Abstract
The DNA mismatch repair system (MMR) identifies replication errors and damaged bases in DNA and functions to preserve genomic integrity. MutS performs the task of locating mismatched base pairs, loops and lesions and initiating MMR, and the fundamental question of how this protein targets specific sites in DNA is unresolved. To address this question, we examined the interactions between Saccharomyces cerevisiae Msh2-Msh6, a eukaryotic MutS homolog, and DNA in real time. The reaction kinetics reveal that Msh2-Msh6 binds a variety of sites at similarly fast rates (kON ∼ 107 M-1 s-1), and its selectivity manifests in differential dissociation rates; e.g., the protein releases a 2-Aminopurine:T base pair approximately 90-fold faster than a G:T mismatch. On releasing the 2-Ap:T site, Msh2-Msh6 is able to move laterally on DNA to locate a nearby G:T site. The long-lived Msh2-Msh6·G:T complex triggers the next step in MMR—formation of an ATP-bound clamp—more effectively than the short-lived Msh2-Msh6·2-Ap:T complex. Mutation of Glu in the conserved Phe-X-Glu DNA binding motif stabilizes Msh2-Msh6E339A·2-Ap:T complex, and the mutant can signal 2-Ap:T repair as effectively as wild-type Msh2-Msh6 signals G:T repair. These findings suggest a targeting mechanism whereby Msh2-Msh6 scans DNA, interrogating base pairs by transient contacts and pausing at potential target sites, and the longer the pause the greater the likelihood of MMR.
Keywords: ATPase activity, pre-steady-state kinetics
DNA mismatch repair (MMR) is responsible for resolving various base pair mismatches and insertion/deletion loops (IDL) that arise in DNA due to replication and recombination errors (1, 2). The MMR protein MutS initiates repair by locating an error, which leads to MutL nicking the DNA strand in its vicinity (3); in some prokaryotes, such as Escherichia coli, MutL induces a third protein, MutH, to nick DNA. Subsequently, the nicked strand is excised and the replication machinery resynthesizes DNA to complete repair. MMR is also implicated in signaling cellular responses to DNA damage. MutS initiates this process by locating lesions in DNA, but the mechanism by which lesion recognition triggers cell cycle checkpoints and apoptosis is not yet resolved (4, 5).
The core MMR proteins have been conserved through evolution, and eukaryotes possess several MutS (Msh) and MutL (Mlh/Pms) homologs that process errors and lesions in DNA. Not surprisingly, defects in their function cause high mutator phenotypes and genomic instability; in humans, hundreds of hMSH2, hMSH6, hMLH1, and hPMS2 variants have been linked to hereditary nonpolyposis colon cancer and sporadic cancers (6) (http://www.insight-group.org).
One of the more intriguing questions regarding MMR centers on the mechanism by which MutS distinguishes the occasional mismatch, IDL, or lesion from excess Watson–Crick base pairs in DNA. This crucial, initial step in MMR requires that MutS employ efficient strategies to interrogate base pairs and recognize a broad spectrum of discrepancies in the structure and/or dynamics of the double helix. A breakthrough in our understanding of this process occurred when crystal structures of E. coli and Thermus aquaticus MutS homodimers (7, 8) and the human Msh2-Msh6 heterodimer (9) bound to different errors/lesions were solved. All structures show the protein encircled around DNA, making sequence-independent hydrogen bonding and van der Waals contacts in the vicinity of the mismatch/IDL/lesion as well as direct contacts with the base in question mainly via a conserved Phe-X-Glu motif from one subunit (Msh6 in eukaryotes). The DNA is kinked toward the major groove (45–60°), with Phe stacked against the base and Glu hydrogen bonded to it. The striking similarities among these structures suggest that the kinked DNA complex is a significant species in the pathway leading to initiation of MMR. However, since no structure of MutS/Msh2-Msh6 bound to fully matched DNA has been reported, the mechanism by which these proteins interact with DNA and recognize their target sites is unclear.
