Abstract
A unique phenomenon of mitochondria-targeted protonophores is described. It consists in a transmembrane H+-conducting fatty acid cycling mediated by penetrating cations such as 10-(6’-plastoquinonyl)decyltriphenylphosphonium (SkQ1) or dodecyltriphenylphosphonium (C12TPP). The phenomenon has been modeled by molecular dynamics and directly proved by experiments on bilayer planar phospholipid membrane, liposomes, isolated mitochondria, and yeast cells. In bilayer planar phospholipid membrane, the concerted action of penetrating cations and fatty acids is found to result in conversion of a pH gradient (ΔpH) to a membrane potential (Δψ) of the Nernstian value (about 60 mV Δψ at ΔpH = 1). A hydrophobic cation with localized charge (cetyltrimethylammonium) failed to substitute for hydrophobic cations with delocalized charge. In isolated mitochondria, SkQ1 and C12TPP, but not cetyltrimethylammonium, potentiated fatty acid-induced (i) uncoupling of respiration and phosphorylation, and (ii) inhibition of H2O2 formation. In intact yeast cells, C12TPP stimulated respiration regardless of the extracellular pH value, whereas a nontargeted protonophorous uncoupler (trifluoromethoxycarbonylcyanide phenylhydrazone) stimulated respiration at pH 5 but not at pH 3. Hydrophobic penetrating cations might be promising to treat obesity, senescence, and some kinds of cancer that require mitochondrial hyperpolarization.
Keywords: mild uncoupling, membrane, Mitochondria-targeted uncoupler, penetrating ion, antioxidant
Some decrease in mitochondrial membrane potential (Δψ) in the resting state may be favorable in treating obesity and hypothyroidism as well as in preventing senescence and certain types of cancer [for reviews, see refs. 1, 2]. In the first two cases, Δψ lowering stimulates respiratory metabolism. As to senescence and cancer, such an effect seems to be related to a decrease in production of reactive oxygen species (ROS) in mitochondria. ROS, in turn, were assumed to mediate senescence and some steps of cancerogenesis (2, 3). As was shown in our group (4), there is a very steep dependence of mitochondrial ROS formation on Δψ. Small (10–15%) lowering of Δψ resulted in ten-fold decrease in the ROS production rate (4). In isolated mitochondria, this can be achieved by adding a low concentration of a protonophorous uncoupler (4–6). This approach, called “mild uncoupling” (4, 6), was recently used by Padalko (7) and by Kowaltowski and coworkers (8) to prolong the lifespan of Drosophila and mice, respectively. However, long-term treatment of animals with uncouplers results in toxic side effects (9).
In this paper, we put forward an alternative approach based on the use of synthetic cations that easily penetrate through biological membranes. Penetrating ions were suggested by our group to reveal electric potential difference across mitochondrial membrane (9, 10). In tetraphenylphosphonium (TPP), a typical representative of such ions, the positive charge is strongly displaced over four phenyl residues. As a result, water dipoles cannot be held by the cation and, hence, do not form an aqueous shell preventing penetration of the ion through a membrane. The interior of mitochondria is the only intracellular compartment which is negatively charged relative to its environment (the cytosol) (9, 10). Therefore, on entering the cell, a penetrating cation will be specifically accumulated within mitochondria. This accumulation should be described by the Nernst equation. When mitochondrial Δψ is ∼180 mV, the penetrating cation concentration in the mitochondrial matrix is 1,000 times higher than in the cytosol (2, 9, 10).
With this consideration as the background, we assumed that penetrating cations could be used as “molecular electric locomotives” to target to mitochondria other substances combined with these cations (9, 11). Such a principle was successfully used by Murphy and coworkers for delivery into mitochondria of antioxidants, e.g., ubiquinone (12). The so-called MitoQ composed of ubiquinone and decyltriphenylphosphonium seemed to be promising as a rechargeable antioxidant regenerated by the respiratory chain. Murphy and his group showed that MitoQ was accumulated and reduced by mitochondria, protecting them and also cell cultures from oxidative stress (for review, see ref. 13).
We confirmed the data of Murphy and coworkers on the antioxidant activity of MitoQ but found that it turns to prooxidant one when the concentration was slightly increased (14) (see also refs. 15–17), thus making risky the use of MitoQ as an antioxidant. Therefore, we searched for mitochondria-targeted antioxidants with wider antioxidant concentration “window” than that of MitoQ. Cationic derivatives of plastoquinone (SkQs) were found to have the desired property (2, 14).
We synthesized a series of plastoquinone derivatives containing triphenylphosphonium (SkQ1) or rhodamine 19 (SkQR1) as cations. Moreover, an SkQ1 analog containing two methylene groups instead of plastoquinone (dodecyltriphenylphosphonium, C12TPP) was synthesized (14). (For formulas, see Supplemental Information, Fig. S1).
