Abstract
Viral infection causes activation of the transcription factors NF-κB and IRF3, which collaborate to induce type I interferons (IFNs) and cellular antiviral response. The mitochondrial outer membrane protein VISA acts as a critical adapter for assembling a virus-induced complex that signals NF-κB and IRF3 activation. Using a biochemical purification approach, we identified the WD repeat protein WDR5 as a VISA-associated protein. WDR5 was recruited to VISA in a viral infection dependent manner. Viral infection also caused translocation of WDR5 from the nucleus to mitochondria. Knockdown of WDR5 impaired the formation of virus-induced VISA-associated complex. Consistently, knockdown of WDR5 inhibited virus-triggered activation of IRF3 and NF-κB as well as transcription of the IFNB1 gene. These findings suggest that WDR5 is essential in assembling a virus-induced VISA-associated complex and plays an important role in virus-triggered induction of type I IFNs.
Keywords: innate immunity, interferon, mitochondrial signal transduction
Viral infections are sensed by pattern-recognition receptors (PRRs) of the innate immune system that recognize pathogen-associated molecular patterns (PAMPs). Viral RNAs as PAMPs are recognized by membrane-bound toll-like receptors (TLRs), such as TLR3, 7, and 8, as well as the cytosolic RIG-I-like receptor (RLR) family members RIG-I, MDA5, and Lgp2 (1–4). Recognition of viral RNAs by the PRRs triggers a series of signaling events that lead to induction of type I interferons (IFNs). Type I IFNs activate the JAK-STAT signal transduction pathways, leading to transcriptional induction of a wide range of downstream antiviral genes and subsequent innate antiviral response (5–8).
Transcriptional induction of type I IFN genes requires the coordinate activation of multiple transcription factors and their cooperative assembly into transcriptional enhancer complexes in vivo. For example, the IFNB1 gene promoter contains conserved enhancer elements recognized by NF-κB (κB site) and phosphorylated IRF3 (ISRE site, also known as PRDIII or IRF-E). It has been shown that transcriptional activation of the IFNB1 gene requires coordinate and cooperative assembly of an enhanceosome that contains all of these transcription factors (7, 9).
In the past several years, considerable progress has been achieved on the molecular mechanisms of RLR-mediated induction of type I IFNs. Both RIG-I and MDA5 contain two CARD modules at their N terminus and a DexD/H-box RNA helicase domain at their C terminus (10, 11). Upon viral infection, the RNA helicase domains of RIG-I and MDA5 serve as intracellular viral RNA receptors, whereas their CARD modules are associated with the downstream CARD-containing adapter protein VISA (also known as MAVS, IPS-1, and Cardif) (12–16). VISA-deficient mouse embryonic fibroblasts and conventional dendritic cells (DCs) are defective in producing type I IFNs and proinflammatory cytokines in response to viruses and VISA-deficient mice were susceptible to infection with various viruses, suggesting an essential role for VISA in virus-triggered innate antiviral response (12, 17). The C terminus of VISA contains a transmembrane domain that anchors VISA to the outer membrane of mitochondria, implying an important role of mitochondria in innate antiviral immunity (15). The importance of VISA in innate antiviral response is also illustrated by the observation that HCV encoded NS3/4A protease cleaves VISA off the mitochondria, resulting in abrogation of type I IFN induction and chronic HCV infection (14).
On the outer membrane of mitochondria, VISA acts as a central adapter for assembling a virus-induced complex that activates distinct signaling pathways leading to IRF3 and NF-κB activation. VISA is constitutively associated with another mitochondrion-associated adapter protein MITA/STING, which is also essential for virus-triggered signaling (18–20). It has also been reported that VISA is associated with NLRX1, a nucleotide-binding domain and leucine-rich-repeat containing family member, which functions as a brake to regulate virus-triggered signaling (21). VISA is associated with TRAF2 and TRAF6 through its TRAF interaction motifs (16). It has been shown that TRAF2 and TRAF6 facilitate K63-linked polyubiquitination of RIP and NEMO respectively. These processes lead to activation of IKK and subsequent NF-κB (22). VISA is also associated with TRAF3, another member of the TRAF protein family. Gene knockout studies have demonstrated that TRAF3 is essential in virus-triggered IRF3 activation (23–25).
In this report, we identified WDR5 as a component of the VISA-associated complex. Viral infection caused translocation of WDR5 to the mitochondria and association with VISA. Knockdown of WDR5 by RNAi impaired formation of virus-induced VISA-associated complex and inhibited virus-triggered NF-κB and IRF3 activation. Our findings suggest that WDR5 is essential for virus-induced assembly of the VISA-associated complex and induction of type I IFNs.
Results
Identification of WDR5 as a VISA-Associated Protein.
