Abstract
TGF-β–activated kinase 1 (TAK1) is a MAP3K family member that activates NF-κB and JNK via Toll-like receptors and the receptors for IL-1, TNF-α, and TGF-β. Because the TAK1 downstream molecules NF-κB and JNK have opposite effects on cell death and carcinogenesis, the role of TAK1 in the liver is unpredictable. To address this issue, we generated hepatocyte-specific Tak1-deficient (Tak1ΔHEP) mice. The Tak1ΔHEP mice displayed spontaneous hepatocyte death, compensatory proliferation, inflammatory cell infiltration, and perisinusoidal fibrosis at age 1 month. Older Tak1ΔHEP mice developed multiple cancer nodules characterized by increased expression of fetal liver genes including α-fetoprotein. Cultures of primary hepatocytes deficient in Tak1 exhibited spontaneous cell death that was further increased in response to TNF-α. TNF-α increased caspase-3 activity but activated neither NF-κB nor JNK in Tak1-deficient hepatocytes. Genetic abrogation of TNF receptor type I (TNFRI) in Tak1ΔHEP mice reduced liver damage, inflammation, and fibrosis compared with unmodified Tak1ΔHEP mice. In conclusion, hepatocyte-specific deletion of TAK1 in mice resulted in spontaneous hepatocyte death, inflammation, fibrosis, and carcinogenesis that was partially mediated by TNFR signaling, indicating that TAK1 is an essential component for cellular homeostasis in the liver.
Keywords: apoptosis, JNK, liver cancer, NF-kB, TNF-α
TGF-β–activated kinase-1 (TAK1) is a member of MAP3K family and a crucial intermediate component in the signaling of Toll-like receptors (TLRs), IL-1 receptor (IL-1R), TNF receptor (TNFR), and TGF-β receptor (1, 2). On activation by corresponding ligands, TLR/IL-1R and TNFR recruit and phosphorylate TAK1 through TNFR-associated factor (TRAF) 6 and TRAF2, respectively. Phosphorylated TAK1 subsequently activates IκB kinase (IKK) and MAP kinase kinase (MKK) 4/7, leading to the activation of NF-κB and JNK, respectively. NF-κB and JNK are essential for the development of immune response and inflammation (3); in particular, they are master regulators of cell death, proliferation, and carcinogenesis in hepatocytes (3,4–5). In B cells and keratinocytes, TAK1 is required for the activation of NF-κB and JNK in response to IL-1, TNF, and TLR ligands (6, 7). B-cell receptors (BCRs) activate JNK, but not NF-κB, through TAK1 (6). In contrast, TAK1 is required for both NF-κB and JNK activation induced by T-cell receptor (TCR) signaling (8, 9). Thus, TAK1 regulates NF-κB and JNK activation in a cell-specific and receptor-specific manner.
Because TAK1-deficient mice are embryonic lethal, the study on TAK1 is limited to mouse embryonic fibroblasts (2). Mice with cell type–specific deletion of TAK1 were generated by crossing mice carrying floxed alleles of TAK1 with transgenic mice expressing Cre recombinase under the control of cell-specific promoters (6, 8, 9). Specific deletion of TAK1 in B cells or T cells defects the development and function of B cells or T cells (6, 8, 9). Mice with deletion of TAK1 in intestinal epithelial cells and keratinocytes display lethal inflammatory bowel disease and dermatitis and die within 1 day and 7 days after birth, respectively (10, 11). A recent study found that ablation of TAK1 in cartilage using Col2-Cre Tg mice resulted in abnormal cartilage development and growth retardation and death within 2–3 weeks after birth (12). Thus, TAK1 is a crucial component for maintaining homeostasis in immune cells, skin, intestine, and cartilage.
In the liver, inactivation of NF-κB in hepatocytes by gene targeting of IKKβ or NF-κB essential modulator (NEMO) increases the susceptibility to cancer development (4, 13). In contrast, inactivation of NF-κB in both Kupffer cells and hepatocytes by crossing IKKβflox/flox with Mx1-Cre Tg mice prevents cancer development in response to N-nitrosodiethylamine (DEN) (4). In addition, hepatocyte-specific c-Jun–deficient or JNK1-deficient mice exhibit decreased liver cancer following DEN administration (5, 14, 15). These findings indicate that NF-κB and JNK/AP-1 pathways play opposing roles in hepatocarcinogenesis. TAK1 regulates both NF-κB and JNK/AP-1 activation, and the mechanism through which inactivation of TAK1 influences liver pathology, including hepatocarcinogenesis, is unclear. The present study demonstrates that Tak1 deletion in hepatocytes causes spontaneous hepatocyte death and subsequent compensatory hepatocyte proliferation, inflammation, fibrosis, and carcinogenesis.
Results
Deletion of TAK1 in Hepatocytes Causes Development of Hepatocellular Carcinoma.
To address the function of TAK1 in hepatocytes, hepatocyte-specific Tak1-deficient (Tak1ΔHEP) mice were generated by crossing Tak1flox/flox mice with Albumin-Cre Tg mice. To confirm the efficient deletion of Tak1 in hepatocytes of these mice, protein expression of TAK1 in whole liver and isolated hepatocytes was determined by immunoblot analysis. Expression of full-length TAK1 was sufficiently deleted in whole livers and hepatocytes of Tak1ΔHEP mice at age 1 month (Fig. 1 A and B). Weak expression of a truncated, nonfunctional form of TAK1 (ΔTAK1) was detected (Fig. 1 A and B) (6, 7). Furthermore, protein levels of TAK1 in Kupffer cells and hepatic stellate cells were not affected, suggesting deletion of TAK1 specifically in hepatocytes (Fig. S1A).
