Abstract
Single-nucleotide changes are the most common cause of natural genetic variation among members of the same species, but there is remarkably little information bearing on how they alter bacterial virulence. We recently discovered a single-nucleotide mutation in the group A Streptococcus genome that is epidemiologically associated with decreased human necrotizing fasciitis (“flesh-eating disease”). Working from this clinical observation, we find that wild-type mtsR function is required for group A Streptococcus to cause necrotizing fasciitis in mice and nonhuman primates. Expression microarray analysis revealed that mtsR inactivation results in overexpression of PrsA, a chaperonin involved in posttranslational maturation of SpeB, an extracellular cysteine protease. Isogenic mutant strains that overexpress prsA or lack speB had decreased secreted protease activity in vivo and recapitulated the necrotizing fasciitis-negative phenotype of the ΔmtsR mutant strain in mice and monkeys. mtsR inactivation results in increased PrsA expression, which in turn causes decreased SpeB secreted protease activity and reduced necrotizing fasciitis capacity. Thus, a naturally occurring single-nucleotide mutation dramatically alters virulence by dysregulating a multiple gene virulence axis. Our discovery has broad implications for the confluence of population genomics and molecular pathogenesis research.
Keywords: group A streptococcus, invasive infection, molecular epidemiology of strain genotype patient phenotype relationships, nonhuman primate
Single-nucleotide mutations are the most abundant cause of genetic variation among members of the same species (1, 2). However, in striking contrast to humans, who have been studied extensively, our understanding of how naturally occurring single-nucleotide mutations alter bacterial phenotypes is rudimentary. Most prokaryotic pathogenesis research efforts have focused intensively on large regions of genetic difference, such as pathogenicity islands and prophages. Thus, there is little information that directly bears on the relationship between particular single-nucleotide changes, their direct or indirect effect on virulence factor expression, and the manifestation of medically important traits such as strain virulence and infection specificity.
Recently, we have investigated the molecular genomic landscape of infection phenotype-strain genotype relationships in human patients at the nucleotide level in group A Streptococcus (GAS), a bacterial pathogen that is a major cause of human morbidity and mortality worldwide (1, 3–5). These studies were made possible by the availability of the 1.9-Mb genome sequences of 12 GAS strains cultured from patients with well-defined clinical syndromes such as pharyngitis, acute rheumatic fever, and necrotizing fasciitis (also known as “flesh-eating” disease) (1, 5). The core genome of strains of distinct M protein serotype differed, on average, by 14,475 SNPs (1, 3). In contrast, strains with the same M protein serotype were far less variable, differing overall by less than several hundred SNPs (1, 3). This restricted level of intra-M serotype SNP variation significantly reduces the complexity of studies designed to understand patient infection phenotype-strain genotype relationships.
Serotype M3 GAS strains commonly cause pharyngitis and severe invasive infections such as necrotizing fasciitis, and they display epidemic behavior (4, 6, 7). We recently analyzed 255 serotype M3 invasive isolates collected in an 11-year comprehensive population-based study conducted in Ontario, Canada (4, 6, 7). Genome-wide molecular analysis of all 255 strains representing distinct strain genotypes and patient phenotypes discovered that a naturally occurring single-nucleotide insertion in the mtsR (metal transporter of streptococcus regulator) gene was epidemiologically associated with a significantly decreased number of human necrotizing fasciitis cases (3, 4, 8, 9). This mtsR mutation creates a stop codon, resulting in premature termination of the MtsR protein (3). Importantly, strains with this mtsR mutation caused normal levels of other human invasive infections (3). The goal of the present study was to test the hypothesis that mtsR inactivation is responsible for the decreased necrotizing fasciitis phenotype observed in human patients. We used an integrated molecular approach with comparative genome sequencing, iterative expression microarray analysis, and a newly developed monkey necrotizing fasciitis model to compare various wild-type and naturally occurring or isogenic mutant GAS strains. The mtsR mutation significantly decreases the necrotizing fasciitis (flesh-eating) capacity of GAS by altering a multiple gene virulence axis to disrupt the in vivo enzymatic activity of SpeB, a secreted broad-spectrum cysteine protease. Our study provides a model strategy to understand the molecular mechanisms underlying unique patient infection phenotype-bacterial strain genotype relationships observed in human infectious disease research.
Results
Serotype M3 Strain MGAS9887 (Naturally Occurring mtsR Mutant) Is Less Virulent for Mice than Wild-Type Strain MGAS315 in an Invasive Infection Model.
To begin testing the hypothesis that mtsR inactivation alters GAS necrotizing fasciitis capacity, we compared the virulence of strain MGAS9887 (a human isolate representative of strains with a naturally occurring mtsR mutant gene) (3, 4) and MGAS315 (representative of mtsR wild-type strains) in a mouse model. Consistent with the hypothesis, we found that strain MGAS9887 was significantly less virulent for mice when injected intramuscularly (Fig. 1A).
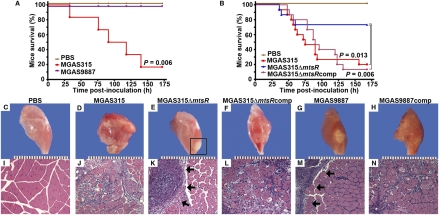
Fig. 1.