VISA plays a central role in assembling a complex mediating virus-triggered IRF3 and NF-κB activation. To identify potential new components associated with VISA, we performed Tandem Affinity Purification (TAP) experiments with VISA as a bait protein. An expression plasmid for VISA tagged with Calmodulin Binding Peptide (CBP) and Streptavidin Binding Peptide (SBP) was stably transfected into 293 cells, and VISA-associated proteins were purified by a TAP purification system. The eluted proteins were identified by a shot-gun mass spectrum analysis method. By comparing with other nonrelated purifications using the same method, we identified WDR5 as a protein specifically associated with VISA (Fig. S1). WDR5 is a WD repeat protein that has been shown to be a component of the mixed lineage leukemia (MLL) complex, which methylates lysine 4 of histone H3 (26–28). It has also been demonstrated that WDR5 is developmentally expressed in osteoblasts and required for osteoblast differentiation (29, 30). EST profile analysis using the GenBank databases indicated that WDR5 was ubiquitously expressed in most tissues, suggesting a broader role for WDR5 in addition to osteoblast differentiation.
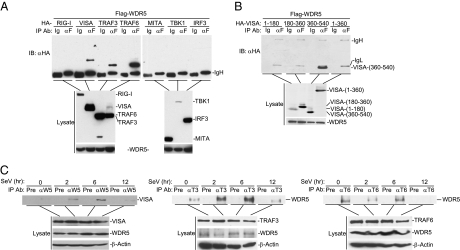
To determine whether WDR5 indeed interacts with VISA, we performed transient transfection and coimmunoprecipitation experiments. The results indicated that WDR5 could interact with VISA (Fig. 1A). In the same experiments, WDR5 also interacted with TRAF3 and TRAF6 but not RIG-I, MITA, TBK1, or IRF3 (Fig. 1A). Interestingly, the C terminus of VISA, which contains its transmembrane domain, was required and sufficient for its interaction with WDR5 (Fig. 1B). Previous studies suggest a nuclear function for WDR5 as a component of the MLL complex, and it is unknown whether WDR5 has a role in the cytoplasm. We isolated nuclear and cytoplasmic fractions and found surprisingly that WDR5 was more abundantly localized in the cytoplasm than in the nucleus (Fig. S2). Endogenous coimmunoprecipitation experiments indicated that WDR5 barely interacted with VISA under physiological conditions, but their interaction was markedly increased at 2 and 6 h after Sendai virus (SeV) infection (Fig. 1C). In similar experiments, the association of WDR5 with TRAF3 was also increased at 2 and 6 h after SeV infection whereas WDR5 constitutively interacted with TRAF6 (Fig. 1C). The associations of WDR5 with VISA, TRAF3, and TRAF6 were diminished at 12 h after viral infection (Fig. 1C). These results suggest that viral infection promotes the association of WDR5 with VISA or TRAF3, whereas WDR5 constitutively interacts with TRAF6.
Fig. 1.
WDR5 is associated with VISA, TRAF3, and TRAF6. (A) WDR5 interacts with VISA, TRAF3, and TRAF6 but not RIG-I, MITA, TBK1, or IRF3 in overexpression system; 293 cells (2 × 106) were transfected with the indicated plasmids (5 μg each). Coimmunoprecipitation and immunoblot analysis were performed with the indicated antibodies (Upper). Expression of the transfected proteins were analyzed by immunoblots with anti-HA or anti-Flag (Lower). (B) Domain mapping of the VISA-WDR5 interaction. 293 cells (2 × 106) were transfected with the indicated plasmids (5 μg each). Coimmunoprecipitation was performed with anti-Flag or control IgG. The immunoprecipitates were analyzed by immunoblot with anti-HA (Top). Expression of the transfected proteins were analyzed by immunoblots with anti-HA (Middle) or anti-Flag (Bottom). (C) WDR5 interacts with VISA, TRAF3, and TRAF6 in untransfected cells; 293 cells (5 × 107) were infected with SeV for the indicated time or left uninfected. Cell lysates were immunoprecipitated with the indicated antisera or control serum. The immunoprecipitates were analyzed by immunoblots with anti-VISA or anti-WDR5 as indicated (Upper). The levels of the proteins were analyzed by immunoblots with the indicated antibodies (Lower). Ig, control mouse IgG; αF, anti-Flag; Pre, preimmune serum; αT3, anti-TRAF3; αT6, anti-TRAF6.
Viral Infection Causes Translocation of WDR5 to the Mitochondria.