This question was tackled recently by a single-molecule study in which Saccharomyces cerevisiae Msh2-Msh6 movement on λ DNA was visualized by total internal reflection microscopy (10). The data indicated that Msh2-Msh6 can slide on the DNA helical axis by 1D diffusion (estimated diffusion coefficient = 0.25 μm2 s-1). It was proposed that such lateral movement could enable Msh2-Msh6 to scan the duplex, perhaps in contact with the replisome (e.g., bound to proliferating cell nuclear antigen), and that sites with higher local flexibility or altered structure could trap the protein and form specific recognition complexes. This is an intriguing model of Msh2-Msh6 actions during the search phase of MMR that could account for its ability to rapidly locate non-Watson–Crick sites in DNA and signal an appropriate response. Thus far, however, there is not enough evidence at base pair–level resolution to address essential features of the model. As noted earlier, there is no structure of MutS/Msh2-Msh6 bound to fully matched DNA. Also, the single-molecule data were at low resolution (approximately 300 base pairs), and the λ DNA was not engineered with MMR target sites (10); therefore, critical information on nonspecific interactions between Msh2-Msh6 and DNA and the transition to specific Msh2-Msh6·DNA recognition complexes remains unknown.
In order to measure transient Msh2-Msh6-DNA interactions at higher resolution, both with respect to the location and timing of binding events, we developed stopped-flow assays with an on-site fluorescence reporter to detect Msh2-Msh6 arrival at and departure from particular sites on DNA. Comparison of the kinetic parameters reveals striking differences in Msh2-Msh6 interactions with various sites, and illuminates the process by which this protein targets mismatched base pairs and other defects in DNA and signals MMR.
Results
Msh2-Msh6·DNA Complex Stability Underlies MMR Signaling Efficacy.
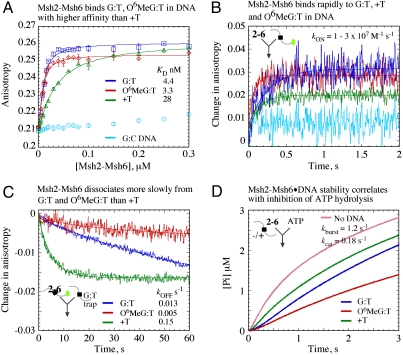
S. cerevisiae Msh2-Msh6 binding to MMR target sites was measured in solution by fluorescence anisotropy of a 37-bp duplex DNA with a mismatch (G:T), a mismatch plus lesion (O6Methylguanine:T), or a base insertion (+T) in the center and a TAMRA label [5-(6)-carboxytetramethylrhodamine] at one end. According to equilibrium binding data shown in Fig. 1A, anisotropy of G:TTAMRA, O6MeG:TTAMRA and +TTAMRA DNA increases with Msh2-Msh6 and yields KD = 4.4, 3.3, and 28 nM, respectively, for the interaction; in contrast, there is barely any change in signal with fully matched G:CTAMRA DNA. The data are consistent with the reported high selectivity of Msh2-Msh6 for mismatches/IDLs/lesions over matched DNA (11–14), and indicate preference for a mismatched base pair (-/+ lesion) over a base insertion.
Fig. 1.
Msh2-Msh6 binds MMR targets rapidly and with varying affinities. (A) Equilibrium fluorescence anisotropy measurements with TAMRA (green dot in scheme) end-labeled DNAs yield KD = 4.4 ± 0.5, 3.3 ± 0.5, and 28 ± 1.6 nM for G:TTAMRA (dark blue), O6MeG:TTAMRA (red), and +TTAMRA (green), respectively, and almost no binding to G:CTAMRA (light blue). (B) Kinetic measurements after mixing Msh2-Msh6 with DNA yield kON = 0.95, 3, and 1.7 × 107 M-1 s-1, respectively, for the three DNAs, and no detectable binding to G:CTAMRA. (C) Mixing Msh2-Msh6·DNATAMRA complexes with unlabeled G:T trap yields kOFF = 0.013, 0.005, and 0.15 s-1, respectively. (D) Free Msh2-Msh6 catalyzes a burst of ATP hydrolysis and Pi release (kburst = 1.2 s-1, kcat = 0.18 s-1) and the DNAs inhibit ATP hydrolysis to varying degrees.