Fig. 1.
Tak1-deficient hepatocytes develop hepatocellular carcinoma. (A and B) TAK1 protein expression in whole liver (A) and primary hepatocytes (B) from WT and Tak1ΔHEP mice was determined by immunoblot analysis. ΔTAK1 indicates a truncated form of TAK1. (C) Livers of 12-month-old WT and Tak1ΔHEP mice. (D and E) Histological findings of 9-month-old WT (D) and Tak1ΔHEP mice (E) by H&E staining. (F) Expression of α-fetoprotein in tumors of Tak1ΔHEP mice assessed by immunohistochemistry. (G and H) Fetal liver gene expression, including Afp (G) and H19, Igf2, Dlk1, and Rex3 (H) in liver tissue from 9-month-old WT (n = 4), and nontumor liver (n = 6) and tumors (n = 6) from Tak1ΔHEP mice, determined by real-time PCR. NT, nontumor liver, T, tumors. Data are reported as mean ± SEM. *P < .05; **P < .01. (Scale bar: 1 cm in C, 20 μm in D–F.)
We found no mortality in the Tak1ΔHEP mice up to age 12 months, at which time multiple large tumors were observed (Fig. 1C). The Tak1ΔHEP mice began to develop liver tumors at age 4 months, and 80% (8/10 mice) of 9 month-old Tak1ΔHEP mice displayed macroscopic tumor nodules in the liver. In contrast, no tumors were detected in the 12-month-old WT littermates. Microscopically, the tumors formed discrete nodules surrounded by normal liver tissue (Fig. 1 D–F). The liver tumors exhibited severe dysplasia with an increased nuclear-to-cytoplasmic index, enlarged and hyperchromatic nuclei, expansive growth, and glycogen deposition. Normal liver architecture, such as bile duct and portal tract formation, was lost.
α-fetoprotein is the most widely used serum marker for diagnosing hepatocellular carcinoma in clinical practice (16). Several tumors expressed α-fetoprotein as determined by immunohistochemistry (Fig. 1F). mRNA levels of a-fetoprotein were significantly increased in tumors, but a moderate up-regulation also was seen in nontumor liver of Tak1ΔHEP mice compared with WT liver (Fig. 1G). To further investigate the malignant potential of tumors in Tak1ΔHEP mice, the expression of fetal stage–specific liver genes, such as H19, insulin-like growth factor 2, delta-like 1 homolog, and reduced in expression 3 (encoded by H19, Igf2, Dlk1, and Rex3, respectively) were measured in tumors and nontumor liver from 9 month-old Tak1ΔHEP mice (17–21). High expression of fetal liver genes was seen exclusively in tumors and not in nontumor liver of Tak1ΔHEP mice or WT liver (Fig. 1H). These results demonstrate that hepatocyte-specific TAK1-deficient mice develop liver tumors spontaneously. The high expression of α-fetoprotein and oncofetal liver genes strongly supports the malignant potential of these tumors.
Spontaneous Hepatocyte Injury and Compensatory Proliferation in Liver of Tak1ΔHEP Mice.
To examine early effects of hepatic TAK1 deletion, serum alanine aminotransferase (ALT) levels were monitored. ALT levels were significantly increased, up to 1,668 ± 118 U/L, in 1-month-old mice and decreased to 312 ± 59 U/L in 4-month-old Tak1ΔHEP mice (Fig. 2A). Consistent with elevated ALT levels, increased hepatocyte apoptosis was observed in Tak1ΔHEP mice, as determined by immunoblot analysis for cleaved caspase-3 and TUNEL staining (Fig. 2 B and C). There was no gender disparity in ALT level or the number of TUNEL-positive cells in Tak1ΔHEP mice (Fig. S2 A and B). Because compensatory hepatocyte proliferation following massive liver cell death has been suggested to promote hepatocarcinogenesis (4), we investigated the regenerative responses in Tak1ΔHEP mice. Cyclin D1 expression and proliferation cell nuclear antigen (PCNA)-positive cells were increased significantly in Tak1ΔHEP livers compared with WT livers (Fig. 2 B and D). These findings suggest that the loss of TAK1 in hepatocytes induces spontaneous liver cell death and subsequent compensatory liver cell proliferation that drives carcinogenesis in the liver.
Fig. 2.
Loss of Tak1 in hepatocytes induces spontaneous apoptosis and compensatory regeneration in the liver. (A) Serum ALT levels were measured in WT and Tak1ΔHEP mice at the age 1, 4, and 9 months (n = 10 at the each time point). (B–D) WT (n = 10) and Tak1ΔHEP (n = 10) mice at age 1 month were analyzed. (B) Immunoblots of full-length and cleaved caspase-3 and cyclin D1. (C) Apoptotic hepatocytes evaluated by TUNEL staining. (D) Proliferating hepatocytes evaluated by immunohistochemistry for PCNA. Data are reported as mean ± SEM. *P < .05; **P < .01 for Tak1ΔHEP mice versus WT mice. (Scale bar: 20 μm in C and F.)
Hepatocyte-Specific TAK1 Deficiency Causes Hepatic Inflammation.