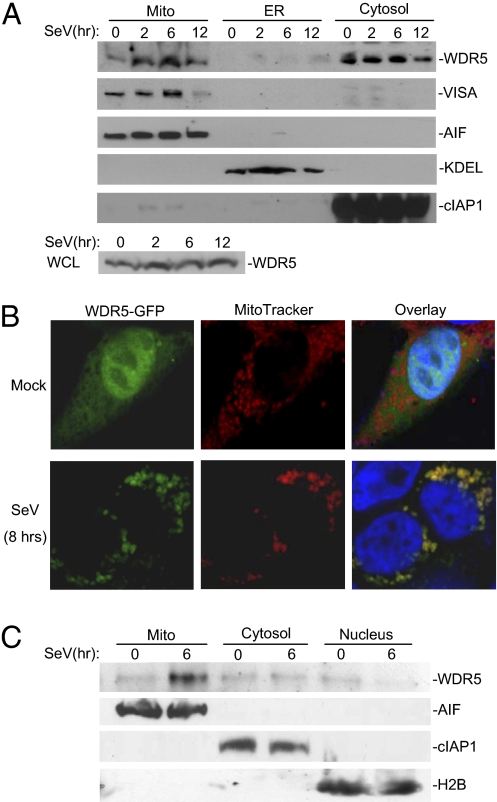
Previously, it has been demonstrated that VISA is localized to the outer membrane of mitochondria (15). Because WDR5 is associated with VISA following viral infection, we determined whether WDR5 is translocated to the mitochondria following viral infection. We performed cellular fractionation experiments and found that in the absence of viral infection, WDR5 was mostly localized in the cytosol and only trace amount of WDR5 was detected in the mitochondrial fraction. However, after SeV infection, mitochondrion-associated WDR5 was markedly increased, whereas the cytosolic and whole cell WDR5 levels were not markedly changed after viral infection (Fig. 2A). Confocal microscopy and cellular fractionation experiments further indicated that viral infection caused marked translocation of WDR5 to the mitochondria, which was probably originated from the nucleus because viral infection caused decrease of WDR5 in the nucleus (Fig. 2 B and C). These results suggest that viral infection caused recruitment of WDR5 to the mitochondria.
Fig. 2.
WDR5 is translocated to the mitochondria after viral infection. (A) Cell fractionation and immunoblot analysis of the subcellular fractions; 293 cells were infected with SeV for the indicated time or left uninfected. Cell fractionations were performed; the fractions were equilibrated to equal volumes and analyzed by immunoblots with the indicated antibodies (Upper). The whole cellular levels of WDR5 upon viral infection were analyzed by immunoblot with anti-WDR5 (Lower). (B) Confocal microscopy of the cellular localization of WDR5; 293 cells were transfected with C-terminal GFP-tagged WDR5. Transfected cells were infected with SeV for 8 h or left uninfected before they were stained with the MitoTracker Red and observed under a confocal microscope. (C) Immunoblot analysis of the cellular localization of WDR5; 293 cells were infected with SeV for 6 h or left uninfected. The cells were fractionated and the subcellular fractions, including mitochondria, cytosolic fraction (cytoplasm without mitochondria and membranes) and nucleus, were equilibrated to equal volumes and analyzed by immunoblots with the indicated antibodies. WCL, whole cell lysate; Mito, mitochondria.
WDR5 Is Required for Virus-Triggered Activation of IRF3 and NF-κB and Transcription of the IFNB1 Gene.
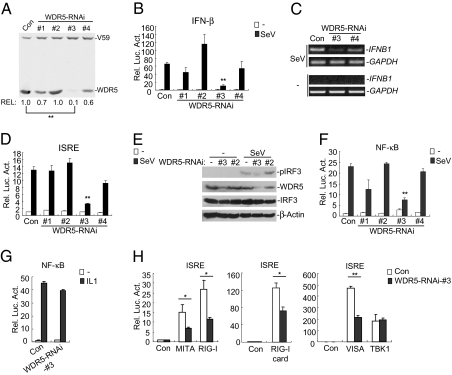
Because WDR5 is translocated to the mitochondria and associated with VISA after viral infection, we determined whether WDR5 is involved in virus-triggered type I IFN signaling. We made four WDR5 RNAi constructs and determined their effects on knockdown of WDR5. As shown in Fig. 3A, the #3 WDR5-RNAi construct could inhibit expression of transfected WDR5 to 10% of the control sample (P < 0.01). The #1 and #4 RNAi constructs could reduce WDR5 levels to 70% and 60% of the control sample, respectively, whereas the #2 RNAi plasmid had no effect. The #3 WDR5-RNAi construct also markedly inhibited expression of endogenous WDR5 protein either without or with SeV infection (Fig. S3). As shown in Fig. 3B, the #3 RNAi construct could significantly inhibit SeV-induced activation of the IFN-β promoter in reporter assays (P < 0.01). Consistent with their effects on WDR5 expression, the #1 and #4 RNAi constructs had minor effects whereas the #2 RNAi construct had no inhibitory effects on SeV-induced activation of the IFN-β promoter (Fig. 3B). Furthermore, knockdown of WDR5 also inhibited transcription of endogenous IFNB1 gene (Fig. 3C). These results suggest that WDR5 is required for virus-triggered IFN-β induction.