Msh2-Msh6 binding to DNA was also monitored in real time by mixing the two reactants in a stopped-flow instrument. Increase in fluorescence anisotropy reflected rapid association of Msh2-Msh6 with G:TTAMRA, O6MeG:TTAMRA, and +TTAMRA DNA, and the data fit to a single exponential function yielded apparent binding rates (kobserved = 3.8, 12, and 7.1 s-1, respectively); there was no detectable interaction with G:CTAMRA DNA (Fig. 1B). In complementary experiments, DNA dissociation was measured by mixing preformed Msh2-Msh6·DNA complexes with excess unlabeled G:T DNA trap and monitoring decrease in anisotropy over time. The data fit to a single exponential yielded kOFF = 0.013, 0.005, and 0.15 s-1 for G:TTAMRA, O6MeG:TTAMRA, and +TTAMRA DNA, respectively (Fig. 1C), indicating significant differences in the stabilities of these complexes (t1/2 = 53, 138, and 4.6 s). Assuming that the kinetics reflect one-step reversible interaction between Msh2-Msh6 and DNA, we calculated bimolecular binding constants, kON = 1, 3 and 1.7 × 107 M-1 s-1 for G:TTAMRA, O6MeG:TTAMRA, and +TTAMRA DNA, respectively (kobserved = kON[Msh2-Msh6] + kOFF). Thus, the transient kinetic data reveal that Msh2-Msh6 binds at similarly rapid rates to DNAs containing a mismatch (-/+ lesion) or base insertion but dissociates at different rates, resulting in complexes with long but varied lifetimes. In contrast, Msh2-Msh6 binding to fully matched DNA is not detectable, likely because of fast dissociation (Msh2-Msh6 would diffuse over 37 bp within 0.6 ms based on a 0.25 μm2 s-1 diffusion coefficient) (10, 15). It should be noted that our in-solution method yields rate constants that are an order of magnitude faster than those obtained from surface-based methods such as surface plasmon resonance, despite the use of similar-sized DNAs (16, 17); variables such as DNA surface density and mass transport effects may underlie apparently slow kinetics in the latter case (18).
We have shown previously that in the absence of DNA one subunit of the MutS/Msh2-Msh6 dimer catalyzes rapid ATP hydrolysis and phosphate (Pi) release, then a slow step in the reaction limits steady-state turnover (i.e., Msh2-Msh6 exists predominantly in an ADP-bound state). On binding a mismatch/IDL, ATP hydrolysis is suppressed, and Msh2-Msh6 persists in an ATP-bound state (19–21). Past reports from several research groups indicate that ATP-bound Msh2-Msh6 is responsible for signaling MMR (14, 16, 22, 23). Given the above data on Msh2-Msh6 DNA-binding kinetics, we asked whether variation in Msh2-Msh6·DNA complex lifetimes has any impact on ATPase kinetics. Fig. 1D shows Msh2-Msh6 catalyzed Pi release in the absence and presence of target DNAs [measured by change in fluorescence of 7-diethylamino-3-((((2-maleimidyl)ethyl)amino)carbonyl) coumarin)-labeled phosphate binding protein (PBP) on binding Pi] (20, 24). Free Msh2-Msh6 catalyzes a burst of ATP hydrolysis at 1.2 s-1, and the subsequent turnover rate is 0.18 s-1 (burst amplitude = 1.9 μM or 1 site per dimer). G:T, O6MeG:T, and +T all suppress ATP hydrolysis but with differing efficiencies: O6MeG:T > G:T > +T (Fig. 1D). These data indicate positive correlation between Msh2-Msh6·DNA stability and the next step in MMR: formation of a ternary Msh2-Msh6·ATP·DNA complex. Analysis of larger IDLs, which are poor Msh2-Msh6 targets (25), supports this hypothesis, as +2T and +3T-bound complexes exhibit shorter lifetimes (kOFF = 0.4 and 2 s-1) and correspondingly poor suppression of ATP hydrolysis (Fig. S1).