Inactivation of mtsR significantly decreases GAS necrotizing fasciitis capacity in mice. (A) Mice were inoculated intramuscularly in the hindlimb with wild-type strain MGAS315 or naturally occurring mtsR mutant strain MGAS9887 (actual dose: MGAS315 1.04 × 106 cfu; MGAS9887 1.02 × 106 cfu). Survival is shown with P value for Kaplan–Meier analysis. (B) Kaplan–Meier survival curves for mice inoculated intramuscularly with wild-type ΔmtsR isogenic mutant or complemented mutant strains (actual dose: MGAS315 1.10 × 106 cfu; MGAS315ΔmtsR 1.12 × 106 cfu; MGAS315ΔmtsRcomp 1.10 × 106 cfu). Gross (C–H) and microscopic (I–N) histopathology (original magnification 4×) of mouse hindlimb lesions at 60 h postinfection. PBS-treated animals had normal hindlimb appearance (C and I). Animals given wild-type strain MGAS315 had extensive myonecrosis (D and J), whereas lesions from animals inoculated with isogenic mtsR mutant strain MGAS315ΔmtsR were confined to the inoculation site (E and K). The boxed area and arrows denote a circumscribed, walled-off lesion. Gene complementation restored the wild-type tissue pathology phenotype (F and L). Lesions from mice inoculated with naturally occurring mtsR mutant strain MGAS9887 have features identical to those in MGAS315ΔmtsR (G and M), and providing a wild-type copy of mtsR to create strain MGAS9887comp restored the wild-type tissue pathology phenotype (H and N). Micrographs show tissue taken from the inoculation site.
Virulence of Wild-Type, Isogenic ΔmtsR Mutant and Complemented GAS Strains in a Mouse Model of Necrotizing Fasciitis.
Compared with reference strain MGAS315, which is genetically representative of serotype M3 strains commonly causing necrotizing fasciitis in humans (3), strain MGAS9887 has 60 other single-nucleotide mutations, thereby prohibiting us from concluding that the naturally occurring inactivation of MtsR alone altered its necrotizing fasciitis capacity. Thus, to further test our hypothesis, we constructed an isogenic mtsR deletion-mutant strain (MGAS315ΔmtsR) from wild-type strain MGAS315 (Fig. S1). In addition, we complemented the mutated gene in strain MGAS315ΔmtsR to create strain MGAS315ΔmtsRcomp (Fig. S1). Compared with the wild-type organism, significantly fewer mice inoculated intramuscularly with MGAS315ΔmtsR died (Fig. 1B). The genetically complemented strain was fully virulent (Fig. 1B). These findings are consistent with the hypothesis that inactivation of the mtsR gene significantly altered virulence.
Pathologic Features of GAS-Infected Mouse Muscle.
We next hypothesized that the attenuated virulence of the mtsR mutant strain would be manifested by significant alteration of the pathologic characteristics of the resulting tissue lesions. Visual inspection of infected mouse hindlimbs revealed extensive differences between animals inoculated with the wild-type or naturally occurring and isogenic ΔmtsR mutant strains (Fig. 1 C–N). For example, the hindlimbs from animals infected with wild-type strain MGAS315 or the complemented mutant strain MGAS315ΔmtsRcomp had extensive liquifactive necrosis centered at the inoculation site that extended throughout the muscle (Fig. 1 D and F). In contrast, mice inoculated with mutant strain MGAS315ΔmtsR had a demarcated abscess centered at, and confined to, the inoculation site. The surrounding tissue was pathologically unremarkable, and the remainder of the extremity remained viable (Fig. 1E). A similar necrotizing fasciitis-negative tissue phenotype was observed in mice inoculated with the naturally occurring mtsR mutant strain MGAS9887 (Fig. 1G), and gene complementation (creating strain MGAS9887comp) restored wild-type mtsR tissue pathology (Fig. 1H). Microscopic analysis of the infected tissue confirmed the gross pathologic features (Fig. 1 I–N).
Growth and Distribution of Wild-Type and mtsR Mutant GAS Strain in Mouse Tissue.
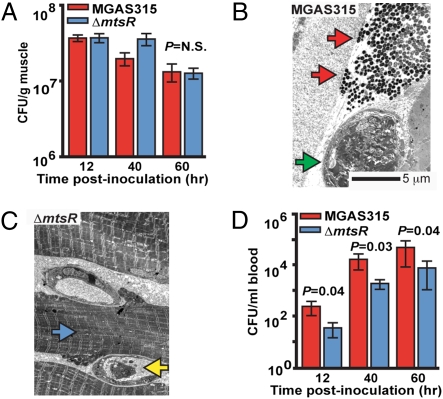
One explanation for the altered virulence of GAS strains with a mutated mtsR gene is a decreased ability to replicate in situ in muscle. To test this hypothesis, we assessed GAS burden in the mouse muscle after intramuscular inoculation. There was no significant difference in the cfu’s recovered per gram of total hindlimb tissue between the experimental groups at any time point (Fig. 2A and Fig. S2A). This finding suggested that any preferential replication of the mtsR wild-type strain or killing of the mutant strain did not cause the observed significant differences in survival and pathology. However, consistent with our visual and microscopic examination, electron micrographs of limb tissue adjacent to the inoculation site showed that wild-type strain organisms were present throughout the muscle, whereas only rare mutant strain organisms were identified outside of the central abscess (Fig. 2 B and C). Taken together, these findings suggest that the mtsR mutant strain has an impaired ability to disseminate through fascial tissue.
Fig. 2.