Fig. 3.
Knockdown of WDR5 inhibits SeV-induced activation of IRF3, NF-κB and the IFN-β promoter (A) Effects of WDR5-RNAi plasmids on the expression of WDR5. 293 cells (2 × 105) were transfected with expression plasmids for Flag-WDR5 and Flag-V59 (0.1 μg each), and the control or indicated WDR5 RNAi plasmids (1 μg). Twenty-four hours after transfection, cell lysates were analyzed by immunoblot with anti-Flag. The WDR5 bands from three independent experiments were quantitated using the Bio-Rad Quantity One Program and normalized by levels of the control protein V-59. The average levels of WDR5 from the three experiments are shown at the bottom of the blot. **, P < 0.01, n = 3. (B, D, and F) Effects of WDR5-RNAi plasmids on SeV-induced activation of the IFN-β promoter (B), ISRE (D), and NF-κB (F); 293 cells (2 × 105) were transfected with the indicated RNAi plasmids together with the indicated reporter plasmids. Cells were left uninfected or infected with SeV for 8 h before luciferase assays were performed. Graphs show mean ± SD, n = 3. **, P < 0.01. (C) Effects of WDR5 knockdown on transcription of endogenous IFNB1 gene. 293 cells (2 × 105) were transfected with a control or the indicated WDR5-RNAi plasmids. Twenty-four hours after transfection, cells were infected with SeV for 6 h or left uninfected before RT-PCRs were performed with the indicated primers. (E) Knockdown of WDR5 inhibits SeV-induced IRF3 phosphorylation; 293 cells (2 × 105) were transfected with a control or the indicated WDR5-RNAi plasmids. Twelve hours after transfection, cells were selected with puromycin (1 μg/mL) for 24 h, then infected with SeV for 6 h or left uninfected. Cell lysates were analyzed by immunoblots with the indicated antibodies. (G) WDR5-RNAi does not inhibit IL-1-induced NF-κB activation. 293 cells (2 × 105) were transfected with NF-κB reporter plasmid together with a control or WDR5-RNAi plasmid. Twenty-four hours after transfection, cells were left untreated or treated with IL-1 (20 ng/mL) for 8 h before luciferase assays were performed. Graphs show mean ± SD, n = 3. (H) Knockdown of WDR5 inhibits RIG-I-, RIG-I-CARD, VISA-, MITA- but not TBK1-mediated ISRE activation. 293 cells (2 × 105) were firstly transfected with a WDR5-RNAi or control plasmid (1 μg). Twenty-four hours later, cells were selected with puromycin (1 μg/mL) for 24 h and then retransfected with ISRE reporter and the indicated expression plasmids (0.1 μg each). Luciferase assays were performed 24 h after the second transfection. Graphs show mean ± SD, n = 3. *, P < 0.05; **, P < 0.01.
Activation of the IFN-β promoter requires cooperative actions of IRF3 and NF-κB. Consistently, knockdown of WDR5 by the #3 RNAi construct significantly inhibited SeV-induced ISRE activation in reporter assays (P < 0.01) (Fig. 3D) and IRF3 phosphorylation (Fig. 3E), which are hallmarks of IRF3 activation. Knockdown of WDR5 also inhibited SeV- but not IL1-induced NF-κB activation (Fig. 3 F and G). These results suggest that WDR5 is specifically involved in SeV-induced IRF3 and NF-κB activation pathways.
To determine the molecular order of WDR5 in the virus-triggered signaling pathways, we examined the effects of WDR5 knockdown on ISRE activation mediated by components of the virus-triggered pathways. The results indicated that knockdown of WDR5 inhibited RIG-I-, RIG-I-CARD-, VISA-, and MITA- but not TBK1-mediated ISRE activation (Fig. 3H), suggesting that WDR5 acts downstream of RIG-I, VISA, and MITA but upstream of TBK1.
WDR5 Is Essential for Assembly of the VISA-Associated Complex at the Mitochondria.