In studies with a fungus, a crustacean, a fly, a fish, and mice, SkQ1 was shown to increase median lifespan (2, 14). This effect was accompanied by retardation of the development of many age-related diseases and traits (2, 14, 18–21). When studying the molecular mechanism of the effects of SkQs, we usually employed C12TPP as the control compound. In a majority of the cases listed above, C12TPP failed to effectively replace SkQ. However, in certain systems, C12TPP was also active, e.g., in D. melanogaster (2, 21) and in some plant cells (A. B. Vartapetian et al., in preparation).
In this work, we studied a mechanism of biological action of SkQ1 and C12TPP and revealed that these compounds can operate as carriers of anions of fatty acids, facilitating fatty acid cycling in the membrane. This cycling can be accounted for by spontaneous influx of protonated-fatty acid to mitochondria and SkQ1- (or C12TPP-) mediated efflux of fatty acid anion in the opposite direction. In fact, our penetrating ions, interacting with fatty acids, operated as mitochondria-targeted protonophorous uncouplers.
Results
Hydrophobic penetrating cations mediate protonophorous activity of fatty acids in bilayer planar phospholipid membranes and liposomes.
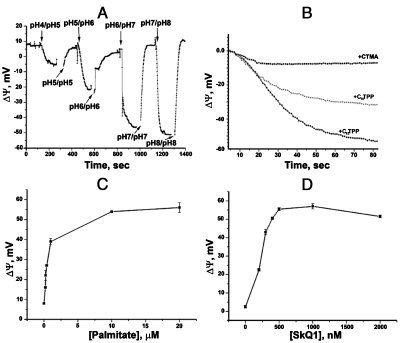
In the first series of experiments, we asked whether penetrating cations can mediate protonophorous activity of fatty acids without assistance of any proteins. To this end, palmitate and penetrating cations SkQ1 and C12TPP were tested in a model system, i.e., bilayer planar phospholipid membrane (BLM) separating two solutions differing in the pH values. In such a system, addition of protonophore (FCCP) resulted in a transmembrane H+ flux generating an electric potential difference across BLM, the more acidic compartment being negatively charged. It was found (Fig. 1) that palmitate and a penetrating cation added together can effectively substitute for FCCP. In these experiments, penetrating cations were added to incubation mixture. As to palmitate, it was added to phospholipid (A, B) or incubation mixture (C, D). As Fig. 1A shows, Δψ increases when pH values of the BLM-separated solutions are higher than the pK value of fatty acid. At pH 4 in one compertment and pH 5 in another compartment, Δψ was as small as 13 mV whereas at pH 6 vs.7 or 7 vs. 8 it reached the theoretical (Nernstian) value, i.e. 60 mV.
Fig. 1.
Penetrating cations mediate protonophorous effect of fatty acids in BLMs. (A,B) The BLM was formed from Ambersep-purified synthetic DPPC supplemented with 1 mg palmitate per 20 mg DPPC. (A) KOH was added to one compartment to create ΔpH and then to the other compartment to equalize the pH values in the two compartments; incubation mixture, 10 mM Tris, 10 mM MES, 10 mM KCl, and 1 µM SkQ1. (B) Comparison of effects of 1 µM C12TPP, 1 µM C10TPP, and 10 μM CTMA on Δψ generation induced by pH shift from pH 6 to 7; for incubation mixture, see (A). (C,D) Palmitate was added in the incubation medium on both sides of the BLM-separated compartments. (C) and (D), Δψ as a function of palmitate and SkQ1 concentrations in the incubation mixture, respectively; for incubation mixture and the pH shift, see Fig. S2E; in (C), 0.5 μM C12TPP was present. In (B), palmitate concentration was 20 μM.
Fig. 1B shows comparison of the Δψ-generating ability by three hydrophobic cations, i.e., C12TPP, decyltriphenylphosphonium (C10TPP), and cetyltrimethylammonium (CTMA). It is seen that C10TPP is less effective than the more hydrophobic C12TPP. As to CTMA, it was nearly ineffective even at concentration ten-fold higher than that of two other cations, most likely because the ionic charge in CTMA is localized, in contrast to the phosphonium derivatives where it is delocalized. Figs. 1C and D demonstrate Δψ dependence upon concentrations of palmitate and SkQ1 when palmitate was added to the incubation mixture.
Details of Δψ measurements in the BLM experiments are shown in Fig. S2A–E. In Fig. S2F, electric current through a BLM was measured. As a penetrating cation, SkQR1 (the rhodamine-containing analog of SkQ1) was employed since its penetrating ability is higher than that of SkQ1 (14, 22). It is seen that level of stationary current appearing when 100 mV Δψ was applied to the BLM was significantly increased if 20 µM palmitate was added to the incubation medium containing 0.5 µM SkQR1.
