Abstract
Endocannabinoids such as anandamide [N-arachidonoylethanolamine (AEA)] and 2-arachidonoyl glycerol (2-AG) are known orexigenic mediators that act via CB1 receptors in hypothalamus and limbic forebrain to induce appetite and stimulate food intake. Circulating endocannabinoid levels inversely correlate with plasma levels of leptin, an anorexigenic mediator that reduces food intake by acting on hypothalamic receptors. Recently, taste has been found to be a peripheral target of leptin. Leptin selectively suppresses sweet taste responses in wild-type mice but not in leptin receptor-deficient db/db mice. Here, we show that endocannabinoids oppose the action of leptin to act as enhancers of sweet taste. We found that administration of AEA or 2-AG increases gustatory nerve responses to sweeteners in a concentration-dependent manner without affecting responses to salty, sour, bitter, and umami compounds. The cannabinoids increase behavioral responses to sweet-bitter mixtures and electrophysiological responses of taste receptor cells to sweet compounds. Mice genetically lacking CB1 receptors show no enhancement by endocannnabinoids of sweet taste responses at cellular, nerve, or behavioral levels. In addition, the effects of endocannabinoids on sweet taste responses of taste cells are diminished by AM251, a CB1 receptor antagonist, but not by AM630, a CB2 receptor antagonist. Immunohistochemistry shows that CB1 receptors are expressed in type II taste cells that also express the T1r3 sweet taste receptor component. Taken together, these observations suggest that the taste organ is a peripheral target of endocannabinoids. Reciprocal regulation of peripheral sweet taste reception by endocannabinoids and leptin may contribute to their opposing actions on food intake and play an important role in regulating energy homeostasis.
Keywords: energy homeostasis, gustation, reciprocal regulation
Endocannabinoids such as anandamide [N-arachidonoylethanolamine (AEA)] and 2-arachidonoyl glycerol (2-AG) are known orexigenic mediators that act via CB1 receptors in hypothalamus and limbic forebrain to induce appetite (1, 2) and stimulate food intake (3). Systemic administration of exogenous cannabinoids or endocannabinoids in rodents causes hyperphagia (4) and increases the preference for palatable substances such as sucrose solution or food pellets (5, 6). These effects are mediated by the CB1 receptor: pretreatment with the CB1 antagonist SR141716 inhibited hyperphagia and reduced consumption of both bland and palatable foods (4–6). The natural “liking” reactions of rats to sweet compounds were amplified by endogenous cannabinoid signals in nucleus accumbens (7). Thus, endocannabinoids may be related to hedonic aspects of sweet taste.
There is growing evidence that taste function can be modulated by hormones or other factors that act on receptors present in the peripheral gustatory system. Leptin, an anorexigenic mediator that reduces food intake by acting on hypothalamic receptors (8), selectively suppresses sweet taste responses and these effects may be mediated by leptin receptor, Ob-Rb (9–11). GLP-1, an incretin that influences glucose transport, metabolism, and homeostasis (12), normally acts to maintain or enhance sweet taste sensitivity by its paracrine activity (13). We sought to determine whether cannabinoids affect peripheral sweet taste reception. In the present study, we investigated neural, behavioral, and cellular responses to taste stimuli before and after administration of endocannabinoids. We demonstrated that sweet taste responses are selectively enhanced by administration of endocannabinoids AEA and 2-AG, and that the sweet enhancing effect of enndocannabinoids was mediated by CB1 receptors, which are coexpressed in taste cells with the sweet receptor component T1r3.
Results and Discussion
Gustatory Nerve Responses.
