analyzes of VZV infection (29). Because GFP is expressed by CMV promoter in the recombinant VZV, the virus particle itself does not contain GFP. Cell-free virus was prepared from VZV-infected MeWo cells by harvesting cells with 0.25% EDTA (2.5 mL per 100-mm dish of infected cells) and resuspending harvested cells in 0.4 mL of SPGA buffer (pH 8.0, 218 mM sucrose, 3.8 mM KH2PO4, 4.9 mM sodium glutamate, and 1% BSA) (36). Suspended cells were sonicated twice on ice for 15 s with a 1 min intervening interval, followed by centrifuge at 12,000 × g at 4°C for 5 min. The resulting supernatant was passed through a 0.45 μm filter and stored at −80°C. Frozen supernatant was thawed immediately before use as cell-free virus. Viral titers were determined by using MAG-transfected OL cells.
Recombinant HSV-1 (strain F) carrying GFP (YK333) (37) were used in this study. Because GFP is expressed by Egr-1 promoter in the recombinant HSV-1, the virus particle itself does not contain GFP. Virus titers were determined by using Vero cells as previously described (37).
Plasmids.
cDNA fragments of human MAG and IDE were amplified from human brain cDNA (Takara Bio), cDNA of HepG2 cells, and cDNA of Plat-E cells, respectively, and were subcloned into pME18S, pMXs-puro, and pMXs-IRES-TurboRFP expression vectors. Human MPRci were amplified from cDNA of Plat-E cells. The human PILRα-expressing vector has been previously described (20). cDNAs for VZV glycoproteins, gB, gE, gC, gH, gL, and gI were amplified from genomic DNA of VZV-infected MeWo cells and subcloned into pcDNA3.1 vector (Invitrogen) or pME18S expression vector. gH lacking the C-terminus tail (amino acid residues 834–841) was generated by QuikChange Site-Directed Mutagenesis Kit (Stratagene). cDNA fragments of human Siglec-2 and Siglec-5 were amplified from cDNA of human peripheral blood mononuclear cells and HL-60 cells, respectively. IMAGE human cDNA clone for human Siglec-3 that was subcloned into a pCMV-SPORT6 vector was purchased from Open Biosystems. A cDNA clone for human Siglec-1 was kindly provided by T. Angata (National Institute of Advanced Industrial Science and Technology, Japan) and subcloned into a pCIneo expression vector (Promega). HSV-1-gB, -gD. -gH and -gL expression vectors were kindly provided by P. G. Spear (Northwestern University). PCR primers and expression vectors used in this study are listed in Table S1.
Transfection.
COS-7 cells or 293T cells were transfected with 293 Fectin (Invitrogen). Stable transfectants expressing human MAG or human IDE were generated by a retroviral transfection system using a pMxs-puro or pMxs-IRES-TurboRFP retroviral vector, as previously described (38, 39).
Ig-Fusion Protein.
Plasmids for Ig fusion proteins were constructed as described above. COS-7 cells were transfected transiently with expression vectors for Ig fusion proteins and the culture supernatants collected; purified human CD85J-Ig fusion protein was used as a control (40). Ig fusion proteins were purified on protein A-conjugated Sepharose. VZV gE-IgG Fc fusion protein (kindly provided by J. I. Cohen, National Institutes of Health) has been described previously (17).
Immunoprecipitation and Immunoblotting.
Cells were disrupted in lysis buffer (20 mM Tris, 150 mM NaCl, and pH 7.5) containing 1% Brij 98 (Sigma) and lysates immunoprecipitated with human MAG-Ig or control-Ig. The immunoprecipitates were eluted by boiling in SDS/PAGE sample buffer and separated on 5 to 20% polyacrylamide gels. Proteins were transferred onto PVDF membranes (Millipore) and blotted with anti-gB (SG2), anti-gE (SG1-1), or anti-gH (SG3) mAbs.
Flow Cytometry.
Cells were incubated with Ig fusion proteins or primary mAbs, followed by PE- or APC-conjugated anti-human IgG or anti-mouse IgG Ab (Jackson Immunoresearch). Expression of viral glycoproteins, Siglecs, and MPRci were analyzed using mAbs as described above. For intracellular staining, cells were fixed with 4% paraformaldehyde in PBS for 15 min at room temperature and permeabilized with permeabilization buffer (150 mM NaCl, 5 mM EDTA, 50 mM Tris-Cl, and pH 7.5) containing 0.02% NaN3 and 0.5% Triton X-100 for 10 min on ice. Permeabilized cells were stained with anti-IE62 mAb and analyzed by using a FACSCalibur (BD Bioscience).