The above data clearly showed that palmitate added together with such hydrophobic penetrating cations as SkQ1 or C12TPP can mediate ΔpH → Δψ transduction in such a protein-free system as BLM [compare with potentiation of fatty-acid-induced uncoupling by hydrophilic tetraphenylphosphonium cation in mitochondria (23), which proved to be sensitive to carboxyatractylate, an inhibitor of the ATP/ADP antiporter (24)]. It was assumed that C12TPP (SkQ1) operates in BLM as carriers of fatty acid anions.
This assumption was confirmed by experiments on liposomes. It was shown that addition of SkQR1 caused a release of carboxyfluorescein (CF) anions from CF-loaded liposomes. Such an effect was absent when doxorubicin cation substituted for CF. Melittin, which induces unspecific increase in the liposome membrane ion conductance, was efficient with both CF- and doxorubicin-loaded liposomes (Fig. S2G). The effect of SkQR1 on CF-loaded liposomes was strongly inhibited by palmitate (Fig. S2H), which can be explained by the competition of the palmitate anions with the CF anions for the SkQR1 cation.
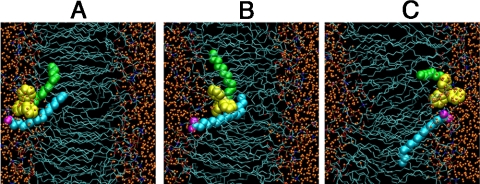
Fig. 2.
Permeation of C12TPP/palmitate ion pair through a POPC membrane (ABF molecular dynamics simulations). Shown are the lipid carbon tails (Thin Cyan Sticks), carbonyls and phosphates (Red and Brown Sticks), cholines (Blue Sticks), water molecules (Small Orange Spheres), C12TPP (phenyl rings, Yellow; dodecyl residue, Green Spheres), palmitate (carboxyl oxygen, Magenta Spheres; hydrocarbon tail, Cyan Spheres). (A) Equilibrium structure obtained after 25 ns of unperturbed MD simulation was used as the starting configuration for the ABF analysis. (B) Structure after 30 ns of ABF simulation (conformation with largest distance between ions). (C) Final structure after 110 ns ABF simulation.
Molecular Dynamics (MD) Modeling of Fatty Acid Anion Transport by Means of C12TPP.
In this part of the study, an MD approach was applied to simulate movement of the C12TPP/palmitate ion pair through a BLM. For the starting point, we placed C12TPP and palmitate ions near each other. After 25 ns equilibration, direct contact between the ions occurred (Fig. 2A).
When the adaptive biasing force (ABF) was applied to C12TPP, its charged headgroup penetrated into the lipid phase. The carboxyl group of palmitate followed the movement of C12TPP through the membrane core, usually remaining at a distance of 4–6 Å apart. In rare cases, however, the distance increased up to 8–10 Å (Fig. 2B; the whole trajectory for the TPP+-COO- distance during ABF simulation is shown in Fig. S3). When C12TPP reached the opposite side of the membrane, an inversion of the ion positions occurred (Fig. 2C). C12TPP/palmitate ion pair movement through the membrane is shown in Movie S1.
Effects of SkQ1 and C12TPP on Mitochondria In Vitro.
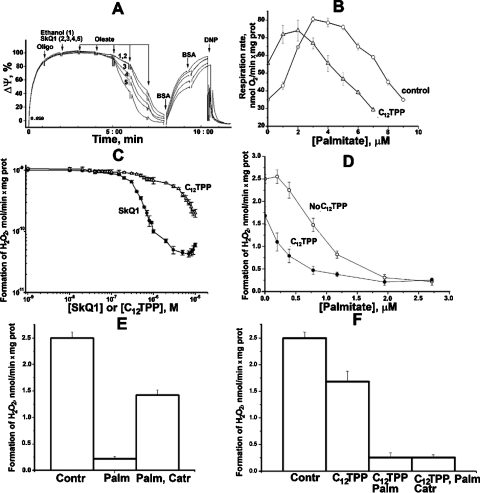
The next question was whether protonophorous effect of fatty acid/penetrating cation pair is realized in mitochondria in vitro and in vivo. As shown by Fig. 3A, SkQ1 potentiates uncoupling activity of a free-fatty acid (oleate) in isolated rat-liver mitochondria. In this experiment, we measured Δψ generated by mitochondria oxidizing succinate. It was found that the same oleate concentrations induced much larger Δψ decrease if 20–80 nM SkQ1 was present. Without oleate, these SkQ1 concentrations did not lower Δψ. The oleate effect was completely reversed when BSA was added to bind the fatty acid. Figs. S4A, B show similar relationships for rat-heart mitochondria. Palmitate was used as an uncoupler. The effect of palmitate on State 4 respiration is shown by Fig. 3B. It is seen that at low palmitate levels, C12TPP stimulates the oxygen consumption, confirming that in this case we deal with uncoupling of respiration and energy conservation. High palmitate concentrations initiated an inhibition of respiratory activity, which is typical for protonophorous uncouplers. Such an inhibition was also stimulated by C12TPP. Figs. 3C–F show the effects of SkQ1 and C12TPP on H2O2 production by rat-heart mitochondria under conditions of reverse electron transfer via complex I. It is shown (Fig. 3C, D and Fig. S5A) that the combination “palmitate + SkQ1(C12TPP)”, like other uncouplers, strongly inhibits generation of H2O2, SkQ1 being more efficient than C12TPP. Carboxyatractylate (Catr), an inhibitor of ATP/ADP antiporter, significantly decreased the effect of palmitate without SkQ1 or C12TPP but was without any influence if one of these cations was added (Figs. 3E and F). Without added palmitate, BSA strongly stimulated H2O2 production if high [SkQ1] was present (Fig. S5B).