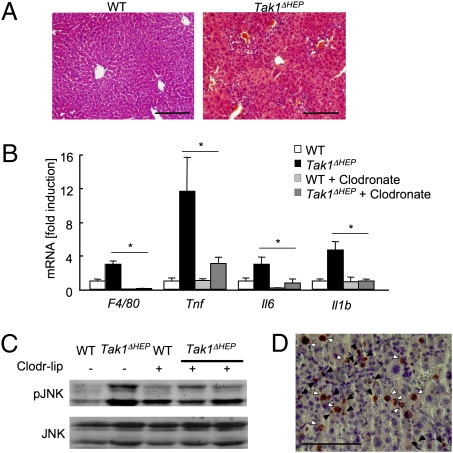
Dying hepatocytes release alarmins that activate resident macrophages (Kupffer cells) to produce various inflammatory cytokines (22). We investigated whether hepatocyte death in Tak1ΔHEP mice triggers hepatic inflammation. Significant inflammatory cell infiltration was seen in Tak1ΔHEP mice at age 1 month (Fig. 3A). Hepatic mRNA expression of inflammatory genes, including Tnf, Il6, and Il1b, was increased significantly in Tak1ΔHEP mice compared with WT littermates (Fig. 3B). Because increased and sustained JNK activation in the liver is associated with inflammation, hepatocyte death, and hepatocarcinogenesis in hepatocyte-specific IKKβ− and NEMO-deficient mice, JNK activation in Tak1ΔHEP mice was evaluated (4, 13). Deletion of TAK1 in hepatocytes resulted in enhanced JNK activation in the liver (Fig. 3C). Activated JNK was observed in both nonparenchymal cells and hepatocytes as evaluated by immunohistochemistry (Fig. 3D). Next, to investigate whether Kupffer cells are the source of increased cytokine production in Tak1ΔHEP liver, Kupffer cells were depleted by liposomal clodronate injection, which efficiently depletes Kupffer cells without damaging other cell types, including hepatocytes and hepatic stellate cells (Fig. S3 A–C) (23). One day after the liposomal clodronate injection, Kupffer cell depletion was confirmed by mRNA and protein expression of F4/80 (Fig. 3B, Fig. S3A). Administration of liposomal clodronate significantly reduced TNF-α, IL-1β, and IL-6 mRNA levels in Tak1ΔHEP liver (Fig. 3B). In addition, depletion of Kupffer cells resulted in a significant reduction of activated JNK in Tak1ΔHEP mice (Fig. 3C), suggesting that Kupffer cells are the major source of activated JNK and proinflammatory cytokines in Tak1ΔHEP mice. These findings indicate that the enhanced hepatocyte death in Tak1ΔHEP mice is associated with hepatic inflammation with JNK activation, and that Kupffer cells are the major source of inflammatory cytokines produced in response to hepatocyte death.
Fig. 3.
Spontaneous hepatitis in Tak1ΔHEP mice. (A–C) WT (n = 10), Tak1ΔHEP (n = 10), and Kupffer cell–depleted Tak1ΔHEP mice (n = 3) were analyzed at age 1 month. Kupffer cells were depleted by liposomal clodronate injection 24 h before death. (A) H&E staining. (B) Hepatic mRNA expression of inflammatory genes including F4/80, Tnf, Il6, and Il1b, determined by real-time PCR. (C) Phospho-JNK and JNK expression in livers from WT, Tak1ΔHEP mice, and Kupffer cell–depleted Tak1ΔHEP mice assessed by immunoblot analysis. (D) Immunohistochemistry for phospho-JNK in Tak1ΔHEP liver. Black and white arrows indicate JNK activation in nonparenchymal cells and hepatocytes, respectively. Data are reported mean ± SEM. *P < .05; **P < .01. (Scale bar: 20 μm in A and D.)
Loss of TAK1 in Hepatocytes Promotes Liver Fibrosis.
Massive hepatocyte death and subsequent inflammation may activate hepatic stellate cells, which are the major source of extracellular matrix proteins, including collagen, that form a fibrotic scar (24, 25). Tak1ΔHEP mice exhibited fibrillar collagen deposition starting as early as age 1 month, as detected by Sirius red staining. The extent of fibrosis was further increased at age 4 months (Fig. 4 A and B). We did not observe a gender disparity in liver fibrosis in Tak1ΔHEP mice (Fig. S2C). The number of αSMA-expressing cells, a marker for activated hepatic stellate cells, was increased significantly in Tak1ΔHEP mice (Fig. 4C). mRNA expression of fibrogenic parameters, including Col1A1, Tgfb1, Acta2, and Timp1, were up-regulated in Tak1ΔHEP mice compared with WT littermates (Fig. 4D). In addition, Tgfb1 mRNA expression was decreased in Kupffer cell–depleted Tak1ΔHEP mice compared with control Tak1ΔHEP mice (Fig. 4E), suggesting that Kupffer cells are the major source of TGF-β1 in Tak1ΔHEP mice. These results indicate that liver injury in Tak1ΔHEP mice is associated with inflammation and that Kupffer cell–derived TGF-β1 drives hepatic stellate cell activation, leading to fibrosis.
Fig. 4.