2-Aminopurine Serves as an On-Site Reporter of Msh2-Msh6 Interactions with Target Sites.
Fluorescence anisotropy measurements with end-labeled DNA provide a generic report of Msh2-Msh6 binding, but do not reveal its specific location on DNA. In order to track Msh2-Msh6 more precisely, we used a 2-Aminopurine:Thymine base pair (2-Ap:T) as an on-site reporter of the interaction. 2-Ap is a fluorescent adenosine analogue that pairs with T in Watson–Crick geometry (26). 2-Ap:T has slightly lower stability (approximately 0.5 kcal mol-1) (27) and, from base pair opening kinetics, slightly shorter lifetime (approximately 6-fold) (28) than A:T. Many proteins, including DNA polymerase, substitute 2-Ap:T for A:T, although the efficiency may vary (e.g., T4 polymerase Km is 25 μM for 2-Ap and 5 μM for A) (29). Since 2-Ap fluorescence is exquisitely sensitive to local environment and is quenched in duplex DNA (30), we positioned 2-Ap:T adjacent to G:T or G:C, anticipating that it would report Msh2-Msh6 binding to the site (24, 31).
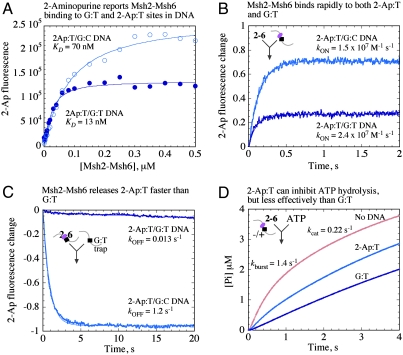
Equilibrium binding experiments show fluorescence intensity of G:T2-Ap DNA increasing with Msh2-Msh6, and the isotherm yields a KD of 13 nM (Fig. 2A), comparable with G:TTAMRA DNA (Fig. 1A). Interestingly, we also observed Msh2-Msh6 binding to the 2-Ap:T site in G:C2-Ap DNA, albeit with much lower affinity (Fig. 2A; KD = 70 nM). Because we did not detect any Msh2-Msh6 binding to fully matched G:CTAMRA (Fig. 1A, 1B), it appears that the protein can distinguish 2-Ap:T from a Watson–Crick base pair, but not as effectively as G:T.
Fig. 2.
An on-site 2-Ap reporter confirms that Msh2-Msh6 dissociation rates underlie target site selectivity. (A) Equilibrium 2-Ap fluorescence measurements yield KD = 13 ± 2 nM for G:T2-Ap (dark blue) and 70 ± 7 nM for G:C2-Ap DNA (light blue); 2-Ap:T (purple dot) is located next to G:T or G:C, respectively. (B) Kinetic measurements yield kON = 2.4 and 1.5 × 107 M-1 s-1 and (C) kOFF = 0.013 and 1.2 s-1 for G:T2-Ap and G:C2-Ap, respectively. (D) ATP hydrolysis is partially suppressed by G:C2-Ap DNA.
Binding kinetics, measured by monitoring increase in 2-Ap fluorescence over time after mixing Msh2-Msh6 and DNA, yielded similar association rates for G:T2-Ap and G:C2-Ap DNAs (Fig. 2B; kON ∼ 2 × 107 M-1 s-1). The dissociation rates, however, are strikingly different. Msh2-Msh6 releases the mismatch in G:T2-Ap slowly (Fig. 2C; kOFF = 0.013 s-1; t1/2 = 53 s), consistent with G:TTAMRA anisotropy data (Fig. 1C); +T2-Ap DNA, which contains a T insertion adjacent to 2-Ap:T, yields similar results as +TTAMRA as well (Fig. 1 and Fig. S2D). In contrast, Msh2-Msh6 release from 2-Ap:T in G:C2-Ap occurs approximately 90-fold faster (Fig. 2C; kOFF = 1.2 s-1; t1/2 = 0.6 s). Thus, the on-site reporter confirms that differences in Msh2-Msh6 affinity for various sites in DNA result, apparently exclusively, from differences in Msh2-Msh6 dissociation kinetics. Moreover, partial inhibition of Msh2-Msh6 catalyzed ATP hydrolysis by G:C2-Ap DNA affirms that Msh2-Msh6·DNA complex stability correlates with formation of the ATP-bound ternary complex (Fig. 2D).