Fig. 3.
Effect of SkQ1 and C12TPP on isolated rat -liver (A) and rat-heart (B–F) mitochondria. (A) SkQ1 potentiates an oleate-induced Δψ decrease in rat-liver mitochondria. At zero time, rat-liver mitochondria (0.7 mg protein/mL) were added. Other additions, oligomycin, 1 μg/mg protein; ethanol, 2.5 μL (curve 1); SkQ1 in 2.5 μL ethanol, final concentrations 20 nM (curve 2), 40 nM (curve 3), 60 nM (curve 4), or 80 nM (curve 5); 3 × 10-6 M oleate (each addition); BSA, 0.2 mg/mL (each addition); 4 × 10-5 M 1,4-dinitrophenol (DNP). Incubation mixture, 250 mM sucrose, 5 mM MOPS-KOH (pH 7.4), 1 mM EGTA, BSA (0.1 mg/mL), 4 mM pyruvate, and 1 × 10-5 M safranin O. (B) C12TPP enhances uncoupling action of palmitate on respiration of rat heart mitochondria. Incubation medium, 250 mM sucrose, 1 mM EGTA, 10 mM MOPS-KOH (pH 7.4), oligomycin (2 μg/mL), 2 μM rotenone, 5 mM succinate, rat heart mitochondria (0.15 mg protein/mL), and, where indicated, 2.5 μM C12TPP. (C–F) SkQ1 and C12TPP effects on H2O2 formation by rat-heart mitochondria. Incubation medium, 250 mM sucrose, 1 mM EGTA, 10 mM MOPS-KOH (pH 7.4), 5 mM succinate, 2 μM Amplex Red, horseradish peroxidase (9 units), and rat-heart mitochondria (0.15 mg protein/mL). In (D), where indicated, 1 μM C12TPP was added. In (E) and (F), additions were 2 μM palmitate, 1 μM C12TPP, and 1 μM Catr.
These data obtained on rat mitochondria were then confirmed when isolated yeast mitochondria were studied (Figs. S6A–H). In Figs. S6A–D, SkQ1 and palmitate were used. Other hydrophobic cations with delocalized charge (SkQ3, MitoQ, and C12TPP) could substitute for SkQ1 (Figs. S6E). This was not the case when a hydrophobic cation with localized charge (CTMA) was added. As to the palmitic acid, it could be replaced by oleic, linoleic, stearic, and decanoic acids. Peroxide of oleic acid as well as acetic acid were inactive.
Thus, one may conclude that protonophorous activity of the penetrating cation/fatty acid pair is inherent in isolated mitochondria in vitro.
Mitochondria-Targeted Uncoupler C12TPP, but Not Untargeted Protonophore FCCP, Stimulates Respiration of Yeast Cells in Acidic Media.
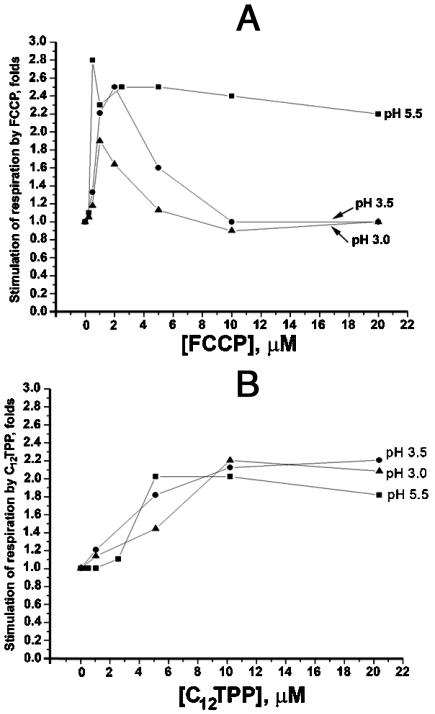
If an intact cell contains a certain level of nonesterified fatty acids, C12TPP should specifically increase H+ conductance of the mitochondrial membrane, other membranes being unaffected. This prediction was verified by experiments on intact S. cerevisiae cells at low pH of the growth medium. As shown in Fig. 4A, lowering of pH in the medium to 3.0 allowed FCCP to stimulate cell respiration only at low uncoupler concentrations. If the concentration was increased, the stimulation disappeared. However, both low and high concentrations of C12TPP caused strong increase in the respiration rate (Fig. 4B). These relationships might be accounted for assuming that high FCCP increased H+ conductance not only of mitochondrial but also of the plasma membrane. This should result in acidification of the cytosol if the pH outside the cell is low. Lowering of intracellular pH should, in turn, inhibit mitochondrial respiration as is seen in mitochondria in vitro (Figs. S6I, J). As to C12TPP, it electrophoretically accumulates in mitochondria and, hence, in cooperation with fatty acids, increases H+ conductance of the mitochondrial (but not of the plasma) membrane. If this is the case, inhibition of respiration at pHout = 3.0 could be achieved by adding a penetrating weak acid instead of the protonophore FCCP. As is seen in Fig. S7, acetate strongly inhibits respiration at pH 3.0 but not at pH 6.0. Collectively, these data demonstrate mitochondria-targeted uncoupling by C12TPP in the living yeast cell.