Inactivation of mtsR significantly decreases the level of bacteremia after intramuscular inoculation. (A) Cfu of GAS per gram of infected hindlimb tissue. (B) Electron micrographs of muscle infected with MGAS315 (original magnification 3000×). At 48 h postinfection, examination of tissue adjacent to the MGAS315 inoculation site shows many wild-type GAS organisms (red arrows) alongside a thrombosed vessel (green arrow) and necrotic fascial debris. (C) Examination of MGAS315ΔmtsR infected tissue adjacent to the central abscess reveals rare ΔmtsR GAS organisms, viable muscle cells that retain their normal ultrastructural features (blue arrow), and a patent vessel containing a PMN (yellow arrow). (D) Cfu of GAS per milliliter of blood.
Dissemination of Wild-Type and mtsR Isogenic Mutant GAS Strain.
Another hypothesis for the reduced lethality of the mtsR mutant strain is a decreased ability to disseminate via the bloodstream and cause systemic disease. To test this hypothesis, we performed quantitative cultures on blood collected from mice inoculated intramuscularly with strain MGAS315, MGAS315ΔmtsR, or MGAS315ΔmtsRcomp. Consistent with the hypothesis, the level of bacteremia in mice infected with the isogenic mtsR mutant strain was significantly lower than animals infected with the mtsR wild-type strain or complemented mutant strain (Fig. 2D and Fig. S2B).
Host PMN Interaction with Wild-Type and mtsR Isogenic Mutant GAS Strain.
As part of the host immune response, PMN leukocytes may contribute to GAS necrotizing fasciitis by elaborating tissue-destructive enzymes or killing bacteria. To test the hypothesis that wild-type strain MGAS315 causes increased PMN recruitment relative to mtsR mutant strain MGAS513ΔmtsR, myeloperoxidase (MPO), a surrogate marker for PMNs and PMN proteases, was measured in the mouse necrotizing fasciitis lesions. However, MPO tissue levels did not significantly differ (Fig. S3A). To test the alternate hypothesis that decreased bacteremia and tissue destruction in mice infected with mtsR mutant strains was caused by decreased GAS survival due to enhanced phagocytosis or susceptibility to intraphagosomal H2O2, strain MGAS315 and MGAS315ΔmtsR were incubated with human PMNs or grown in the presence of hydrogen peroxide. However, survival did not significantly differ (Fig. S3 B–D).
Wild-Type Strain MGAS315 Causes Significantly More Extensive Necrotizing Fasciitis in Cynomolgus Macaques than Isogenic Mutant Strain MGAS315ΔmtsR.
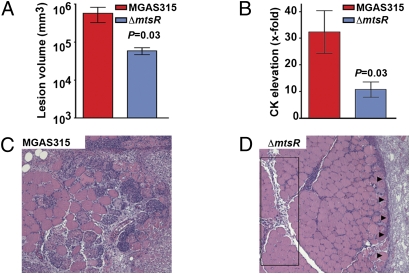
GAS is a host-specialist pathogen, causing natural disease only in humans. Several known and putative GAS virulence factors have modest or no activity against mouse molecules (10, 11), which means that a full understanding of molecular pathogenesis based exclusively on mouse infection models cannot be achieved. The cynomolgus macaque is an excellent model of human GAS pharyngitis, and this model has been successfully employed in several studies to provide new information about GAS pathogenesis (12). Inasmuch as necrotizing fasciitis is a devastating disease with high rates of morbidity and mortality, with little known about pathogenesis, we sought to unambiguously demonstrate that mtsR is involved (8, 9, 13, 14). We intramuscularly inoculated four monkeys each with sterile PBS, wild-type strain MGAS315, or mutant strain MGAS315ΔmtsR to assess virulence. Compared with the wild-type strain, animals infected with the mutant strain had significantly smaller muscle lesions, less myonecrosis, and less muscle-cell enzyme release (Fig. 3). Thus, the monkey data provide crucial and unambiguous evidence that inactivation of mtsR detrimentally alters GAS necrotizing fasciitis capacity in the most relevant nonhuman host available.
Fig. 3.
Inactivation of mtsR significantly decreases GAS necrotizing fasciitis capacity in cynomolgus macaques. (A) Cynomolgus macaques were inoculated intramuscularly in the anterior thigh, and mean lesion areas are shown with P value for Mann–Whitney test. (B) Creatine kinase (CK), a muscle cell enzyme used to measure tissue damage, was significantly elevated. (C) Muscle from animals infected with the wild-type strain MGAS315 shows extensive myonecrosis and tissue destruction (original magnification 4×). (D) Muscle from animals infected with MGAS315ΔmtsR shows relative preservation of tissue, with greatly restricted lesion progression. The box denotes that tissue pathology is limited to the major fascial planes. Arrows denote a circumscribed abscess analogous to the lesion phenotype observed in mice (see Fig. 2).
Expression Microarray Analysis.
The findings described herein are consistent with the idea that mtsR mutant strains are less efficient than wild-type organisms at damaging host tissue and disseminating from the inoculation site. We sought to obtain additional data bearing on the virulence differences by conducting expression microarray analysis. Thus, we compared the transcriptome of strain MGAS315 and isogenic mutant strain MGAS315ΔmtsR grown in vitro. The two strains differed by only 91 transcripts at either mid- or late-exponential growth phase (Fig. S4 and Table S1). The transcriptome analysis confirmed all key findings reported previously comparing strains MGAS315 and MGAS9887 (naturally occurring mtsR mutant) (3), including altered transcript levels of multiple operons involved with metal homeostasis, nucleotide metabolism, and oxidative stress response (Fig. S4). Interestingly, none of the altered transcripts encoded known major GAS virulence factors such as secreted toxins (i.e., SpeA) or degradative enzymes (i.e., SpyCEP).