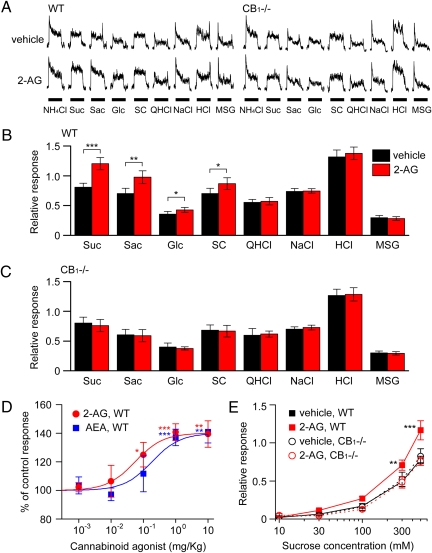
We examined potential effects of endocannabinoids on gustatory nerve responses to various taste stimuli and involvement of CB1 receptors in the effects by using wild-type (WT) and CB1−/− mice (14). Because mouse responses to sweet substances are much larger in the chorda tympani (CT) nerve innervating the anterior tongue than in the glossopharyngeal (GL) nerve innervating the posterior tongue (15, 16) we focused on CT nerve responses. We recorded CT taste responses after administration of vehicle (saline with less than 0.5% ethanol) or cannabinoids AEA and 2-AG. After i.p. injection of 2-AG, CT nerve responses of WT mice to sweeteners increased significantly [Fig. 1A: sucrose (P < 0.001, t test), saccharin (P < 0.01), glucose and SC45647 (P < 0.05)]. After injection of endocannabinoids, increased responses to sweet compounds (∼150% of control for 500 mM sucrose) were observed at 10–30 min postinjection and then recovered to the control level at 60–120 min postinjection (Fig. S1). In marked contrast, 2-AG had no such effect in WT mice on responses to salty (NaCl), bitter (quinine), sour (HCl), or umami [MSG: monosodium l-glutamate (in the presence of 10 μM amiloride, a sodium response inhibitor)] substances (P > 0.1; Fig. 1B and Fig. S2), suggesting that the effect of 2-AG is highly specific for sweet taste. The sweet enhancing effect of 2-AG was absent in CB1−/− mice (P > 0.1; Fig. 1 A and C), indicating that 2-AG is acting on sweet taste via the CB1 receptor. The effect of endocannabinoids on sweet responses in WT mice was dose dependent (Fig. 1D), saturated at ≈1 mg/kg body weight (bw) of the AEA or 2-AG injected. 2-AG has a higher affinity than AEA for the CB1 receptor (17): consistent with the published report, the EC50 of AEA (0.185 mg/kg bw) was approximately 3-fold greater than that of 2-AG (0.055 mg/kg bw), but the maximum effect at the saturating concentration was not significantly different between 2-AG and AEA. At a dose of 1 mg/kg bw of 2-AG, the CT nerve responses to ∼10–500 mM sucrose were significantly enhanced in WT (F(1,79) = 5.68, P < 0.05) but not in CB1−/− mice (F(1,76) = 0.73, P > 0.1; Fig. 1E). Similar effects of 2-AG were observed in GL nerve responses (Fig. S3).
Fig. 1.
Endocannabinoids enhance gustatory nerve responses to sweeteners. (A) Typical examples of CT nerve responses of WT and CB1−/− mice showing the effect of i.p. injection of 1 mg/kg bw of 2-AG (Lower traces) vs. vehicle-injected control (Upper traces). CT nerve responses (normalized to response to 100 mM NH4Cl) of WT (B) and CB1−/− (C) mice stimulated by sweet (Suc, 500 mM sucrose; Sac, 20 mM saccharin; Glc, 500 mM glucose; SC, 1 mM SC45647), bitter (QHCl, 20 mM quinine-HCl), salty (NaCl, 100 mM NaCl), sour (HCl, 10 mM HCl), and umami (MSG, 100 mM monosodium glutamate + 10 μM amiloride) compounds 10–30 min after administration of vehicle (black bars) or 1 mg/kg bw of 2-AG (red bars) (n = 5–10). (D) Dose-dependent effect of AEA (blue symbols) or 2-AG (red symbols) treatment on normalized chorda tympani nerve responses to 500 mM sucrose (n = 5–14). (E) Concentration-dependent responses to sucrose 10–30 min after administration of vehicle (black symbols) or 1 mg/kg bw of 2-AG (red symbols) in WT (squares) (n = 7) and CB1−/− (circles) mice (n = 5). Asterisks indicate significant differences from control (*P < 0.05; **P < 0.01; ***P < 0.001; Fisher’s PLSD post hoc test or t test). All data are presented as the mean ± SEM.
Behavioral Responses.