Fig. 4.
Effect of FCCP (A) and C12TPP (B) on respiration of S. cerevisiae cells at different extracellular pH values. Incubation mixture, 50 mM potassium phosphate, pH 5.5 or 3.5 or 3.0 (H3PO4 was used for acidification), and 0.005% glucose.
Discussion
The data presented in this paper have shown that concerted action of: (i) hydrophobic cations with delocalized charge, and (ii) fatty acids resulted in H+ conductance in model and natural membranes. As to the hydrophilic delocalized cation TPP, it was of much smaller effect. The hydrophobic cation CTMA containing localized charge was also without measurable effect (Fig. 1B). At acidic pH, the effect lowers due to a strong domination of the protonated over deprotonated state of fatty acids.
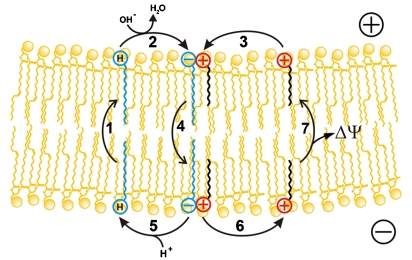
The relationships described above can be illustrated by the scheme shown by Fig. 5. According to this scheme, protonated-fatty acid moves across a membrane by a flip-flop mechanism, transporting H+ from the lower (more acid) compartment to the upper (more alkaline) compartment (stage 1). This stage is fast (27) and does not require any carrier. In the upper membrane/water interface, the fatty acid is deprotonated (stage 2) and the resulting fatty acid anion combines with C12TPP cation (stage 3). Then the C12TPP cation/fatty acid anion pair diffuses across the membrane to the opposite (lower) interface (stage 4). The cycle is completed by protonation of the fatty acid anion in the interface facing the lower (more acidic) compartment (stage 5), accompanied by release of free C12TPP cation (stage 6), which returns to the upper membrane surface (stage 7). It is stage 7 that is electrogenic, thus being responsible for Δψ generation (the upper compartment being positively charged).
Fig. 5.
A scheme illustrating mechanism of protonophorous fatty acid cycling mediated by penetrating cations. Symbols ⊖ and ⊕ represent anionic and cationic groups of fatty acid and C12TPP, respectively; represents protonated carboxyl group of fatty acid.
represents protonated carboxyl group of fatty acid.
The crucial stage 4 was modeled by molecular dynamics calculations (Fig. 2). The remarkable result of this analysis is that during the transmembrane journey of the C12TPP/palmitate ion pair the distance between the cation and anion moieties is not constant. Rather, it varies from 0.3–1.0 nm without irreversible decomposition of the pair. Such a phenomenon can be explained by very low dielectric constant in the hydrophobic-membrane core. This means that direct contact of the cation and anion is not necessary to organize translocation of a penetrating cation/nonpenetrating anion pair through a phospholipid membrane. Therefore, it seems possible that steric difficulties preventing ion pair formation in the aqueous phase can be insignificant in the hydrophobic region of a membrane. In fact, we deal here with a unique type of carrier which facilitates transmembrane movement of an anion by means of a long-distance interaction (like a man keeping a dog on a leash when walking).
Interaction of this type accounts for unspecificity of the cation to the chemical structure of the transported anion. As shown by Fig. S2G,H, SkQR1 is effective in transporting not only such carboxylate monanions as palmitate, and other fatty acids, but also carboxyfluorescein containing three anionic groups. Perhaps, certain biological effects of SkQ1 reproduced by C12TPP are due to facilitation of the transport of some hydrophobic anions rather than to an antioxidant effect. Mild uncoupling is a possibility to explain the situation when both SkQ1 and C12TPP appear to be operative. As was found by our group, mitochondrial ROS generation shows very steep dependence on Δψ (4). Thus, a slight Δψ decrease [“mild uncoupling” (6)] results in manyfold decrease in the rate of ROS formation by mitochondria. One might suggest that uncoupling by SkQ1 (or C12TPP)/fatty acid ion pair mimics the mild uncoupling activity of UCP, ATP/ADP-antiporter, or other mitochondrial anion carriers, also mediated by fatty acids (9).