Tak1ΔHEP mice develop spontaneous liver fibrosis. (A–D) WT and Tak1ΔHEP mice at the age of 1, 4, and 9 months (n = 10 at the each time point) were used for analysis. Fibrillar collagen deposition was determined by Sirius red staining (A) and its quantification (B). Sirius red staining in 4-month-old mice is shown. (C) Expression of αSMA in 1-month-old mice was determined by immunohistochemistry. (D) Hepatic mRNA expression of fibrogenic markers, including Col1A1, Tgfb1, Acta2, and Timp1, was determined by quantitative real-time PCR in 1-month-old WT and Tak1ΔHEP mice. (E) Hepatic mRNA expression of Tgfb1 was determined in Kupffer cell–depleted Tak1ΔHEP mice by real-time PCR. Data are reported as mean ± SEM. *P < .05; **P < .01 for Tak1ΔHEP mice versus WT mice. (Scale bar: 20 μm in A and C.)
TAK1 Deficiency Increases Susceptibility to TNF-α–Mediated Hepatocyte Death.
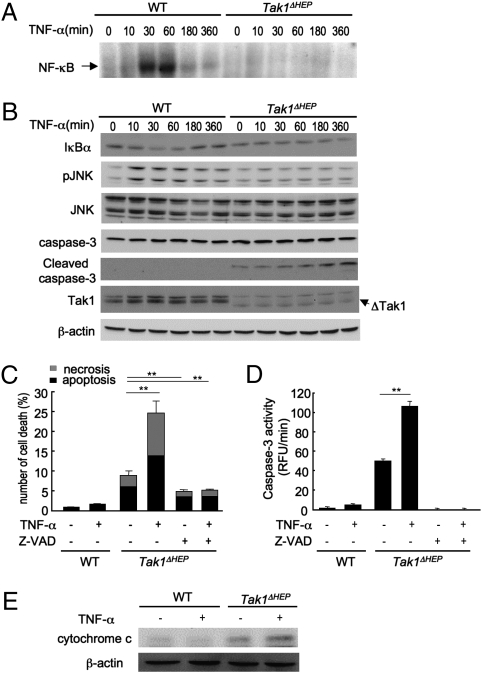
We next investigated the mechanism of spontaneous hepatocyte death in Tak1ΔHEP mice. As shown in Fig. 3B, TNF-α was up-regulated in Tak1ΔHEP liver. Because TNF receptor signaling is a potent activator of cell death (26), we examined cell death in primary hepatocytes isolated from Tak1ΔHEP and WT mice, followed by stimulation with TNF-α. TNF-α stimulation activated NF-κB in WT, but not in Tak1-deficient hepatocytes (Fig. 5A). This was confirmed by the finding of a transient decrease in IκBα expression in TNF-α–stimulated WT hepatocytes but no change in IκBα level in Tak1-deficient hepatocytes (Fig. 5B). JNK phosphorylation was increased in WT, but not in Tak1-deficient hepatocytes after TNF-α stimulation (Fig. 5B). Furthermore, injection of TNF-α in Tak1ΔHEP mice in vivo did not further increase the activation of NF-κB and JNK in hepatocytes, confirming the unresponsiveness of NF-κB and JNK in Tak1-deficient hepatocytes in response to TNF-α in vivo (Fig. S4).
Fig. 5.
Deletion of TAK1 in hepatocytes abolishes JNK and NF-κB activation and confers susceptibility to TNF-mediated cell death. (A–C) Primary hepatocytes from WT and Tak1ΔHEP mice were incubated with TNF-α (20 ng/mL) for the indicated time. (A) NF-κB binding activity was determined. (B) IκBα, phospho-JNK, JNK, caspase-3, cleaved caspase-3, TAK1, and β-actin were determined by immunoblot analysis. (C) Apoptotic and necrotic cells were determined by Hoechst and PI staining, respectively, and counted 8 h after stimulation with 20 ng/mL TNF-α with or without pretreatment with z-VAD-FMK (50 μM) for 30 min. (D) Caspase-3 activity was measured 6 h after stimulation with 20 ng/mL TNF-α with or without pretreatment with z-VAD-FMK (50 μM) for 30 min. Data are reported as mean ± SEM of triplicate cultures. **P < .01. (E) Cytosolic expression of cytochrome c was determined by immunoblot analysis.
Whereas TNF-α did not induce cell death in WT hepatocytes, substantial spontaneous cell death was observed in Tak1-deficient hepatocytes, which was further increased after treatment with TNF-α (Fig. 5C). Consistent with these results, cleaved caspase-3 expression and substantially increased caspase-3 activity were seen in untreated Tak1-deficient hepatocytes, and TNF-α treatment further augmented the expression of cleaved caspase-3 and caspase-3 activity by Tak1 deficiency (Fig. 5 B and D). This cell death was blocked by treatment with pan-caspase inhibitor z-VAD-FMK, indicating that both spontaneous and TNF-α–induced cell death in Tak1-deficient hepatocytes depends largely on caspases (Fig. 5 C and D). Moreover, cytosolic levels of cytochrome c were increased in Tak1-deficient hepatocytes and were further increased after TNF-α treatment (Fig. 5E), indicating that the mitochondria pathway contributes to cell death in Tak1-deficient hepatocytes. Together, the deletion of TAK1 and subsequent failure to activate both NF-κB and JNK in response to TNF-α results in increased susceptibility to cell death through caspases and mitochondria.
Genetic Ablation of TNF Receptor Signaling Ameliorates Liver Injury, inflammation, and Fibrosis in Tak1ΔHEP Mice.