However, one altered transcript was of particular interest. The prsA gene was significantly up-regulated (expressed 1.8-fold higher, P < 0.05) in strain MGAS315ΔmtsR relative to MGAS315, a finding confirmed by TaqMan analysis (Fig. 4A). Previously published data showed that prsA transcription was also up-regulated in strain MGAS9887 compared with strain MGAS315 (3). This gene encodes a peptidyl-prolyl isomerase required for normal maturation and enzymatic activity of streptococcal pyrogenic exotoxin B (SpeB) (15), a critical broad-spectrum secreted protease virulence factor involved in the degradation of extracellular matrix proteins, inactivation of innate immune molecules, and destruction of host tissue (16–22). Although control of prsA transcription is not well understood in GAS (15), dysregulation of prsA homologs (either increased or decreased expression of prsA) in other bacterial species has been shown to detrimentally alter the maturation of secreted proteins that are dependent on PrsA for extracellular processing (23–25). Also, SpeB is abundantly expressed in situ in infected human tissues (26). Thus, we hypothesized that the reduced ability of mtsR mutant GAS strains to cause necrotizing fasciitis is due to the dysregulated overexpression of PrsA and resultant decrease in secreted SpeB enzymatic activity, a secreted enzyme that destroys host extracellular matrix proteins.
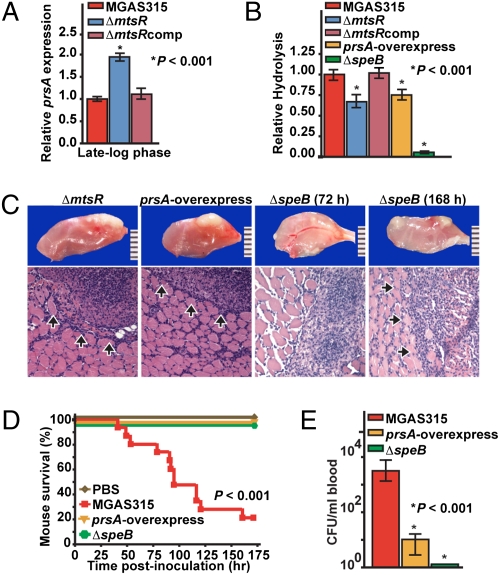
Fig. 4.
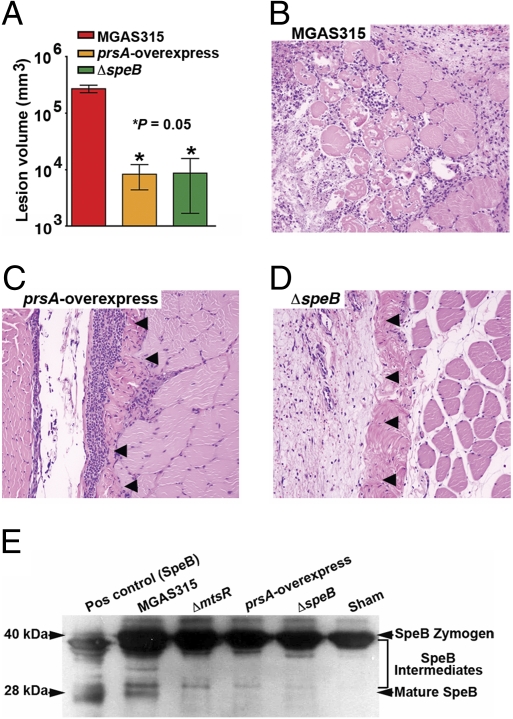
Dysregulated prsA expression and altered extracellular SpeB cysteine protease activity in ΔmtsR mutant strains. (A) TaqMan analysis of prsA transcript level in strains MGAS315, MGAS315ΔmtsR, and MGAS315ΔmtsRcomp. (B) Level of proteolytic activity using a standard milk agar assay for SpeB. Compared with wild-type strain MGAS315, mutant strains MGAS315ΔmtsR, MGAS315prsA-overexpress, and MGAS315ΔspeB had significantly reduced proteolytic activity. (C) Examination of hindlimb lesions from mice infected with strains MGAS315ΔmtsR and MGAS315prsA-overexpress revealed identical features at 72 h postinoculation. Arrows denote a circumscribed lesion (original magnification 4×).The ΔspeB mutant strain caused a similar lesion but took 168 h to form an equivalently sized abscess. (D) Kaplan–Meier survival curves for mice inoculated intramuscularly with wild-type, prsA-overexpressing, or ΔspeB strains. (E) Cfu’s of GAS per milliliter of blood.
prsA Overexpression or speB Inactivation Recapitulates the Decreased Necrotizing Fasciitis Virulence Phenotype of mtsR-Negative Strains.
To test the hypothesis that prsA up-regulation contributes to the significantly attenuated necrotizing fasciitis phenotype of MGAS315ΔmtsR, we constructed an isogenic strain that overexpresses prsA (creating strain MGAS315prsA-overexpress; Fig. S1). Furthermore, to also test the hypothesis that the observed necrotizing fasciitis-negative phenotype of mtsR-negative strains is due to decreased SpeB activity, we studied the virulence phenotype of an isogenic speB-deletion mutant strain (MGAS315ΔspeB). Consistent with these hypotheses, the ΔmtsR and the prsA-overexpressing strains had significantly reduced caseinolytic activity in a standard milk agar assay used to assess the extracellular cysteine protease function of SpeB (20) (Fig. 4B). Importantly, deletion of the speB gene abolished nearly all caseinolytic activity.