Viral Infection.
Cell-free VZV or HSV infection was analyzed by mixing 2 × 104 or 5 × 104 of cells with various amounts of cell-free VZV or HSV in advanced RPMI-1640 (Invitrogen) containing 1% FCS in 96-well tissue culture plates, respectively. Plates were centrifuged at 1,220 × g at 32°C for 2 h, followed by 24 h culture.
Cell-Fusion Assay.
MAG, IDE, MPRci, and DsRed were cotransfected into 293T cells and the resulting transfectants used as target cells. The set of VZV-gB, -gE, -gH, -gL, and GFP or the set of HSV-gB, -gD, gH, gL and GFP was also cotransfected into 293T cells or PILR-negative CHO cells, respectively, and these transfectants were used as effector cells. The total amount of DNA for transfcetion into 2 × 105 cells was 0.8 μg. Glycoprotein VZV-gH that lacked the C-terminus tail was used for fusion assays, as described above, because cell surface expression of mutant gH was higher than that of full-length gH (41). Eighteen to 24 h after transfection with VZV glycoproteins, or 6 h after transfection with HSV glycoproteins, 5 × 104 each of the target and effector cells were cocultured in 96-well plates for 18 h, the cells analyzed by fluorescence microscopy (Carl Zeiss), photographs taken with a D3 digital camera (Nikon), and the images then processed using Canvas software (ACD Systems). To quantify fusion efficiency, a plasmid encoding T7 RNA polymerase (pCAGT7) was cotransfected into target cells instead of a GFP-expressing plasmid, and a plasmid carrying the firefly luciferase gene under control of the T7 promoter (pT7EMCLuc) was cotransfected into effector cells instead of a DsRed-expressing plasmid (28). As an internal control, the Renilla luciferase gene driven by the SV40 promoter (pRL-SV40, Promega) was cotransfected into target or effector cells. The effector and target cells were harvested cocultured as described above. Then, firefly and Renilla luciferase activities were independently quantified by using the Dual-Luciferase Reporter Assay System (Promega). The relative firefly luciferase activity was calculated as follows: [(the firefly luciferase activity / Renilla luciferase activity) × 100] / maximum (the firefly luciferase activity / Renilla luciferase activity). The statistical difference was determined by Student's t-test. Each P-value is shown in figures. Difference with P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Drs. A. M. Arvin, J. I. Cohen, P. G. Spear and T. Angta for kindly providing reagents, and Dr. L. L. Lanier for kind suggestions and Ms. R. Hirohata and M. Matsumoto for technical assistance. This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture, Japan (to T. Suenaga, Y.K., Y.M., and H.A.) and in part by grants from the Takeda Science Foundation (to H.A.), the Uehara Memorial Foundation (to H.A.), and the Naito Foundation (to H.A.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0913351107/DCSupplemental.