Given that TNF-α treatment increases cell death in Tak1-deficient hepatocytes, signaling through TNFR type I apparently contributes to spontaneous liver injury, inflammation, and fibrosis in Tak1ΔHEP mice. To explore this, we generated Tnfr1-deficient Tak1ΔHEP mice. The Tnfr1-deficient Tak1ΔHEP mice exhibited blunted TAK1 expression in the liver, similar to Tak1ΔHEP mice (Fig. 6A). The Tnfr1-deficient Tak1ΔHEP mice had ∼50% lower ALT levels and reduced expression of cleaved caspase-3 compared with Tnfr1-intact Tak1ΔHEP mice (Fig. 6 B and C). We next investigated inflammation and fibrosis as a consequence of hepatocyte death in these mice. Tnfr1-deficient Tak1ΔHEP mice showed an 80% reduction in inflammatory gene expression, including Tnf, Il6, Il1b, and Ccl2, compared with Tak1ΔHEP mice (Fig. 6D). Increased phosphorylation of JNK was attenuated in Tnfr1-deficient Tak1ΔHEP mice (Fig. 6E). Hepatic fibrosis was significantly suppressed in Tnfr1-deficient Tak1ΔHEP mice compared with Tnfr1-intact Tak1ΔHEP mice, as assessed by measurement of Timp1 mRNA expression and quantification of the Sirius red–positive area (Fig. 6 D and F). These results indicate that TNFR signaling is a critical component in the regulation of inflammation, cell death, and fibrosis in Tak1ΔHEP mice.
Fig. 6.
Deletion of TNFRI reduces liver injury, apoptosis, and fibrosis in Tak1ΔHEP mice. WT (n = 10), Tak1ΔHEP (n = 10), Tnfr1−/− (n = 5) and Tnfr1−/− Tak1ΔHEP (n = 7) mice age 1 month were used for the analyses. (A) Expression of TAK1 was determined by immunoblot analysis. (B) Serum ALT levels are shown. (C) Immunoblots of full-length and cleaved caspase-3 are shown. (D) mRNA expression of Tnf, Il1b, Ccl2, Il6, and Timp1 was determined by real-time PCR. (E) Expression of phospho-JNK and JNK in liver was assessed by immunoblot analysis. (F) Collagen deposition was determined by quantification of Sirius red staining. Data are reported as mean ± SEM. *P < .05; **P < .01.
Discussion
TAK1 is a MAP3K that activates NF-κB and JNK, which are key components for immunity, inflammation, regeneration, apoptosis, and carcinogenesis (1, 3, 27). NF-κB plays a protective role against hepatocyte death (28, 29). In contrast, JNK activation is proapoptotic in hepatocytes (26). Similarly, the TAK1 downstream components NF-κB and JNK have contradictory roles in hepatocarcinogenesis (4, 5, 13–15). Thus, whether TAK1 promotes or prevents hepatocarcinogenesis is unclear. The present study demonstrates that hepatocyte-specific deletion of TAK1 inhibits NF-κB and JNK activation and induces spontaneous hepatocyte death. Dying hepatocytes release alarmins that stimulate Kupffer cells and hepatic stellate cells, leading to inflammation and fibrosis, respectively, and ultimately resulting in hepatocarcinogenesis. TAK1-mediated liver disease depends on TNFR signaling (Fig. S5).
Tak1ΔHEP mice spontaneously develop hepatocellular carcinoma associated with the reactivation of oncofetal genes, a common feature in human hepatocellular carcinoma and in murine liver cancer induced by treatment with DEN (17–21). Oncofetal liver genes expressed in the tumors of Tak1ΔHEP mice included α-fetoprotein, H19, insulin-like growth factor 2, delta-like 1 homolog, and reduced expression of 3. In particular, H19 is not up-regulated in regenerating livers, suggesting a specific marker for hepatocellular carcinoma (20). Previous studies have shown that the development of spontaneous liver cancer in hepatocyte-specific NEMO- or Dicer1–deficient mice is associated with hepatocyte death and compensatory regenerative responses (13, 17). Similar findings were observed inTak1ΔHEP mice, suggesting that carcinogenesis in Tak1ΔHEP mice results from a downstream consequence of sustained apoptosis and the emergence of regenerative clones that acquire a dedifferentiated phenotype. On the other hand, TAK1 has been reported to repress transcription of the telomerase reverse-transcriptase gene, suggesting a direct effect of TAK1 in cancer promotion (30). In contrast to the increased susceptibility to hepatocyte death, apoptosis and caspase-3 activation in cancer cells were not enhanced in Tak1ΔHEP mice (Fig. S6). Although DEN-induced hepatocellular carcinoma is the most commonly used murine model of liver cancer, it lacks the hallmarks of human liver cancer: chronic liver injury, inflammation, and fibrosis. In contrast, hepatocarcinogenesis in Tak1ΔHEP mice is accompanied by severe hepatic inflammation and fibrosis, similar to human hepatocellular carcinoma. Thus, Tak1ΔHEP mice will provide a useful model to further investigate the contribution of inflammation and fibrosis to liver cancer.