Also consistent with the proposed role of SpeB in mtsR mutant strains, the prsA-overexpressing and ΔspeB strains had a necrotizing fasciitis virulence phenotype in mice that was indistinguishable from the disease caused by the ΔmtsR strains. Visual and microscopic examination of hindlimb lesions revealed nearly identical features in mice infected with either strain MGAS315prsA-overexpress or MGAS315ΔmtsR (Fig. 4C). However, the tissue pathology caused by the ΔspeB strain was even more strikingly attenuated. The lesions in mice receiving the ΔspeB strain were less severe, taking twice as long (72 h vs. 168 h) to produce equivalently sized abscesses to those occurring in animals given the ΔmtsR or prsA-overexpressing strains (Fig. 4C). Moreover, compared with the wild-type organism that causes significant mortality in our mouse model, no mice died following intramuscular inoculation with strains overexpressing prsA or lacking speB (Fig. 4D). Similarly, the ΔspeB mutant strain caused a significantly lower level of bacteremia than the ΔmtsR and prsA-overexpressing strains (Fig. 4E). These findings are consistent with a complete absence of SpeB function in strain MGAS315ΔspeB compared with a reduced level of extracellular secreted SpeB protease activity in strains MGAS315ΔmtsR or MGAS315prsA-overexpress. An identical attenuation of necrotizing fasciitis phenotype, including significantly reduced mortality and tissue damage, was observed in mice infected with a panel of wild-type and isogenic mutant (ΔmtsR and ΔspeB) serotype M1 strains (Fig. S5).
prsA Overexpression or speB Inactivation Significantly Decreases Necrotizing Fasciitis in Cynomolgus Macaques.
To unequivocally demonstrate that prsA overexpression and SpeB inactivation underlie the decreased necrotizing fasciitis capacity observed in the naturally occurring and isogenic mtsR mutant GAS strains, we performed a second nonhuman primate experiment. Three monkeys each were inoculated intramuscularly with strain MGAS315, MGAS315prsA-overexpress, or MGAS315ΔspeB. Compared with the wild-type strain, monkeys infected with the prsA-overexpressing or speB-inactivated strains had significantly smaller muscle lesions and less myonecrosis (Fig. 5 A–D). Visual and microscopic features of muscle infected with the isogenic mutant strains MGAS315ΔmtsR, MGAS315prsA-overexpress, and MGAS315ΔspeB were indistinguishable (compare Fig. 5 and Fig. 2).
Fig. 5.
Overexpression of prsA or inactivation of speB reproduces the ΔmtsR GAS necrotizing fasciitis phenotype in cynomolgus macaques. (A) Cynomolgus macaques were inoculated intramuscularly in the anterior thigh, and mean lesion areas are shown with P value for Mann–Whitney test. (B) Muscle from animals infected with wild-type strain MGAS315 shows extensive myonecrosis and tissue destruction. (C and D) Muscle from animals infected with prsA-overexpressing or ΔspeB mutant strains shows relative preservation of tissue with greatly restricted lesion progression. Arrows denote a circumscribed abscess analogous to the lesion phenotype observed in mice (original magnification 4×; see Fig. 4). These microscopic features are identical to muscle from monkeys infected with the ΔmtsR mutant strain (see Fig. 3). (E) In vivo SpeB enzymatic activity measured by proteolytic conversion of an autocatalytic-negative Cys192Ser mutant SpeB zymogen (40 kDa) through several intermediates to the mature protein (28 kDa). Compared with muscle taken from monkeys infected with wild-type strain MGAS315, SpeB protease activity is significantly less in the infected muscle of animals receiving the ΔmtsR or prsA-overexpressing strains, and absent in animals receiving the ΔspeB strain or sham inoculation.
SpeB Protease Activity In Vivo.
To confirm that the isogenic mutant GAS strains have altered SpeB protease activity in vivo, zymogen protease assays were performed on infected monkey muscle. Compared with monkeys infected with wild-type strain MGAS315, SpeB protease activity was significantly decreased in the muscle of monkeys receiving the isogenic ΔmtsR or prsA-overexpressing strains and absent in monkeys receiving the ΔspeB strain or sham inoculation (Fig. 5E).
Discussion
Model to Explain Decreased Necrotizing Fasciitis Capacity in mtsR Mutant Gas Strains.