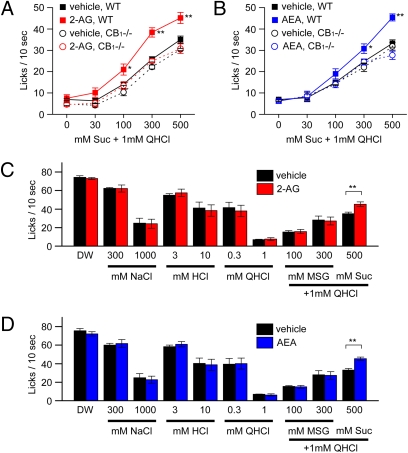
Next, we asked whether the enhancement of sweet responses of gustatory nerves by endocannabinoids would alter behavioral responses of mice to sweet stimuli. We used a short-term lick test and measured the number of licks (per 10 s) for test stimuli after administration of vehicle or 2-AG. Numbers of licks per 10 s for distilled water (DW) and sucrose at various concentrations in water-deprived mice are typically similar, within a range of about 60–80, whereas lick responses for 1 mM quinine are around 10. To more clearly detect concentration-dependent changes in lick rates for sucrose, we used mixtures of 1 mM quinine and ∼30–500 mM sucrose (a sweet-bitter mixture paradigm) (10) as test solutions. As shown in Fig. 2, lick rates for sucrose-quinine mixtures increased with increasing sucrose concentration in both WT and CB1−/− mice (Fig. 2 A and B), indicating clear concentration dependencies. Thirty minutes after i.p. injection of 1 mg/kg bw of 2-AG (Fig. 2A) or AEA (Fig. 2B) in WT mice, mean lick rates for the sucrose-quinine mixtures at different concentrations of sucrose were significantly greater than before injection with 2-AG (F(1,39) = 16.6, P < 0.01) or AEA (F(1,39) = 16.4, P < 0.01). However, administration of 2-AG and AEA in WT mice did not affect lick rates for NaCl (300 and 1,000 mM), HCl (3 and 10 mM), quinine (0.3 and 1 mM), or MSG (100 and 300 mM) + quinine (1 mM) (Fig. 2 C and D), indicating a selective increase in the responses to the sucrose component of the sweet-bitter mixtures. In marked contrast, the sweet enhancing effect of 2-AG and AEA was not observed in CB1−/− mice (F(1,39) = 1.35 for 2-AG, F(1,47) = 0.10 for AEA, P > 0.1, Fig. 2 A and B), again indicating the involvement of CB1 receptors in the 2-AG effect on sweet responses. The time course for the effect of injected 2-AG on lick rates was comparable with that of CT nerve responses (Fig. S4). Lick rates for 500 mM sucrose plus 1 mM quinine started increasing ∼10 min after injection, reached a maximum level of enhancement 10–30 min after injection (about 140% of control for 500 mM sucrose), and recovered to the control level ≈2 h after injection of 2-AG (Fig. S4). Collectively, both nerve and behavioral response measurements indicate that administration of endocannabinoids selectively enhances sweet taste responses and the endocannabinoid effect is mediated by their receptor, CB1.
Fig. 2.
Endocannabinoids enhance behavioral responses to sweeteners. Concentration response relationships to varying concentrations of sucrose in mixtures with 1 mM quinine 30 min after i.p. injection of vehicle (black symbols) or 1 mg/kg bw 2-AG (red symbols) (A) and AEA (blue symbols) (B) in WT (squares) and CB1−/− (circles) mice (n = 5). Lick responses to distilled water (DW), NaCl (300 and 1,000 mM), HCl (3 and 10 mM), quinine (QHCl; 0.3 and 1 mM), MSG + 1 mM quinine (MSG; 100 and 300 mM), and sucrose + 1 mM quinine (Suc; 500 mM) 30 min after administration of vehicle (black bars), 1 mg/kg bw 2-AG (red bars) (C), or AEA (blue bars) (D) in WT mice (n = 5). Asterisks indicate significant differences (*P < 0.05; **P < 0.01; Fisher’s PLSD post hoc test or t test). All data are presented as the mean ± SEM.
Taste Cell Responses.