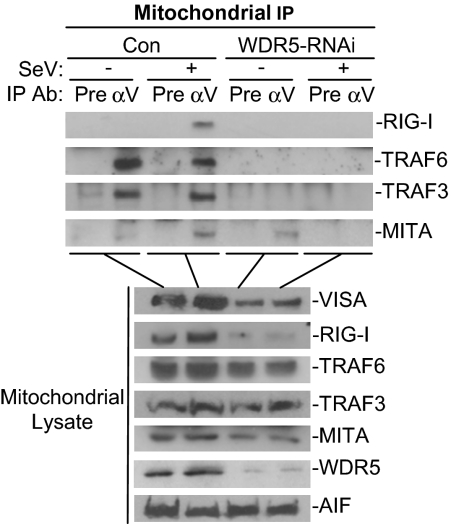
To further explore the mechanisms on the involvement of WDR5 in virus-triggered signaling, we determined the roles of WDR5 on the assembly of VISA-associated complex following viral infection. To do this, 293 cells were transfected with the #3 WDR5-RNAi plasmid and then infected or left uninfected with SeV for 6 h. The mitochondria were isolated and VISA-associated complex at the mitochondria was immunoprecipitated with anti-VISA antibody. Immunoblot analysis indicated that RIG-I was recruited to VISA in a viral infection dependent manner, whereas MITA, TRAF3 and TRAF6 were constitutively associated with VISA. Interestingly, knockdown of WDR5 expression by RNAi impaired virus-triggered association of VISA with RIG-I or MITA, as well as the constitutive association between VISA and TRAF3 or TRAF6 (Fig. 4). The impairment of assembly of VISA-associated complex was correlated with down-regulation of RIG-I and MITA at the mitochondria after WDR5 knockdown (Fig. 4). Because whole cellular levels of RIG-I and MITA were unchanged after WDR5 knockdown (Fig. S3), these results suggest that WDR5 is important for recruiting RIG-I and MITA to the VISA-associated complex on the mitochondrial membrane. Interestingly, the levels of TRAF3 and TRAF6 were not markedly changed after WDR5 knockdown, suggesting that WDR5 is not involved in recruiting TRAF3 and TRAF6 to the mitochondria but instead is important for the interactions between VISA and TRAF3 or TRAF6.
Fig. 4.
Effects of WDR5 knockdown on assembly of the VISA-associated complex on the mitochondria. 293 cells (1 × 108) were transfected with a control or WDR5-RNAi plasmid. Twenty-four hours after transfection, cells were selected with puromycin (1 μg/mL) for 24 h and then infected with SeV for 6 h or left uninfected. After infection, the mitochondria were collected by cell fractionation and the mitochondrial lysates were subjected to immunoprecipitation and immunoblot analysis with the indicated antibodies (Upper). Mitochondrial lysates were analyzed by immunoblots with the indicated antibodies (Lower). Pre, preimmune serum; αV, anti-VISA.
Discussion
In most cell types, infection by RNA viruses is sensed by the cytoplasmic RNA helicase proteins RIG-I and MDA5. Recognition of viral RNAs by these helicase proteins results in induction of type I IFNs and other antiviral genes (10, 11). Elucidation of the molecular events of RIG-I and MDA5-mediated induction of type I IFNs is critical for understanding the complicated mechanisms of cellular antiviral innate immune response. Previous studies have revealed that the mitochondrial outer membrane protein VISA plays a central role in assembling a complex mediating virus-triggered IRF3 and NF-κB activation (13–16). However, the components of VISA-associated complex and their roles in virus-triggered signaling have not been fully defined. In this report, we identified WDR5 as a VISA-associated component required for virus-triggered activation of IRF3 and NF-κB as well as induction of IFN-β.
Previously, it has been shown that WDR5 is a component of the MLL complex, which methylates lysine 4 of histone H3 (26–28). EST profile analysis indicated that WDR5 was ubiquitously expressed in most tissues. Cell fractionation experiments indicated that WDR5 was more abundantly localized in the cytoplasm than in the nucleus. These observations point to a previously unrevealed cytoplasmic function of WDR5. Using a TAP purification approach, we found that WDR5 was associated with VISA. Transient transfection and coimmunoprecipitation experiments indicated that VISA interacted with WDR5 constitutively. However, in untransfected cells, endogenous WDR5 was associated with VISA in a viral infection-dependent manner. These can be explained by two possibilities. Firstly, overexpression of VISA mimics its activation state as suggested by its ability to activate the IFN-β promoter following its overexpression. Second, overexpression may detect weak interaction. In fact, many if not most protein–protein interactions in various signal transduction pathways occur constitutively in overexpression system whereas in untransfected cells the interactions are stimulation-dependent.
Consistent with the observation that the interaction between VISA and WDR5 was viral infection dependent, viral infection caused translocation of WDR5 to the mitochondria, as revealed by both cellular fractionation and confocal microscopy experiments. Interestingly, viral infection caused down-regulation of WDR5 in the nucleus, suggesting that the mitochondrion-associated WDR5 may originate from the nucleus.