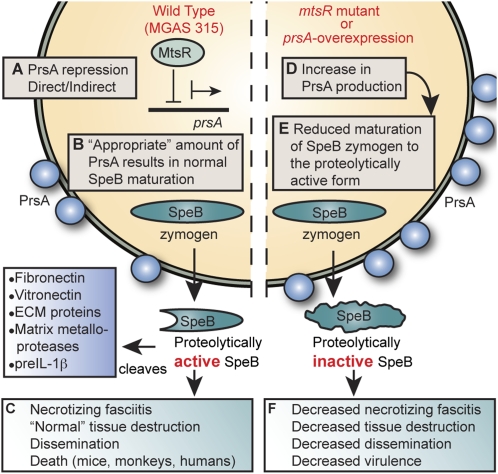
Although our findings do not explain all aspects of the molecular mechanism underlying why a single nucleotide change in the mtsR gene significantly decreases human necrotizing fasciitis capacity in GAS, the data show that mtsR inactivation causes the dysregulated overexpression of prsA, which in turn results in decreased extracellular secreted protease activity of SpeB (Fig. 6). In this regard, we note that the prsA homolog in Bacillus subtilis has been extensively studied, and its dysregulated expression, resulting in either increased or decreased prsA transcription, impairs the secretion and extracellular processing of other proteins (23–25). SpeB is well known to be a critical virulence factor for severe invasive episodes of GAS infection, and its genetic inactivation significantly decreases virulence and tissue destruction (16–22). This secreted broad-spectrum protease has many detrimental effects on host molecules. For example, it degrades inflammatory mediators, such as complement factor C3b and LL-37, and extracellular matrix proteins, such as fibronectin and vitronectin, and it activates host matrix metalloproteases and preinterleukin-1β (16–22). As such, SpeB enhances evasion of innate immunity, propagation of bacteria in vivo, and dissemination to distant anatomic sites (16, 20, 21). These virulence activities are postulated to be crucial for necrotizing fasciitis pathogenesis (27). Consistent with these ideas, SpeB is abundantly present in necrotic human tissue (26), and SpeB enzymatic activity was significantly less in monkeys infected with the isogenic mutant (ΔmtsR, prsA-overexpress, and ΔspeB) strains (Fig. 5E). Thus, any molecular process that decreases the amount of mature SpeB is expected to produce the observed disease phenotype of decreased necrotizing fasciitis. Importantly, the total number of all human infections caused by organisms with the naturally occurring mtsR mutation was not significantly decreased, as shown in our previously published population-based study (3), indicating that mtsR inactivation does not dramatically alter the ability of GAS to cause other types of invasive infections. That is, naturally occurring mtsR mutant GAS strains such as MGAS9887 cause normal levels of pneumonia, bacteremia, and synovitis in humans, so there is no apparent positive or negative selective pressure on mtsR (3) in the context of these diseases. Rather, the MtsR-negative genotype is highly specific for a decreased necrotizing fasciitis disease phenotype (3). This model is supported by our molecular epidemiology studies that show mtsR mutant strains to be a frequent cause of human infections other than necrotizing fasciitis (3), the murine and nonhuman primate data presented herein, and findings by other investigators that show mtsR inactivation does not alter virulence in extramuscular sites (8, 9).
Fig. 6.
Model for the role of the mtsR-prsA-SpeB virulence axis in GAS necrotizing fasciitis. (A) In wild-type GAS strains such MGAS315, MtsR directly or indirectly regulates PrsA expression. (B) PrsA, a peptidyl-prolyl isomerase, is essential for proper maturation of the broad-spectrum cysteine protease SpeB. (C) In this wild-type condition, there is an appropriate stoichiometry between PrsA and SpeB, resulting in a normal level of extracellular SpeB cysteine protease activity and full GAS virulence. (D and E) Dysregulated overexpression of PrsA via either mtsR deletion (MGAS315ΔmtsR and MGAS9887) or overexpression of prsA transcription (MGAS315prsA-overexpress) disrupts the normal stoichiometry between PrsA and SpeB, significantly decreasing extracellular cysteine protease activity. (F) Without the wild-type level of proteolytically active SpeB available to act on its many downstream targets, the mutant GAS organisms (ΔmtsR, prsA-overexpress, and ΔspeB) are rendered considerably less virulent in muscle. They have a reduced ability to destroy soft tissue, disseminate from the infection site, and cause human necrotizing fasciitis and death.
Regardless of whether an organism is a haploid prokaryote or a diploid eukaryote, single-nucleotide changes are the most abundant cause of genetic differences between members of the same species (1, 2). For example, between M protein serotypes in GAS, single-nucleotide changes outnumber large blocks of differences in gene content by greater than ∼500-fold (1, 3, 4). This means that, in principle, single-nucleotide mutations have the capacity to alter host-pathogen interactions, and as a result, significantly influence patient infection phenotype-strain genotype relationships in human infectious disease. However, very little information bearing on this issue is available. Our discovery that a single-nucleotide mutation is responsible for a striking alteration in necrotizing fasciitis capacity by disrupting the mtsR-prsA-SpeB virulence axis was made possible by the confluence of low-cost DNA sequencing, the availability of hundreds of serotype M3 GAS strains from patients with clinically well-characterized invasive infections, and a highly integrated investigative strategy (3, 6, 7). Although pathogenic bacteria have very small genomes relative to higher eukaryotes, study of the relationships between patient infection phenotype and strain genotype at the genome-wide level has lagged considerably behind the study of human complex genetic traits. Our findings suggest that additional research in this area would be fruitful in other microbial pathogens. Importantly, recent data from the study of Staphylococcus aureus and Pseudomonas aeruginosa pathogenesis suggest that interest in this confluence is accelerating (28, 29). Our study serves as a model strategy to describe a specific patient infection phenotype, perform genome sequencing to identify the potential polymorphisms responsible, and elucidate the underlying mechanism of a devastating infectious disease.
Materials and Methods
For further details, see SI Materials and Methods.
Bacterial Strains and Construction of Isogenic Mutants.