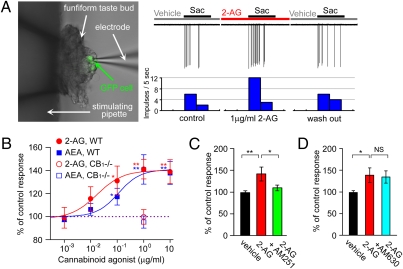
We next sought to determine whether the endocannabinoid effect occurred at the taste cell level. In fungiform taste buds, which are located on the anterior tongue innervated by the CT nerve, we found many taste cells (TCs) that responded to a sweetener (∼1–20 mM saccharin) with action potentials (18). To identify taste cells expressing T1r3, a component of both sweet and umami receptors (19–23), we used transgenic mice that express green fluorescent protein (GFP) from the T1r3 promoter (24) and recorded taste responses from these cells (Fig. 3A). As expected, T1r3-GFP taste cells responded to multiple sweeteners such as sucrose, glucose, saccharin, SC45647, and D-phenylalanine (Fig. S5); responses to saccharin were enhanced by basolateral treatment of 1 μg/mL 2-AG (Fig. 3A). We tested the effect of basolateral application of AEA and 2-AG on responses of T1r3-GFP taste cells and sweet sensitive taste cells in WT mice. In total, 27 of 47 (57%, 22 of 39 in WT, 5 of 8 in T1r3-GFP mice) cells showed enhancement of responses to sweeteners (>120% of control) after application of 1 μg/mL AEA or 2-AG to the basolateral side of taste cell membrane. Responses of TCs to saccharin were significantly increased after application of 1 μg/mL AEA (P < 0.01, n = 28) or 2-AG (P < 0.01, n = 19, t test). The enhancing effects of AEA and 2-AG on sweet responses of TCs in WT mice saturated at ∼1 μg/mL (Fig. 3B). We found the half maximal effective concentration (EC50) for enhancing sweet responses of WT TCs by AEA (0.112 μg/mL) was about 6-fold greater than that of 2-AG (0.017 μg/mL). The effective concentrations of the endocannabinoids are within physiological ranges found in various tissues (25). In CB1−/− mice, sweet responses of TCs were not affected by 1 μg/mL AEA (95.3 ± 5.3%, n = 7, Fig. 3B, open circle) or 2-AG (99.8 ± 6.3%, n = 5, Fig. 3B, open rectangle). A pharmacological blocker of CB1 receptors, AM251, suppressed the sweet enhancing effect of 1 μg/mL 2-AG (P < 0.05, n = 6, Fig. 3C); however, the CB2 receptor blocker AM630 did not (P > 0.1, n = 5, Fig. 3D). These data indicate that endocannabinoids act on CB1 receptors to enhance sweet taste responses of TCs.
Fig. 3.
Endocannabinoids enhance sweet responses of taste bud cells. (A) The effect of 1 μg/mL 2-AG on the response of a T1r3-GFP taste cell in the isolated fungiform taste bud with the epithelium to the sweet compound saccharin. The picture shows a T1r3-GFP taste cell from which taste responses were recorded. In this cell, the response to 5 mM saccharin was increased about 2-fold by bath application of 2-AG for 2 min and returned to the control level 2 min after wash-out of 2-AG. (B) Dose-dependent effect of AEA and 2-AG on responses to saccharin of taste bud cells from WT and T1r3-GFP mice (labeled WT, n = 7–28). Responses to saccharin of taste bud cell in CB1−/− mice were not affected by 1 μg/mL AEA (blue open rectangles, n = 7) or 2-AG (red open circles, n = 5). (C) The CB1 antagonist AM251 inhibited the enhancing effects of 2-AG on responses to saccharin of TCs from WT and T1r3-GFP mice (n = 10). (D) The CB2 antagonist AM630 did not inhibit the enhancing effects of 2-AG on response to saccharin of TCs from WT and T1r3-GFP mice (n = 9). Asterisks indicate significant differences (NS: P > 0.1; *P < 0.05; **P < 0.01, t test). All data are presented as the mean ± SEM.
Expression of the CB1 Receptor.