To investigate the roles of WDR5 in virus-triggered signaling, we determined the effects of WDR5 knockdown on virus-triggered IRF3 and NF-κB activation as well as induction of IFN-β. Our results indicated that knockdown of WDR5 inhibited SeV-induced activation of IRF3, NF-κB as well as IFN-β promoter, suggesting that WDR5 is required for virus-induced type I IFN signaling. Consistent with its association with VISA, knockdown of WDR5 inhibited upstream components RIG-I-, VISA-, and MITA- but not downstream component TBK1-mediated signaling. Because knockdown of WDR5 does not inhibit expression levels of RIG-I and MITA, these results together suggest that WDR5 acts downstream of RIG-I and MITA and upstream of TBK1.
Previous studies suggest that overexpression of some of the signaling components of the virus-triggered pathways can activate IRF3, NF-κB, or both. However, overexpression of WDR5 failed to activate either IRF3 or NF-κB in reporter assays. This observation is not totally unexpected in light of the observations on other molecules in the pathways. For example, it is well established that IKKγ is required for activation of NF-κB, whereas TRAF3 is required for virus-triggered IRF3 activation. However, overexpression of either IKKγ or TRAF3 does not activate NF-κB or IRF3 (23, 24, 31).
In our experiments, we found that knockdown of WDR5 impaired the formation of the VISA-associated complex, providing an explanation of the critical role of WDR5 in virus-triggered type I IFN signaling. Knockdown of WDR5 caused down-regulation of RIG-I and MITA levels in the mitochondria after viral infection, whereas the whole cellular levels of RIG-I and MITA were unchanged after WDR5 knockdown. The simplest explanation for these observations is that WDR5 is important for recruiting RIG-I and MITA to the VISA-associated complex on the mitochondrial membrane. Knockdown of WDR5 also impaired the association of VISA with TRAF3 or TRAF6 at the mitochondria. However, the levels of TRAF3 and TRAF6 in the mitochondria were not markedly changed after WDR5 knockdown. These results suggest that WDR5 is not involved in recruiting TRAF3 and TRAF6 to the mitochondria; instead, it is important for the interactions between VISA and TRAF3 or TRAF6. In conclusion, the identification of WDR5 as a component essential for assembly of VISA-associated complex after viral infection provides insights to the molecular mechanisms of virus-triggered type I IFN signaling and cellular antiviral response.
Methods
Reagents.
IL-1 (R&D Systems); MitoTracker Red (Molecular Probes); mouse monoclonal antibodies against Flag, HA and β-actin (Sigma), cIAP1 (R&D), AIF, KDEL, and H2B (Santa Cruz Biotechnology); rabbit polyclonal antibodies against TRAF3, TRAF6, and IRF3 (Santa Cruz Biotechnology), and phospho-IRF3 (Ser396) (Upstate) were purchased from the indicated manufacturers. SeV, rabbit anti-VISA, and mouse anti-RIG-I were previously described (16, 32, 33). Mouse anti-WDR5 antisera were raised against recombinant human WDR5.
Constructs.
NF-κB, ISRE, and the IFN-β promoter luciferase reporter plasmids, mammalian expression plasmids for HA- or Flag-tagged VISA and its mutants, RIG-I, TBK1,TRADD, FADD, RIP IRF3, TRAF3, TRAF6, and MITA were previously described (16, 18, 32, 33). Mammalian expression plasmids for HA-, Flag-, or GFP-tagged human WDR5 were constructed by standard molecular biology techniques.
Protein Purification and Mass Spectrometry Analysis.
We firstly made a pCTAP-VISA construct, in which VISA cDNA was inframe fused to the C-terminal CBP and SBP tags in the pCTAP-A plasmid (Stratagene); 293 cells (5 × 108) stably transfected with pCTAP-VISA were collected and the cell lysate was subjected to tandem affinity purification procedures with the Interplay Mammalian TAP System (Stratagene). The purified VISA-associated proteins were digested by trypsin in solution. The tryptic peptides were analyzed by HPLC-ESI/MS/MS with a Thermo Finnigan LTQ adapted for nanospray ionization. The tandem spectra were searched against Homo sapiens National Center for Biotechnology Information reference database using the SEQUEST. Results was filtered by Xcorr +1 >1.9, +2 > 2.2, +3 >3.5, sp > 500, Deltcn >0.1, Rsp < = 5.
RNAi.
Double-strand oligonucleotides corresponding to the target sequences were cloned into the pSuper.retro RNAi plasmid (Oligoengine). The target sequences for human WDR5 cDNA were as follows: #1: 5′-GCTGGGAATATCCGATGTA -3′; #2: 5′-GCGTGAGGATATGGGATG -3′; #3: 5′-GAATGAGAAATACTGCATA-3′; #4: 5′-CAGAGGATAACCTTGTTTA-3′.