References
- 1.Cohen JI. Strategies for herpes zoster vaccination of immunocompromised patients. J Infect Dis. 2008;197(Suppl 2):S237–S241. doi: 10.1086/522129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steiner I, Kennedy PG, Pachner AR. The neurotropic herpes viruses: herpes simplex and varicella-zoster. Lancet Neurol. 2007;6:1015–1028. doi: 10.1016/S1474-4422(07)70267-3. [DOI] [PubMed] [Google Scholar]
- 3.Arvin AM. Varicella-zoster virus: molecular virology and virus-host interactions. Curr Opin Microbiol. 2001;4:442–449. doi: 10.1016/s1369-5274(00)00233-2. [DOI] [PubMed] [Google Scholar]
- 4.Marsh M, Helenius A. Virus entry: open sesame. Cell. 2006;124:729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sedý JR, Spear PG, Ware CF. Cross-regulation between herpesviruses and the TNF superfamily members. Nat Rev Immunol. 2008;8:861–873. doi: 10.1038/nri2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole NL, Grose C. Membrane fusion mediated by herpesvirus glycoproteins: the paradigm of varicella-zoster virus. Rev Med Virol. 2003;13:207–222. doi: 10.1002/rmv.377. [DOI] [PubMed] [Google Scholar]
- 7.Gabel CA, et al. Varicella-zoster virus glycoprotein oligosaccharides are phosphorylated during posttranslational maturation. J Virol. 1989;63:4264–4276. doi: 10.1128/jvi.63.10.4264-4276.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu Z, Gershon MD, Ambron R, Gabel C, Gershon AA. Infection of cells by varicella zoster virus: inhibition of viral entry by mannose 6-phosphate and heparin. Proc Natl Acad Sci USA. 1995;92:3546–3550. doi: 10.1073/pnas.92.8.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen JJ, Zhu Z, Gershon AA, Gershon MD. Mannose 6-phosphate receptor dependence of varicella zoster virus infection in vitro and in the epidermis during varicella and zoster. Cell. 2004;119:915–926. doi: 10.1016/j.cell.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh P, Dahms NM, Kornfeld S. Mannose 6-phosphate receptors: new twists in the tale. Nat Rev Mol Cell Biol. 2003;4:202–212. doi: 10.1038/nrm1050. [DOI] [PubMed] [Google Scholar]
- 11.Morgan DO, et al. Insulin-like growth factor II receptor as a multifunctional binding protein. Nature. 1987;329:301–307. doi: 10.1038/329301a0. [DOI] [PubMed] [Google Scholar]
- 12.Mo C, Lee J, Sommer M, Grose C, Arvin AM. The requirement of varicella zoster virus glycoprotein E (gE) for viral replication and effects of glycoprotein I on gE in melanoma cells. Virology. 2002;304:176–186. doi: 10.1006/viro.2002.1556. [DOI] [PubMed] [Google Scholar]
- 13.Berarducci B, et al. Deletion of the first cysteine-rich region of the varicella-zoster virus glycoprotein E ectodomain abolishes the gE and gI interaction and differentially affects cell-cell spread and viral entry. J Virol. 2009;83:228–240. doi: 10.1128/JVI.00913-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mallory S, Sommer M, Arvin AM. Mutational analysis of the role of glycoprotein I in varicella-zoster virus replication and its effects on glycoprotein E conformation and trafficking. J Virol. 1997;71:8279–8288. doi: 10.1128/jvi.71.11.8279-8288.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang ZH, et al. Essential role played by the C-terminal domain of glycoprotein I in envelopment of varicella-zoster virus in the trans-Golgi network: interactions of glycoproteins with tegument. J Virol. 2001;75:323–340. doi: 10.1128/JVI.75.1.323-340.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maresova L, Pasieka TJ, Grose C. Varicella-zoster Virus gB and gE coexpression, but not gB or gE alone, leads to abundant fusion and syncytium formation equivalent to those from gH and gL coexpression. J Virol. 2001;75:9483–9492. doi: 10.1128/JVI.75.19.9483-9492.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Q, Ali MA, Cohen JI. Insulin degrading enzyme is a cellular receptor mediating varicella-zoster virus infection and cell-to-cell spread. Cell. 2006;127:305–316. doi: 10.1016/j.cell.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ali MA, Li Q, Fischer ER, Cohen JI. The insulin degrading enzyme binding domain of varicella-zoster virus (VZV) glycoprotein E is important for cell-to-cell spread and VZV infectivity, while a glycoprotein I binding domain is essential for infection. Virology. 2009;386:270–279. doi: 10.1016/j.virol.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spear PG. Herpes simplex virus: receptors and ligands for cell entry. Cell Microbiol. 2004;6:401–410. doi: 10.1111/j.1462-5822.