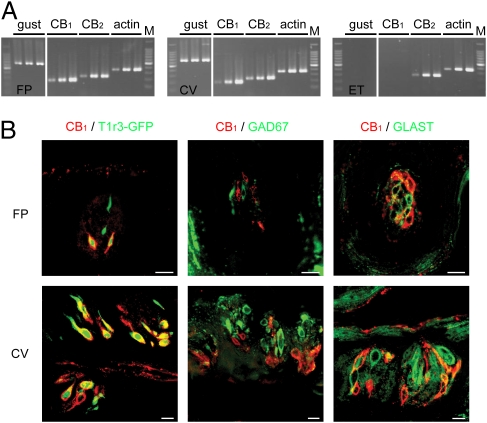
We next tested whether TCs express the CB1 receptor. In RT-PCR experiments (Fig. 4A), the mRNAs for α-gustducin (a taste selective G protein α-subunit) (26), CB1 and CB2 receptors were all expressed in taste buds in both fungiform papillae (FP) and circumvallate (CV) papillae. The mRNA for CB2, but not those for CB1 and gustducin, were expressed in epithelial tissues (ET) adjacent to taste buds. Control experiments in which reverse transcriptase was omitted yielded no specific products (Fig S6). We used immunohistochemistry to determine whether CB1 receptors were coexpressed with T1r3, a component of sweet and umami receptors. In WT mice about 70% of TCs in both FP and CV expressing CB1 receptors coexpressed T1r3; ∼60% of TCs expressing T1r3 also expressed CB1 (Fig. 4B, Fig. S7, and Table S1). In CB1−/− mice CB1 immunoreactivity in TCs was absent (Fig. S7E). The average number of CB1 positive taste cells in a fungiform or circumvallate taste bud in our slice preparation was 1.67 ± 0.08 (n = 67) and 1.73 ± 0.08 (n = 137), respectively: there is no significant difference between these numbers (P > 0.1).
Fig. 4.
CB1 and T1r3 are coexpressed in taste bud cells. (A) Expression of gustducin (40, 45, and 50 cycles), CB1 (40, 45, and 50 cycles), CB2 (40, 45, and 50 cycles), and β-actin (25, 30, and 35 cycles) mRNAs in fungiform taste buds (FP), circumvallate taste buds (CV), and tongue epithelium devoid of TCs (ET). M, 100-bp marker. (B) Coexpression patterns of CB1 with: T1r3 (Left), GAD67 (Middle), and GLAST (Right) in FP and CV of T1r3-GFP or WT mice. Immunostaining for CB1 is shown in red. T1r3-GFP expression and immunostaining for GAD and GLAST are shown in green. (Scale bar, 10 μm.) Negative control and immunostaining in CB1−/− mice are shown in Fig. S7.
In the central nervous system, CB1 receptors are expressed in presynaptic cells and underlie modulation (inhibition) of transmitter release from presynaptic cells (27). In the peripheral taste organ, CB1 immnoreactivity was observed in fewer than 12% of GAD67-expressing TCs, which in mice are thought to be presynaptic cells (28) (Fig. 4B and Table S1). GAD67-expressing presynaptic cells are reported to be primarily sensitive to sour taste stimuli (29, 30). Endocannabinoids did not affect sour taste responses, indicating that presynaptic cells are not the major target for endocannabinoids in the taste organ. Instead, the majority of TCs expressing CB1 receptors are sweet-sensitive cells expressing T1r3: endocannabinoids act to enhance sweet taste responses through these type II taste receptor cells known to lack well-elaborated synapses.
To date, leptin (9–11), CCK (31, 32), VIP (32), NPY (33), and GLP-1 (13) are implicated in the modulation of peripheral taste sensitivity. Leptin and GLP-1 are known to be modulators for sweet taste. Leptin specifically suppresses sweet taste responses and these effects may be mediated by leptin receptors (Ob-Rb) on TCs (9–11). GLP-1 signaling increases sweet and sour taste sensitivity and these effects may be mediated by GLP-1 receptors on adjacent intragemmal afferent nerve fibers (13). Our findings indicate that endocannabinoids selectively enhance sweet taste sensitivity via CB1 receptors on the TCs. Both endocannabinoids and GLP-1 enhance sweet taste but their specificity (sweet vs. sweet-and-sour) and targets (TCs vs. afferent fibers) differ, suggesting that these modulators have different roles in modulating sweet taste. Circulating endocannabinoid levels inversely correlate with plasma levels of leptin (34). Both endocannabinoids and leptin affect responses of TCs via their cognate receptors. Therefore, endocannabinoids and leptin may reciprocally regulate peripheral sweet taste sensitivity.