Msh2-Msh6 Moves on DNA in Search of MMR Target Sites.
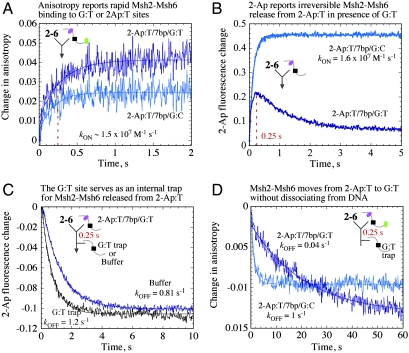
Since 2-Aminopurine is an effective on-site reporter for Msh2-Msh6 DNA binding/release, we could use it to ask how the protein locates particular sites on DNA. A DNA substrate was designed in which a 2-Ap:T base pair and G:T mismatch were positioned 7 bp apart, so as to allow binding of only one Msh2-Msh6 per DNA, to either site (2-Ap:T/7 bp/G:TTAMRA); human Msh2-Msh6 contacts are detected 6 and 4 bp from G:T in the crystal structure (9). The substrate was also end-labeled with TAMRA, in order to detect Msh2-Msh6 binding by both fluorescence anisotropy (generic signal) and 2-Ap fluorescence (site-specific signal) and monitor transient interactions that facilitate selection of one site over the other.
Kinetic anisotropy measurements show that Msh2-Msh6 binds both 2-Ap:T/7 bp/G:TTAMRA DNA and the control DNA with no G:T (2-Ap:T/7 bp/G:CTAMRA), at similar rapid rates (Fig. 3A; kON ∼ 1.5 × 107 M-1 s-1), consistent with previous data (Figs. 1B and 2B). Lower amplitude of the 2-Ap:T/7 bp/G:CTAMRA trace is due to relatively rapid dissociation of Msh2-Msh6 from 2-Ap:T (Fig. 2C), resulting in less Msh2-Msh6·DNA complex at equilibrium. 2-Ap fluorescence measurements also show Msh2-Msh6 binding 2-Ap:T/7 bp/G:CTAMRA with apparent kON = 1.6 × 107 M-1 s-1 (Fig. 3B). However, in case of the G:T mismatch-containing 2-Ap:T/7 bp/G:TTAMRA, 2-Ap fluorescence increases rapidly and then decreases nearly to the baseline (Fig. 3B). One possible explanation for the biphasic kinetics is that any Msh2-Msh6 bound to the 2-Ap:T site releases it rapidly (Fig. 2C; t1/2 = 0.6 s), and the G:T site on the DNA serves as an endogenous trap for the protein (Fig. 2C; t1/2 = 53 s), preventing its return to 2-Ap:T.
Fig. 3.
Msh2-Msh6 moves from 2-Ap:T to G:T without dissociating from DNA. (A) Fluorescence anisotropy shows Msh2-Msh6 binding mismatched 2-Ap:T/7 bp/G:TTAMRA and matched 2-Ap:T/7 bp/G:CTAMRA DNA rapidly (kON = 1.35 and 1.4 × 107 M-1 s-1, respectively). (B) 2-Ap fluorescence yields the same rate for 2-Ap:T/7 bp/G:CTAMRA, but 2-Ap:T/7 bp/G:TTAMRA shows biphasic kinetics. (C) 2-Ap fluorescence reports rapid release of Msh2-Msh6 from 2-Ap:T in 2-Ap:T/7 bp/G:TTAMRA with an external trap (unlabeled G:T) or with only buffer added after 0.25 s (kOFF = 0.8–1.2 s-1). (D) Corresponding TAMRA anisotropy shows slow release of Msh2-Msh6 from 2-Ap:T/7 bp/G:TTAMRA (kOFF = 0.044 s-1).