It should be stressed that an effective mechanism of mild uncoupling must be organized in a way preventing its escalation to strong uncoupling when Δψ drops below the level critical for ATP synthesis. The mild uncoupling mechanism in its optimal version should only slightly decrease Δψ, preventing ROS formation but leaving the resting Δψ at the level still sufficient to support operation of H+-ATP-synthase. Such a requirement is fulfilled when concentrations of free-fatty acids or penetrating cations increase. This increase should result in lowering of Δψ, the driving force for accumulation of cations in mitochondria. Hence, a Δψ decrease lowers, say, the C12TPP concentration in mitochondria, which in turn prevents further Δψ decrease*. Thus, C12TPP and related compounds might be promising tools to prevent mitochondrial hyperpolarization which was assumed to be inherent in obesity, hypothyroidism, certain cases of cancer, etc. (9, 27–29), without a risk of such a dramatic side effect as inhibition of oxidative phosphorylation. Comparing SkQ1 and C12TPP as mild uncouplers, one can conclude that the latter looks like a better mediator of this effect. C12TPP, in contrast to SkQ1, lacks prooxidant activity even at its high concentrations [see ref. 14 and Fig. 3C].
An interesting observation was made when Catr was tested in palmitate-treated mitochondria. Without C12TPP, Catr prevented palmitate-induced decrease in H2O2 formation by mitochondria (Fig. 3E), thus confirming an original observation made by our group (30). Similar action of Catr was revealed when fatty acid uncoupling was potentiated by TPP (24). However, the effect of C12TPP was Catr resistant (Fig. 3F). Apparently, C12TPP, being much more hydrophobic than TPP, does not require assistance by ATP/ADP-antiporter to transport palmitate.
The final series of experiments described above directly showed an advantage of a mitochondria-targeted uncoupler (C12TPP) over a nontargeted one FCCP. It was found that low (2.5 - 5 × 10-7 M) FCCP stimulated the cell respiration at extracellular pH 5.5 as well as at pH 3.0. However, at higher FCCP the stimulation was observed only at pH 5.5 (Fig. 4A). This can be easily explained assuming that low FCCP concentrations slightly increased mitochondrial H+ conductance, which is originally very low (9), and such an increase appears to be sufficient to stimulate respiration. As to plasmalemma, initially having higher H+ conductance than mitochondrial membrane, such a low FCCP concentrations fail to significantly increase the conductance. As a result, the pH gradient between the incubation medium and cytosol remains unaffected by submicromolar FCCP concentrations and yeast can respire at extracellular pH 3.0 in spite of FCCP addition. At higher FCCP concentrations, the conductance is increased not only in mitochondrial membrane but also in plasmalemma, so cytosolic pH lowers when the outer pH is as low as 3.0. This results in inhibition of respiration. In contrast to FCCP, C12TPP accumulates only in mitochondria and even its excess fails to lower cytosolic pH at outer pH 3.0. Therefore, no inhibition of respiration is seen at high C12TPP concentrations (Figs. 4B and S8).
In conclusion, a class of small molecules is described that are, in cooperation with free-fatty acids, competent in increasing mitochondria-specific H+ conductance. These molecules are hydrophobic cations with delocalized charges.
Materials and Methods
BLM
BLM was formed from a 2% decane solution of the E. coli phospholipids or diphytanoylphosphatidylcholine (DPPC) on a 0.6 mm aperture in a Teflon septum separating the experimental chamber into two compartments of equal size. Electrical parameters were measured with two AgCl electrodes placed into the solutions on two sides of the BLM.
Molecular Dynamics.
Our simulation system comprised 60 molecules of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylcholine (POPC), 991 water molecules, and a single pair of C12TPP and palmitate (the total number of atoms in the system was 11, 133).
All MD and ab initio calculations were performed using the SKIF “Chebyshev” supercomputer at the Computation Center, Moscow State University (details in SI Text).
Isolation of Mitochondria.
Rat-heart and liver mitochondria were isolated as described elsewhere (14). Isolated mitochondria were suspended in medium containing 250 mM sucrose, 10 mM MOPS KOH (pH 7.4), 1 mM EGTA, and 0.1 or 0.3 % of defatted BSA (for heart or liver mitochondria, respectively).
The Cell or Mitochondrial Respiration.
The cell or mitochondrial respiration was measured using a standard polarographic technique with a Clark-type oxygen electrode. Incubation medium for yeast cells contained 50 mM KH2PO4 (pH 5.5, 3.5, or 3.0; H3PO4 was used for acidification of the media) and 0.005% glucose. Incubation medium for yeast mitochondria contained 0.6 M mannitol, 10 mM TRIS-HCl, 2 mM H3PO4 (pH 7.4, 6.8 or 6.0), 4 mM pyruvate, and 1 mM malate.