Exogenous cannabinoid agonists and antagonists are known to affect the preference for sweet compounds. Administration of cannabinoid agonists increase the intake of sucrose solutions (5). Systemic administration of the CB1 antagonist SR141716A decreases intake of a sweet milk diet (35), sucrose solution (5, 36), and sweetened pellets without affecting the intake of normal pellets (37, 38). These results suggest an interaction of cannabinoid-induced modifications in feeding behavior with the sensation of palatability component (i.e., sweet taste) of food stimuli. Infusions of AEA into the nucleus accumbens enhance taste reactivity to sucrose, although standard chow intake is also enhanced (7). Infusions of 2-AG into the pontine parabrachial nucleus that contains third order gustatory neurons increase intake of sweet food without affecting the intake of normal chow during the first 30 min after infusion (39). Our findings provide evidence that the peripheral taste organ is also a target of cannabinoids. Increases in taste cell responses, nerve responses, and lick responses to sucrose especially at its higher (more palatable) concentrations found in this study are in line with the previous findings mentioned above. This modulation of peripheral sweet taste sensitivities by endocannabinoids may play a significant role in regulating feeding behavior.
In conclusion, we have identified endocannabinoids as modulators of the peripheral components of sweet taste. The positive effect of endocannabinoids on sweet sensitivity was opposed to that of leptin, which suppresses sweet sensitivity (9–11). Endocannabinoids, therefore, not only stimulate food intake via central systems but also may increase palatability of foods by enhancing peripheral sweet taste responses. We found that the sweet enhancing effect of enndocannabinoids was mediated by CB1 receptors, which were coexpressed with the sweet receptor component T1r3 in TCs. Orexigenic and anorexigenic factors such as endocannnabinoids and leptin may affect energy homeostasis by regulating taste sensitivity.
Methods
Full methods are in SIMethods.
All experimental procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the committee for Laboratory Animal Care and Use at Kyushu University, Japan.
Animals.
T1r3-GFP mice were as described previously (24). CB1-KO mice on a CD1 background (14) were backcrossed to C57BL/6N mice for five generations to breed heterozygous mice. These mice were interbred to generate CB1−/− mice.
Nerve Recordings.
Whole nerve responses to lingual application of tastants were recorded from the CT and the GL nerve as described (9, 15, 16). Responses to tastants were normalized to responses to NH4Cl. A series of taste responses was recorded before and ∼5–120 min after i.p. injection of AEA, 2-AG, or vehicle (physiological saline/ethanol, 99:1). Significant effects of AEA or 2-AG in neural and behavioral experiments were tested with repeated ANOVA, the Fisher’s PLSD post hoc test and Student’s t test. All data are presented as the mean ± SEM.
Behavioral Tests.
Taste behavior was assayed by a short-term lick test with sweet-bitter mixtures as test stimuli (10). On training days (from the first to the fifth day), the mouse was placed in the test cage and trained to drink distilled water on an interval schedule (10-s DW presentation, 20-s interval) for 1-h session after 23-h water deprivation. On the test day, the number of licks for each test stimulus and DW was counted during the first 10 s after the animal’s first lick before and ∼5–120 min after administration of AEA, 2-AG, or vehicle.
Taste Cell Recordings.
Taste responses of fungiform TCs were recorded as previously described (18, 30). Action potentials of TCs in isolated taste buds were recorded extracellularly from the basolateral side at room temperature (25°C). TCs were adapted to vehicle (Tyrode with <0.1% ethanol). Numbers of impulses/10 s subtracting spontaneous activities were used to assess the effect of 2-AG, AEA, AM251, and AM630.
RT-PCR and Immunostaining.
RT-PCR and immunostaining were as described previously (9, 10). Primer sequences for each PCR are listed in Table S2. Antibodies for immunostaining were obtained from commercial sources (Table S3).