NF-κB is a key component for hepatocyte survival by transcription of antiapoptotic proteins such as Bcl-XL, GADD45, A1/Bfl1, cIAPs, and c-FLIP (26). JNK promotes hepatocyte apoptosis (5, 15, 26). Hepatocytes are highly resistant to TNF-mediated cell death, including apoptosis, by inducing NF-κB–dependent survival signals (26). Accordingly, the blocking of NF-κB–dependent survival signals by genetic deletion of NEMO- or NF-κBp65 in hepatocytes renders them highly sensitive to TNF-induced cell death (28, 29). TAK1 regulates JNK activation in response to TNF-α in hepatocytes and hepatic stellate cells, as demonstrated by adenoviral overexpression of a dominant negative form of TAK1 (31, 32). The present study demonstrates that TAK1 regulates the activation of both NF-κB and JNK in hepatocytes (Fig. 5, Fig. S4). Although NF-κB and JNK play opposing roles in cell death, genetic inactivation of TAK1 induces increased sensitivity to cell death in hepatocytes. In addition, TAK1 deletion alone induces substantial cell death, which is further increased in response to TNF-α. In response to TNF-α treatment, NEMO- or NF-κBp65–deficient hepatocytes show blunted NF-κB activation but sustained JNK activation (28, 29). In contrast, TAK1 deficiency abolishes both NF-κB and JNK activation in hepatocytes treated with TNF-α. JNK is a potent proapoptotic inducer through JNK1-mediated cFLIP degradation or JNK2-mediated caspase-8 activation (26). Pharmacologic inhibition of JNK reduces TNF-mediated cell death in NF-κB–inactivated hepatocytes (26). These findings suggest that cell death in TAK1-deficient cells is due mainly to inhibition of an NF-κB–dependent survival effect.
In contrast to the blunted TNF-mediated JNK activation in TAK1-deficient hepatocytes in vitro, JNK was activated in both nonparenchymal cells and hepatocytes of Tak1ΔHEP mice (Figs. 3 and 5). We and others have recently shown that JNK in hematopoietic cells, including Kupffer cells, is important for liver inflammation and cytokine production in nonalcoholic steatohepatitis and in concanavalin A–mediated liver injury (33, 34). In addition, the activation of JNK in hepatic stellate cells contributes to the development of liver fibrosis (35). Thus, Kupffer cells and hepatic stellate cells account for JNK activation in nonparenchymal cells of Tak1ΔHEP mice. Considering that JNK activation was significantly reduced by Kupffer cell depletion in Tak1ΔHEP mice (Figs. 3 and 6), JNK is activated in Kupffer cells and in hepatocytes or stellate cells by the factors derived from Kupffer cells (Fig. 3D). Genetic ablation of TNFR in Tak1ΔHEP mice attenuated JNK activation. In addition, Tnfr1−/−Tak1ΔHEP mice had lower levels of inflammatory and fibrogenic mediators (Fig. 6). Moreover, TNF-α directly up-regulates Timp-1 expression in hepatic stellate cells (Fig. S7). Thus, TNF signaling mediates inflammation and fibrosis in nonparenchymal cells, including Kupffer cells and hepatic stellate cells, as well as hepatocyte death in Tak1ΔHEP mice (Fig. S5).
p38 MAPK is downstream of TAK1 in several cell types (6, 9). The role of p38 MAPK for hepatocyte injury remains controversial. The loss of p38 has been reported to enhance liver injury and cancer after DEN treatment (22, 36). On the other hand, another study found that liver-specific ablation of p38 increased JNK activation in response to TNF-α induced by lipopolysaccharide challenge without enhancing liver injury (37). Our data demonstrate that p38 activation was increased in TAK1-deficient hepatocytes treated with TNF-α (Fig. S8A). Our data, along with results from Heinrichsdorff et al. (37) suggest that (i) activation of p38 in hepatocytes does not require TAK1, and (ii) p38 plays only a minor role in TNF-mediated hepatocyte death (Fig. S8A). Increased p38 activation in Tak1ΔHEP mice was diminished by the depletion of Kupffer cell or TNFR deletion (Fig. S8 B and C). Taken together, our results suggest that p38 in TAK1-deficient hepatocytes is not involved in liver injury in Tak1ΔHEP mice.
In conclusion, specific ablation of TAK1 in hepatocytes causes spontaneous cell death, inflammation, and fibrosis and ultimately results in cancer development. Thus, TAK1 is a master regulator of liver homeostasis that prevents liver damage, inflammation, fibrosis, and carcinogenesis.
Materials and Methods
Mice.
Albumin promoter–driven Cre recombinase transgenic mice (Albumin-Cre Tg mice) and TNFRI-deficient mice were purchased from Jackson Laboratories. Mice carrying the floxed allele of Tak1 (Tak1flox/flox mice) have been described previously (6). Tak1flox/flox mice were crossed with Albumin-Cre Tg mice to generate hepatocyte-specific Tak1-deficient mice (Tak1ΔHEP mice) that express a truncated form of TAK1 lacking the kinase domain including the ATP-binding site (K63) generated by Cre excision of floxed exon 2 of TAK1 (ΔTAK1) selectively in hepatocytes (Figs. 1 A and B and 5B), which does not have a kinase activity, as described previously (6, 7). Tak1flox/flox littermates without the Cre transgene were used as WT controls. Kupffer cell–depleted mice were generated by i.v. infusion of liposomal clodronate (10 μL/g mouse weight) (23, 38). No adverse effects of liposomal clodronate on hepatic stellate cells and hepatocytes were confirmed (Fig. S2). Mice received humane care according to National Institutes of Health recommendations outlined in their Guide for the Care and Use of Laboratory Animals. All animal experiments were approved by the University of California San Diego Institutional Animal Care and Use Committee.
Histological Examination.