To test this hypothesis, we designed a sequential mixing experiment that would monitor 2-Ap:T-bound Msh2-Msh6 over time. The protein was initially mixed with 2-Ap:T/7 bp/G:TTAMRA for 250 ms (peak fraction of Msh2-Msh6 bound to 2-Ap:T; Fig. 3B), followed by addition of excess unlabeled G:T DNA as an exogenous trap for free Msh2-Msh6. A similar experiment was performed in which only buffer was added to the reaction after 250 ms, in order to test whether the G:T site on 2-Ap:T/7 bp/G:TTAMRA serves as a comparable endogenous trap. Data collected after the second mixing step show rapid decrease in 2-Ap fluorescence as Msh2-Msh6 releases 2-Ap:T and is trapped by G:T. The kinetics are similar whether G:T is present in cis (2-Ap:T/7 bp/G:TTAMRA) or in trans (excess unlabeled G:T) (Fig. 3C; kOFF = 0.8–1.2 s-1); in the absence of any G:T, Msh2-Msh6 rebinds 2-Ap:T, and fluorescence increases (Fig. 2B. These results confirm the hypothesis that after leaving 2-Ap:T, Msh2-Ms6 can be trapped by a G:T site present on the same DNA.
We could now query the mode of Msh2-Msh6 transfer from 2-Ap:T to G:T. The sequential mixing experiment was performed again by incubating Msh2-Msh6 with 2-Ap:T/7 bp/G:TTAMRA for 250 ms followed by addition of the G:T trap, except this time the interaction was monitored by fluorescence anisotropy. According to these data, most of the bound Msh2-Msh6 dissociates at a slow rate from 2-Ap:T/7 bp/G:TTAMRA DNA (Fig. 3D; kOFF = 0.04 s-1); without the trap, 2-Ap:T/7 bp/G:TTAMRA anisotropy increases as Msh2-Msh6 continues binding G:T until equilibrium is reached (Fig. 1B). An analogous experiment performed with 2-Ap:T/7 bp/G:CTAMRA confirms rapid Msh2-Msh6 dissociation in the absence of an internal G:T site (Fig. 3D; kOFF = 1 s-1). Thus, we can conclude that on releasing 2Ap:T (at approximately 1 s-1), Msh2-Msh6 does not dissociate from DNA; rather, it moves laterally on the duplex and is trapped by the G:T mismatch, and then dissociates slowly as expected for Msh2-Msh6·G:T complex (Fig. 1C). These results provide direct evidence for Msh2-Msh6 movement on DNA en route to formation of a stable complex with an MMR target site.
Effects of Nucleotide Cofactors on Msh2-Msh6-DNA Interactions During the Search Phase of MMR.
As noted earlier, MutS/Msh2-Msh6 ATPase kinetics suggest that the proteins exist predominantly in ADP-bound state when not bound to a mismatch/IDL (19, 20, 32); therefore, we examined Msh2-Msh6 interactions with DNA in the presence of ADP. Under these conditions, Msh2-Msh6 binds at kON ∼ 3 × 107 M-1 s-1 and dissociates at kOFF = 0.04, 0.35, and 1.5 s-1 from G:T, +T and 2-Ap:T, respectively (Table 1; Fig. S2). Thus, both nucleotide-free and ADP-bound Msh2-Msh6 exhibit similar DNA binding kinetics. Previous studies have also shown that ATP binding weakens MutS/Msh2-Msh6 interactions with DNA (32), and it has been proposed that differential ATP-induced loss of affinity for matched versus mismatched DNA yields net increase in MutS/Msh2-Msh6 specificity (33). We find that preincubation of Msh2-Msh6 with ATPγS results in no detectable binding to either DNA (Fig. S2). Mixing Msh2-Msh6·DNA with ATP and G:T trap leads to faster Msh2-Msh6 dissociation from G:T (kOFF = 0.4 s-1) but not +T (kOFF = 0.5 s-1) or 2-Ap:T (kOFF = 2 s-1) (Table 1; Fig. S2). These data do not support the above hypothesis, in that ATP-bound Msh2-Msh6 does not have higher affinity for G:T over 2-Ap:T. It remains possible that ATP binding has a more subtle effect on Msh2-Msh6; for instance, switching of ATP-bound Msh2-Msh6 to a conformation that slides away from a mismatch/IDL is considered a key step in the reaction (34, 35). Previous studies indicate that this switch may be specific to Msh2-Msh6·ATP·DNA complex formed at a mismatch (12, 35). The finding that ATP-bound Msh2-Msh6 releases G:T and +T sites at similar rates (approximately 0.5 s-1) raises the possibility of a common Msh2-Msh6 conformation triggered by MMR target sites.