Supplementary Material
Acknowledgments
This work was supported by Japan Society for the Promotion of Science, Grant-in-Aid for Scientific Research (KAKENHI) 18109013 and 18077004 (to Y.N.) and National Institutes of Health DC03155 (to R.F.M.).
Footnotes
Conflict of interest statement: R.F.M. has a personal financial interest in the form of stock ownsership in the Redpoint Bio company and is an inventor on patents and patent applications, which have been licensed to Redpoint Bio. All other authors declare no competing financial interests.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912048107/DCSupplemental.
References
- 1.Jamshidi N, Taylor DA. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br J Pharmacol. 2001;134:1151–1154. doi: 10.1038/sj.bjp.0704379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cota D, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: Stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136:550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams CM, Kirkham TC. Anandamide induces overeating: Mediation by central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1999;143:315–317. doi: 10.1007/s002130050953. [DOI] [PubMed] [Google Scholar]
- 5.Higgs S, Williams CM, Kirkham TC. Cannabinoid influences on palatability: Microstructural analysis of sucrose drinking after delta(9)-tetrahydrocannabinol, anandamide, 2-arachidonoyl glycerol and SR141716. Psychopharmacology (Berl) 2003;165:370–377. doi: 10.1007/s00213-002-1263-3. [DOI] [PubMed] [Google Scholar]
- 6.Jarrett MM, Limebeer CL, Parker LA. Effect of Delta9-tetrahydrocannabinol on sucrose palatability as measured by the taste reactivity test. Physiol Behav. 2005;86:475–479. doi: 10.1016/j.physbeh.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 7.Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: Anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology. 2007;32:2267–2278. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- 8.Friedman JM. Modern science versus the stigma of obesity. Nat Med. 2004;10:563–569. doi: 10.1038/nm0604-563. [DOI] [PubMed] [Google Scholar]
- 9.Kawai K, Sugimoto K, Nakashima K, Miura H, Ninomiya Y. Leptin as a modulator of sweet taste sensitivities in mice. Proc Natl Acad Sci USA. 2000;97:11044–11049. doi: 10.1073/pnas.190066697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shigemura N, et al. Leptin modulates behavioral responses to sweet substances by influencing peripheral taste structures. Endocrinology. 2004;145:839–847. doi: 10.1210/en.2003-0602. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura Y, et al. Diurnal variation of human sweet taste recognition thresholds is correlated with plasma leptin levels. Diabetes. 2008;57:2661–2665. doi: 10.2337/db07-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rehfeld JF. The new biology of gastrointestinal hormones. Physiol Rev. 1998;78:1087–1108. doi: 10.1152/physrev.1998.78.4.1087. [DOI] [PubMed] [Google Scholar]
- 13.Shin YK, et al. Modulation of taste sensitivity by GLP-1 signaling. J Neurochem. 2008;106:455–463. doi: 10.1111/j.1471-4159.2008.05397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ledent C, et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- 15.Damak S, et al. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301:850–853. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- 16.Talavera K, et al. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature. 2005;438:1022–1025. doi: 10.1038/nature04248. [DOI] [PubMed] [Google Scholar]
- 17.Luk T, et al. Identification of a potent and highly efficacious, yet slowly desensitizing CB1 cannabinoid receptor agonist. Br J Pharmacol. 2004;142:495–500. doi: 10.1038/sj.bjp.0705792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida R, et al. Taste responsiveness of fungiform taste cells with action potentials. J Neurophysiol. 2006;96:3088–3095. doi: 10.1152/jn.00409.2006. [DOI] [PubMed] [Google Scholar]
- 19.Kitagawa M, Kusakabe Y, Miura H, Ninomiya Y, Hino A. Molecular genetic identification of a candidate receptor gene for sweet taste. Biochem Biophys Res Commun. 2001;283:236–242. doi: 10.1006/bbrc.2001.4760. [DOI] [PubMed] [Google Scholar]