Measurement of Hydrogen Peroxide.
Amplex Red reagent (10-acetyl-3,7-dihydroxyphenoxazine, Invitrogen) was used to detect the release of H2O2 from mitochondria. Amplex Red oxidation was measured fluorometrically (excitation/emission maxima, 550/595 nm) with a Hitachi MPF4 spectrophotometer. Mitochondrial suspension was added to a 1 ml plastic cuvette to be diluted by isolation medium without BSA (see above) to protein concentration of 0.25 mg/mL. Then horseradish peroxidase (final content, 1 U/mL) and 4 μL 8 mM Amplex Red solution in dimethyl sulfoxide were added.
Mitochondrial Membrane Potential Measurement.
Safranin O was used as a membrane potential probe. The 555–523-nm light absorption ratio was measured with an Aminco DW-2000 spectrophotometer (dual-wavelength regime). The medium for measurement contained 250 mM sucrose, 10 mM MOPS KOH, pH 7.4, 0.1 mM EGTA, 5 mM succinate, 2 μM rotenone, and 15 μM safranin O. Mitochondrial protein concentration was 0.7 mg/mL.
Supplementary Material
Acknowledgments.
The authors are grateful to Professor V.A. Sadovnichii for attention to this study, to Drs. L.S. Khailova, S.S. Klishin, K.G. Lyamzaev, R.A. Simonyan, E.V. Sukhanova, V. N. Tashlitsky, and R.A. Zvyagilskaya for participation in certain experiments and N. S. Melik-Nubarov for useful advice. This work was supported by Mr. A.V. Chikunov and the Rostok Group, the Russian Foundation for Basic Research Grant 09-04-01238), and the Russian Ministry of Education and Science Grant 5762.2008.4 and goskontract N 02.740.11.0097.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910216107/DCSupplemental.
*The idea of a “self-limiting mitochondrial protonophore” was discussed by Blaikie at al. (26) who connected dinitrophenol and triphenylphosphonium with propyl linker. The compound was accumulated by mitochondria but did not uncouple (most probably due to impermeability of its zwitterionic form).
References
- 1.Skulachev VP. Uncoupling: New approaches to an old problem of bioenergetics. Biochim Biophys Acta. 1998;1363:100–124. doi: 10.1016/s0005-2728(97)00091-1. [DOI] [PubMed] [Google Scholar]
- 2.Skulachev VP, et al. An attempt to prevent senescence: A mitochondrial approach. Biochim Biophys Acta. 2009;1787:437–461. doi: 10.1016/j.bbabio.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Longo VD, Mitteldorf J, Skulachev VP. Programmed and altruistic ageing. Nat Rev Genet. 2005;6:866–872. doi: 10.1038/nrg1706. [DOI] [PubMed] [Google Scholar]
- 4.Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- 5.Skulachev VP, Sharaf AA, Liberman EA. Proton conductors in the respiratory chain and artificial membranes. Nature. 1967;216:718–719. doi: 10.1038/216718a0. [DOI] [PubMed] [Google Scholar]
- 6.Skulachev VP. Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Q Rev Biophys. 1996;29:169–202. doi: 10.1017/s0033583500005795. [DOI] [PubMed] [Google Scholar]
- 7.Padalko VI. Uncoupler of oxidative phosphorylation prolongs the lifespan of Drosophila. Biochemistry Moscow. 2005;70:986–989. doi: 10.1007/s10541-005-0213-1. [DOI] [PubMed] [Google Scholar]
- 8.Caldeira da Silva CC, Cerqueira FM, Barbosa LF, Medeiros MH, Kowaltowski AJ. Mild mitochondrial uncoupling in mice affects energy metabolism, redox balance and longevity. Aging Cell. 2008;7:552–560. doi: 10.1111/j.1474-9726.2008.00407.x. [DOI] [PubMed] [Google Scholar]
- 9.Skulachev VP. Membrane bioenergetics. Berlin: Springer-Verlag; 1988. p. 442. [Google Scholar]
- 10.Liberman EA, Topaly VP, Tsofina LM, Jasaitis AA, Skulachev VP. Mechanism of coupling of oxidative phosphorylation and the membrane potential of mitochondria. Nature. 1969;222:1076–1078. doi: 10.1038/2221076a0. [DOI] [PubMed] [Google Scholar]
- 11.Severin SE, Skulachev VP, Yaguzhinskiy LS. [Possible role of carnitine in the transport of fatty acids through the mitochondrial membrane] Biochemistry Moscow. 1970;35:1250–1253. Russian. [PubMed] [Google Scholar]
- 12.Kelso GF, et al. Selective targeting of a redox-active ubiquinone to mitochondria within cells: Antioxidant and antiapoptotic properties. J Biol Chem. 2001;276:4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- 13.Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 14.Antonenko YN, et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 1. Cationic plastoquinone derivatives: Synthesis and in vitro studies. Biochemistry Moscow. 2008;73:1273–1287. doi: 10.1134/s0006297908120018. [DOI] [PubMed] [Google Scholar]