Table 1.
Kinetic parameters for Msh2-Msh6-DNA interactions
| Protein (nucleotide) | kON( × 107 M-1 s-1) | kOFF(s-1) | KD, nM | ||||||
| G:T | +T | 2-Ap:T | G:T | +T | 2-Ap:T | G:T | +T | 2-Ap:T | |
| Msh2-6 (none) | 1 | 1.7 | 1.5 | 0.013 | 0.15 | 1.2 | 1.3 | 8.8 | 80 |
| Msh2-6 (ADP) | 3 | 3.7 | 3 | 0.04 | 0.35 | 1.5 | 1.3 | 9.4 | 50 |
| Msh2-6 (ATP/ATPγS) | 0.4 | 0.5 | 2 | ||||||
| Msh2-6E339A (none) | 5.7 | 2 | 6.8 | 0.06 | 0.13 | 0.14 | 1 | 6.5 | 2 |
Impact of Msh6 Glu339 on Recognition of MMR Target Sites.
Glutamate in the conserved Phe-X-Glu DNA binding motif of MutS/Msh2-Msh6 proteins hydrogen bonds with the mispaired or inserted base in DNA (8, 9, 36). We assessed the contribution of S. cerevisiae Msh6 Glu339 to mismatch recognition by examining an alanine mutant, Msh2-Msh6E339A. The mutant binds G:T and 2-Ap:T at slightly faster rates than wild-type Msh2-Msh6 (kON ∼ 6 × 107 M-1 s-1). More significant differences manifest during DNA dissociation as Msh2-Msh6E339A releases 2-Ap:T at approximately 7-fold slower rate (kOFF = 0.14 s-1) and G:T at approximately 4-fold faster rate (kOFF = 0.06 s-1) than Msh2-Msh6 (Table 1; Fig. S3); +T binding kinetics appear to be unaffected. The ATPase analysis shows that free Msh2-Msh6E339A catalyzes a burst of ATP hydrolysis at 1.4 s-1 and kcat 0.25 s-1 like the wild-type Msh2-Msh6; however, in case of the mutant, 2-Ap:T binding inhibits ATP hydrolysis as strongly as G:T (Fig. S3D), consistent with the longer lifetime of Msh2-Msh6E339A·2-Ap:T complex. These data indicate that Msh6 Glu339 alters Msh2-Msh6·DNA complex dissociation rates and thereby influences selection of MMR target sites in DNA.