- 20.Max M, et al. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet. 2001;28:58–63. doi: 10.1038/ng0501-58. [DOI] [PubMed] [Google Scholar]
- 21.Montmayeur JP, Liberles SD, Matsunami H, Buck LB. A candidate taste receptor gene near a sweet taste locus. Nat Neurosci. 2001;4:492–498. doi: 10.1038/87440. [DOI] [PubMed] [Google Scholar]
- 22.Nelson G, et al. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 23. Nelson G, et al. (2002) An amino-acid taste receptor. Nature 416:199–202. [DOI] [PubMed]
- 24.Damak S, Mosinger B, Margolskee RF. Transsynaptic transport of wheat germ agglutinin expressed in a subset of type II taste cells of transgenic mice. BMC Neurosci. 2008;9:96. doi: 10.1186/1471-2202-9-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugiura T, Kobayashi Y, Oka S, Waku K. Biosynthesis and degradation of anandamide and 2-arachidonoylglycerol and their possible physiological significance. Prostaglandins Leukot Essent Fatty Acids. 2002;66:173–192. doi: 10.1054/plef.2001.0356. [DOI] [PubMed] [Google Scholar]
- 26.McLaughlin SK, McKinnon PJ, Margolskee RF. Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature. 1992;357:563–569. doi: 10.1038/357563a0. [DOI] [PubMed] [Google Scholar]
- 27.Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- 28.DeFazio RA, et al. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci. 2006;26:3971–3980. doi: 10.1523/JNEUROSCI.0515-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang YA, Maruyama Y, Stimac R, Roper SD. Presynaptic (Type III) cells in mouse taste buds sense sour (acid) taste. J Physiol. 2008;586:2903–2912. doi: 10.1113/jphysiol.2008.151233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida R, et al. Discrimination of taste qualities among mouse fungiform taste bud cells. J Physiol. 2009;587:4425–4439. doi: 10.1113/jphysiol.2009.175075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herness S, Zhao FL, Lu SG, Kaya N, Shen T. Expression and physiological actions of cholecystokinin in rat taste receptor cells. J Neurosci. 2002;22:10018–10029. doi: 10.1523/JNEUROSCI.22-22-10018.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen T, et al. Co-expression patterns of the neuropeptides vasoactive intestinal peptide and cholecystokinin with the transduction molecules alpha-gustducin and T1R2 in rat taste receptor cells. Neuroscience. 2005;130:229–238. doi: 10.1016/j.neuroscience.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 33.Zhao FL, et al. Expression, physiological action, and coexpression patterns of neuropeptide Y in rat taste-bud cells. Proc Natl Acad Sci USA. 2005;102:11100–11105. doi: 10.1073/pnas.0501988102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monteleone P, et al. Blood levels of the endocannabinoid anandamide are increased in anorexia nervosa and in binge-eating disorder, but not in bulimia nervosa. Neuropsychopharmacology. 2005;30:1216–1221. doi: 10.1038/sj.npp.1300695. [DOI] [PubMed] [Google Scholar]
- 35.Rowland NE, Mukherjee M, Robertson K. Effects of the cannabinoid receptor antagonist SR 141716, alone and in combination with dexfenfluramine or naloxone, on food intake in rats. Psychopharmacology (Berl) 2001;159:111–116. doi: 10.1007/s002130100910. [DOI] [PubMed] [Google Scholar]
- 36.Thornton-Jones ZD, Kennett GA, Vickers SP, Clifton PG. A comparison of the effects of the CB(1) receptor antagonist SR141716A, pre-feeding and changed palatability on the microstructure of ingestive behaviour. Psychopharmacology (Berl) 2007;193:1–9. doi: 10.1007/s00213-007-0745-8. [DOI] [PubMed] [Google Scholar]
- 37.Simiand J, Keane M, Keane PE, Soubrié P. SR 141716, a CB1 cannabinoid receptor antagonist, selectively reduces sweet food intake in marmoset. Behav Pharmacol. 1998;9:179–181. [PubMed] [Google Scholar]
- 38.Arnone M, et al. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- 39.DiPatrizio NV, Simansky KJ. Activating parabrachial cannabinoid CB1 receptors selectively stimulates feeding of palatable foods in rats. J Neurosci. 2008;28:9702–9709. doi: 10.1523/JNEUROSCI.1171-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.