Transfection and Reporter Gene Assays.
293 cells (approximately 1 × 105) were seeded on 24-well plates and transfected the following day by standard calcium phosphate precipitation. To normalize for transfection efficiency, 0.01 μg of pRL-TK or pRL-SV40 Renilla luciferase reporter plasmid was added to each transfection. Luciferase assays were performed using a dual-specific luciferase assay kit (Promega). Firefly luciferase activities were normalized based on Renilla luciferase activities. All reporter assays were repeated at least three times. Data shown were average values ± SD from one representative experiment.
RT-PCR.
Total RNA was isolated from 293 cells using TRIzol reagent (Invitrogen) and subjected to RT–PCR analysis to measure the expression of IFNB1 and GAPDH. Gene-specific primer sequences were as follows.
IFNB1: 5′-CAGCAATTTTCAGTGTCAGCAAGCT-3′ and 5′-TCATCCTGTCCTTGAGGCAGTAT-3′;
GAPDH: 5-AAAATCAAGTGGGGCGATGCT-3′ and 5′-GGGCAGAGATGATGACCCTTT-3′.
Coimmunoprecipitation and Immunoblot Analysis.
For transient transfection and coimmunoprecipitation experiments, 293 cells (approximately 1 × 106) were transfected for 24 h. The transfected cells were lysed in 1 mL of lysis buffer (20 mM Tris pH 7.5, 150 mM NaCl, 1% Triton, 1 mM EDTA, 10 μg/mL aprotinin, 10 μg/mL leupeptin, 1 mM phenylmethylsulfonyl fluoride). For each immunoprecipitation, 0.4 mL aliquot of lysate was incubated with 0.5 μg of the indicated antibody or control IgG and 30 μL of a 1:1 slurry of Protein G Sepharose (GE Healthcare) for 2 h. The Sepharose beads were washed three times with 1 mL of lysis buffer containing 500 mM NaCl. The precipitates were analyzed by standard immunoblot procedures.
For endogenous coimmunoprecipitation experiments, 293 cells (5 × 107) were infected with SeV for the indicated times or left uninfected. The coimmunoprecipitation and immunoblot experiments were performed as described above.
Subcellular Fractionation.
Isolation of mitochondrial, membrane, and cytosolic fraction: 293 cells (5 × 107) infected with SeV or left uninfected were washed with PBS and lysed by douncing 40 times in 2 mL homogenization buffer (ApplyGen). The homogenate was centrifuged at 500 × g for 10 min. The supernatant (S5) was centrifuged at 5,000 × g for 10 min to precipitate mitochondria (P5K). The supernatant from this step (S5K) was further centrifuged at 50,000 × g for 30 min to yield the P50K, which contains the membrane fraction and the S50K, which mainly consists of cytosol.
Isolation of nuclear fraction: 293 cells (5 × 107) infected with SeV or left uninfected were washed with PBS and lysed by douncing for 40 times in 1 mL CER buffer (ApplyGen). The homogenate was centrifuged at 500 × g for 5 min and the pellet was saved as the crude nuclei, which was then washed twice with 500 μL NER buffer (ApplyGen).
Immunofluorescent Confocal Microscopy.
The 293transfected cells were left uninfected or infected with SeV for the indicated time. Cells were incubated with the MitoTracker Red (Molecular Probes) 30 min before the harvest. The cells were then fixed with 4% paraformaldehyde for 10 min and observed with a Leica confocal microscope under a ×100 oil objective.
Supplementary Material
Acknowledgments
We thank Dr. Fuquan Yang and Mr. Peng Xue from the Institute of Biophysics at Chinese Academy of Sciences for their help with mass spectrometry. This work was supported by grants from the Chinese 973 Program (2006CB504301, 2010CB911802), the National Natural Science Foundation of China (30630019, 30700431, and 30921001), the Chinese Science and Technology Key Project (2008ZX10002-014) and the Chinese 863 program (2006AA02A306).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908967107/DCSupplemental.