Strain MGAS315 (ATCC-BAA595) was isolated from a patient with streptococcal toxic shock syndrome. Strain MGAS9887, a clinical isolate with a naturally occurring mtsR mutation (3, 4), was collected in accordance with a protocol approved by the Institutional Review Board, Human Subjects Review Committee, University of Toronto. Genome sequences of both strains have been reported (3, 4). We generated isogenic mutant strain MGAS315ΔmtsR by gene replacement (9), and mtsR complemented and prsA overexpressing strains by providing wild-type genes in trans on a low-copy-number plasmid (Fig. S1). The ΔspeB strains were previously described. No differences in growth were observed among the isogenic strains (Fig. S1).
Mouse Infection.
Five- to 6-week-old (20-25 g) outbred immunocompetent female CD1 mice (Harlan Laboratories) were used for all studies. Animals were randomly assigned to the indicated strain treatment groups and inoculated intramuscularly in the right hindlimb with 1 × 106 cfu of GAS in 100 μL PBS. Doses were prepared at the time of inoculation from previously quantified frozen stocks, and the actual dose given was confirmed by counting cfu’s. Control animals were injected with PBS. For survival experiments (n = 15 per group), near mortality was determined by observation. For the quantitative culture experiment (n = 20 per group per time point), limbs were homogenized (Omni International) and cfu’s were determined by culturing serial dilutions. For histology, limbs were processed using standard methods. All mouse experimental protocols were approved by the Institutional Animal Care and Use Committee, Methodist Hospital Research Institute.
Isolation of Human PMNs and Bactericidal Assays.
Human PMNs were isolated from venous blood of healthy individuals in accordance with a protocol approved by the Institutional Review Board for Human Subjects, National Institute of Allergy and Infectious Diseases.
Monkey Infection.
Adult male cynomolgus macaques (Macaca fasicularis) (Charles River BRF) were used for all studies. In the first experiment, we inoculated each animal (n = 4 per group) with sterile PBS, MGAS315, or MGAS315ΔmtsR. In the second experiment, we inoculated each animal (n = 3 per group) with MGAS315, MGAS315prsA-overexpress, or MGAS315ΔspeB. Each animal was anesthetized, outfitted with a transdermal fentanyl patch, and inoculated intramuscularly in the anterior thigh. Animals were observed continuously, killed in pairs when judged to be near death, and necropsied. The study protocol was approved by the Animal Care and Use Committee, University of Houston.
In Vitro Expression Microarray and TaqMan Analysis.
We performed expression microarray analysis as described previously (Table S2) (3). Briefly, bacteria were grown in triplicate and harvested at the indicated growth phase, and transcripts were analyzed using a custom Affymetrix GeneChip. Data have been deposited in the GEO database at National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/geo/). TaqMan real-time quantitative reverse transcription PCR (QRT-PCR) was performed in quadruplicate reactions made from duplicate cultures using an ABI Thermocycler 7700 (Applied Biosystems).
SpeB Activity.
We assessed GAS extracellular protease activity by casein hydrolysis using milk agar as described previously. We assessed SpeB activity in vivo from homogenized monkey muscle using a zymogen activation assay as described previously.
Supplementary Material
Acknowledgments
We thank K. Stockbauer for critical review of the manuscript, A. Tart for contributions to experimental design, R. Gonzalez and A. Dayton for technical expertise, and D. Sturdevant, P. Sumby, and S. A. Shelburne III for discussions. This work was supported by American Heart Association Grant AHA-0775045N and the Intramural Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
Data deposition: Expression microarray data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, http://www.ncbi.nlm.nih.gov/geo/#GSE10252.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911811107/DCSupplemental.
References
- 1.Beres SB, Musser JM. Contribution of exogenous genetic elements to the group A Streptococcus metagenome. PLoS One. 2007;2:e800. doi: 10.1371/journal.pone.0000800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jakobsson M, et al. Genotype, haplotype and copy-number variation in worldwide human populations. Nature. 2008;451:998–1003. doi: 10.1038/nature06742. [DOI] [PubMed] [Google Scholar]
- 3.Beres SB, et al. Molecular genetic anatomy of inter- and intraserotype variation in the human bacterial pathogen group A Streptococcus. Proc Natl Acad Sci USA. 2006;103:7059–7064. doi: 10.1073/pnas.0510279103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beres SB, et al. Genome-wide molecular dissection of serotype M3 group A Streptococcus strains causing two epidemics of invasive infections. Proc Natl Acad Sci USA. 2004;101:11833–11838. doi: 10.1073/pnas.0404163101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musser JM, Shelburne SA., III A decade of molecular pathogenomic analysis of group A Streptococcus. J Clin Invest. 2009;119:2455–2463. doi: 10.1172/JCI38095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies HD, et al. Ontario Group A Streptococcal Study Group. Invasive group A streptococcal infections in Ontario, Canada. N Engl J Med. 1996;335:547–554. doi: 10.1056/NEJM199608223350803. [DOI] [PubMed] [Google Scholar]
- 7.Sharkawy A, et al. Ontario Group A Streptococcal Study Group. Severe group a streptococcal soft-tissue infections in Ontario: 1992-1996. Clin Infect Dis. 2002;34:454–460. doi: 10.1086/338466. [DOI] [PubMed] [Google Scholar]