- 15.James AM, Cocheme HM, Smith RA, Murphy MP. Interactions of mitochondria-targeted and untargeted ubiquinones with the mitochondrial respiratory chain and reactive oxygen species. Implications for the use of exogenous ubiquinones as therapies and experimental tools. J Biol Chem. 2005;280:21295–21312. doi: 10.1074/jbc.M501527200. [DOI] [PubMed] [Google Scholar]
- 16.O'Malley Y, Fink BD, Ross NC, Prisinzano TE, Sivitz WI. Reactive oxygen and targeted antioxidant administration in endothelial cell mitochondria. J Biol Chem. 2006;281:39766–39775. doi: 10.1074/jbc.M608268200. [DOI] [PubMed] [Google Scholar]
- 17.Doughan AK, Dikalov SI. Mitochondrial redox cycling of mitoquinone leads to superoxide production and cellular apoptosis. Antioxid Redox Signaling. 2007;9:1825–1836. doi: 10.1089/ars.2007.1693. [DOI] [PubMed] [Google Scholar]
- 18.Bakeeva LE, et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 2. Treatment of some ROS- and age-related diseases (heart arrhythmia, heart infarctions, kidney ischemia, and stroke) Biochemistry Moscow. 2008;73:1288–1299. doi: 10.1134/s000629790812002x. [DOI] [PubMed] [Google Scholar]
- 19.Agapova LS, et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 3. Inhibitory effect of SkQ1 on tumor development from p53-deficient cells. Biochemistry Moscow. 2008;73:1300–1316. doi: 10.1134/s0006297908120031. [DOI] [PubMed] [Google Scholar]
- 20.Neroev VV, et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 4. Age-related eye disease. SkQ1 returns vision to blind animals. Biochemistry Moscow. 2008;73:1317–1328. doi: 10.1134/s0006297908120043. [DOI] [PubMed] [Google Scholar]
- 21.Anisimov VN, et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 5. SkQ1 prolongs lifespan and prevents development of traits of senescence. Biochemistry Moscow. 2008;73:1329–1342. doi: 10.1134/s0006297908120055. [DOI] [PubMed] [Google Scholar]
- 22.Rokitskaya TI, Klishin SS, Severina, Skulachev VP, Antonenko YN. Kinetic analysis of permeation of mitochondria-targeted antioxidants across bilayer lipid membranes. J Membr Biol. 2008;224:9–19. doi: 10.1007/s00232-008-9124-6. [DOI] [PubMed] [Google Scholar]
- 23.Schőnfeld P. Anion permeation limits the uncoupling activity of fatty acids in mitochondria. FEBS Lett. 1992;303:190–192. doi: 10.1016/0014-5793(92)80516-j. [DOI] [PubMed] [Google Scholar]
- 24.Dedukhova VI, Mokhova EN, Starkov AA, Leikin Yu N. Carboxyatractylate inhibits the potentiating effect of lipophylic cation TPP+ on uncoupling activity of fatty acid. Biochem Mol Biol Int. 1993;30:1161–1167. [PubMed] [Google Scholar]
- 25.Simard JR, Pillai BK, Hamilton JA. Fatty acid flip-flop in a model membrane is faster than desorption into the aqueous phase. Biochemistry. 2008;47:9081–9089. doi: 10.1021/bi800697q. [DOI] [PubMed] [Google Scholar]
- 26.Blaikie FH, et al. Targeting dinitrophenol to mitochondria: Limitations to the development of a self-limiting mitochondrial protonophore. Biosci Rep. 2006;26:231–243. doi: 10.1007/s10540-006-9018-8. [DOI] [PubMed] [Google Scholar]
- 27.Horvath TL, et al. Uncoupling proteins-2 and 3 influence obesity and inflammation in transgenic mice. Int J Obesity Related Metabolism Disorders. 2003;27:433–442. doi: 10.1038/sj.ijo.0802257. [DOI] [PubMed] [Google Scholar]
- 28.Nogueira V, et al. Thyroid status is a key regulator of both flux and efficiency of oxidative phosphorylation in rat hepatocytes. J Bioenerg Biomembr. 2002;34:55–66. doi: 10.1023/a:1013822820840. [DOI] [PubMed] [Google Scholar]
- 29.Lanni A, et al. 3,5-diiodo-L-thyronine powerfully reduces adiposity in rats by increasing the burning of fats. FASEB J. 2005;19:1552–1554. doi: 10.1096/fj.05-3977fje. [DOI] [PubMed] [Google Scholar]
- 30.Andreyev A, et al. The ATP/ADP-antiporter is involved in the uncoupling effect of fatty acids on mitochondria. Eur J Biochem. 1989;182:585–592. doi: 10.1111/j.1432-1033.1989.tb14867.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.