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- 20.Satoh T, et al. PILRalpha is a herpes simplex virus-1 entry coreceptor that associates with glycoprotein B. Cell. 2008;132:935–944. doi: 10.1016/j.cell.2008.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliver SL, et al. Mutagenesis of varicella-zoster virus glycoprotein B: putative fusion loop residues are essential for viral replication, and the furin cleavage motif contributes to pathogenesis in skin tissue in vivo. J Virol. 2009;83:7495–7506. doi: 10.1128/JVI.00400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang KC, et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- 23.McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu BP, Fournier A, GrandPré T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- 25.Atwal JK, et al. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- 26.Arase H, Lanier LL. Specific recognition of virus-infected cells by paired NK receptors. Rev Med Virol. 2004;14:83–93. doi: 10.1002/rmv.422. [DOI] [PubMed] [Google Scholar]
- 27.Varki A, Angata T. Siglecs—the major subfamily of I-type lectins. Glycobiology. 2006;16:1R–27R. doi: 10.1093/glycob/cwj008. [DOI] [PubMed] [Google Scholar]
- 28.Okuma K, Nakamura M, Nakano S, Niho Y, Matsuura Y. Host range of human T-cell leukemia virus type I analyzed by a cell fusion-dependent reporter gene activation assay. Virology. 1999;254:235–244. doi: 10.1006/viro.1998.9530. [DOI] [PubMed] [Google Scholar]
- 29.Zerboni L, Sommer M, Ware CF, Arvin AM. Varicella-zoster virus infection of a human CD4-positive T-cell line. Virology. 2000;270:278–285. doi: 10.1006/viro.2000.0304. [DOI] [PubMed] [Google Scholar]
- 30.Quarles RH. Myelin-associated glycoprotein (MAG): past, present and beyond. J Neurochem. 2007;100:1431–1448. doi: 10.1111/j.1471-4159.2006.04319.x. [DOI] [PubMed] [Google Scholar]
- 31.Carpenter JE, Henderson EP, Grose C. Enumeration of an extremely high particle-to-PFU ratio for Varicella-zoster virus. J Virol. 2009;83:6917–6921. doi: 10.1128/JVI.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heldwein EE, et al. Crystal structure of glycoprotein B from herpes simplex virus 1. Science. 2006;313:217–220. doi: 10.1126/science.1126548. [DOI] [PubMed] [Google Scholar]
- 33.Reichelt M, Zerboni L, Arvin AM. Mechanisms of varicella-zoster virus neuropathogenesis in human dorsal root ganglia. J Virol. 2008;82:3971–3983. doi: 10.1128/JVI.02592-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Assouline JG, et al. Varicella-zoster virus infection of human astrocytes, Schwann cells, and neurons. Virology. 1990;179:834–844. doi: 10.1016/0042-6822(90)90152-h. [DOI] [PubMed] [Google Scholar]
- 35.Yamagishi Y, et al. Varicella-zoster virus glycoprotein M homolog is glycosylated, is expressed on the viral envelope, and functions in virus cell-to-cell spread. J Virol. 2008;82:795–804. doi: 10.1128/JVI.01722-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kinchington PR, Fite K, Turse SE. Nuclear accumulation of IE62, the varicella-zoster virus (VZV) major transcriptional regulatory protein, is inhibited by phosphorylation mediated by the VZV open reading frame 66 protein kinase. J Virol. 2000;74:2265–2277. doi: 10.1128/jvi.74.5.2265-2277.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka M, Kodaira H, Nishiyama Y, Sata T, Kawaguchi Y. Construction of recombinant herpes simplex virus type I expressing green fluorescent protein without loss of any viral genes. Microbes Infect. 2004;6:485–493. doi: 10.1016/j.micinf.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 38.Kitamura T, et al. Retrovirus-mediated gene transfer and expression cloning: powerful tools in functional genomics. Exp Hematol. 2003;31:1007–1014. [PubMed] [Google Scholar]
- 39.Suenaga T, et al. Cloning of B cell-specific membrane tetraspanning molecule BTS possessing B cell proliferation-inhibitory function. Eur J Immunol. 2007;37:3197–3207. doi: 10.1002/eji.200737052. [DOI] [PubMed] [Google Scholar]
- 40.Shiratori I, et al. Down-regulation of basophil function by human CD200 and human herpesvirus-8 CD200. J Immunol. 2005;175:4441–4449. doi: 10.4049/jimmunol.175.7.4441. [DOI] [PubMed] [Google Scholar]
- 41.Pasieka TJ, Maresova L, Grose C. A functional YNKI motif in the short cytoplasmic tail of varicella-zoster virus glycoprotein gH mediates clathrin-dependent and antibody-independent endocytosis. J Virol. 2003;77:4191–4204. doi: 10.1128/JVI.77.7.4191-4204.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.