- 8.Bates CS, Toukoki C, Neely MN, Eichenbaum Z. Characterization of MtsR, a new metal regulator in group A streptococcus, involved in iron acquisition and virulence. Infect Immun. 2005;73:5743–5753. doi: 10.1128/IAI.73.9.5743-5753.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanks TS, et al. Differential regulation of iron- and manganese-specific MtsABC and heme-specific HtsABC transporters by the metalloregulator MtsR of group A Streptococcus. Infect Immun. 2006;74:5132–5139. doi: 10.1128/IAI.00176-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agniswamy J, Lei B, Musser JM, Sun PD. Insight of host immune evasion mediated by two variants of group a Streptococcus Mac protein. J Biol Chem. 2004;279:52789–52796. doi: 10.1074/jbc.M410698200. [DOI] [PubMed] [Google Scholar]
- 11.Sumby P, et al. A chemokine-degrading extracellular protease made by group A Streptococcus alters pathogenesis by enhancing evasion of the innate immune response. Infect Immun. 2008;76:978–985. doi: 10.1128/IAI.01354-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sumby P, Tart AH, Musser JM. A non-human primate model of acute group a Streptococcus pharyngitis. Methods Mol Biol. 2008;431:255–267. doi: 10.1007/978-1-60327-032-8_20. [DOI] [PubMed] [Google Scholar]
- 13.Bryant AE, Bayer CR, Huntington JD, Stevens DL. Group A streptococcal myonecrosis: Increased vimentin expression after skeletal-muscle injury mediates the binding of Streptococcus pyogenes. J Infect Dis. 2006;193:1685–1692. doi: 10.1086/504261. [DOI] [PubMed] [Google Scholar]
- 14.Young MH, Aronoff DM, Engleberg NC. Necrotizing fasciitis: Pathogenesis and treatment. Expert Rev Anti Infect Ther. 2005;3:279–294. doi: 10.1586/14787210.3.2.279. [DOI] [PubMed] [Google Scholar]
- 15.Ma Y, et al. Identification and characterization of bicistronic speB and prsA gene expression in the group A Streptococcus. J Bacteriol. 2006;188:7626–7634. doi: 10.1128/JB.01059-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuo CF, et al. Role of streptococcal pyrogenic exotoxin B in the mouse model of group A streptococcal infection. Infect Immun. 1998;66:3931–3935. doi: 10.1128/iai.66.8.3931-3935.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burns EH, Jr, Marciel AM, Musser JM. Activation of a 66-kilodalton human endothelial cell matrix metalloprotease by Streptococcus pyogenes extracellular cysteine protease. Infect Immun. 1996;64:4744–4750. doi: 10.1128/iai.64.11.4744-4750.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapur V, et al. A conserved Streptococcus pyogenes extracellular cysteine protease cleaves human fibronectin and degrades vitronectin. Microb Pathog. 1993;15:327–346. doi: 10.1006/mpat.1993.1083. [DOI] [PubMed] [Google Scholar]
- 19.Kuo CF, Lin YS, Chuang WJ, Wu JJ, Tsao N. Degradation of complement 3 by streptococcal pyrogenic exotoxin B inhibits complement activation and neutrophil opsonophagocytosis. Infect Immun. 2008;76:1163–1169. doi: 10.1128/IAI.01116-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lukomski S, et al. Inactivation of Streptococcus pyogenes extracellular cysteine protease significantly decreases mouse lethality of serotype M3 and M49 strains. J Clin Invest. 1997;99:2574–2580. doi: 10.1172/JCI119445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Svensson MD, et al. Role for a secreted cysteine proteinase in the establishment of host tissue tropism by group A streptococci. Mol Microbiol. 2000;38:242–253. doi: 10.1046/j.1365-2958.2000.02144.x. [DOI] [PubMed] [Google Scholar]
- 22.Terao Y, et al. Group A streptococcal cysteine protease degrades C3 (C3b) and contributes to evasion of innate immunity. J Biol Chem. 2008;283:6253–6260. doi: 10.1074/jbc.M704821200. [DOI] [PubMed] [Google Scholar]
- 23.Wu SC, Ye R, Wu XC, Ng SC, Wong SL. Enhanced secretory production of a single-chain antibody fragment from Bacillus subtilis by coproduction of molecular chaperones. J Bacteriol. 1998;180:2830–2835. doi: 10.1128/jb.180.11.2830-2835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vitikainen M, et al. Quantitation of the capacity of the secretion apparatus and requirement for PrsA in growth and secretion of alpha-amylase in Bacillus subtilis. J Bacteriol. 2001;183:1881–1890. doi: 10.1128/JB.183.6.1881-1890.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vitikainen M, Hyyryläinen HL, Kivimäki A, Kontinen VP, Sarvas M. Secretion of heterologous proteins in Bacillus subtilis can be improved by engineering cell components affecting post-translocational protein folding and degradation. J Appl Microbiol. 2005;99:363–375. doi: 10.1111/j.1365-2672.2005.02572.x. [DOI] [PubMed] [Google Scholar]
- 26.Johansson L, et al. Cathelicidin LL-37 in severe Streptococcus pyogenes soft tissue infections in humans. Infect Immun. 2008;76:3399–3404. doi: 10.1128/IAI.01392-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olsen RJ, Shelburne SA, Musser JM. Molecular mechanisms underlying group A streptococcal pathogenesis. Cell Microbiol. 2009;11:1–12. doi: 10.1111/j.1462-5822.2008.01225.x. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy AD, et al. Epidemic community-associated methicillin-resistant Staphylococcus aureus: Recent clonal expansion and diversification. Proc Natl Acad Sci USA. 2008;105:1327–1332. doi: 10.1073/pnas.0710217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith EE, et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci USA. 2006;103:8487–8492. doi: 10.1073/pnas.0602138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.