Liver specimens were fixed in 10% buffered formalin. Sections were incubated with a monoclonal antibody to αSMA (DakoCytomation) and PCNA (Biolegend) using the MOM kit (Vector Laboratories), goat anti-AFP antibody, goat NF-κBp65 antibody (Santa Cruz Biotechnology), and rabbit anti-active JNK (Promega) (23). H&E, TUNEL, and Sirius red staining were performed as described previously (39, 40). TUNEL- and PCNA- positive cells were counted on 10 fields of 100× and 200× magnification per slide, respectively. Sirius red– positive area was measured for 10 low-power (100×) fields per slide and quantified using National Institutes of Health imaging software.
Western Blot Analysis.
Protein extracts were electrophoresed and then blotted. Blots were incubated with antibodies for caspase-3, cleaved caspase-3, pJNK, pp38, TAK1, cytochrome c (Cell Signaling), β-actin (Sigma), cyclin D1, JNK, p38, and IκBα (Santa Cruz Biotechnology) with secondary HRP-conjugated antibody and visualized (41). A cytosolic fraction for cytochrome c immunoblotting was prepared as described previously (42).
Quantitative Real-Time PCR Analysis.
RNA extracted from liver was subjected to reverse transcription and subsequent PCR using an ABI PRISM 7000 Sequence Detector (Applied Biosystems) (41). PCR primer sequences are listed in Table S1. The expression of respective genes was normalized to 18S RNA as an internal control.
Cell Isolation and Treatment.
Hepatocytes were isolated from Tak1ΔHEP and WT mice (39). After cell attachment, hepatocytes were serum-starved for 6 h followed by treatment with 20 ng/mL murine TNF-α (R&D Systems) for prespecified time periods. Apoptosis and necrosis were examined using Hoeschst 33342 (Invitrogen) and propidium iodide staining, in which nucleus fragmentation and positive cells were defined as apoptosis and necrosis, respectively (39). Caspase-3 activity was measured using a caspase-3 assay kit (AnaSpec) (39). Z-VAD-FMK (50 μM) was applied for 30 min before TNF-α treatment.
Electrophoretic Mobility Shift Assay (EMSA).
A double-stranded, NF-κB–specific oligonucleotide probe (consensus sequence 5′-ATCAG GGACTTTCCGCTGGGGACTTTCCG-3′ and 5′-GGCGGAAAGTCCCCAGCGGAAAGTCC CTGAT-3′) was labeled with [32P] dCTP by Klenow fragment. Nuclear extracts were incubated with labeled probe as described previously (43).
Statistical Analysis.
Differences between two groups were compared using the Mann-Whitney U test or two-tailed unpaired Student t test. Differences between multiple groups were compared using one-way ANOVA (GraphPad Prism 4.02; GraphPad Software); P < .05 was considered significant.
Supplementary Material
Acknowledgments
We thank Rie Seki and Karin Diggle (University of California San Diego) for excellent technical assistance. This study is supported by a Liver Scholar Award from the American Association for the Study of Liver Diseases/American Liver Foundation and by a pilot project from the Southern California Research Center for ALPD and Cirrhosis (P50 AA11999) funded by the National Institute on Alcohol Abuse and Alcoholism (both to E.S.), and by National Institutes of Health Grant 5R01GM041804 (to D.A.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909781107/DCSupplemental.
References
- 1.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 2.Shim JH, et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 4.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Sakurai T, Maeda S, Chang L, Karin M. Loss of hepatic NF-kappa B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc Natl Acad Sci USA. 2006;103:10544–10551. doi: 10.1073/pnas.0603499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato S, et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- 7.Omori E, Morioka S, Matsumoto K, Ninomiya-Tsuji J. TAK1 regulates reactive oxygen species and cell death in keratinocytes, which is essential for skin integrity. J Biol Chem. 2008;283:26161–26168. doi: 10.1074/jbc.M804513200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu HH, Xie M, Schneider MD, Chen ZJ. Essential role of TAK1 in thymocyte development and activation. Proc Natl Acad Sci USA. 2006;103:11677–11682. doi: 10.1073/pnas.0603089103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan YY, Chi H, Xie M, Schneider MD, Flavell RA. The kinase TAK1 integrates antigen and cytokine receptor signaling for T cell development, survival and function. Nat Immunol. 2006;7:851–858. doi: 10.1038/ni1355. [DOI] [PubMed] [Google Scholar]
- 10.Omori E, et al. TAK1 is a master regulator of epidermal homeostasis involving skin inflammation and apoptosis. J Biol Chem. 2006;281:19610–19617. doi: 10.1074/jbc.M603384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kajino-Sakamoto R, et al. Enterocyte-derived TAK1 signaling prevents epithelium apoptosis and the development of ileitis and colitis. J Immunol. 2008;181:1143–1152. doi: 10.4049/jimmunol.181.2.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shim JH, et al. TAK1 is an essential regulator of BMP signaling in cartilage. EMBO J. 2009;28:2028–2041. doi: 10.1038/emboj.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luedde T, et al. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11:119–132. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Eferl R, et al. Liver tumor development: c-Jun antagonizes the proapoptotic activity of p53. Cell. 2003;112:181–192. doi: 10.1016/s0092-8674(03)00042-4. [DOI] [PubMed] [Google Scholar]