References
- 1.Hiscott J. Triggering the innate antiviral response through IRF-3 activation. J Biol Chem. 2007;282:15325–15329. doi: 10.1074/jbc.R700002200. [DOI] [PubMed] [Google Scholar]
- 2.Ishii KJ, Koyama S, Nakagawa A, Coban C, Akira S. Host innate immune receptors and beyond: making sense of microbial infections. Cell Host Microbe. 2008;3:352–363. doi: 10.1016/j.chom.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Yoneyama M, Fujita T. Structural mechanism of RNA recognition by the RIG-I-like receptors. Immunity. 2008;29:178–181. doi: 10.1016/j.immuni.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Seth RB, Sun L, Chen ZJ. Antiviral innate immunity pathways. Cell Res. 2006;16:141–147. doi: 10.1038/sj.cr.7310019. [DOI] [PubMed] [Google Scholar]
- 5.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Hiscott J. Convergence of the NF-kappaB and IRF pathways in the regulation of the innate antiviral response. Cytokine Growth Factor Rev. 2007;18:483–490. doi: 10.1016/j.cytogfr.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Honda K, Takaoka A, Taniguchi T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Durbin JE, et al. Type I IFN modulates innate and specific antiviral immunity. J Immunol. 2000;164:4220–4228. doi: 10.4049/jimmunol.164.8.4220. [DOI] [PubMed] [Google Scholar]
- 9.Maniatis T, et al. Structure and function of the interferon-beta enhanceosome. Cold Spring Harb Symp Quant Biol. 1998;63:609–620. doi: 10.1101/sqb.1998.63.609. [DOI] [PubMed] [Google Scholar]
- 10.Andrejeva J, et al. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc Natl Acad Sci USA. 2004;101:17264–17269. doi: 10.1073/pnas.0407639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoneyama M, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 12.Sun QL, et al. The specific and essential role of MAVS in antiviral innate immune responses. Immunity. 2006;24:633–642. doi: 10.1016/j.immuni.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Kawai TK, et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 14.Meylan EJ, et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 15.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Xu LG, et al. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol Cell. 2005;19:727–740. doi: 10.1016/j.molcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Kumar H, et al. Essential role of IPS-1 in innate immune responses against RNA viruses. J Exp Med. 2006;203:1795–1803. doi: 10.1084/jem.20060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong B, et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29:538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin L, et al. MPYS, a novel membrane tetraspanner, is associated with major histocompatibility complex class II and mediates transduction of apoptotic signals. Mol Cell Biol. 2008;28:5014–5026. doi: 10.1128/MCB.00640-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore CB, et al. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature. 2008;451:573–577. doi: 10.1038/nature06501. [DOI] [PubMed] [Google Scholar]
- 22.Zhao T, et al. The NEMO adaptor bridges the nuclear factor-kappaB and interferon regulatory factor signaling pathways. Nat Immunol. 2007;8:592–600. doi: 10.1038/ni1465. [DOI] [PubMed] [Google Scholar]
- 23.Saha SK, et al. Regulation of antiviral responses by a direct and specific interaction between TRAF3 and Cardif. EMBO J. 2006;25:3257–3263. doi: 10.1038/sj.emboj.7601220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oganesyan G, et al. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature. 2006;439:208–211. doi: 10.1038/nature04374. [DOI] [PubMed] [Google Scholar]
- 25.Häcker H, et al. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006;439:204–207. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- 26.Trievel RC, Shilatifard A. WDR5, a complexed protein. Nat Struct Mol Biol. 2009;16:678–680. doi: 10.1038/nsmb0709-678. [DOI] [PubMed] [Google Scholar]
- 27.Wysocka J, et al. WDR5 associates with histone H3 methylated at K4 and is essential for H3 K4 methylation and vertebrate development. Cell. 2005;121:859–872. doi: 10.1016/j.cell.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 28.Yokoyama A, et al. Leukemia proto-oncoprotein MLL forms a SET1-like histone methyltransferase complex with menin to regulate Hox gene expression. Mol Cell Biol. 2004;24:5639–5649. doi: 10.1128/MCB.24.13.5639-5649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu ED, Demay MB, Gori F. Wdr5 is essential for osteoblast differentiation. J Biol Chem. 2008;283:7361–7367. doi: 10.1074/jbc.M703304200. [DOI] [PubMed] [Google Scholar]
- 30.Gori F, Divieti P, Demay MB. Cloning and characterization of a novel WD-40 repeat protein that dramatically accelerates osteoblastic differentiation. J Biol Chem. 2001;276:46515–46522. doi: 10.1074/jbc.M105757200. [DOI] [PubMed] [Google Scholar]
- 31.Rothwarf DM, Zandi E, Natoli G, Karin M. IKK-gamma is an essential regulatory subunit of the IkappaB kinase complex. Nature. 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 32.Diao F, et al. Negative regulation of MDA5- but not RIG-I-mediated innate antiviral signaling by the dihydroxyacetone kinase. Proc Natl Acad Sci USA. 2007;104:11706–11711. doi: 10.1073/pnas.0700544104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang J, et al. SIKE is an IKK epsilon/TBK1-associated suppressor of TLR3- and virus-triggered IRF-3 activation pathways. EMBO J. 2005;24:4018–4028. doi: 10.1038/sj.emboj.7600863. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.