- 15.Hui L, Zatloukal K, Scheuch H, Stepniak E, Wagner EF. Proliferation of human HCC cells and chemically induced mouse liver cancers requires JNK1-dependent p21 down-regulation. J Clin Invest. 2008;118:3943–3953. doi: 10.1172/JCI37156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson PJ. The role of serum alpha-fetoprotein estimation in the diagnosis and management of hepatocellular carcinoma. Clin Liver Dis. 2001;5:145–159. doi: 10.1016/s1089-3261(05)70158-6. [DOI] [PubMed] [Google Scholar]
- 17.Sekine S, et al. Disruption of Dicer1 induces dysregulated fetal gene expression and promotes hepatocarcinogenesis. Gastroenterology. 2009;136:2304–2315. doi: 10.1053/j.gastro.2009.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao X, et al. A methylated oligonucleotide inhibits IGF2 expression and enhances survival in a model of hepatocellular carcinoma. J Clin Invest. 2003;111:265–273. doi: 10.1172/JCI15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J, et al. Up-regulation of DLK1 as an imprinted gene could contribute to human hepatocellular carcinoma. Carcinogenesis. 2007;28:1094–1103. doi: 10.1093/carcin/bgl215. [DOI] [PubMed] [Google Scholar]
- 20.Graveel CR, Jatkoe T, Madore SJ, Holt AL, Farnham PJ. Expression profiling and identification of novel genes in hepatocellular carcinomas. Oncogene. 2001;20:2704–2712. doi: 10.1038/sj.onc.1204391. [DOI] [PubMed] [Google Scholar]
- 21.Braeuning A, Jaworski M, Schwarz M, Köhle C. Rex3 (reduced in expression 3) as a new tumor marker in mouse hepatocarcinogenesis. Toxicology. 2006;227:127–135. doi: 10.1016/j.tox.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Sakurai T, et al. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14:156–165. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seki E, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 24.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwabe RF, Brenner DA. Mechanisms of liver injury, I. TNF-alpha–induced liver injury: Role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G583–G589. doi: 10.1152/ajpgi.00422.2005. [DOI] [PubMed] [Google Scholar]
- 27.Ninomiya-Tsuji J, et al. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signaling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 28.Beraza N, et al. Hepatocyte-specific IKK gamma/NEMO expression determines the degree of liver injury. Gastroenterology. 2007;132:2504–2517. doi: 10.1053/j.gastro.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 29.Geisler F, Algül H, Paxian S, Schmid RM. Genetic inactivation of RelA/p65 sensitizes adult mouse hepatocytes to TNF-induced apoptosis in vivo and in vitro. Gastroenterology. 2007;132:2489–2503. doi: 10.1053/j.gastro.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 30.Fujiki T, et al. TAK1 represses transcription of the human telomerase reverse- transcriptase gene. Oncogene. 2007;26:5258–5266. doi: 10.1038/sj.onc.1210331. [DOI] [PubMed] [Google Scholar]
- 31.Liedtke C, et al. Jun kinase modulates tumor necrosis factor–dependent apoptosis in liver cells. Hepatology. 2002;36:315–325. doi: 10.1053/jhep.2002.34615. [DOI] [PubMed] [Google Scholar]
- 32.Schnabl B, et al. TAK1/JNK and p38 have opposite effects on rat hepatic stellate cells. Hepatology. 2001;34:953–963. doi: 10.1053/jhep.2001.28790. [DOI] [PubMed] [Google Scholar]
- 33.Kodama Y, et al. c-Jun N-terminal kinase-1 from hematopoietic cells mediates progression from hepatic steatosis to steatohepatitis and fibrosis in mice. Gastroenterology. 2009;137:1467–1477. doi: 10.1053/j.gastro.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Das M, et al. Induction of hepatitis by JNK-mediated expression of TNF-alpha. Cell. 2009;136:249–260. doi: 10.1016/j.cell.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kluwe J, et al. Modulation of hepatic fibrosis by c-Jun-N-terminal kinase inhibition. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hui L, et al. p38alpha suppresses normal and cancer cell proliferation by antagonizing the JNK-c-Jun pathway. Nat Genet. 2007;39:741–749. doi: 10.1038/ng2033. [DOI] [PubMed] [Google Scholar]
- 37.Heinrichsdorff J, Luedde T, Perdiguero E, Nebreda AR, Pasparakis M. p38 alpha MAPK inhibits JNK activation and collaborates with IkappaB kinase 2 to prevent endotoxin-induced liver failure. EMBO Rep. 2008;9:1048–1054. doi: 10.1038/embor.2008.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Rooijen N, Sanders A. Liposome-mediated depletion of macrophages: Mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 39.Kodama Y, et al. Antiapoptotic effect of c-Jun N-terminal kinase-1 through Mcl-1 stabilization in TNF-induced hepatocyte apoptosis. Gastroenterology. 2009;136:1423–1434. doi: 10.1053/j.gastro.2008.12.064. [DOI] [PubMed] [Google Scholar]
- 40.Seki E, et al. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009;119:1858–1870. doi: 10.1172/JCI37444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seki E, et al. CCR2 promotes hepatic fibrosis in mice. Hepatology. 2009;50:185–197. doi: 10.1002/hep.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osawa Y, et al. Caspase activation during hepatocyte apoptosis induced by tumor necrosis factor-alpha in galactosamine-sensitized mice. Liver. 2001;21:309–319. doi: 10.1034/j.1600-0676.2001.210503.x. [DOI] [PubMed] [Google Scholar]
- 43.Seki E, et al. Contribution of Toll-like receptor/myeloid differentiation factor 88 signaling to murine liver regeneration. Hepatology. 2005;41:443–450. doi: 10.1002/hep.20603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.