Discussion
MutS proteins perform the essential task of locating replication errors and damage lesions in DNA for MMR (1, 2). In this study, we tackled the question of how S. cerevisiae Msh2-Msh6 recognizes target sites in DNA and signals initiation of MMR. In-solution kinetic analysis of Msh2-Msh6-DNA interactions indicates that the protein binds different sites on DNA rapidly and forms complexes of varying stability. All DNAs are bound at similar rates (1–4 × 107 M-1 s-1), but the dissociation rates vary over a hundredfold such that G:T, O6MeG:T form the most stable and 2-Ap:T, +3T form the least stable complexes with Msh2-Msh6. Both G:T and O6MeG:T are well-known targets of Msh2-Msh6 (4, 5). In case of IDLs, there is functional overlap between Msh2-Msh6, which processes short loops, and Msh2-Msh3, which processes short and long loops in DNA (25). Lastly, 2-Ap:T is similar enough to A:T to serve as a mimic for DNA metabolic enzymes (27–29). Formation of long-lived Msh2-Msh6 complexes with strong MMR targets (G:T, O6MeG:T) and short-lived complexes with weak MMR targets (+3T, 2-Ap:T) suggests that stability of interaction is a defining feature of sites that trigger MMR. The kinetic data also revealed that on releasing a weak site (2-Ap:T), Msh2-Msh6 can move on DNA to locate a strong site (G:T). Based on the above findings, we hypothesized that as Msh2-Msh6 scans DNA for discrepancies, it pauses at potential for MMR, and a longer pause increases the likelihood of signaling MMR.
We tested this hypothesis by measuring the effects of different DNA substrates on Msh2-Msh6 ATPase activity. Several past reports indicate that the MutS/Msh2-Msh6·ATP·DNA complex is important for initiation of MMR (14, 16, 22, 23), and we have shown that Msh2-Msh6 binding to G:T inhibits ATP hydrolysis (not ATP binding), facilitating formation of the ternary complex (19–21). According to pre–steady-state ATPase kinetics, the DNAs suppress ATP hydrolysis to varying degrees; O6MeG:T is a robust inhibitor like G:T, whereas the +T loops and 2-Ap:T are partial inhibitors. Thus, strong sites lead to more Msh6·ATP·DNA complex, but it appears that the fraction of Msh2-Msh6 bound at weak sites can also form the ternary complex and possibly trigger MMR as well. Consistent with these data, 2-Ap treatment activates MMR in E. coli (37, 38) and increases the number of MutS·MutL complexes bound to DNA in Bacillus subtilis (39). The finding that Msh2-Msh6 interaction with +T is less stable than G:T is interesting though not without precedent (40), and its physiological impact may be minimal since Msh2-Msh6 and Msh2-Msh3 have overlapping IDL recognition functions (25). We also noted that in a different sequence context +T can strongly inhibit ATP hydrolysis (Fig. S1C) (19), whereas sequence context does not appear to affect G:T mismatch recognition (17, 19).
We further tested the hypothesis that longer-lived Msh2-Msh6·DNA complexes are more likely to signal MMR by analyzing a DNA binding mutant, Msh2-Msh6E339A. Mutation of Glu in the Phe-X-Glu motif reportedly reduces MutS/Msh2-Msh6 selectivity (36, 41, 42). Msh2-Msh6E339A forms a more stable complex with 2-Ap:T than wild-type protein, and its ATPase activity is correspondingly inhibited to a greater extent (Table 1; Fig. S3). Thus, Msh6 Glu339 appears to destabilize Msh2-Msh6 binding to with weak sites and reduce promiscuous signaling of MMR. We also detected less stable interaction between Msh2-Msh6E339A and G:T, while +T was unaffected. These observations are consistent with in vivo data indicating that Msh6 Glu339 is involved in repair of mismatched base pairs, but is dispensable for single base IDLs (43). In E. coli MutS, E38A mutation causes near loss of MMR (36, 41, 43); it has been hypothesized that MutSE38A is unable to form ATP-bound complexes with DNA (36) or its poor selectivity limits MMR at bona fide targets (42), which are both likely mechanisms based on our study of Msh2-Msh6E339A.
In summary, we have found that nucleotide-free or ADP-bound Msh2-Msh6 makes rapid, transient contact with potential target sites in DNA during the search phase of MMR. It pauses for long periods at strong sites such as G:T or O6MeG:T, which facilitates formation of ATP-bound ternary complexes and signals MMR. Msh2-Msh6 also pauses briefly at other sites that have altered structure and/or dynamics, which may trigger MMR as well, albeit less effectively (Fig. S4). Such promiscuity may be an unavoidable side effect of the requirement that Msh2-Msh6 recognize a broad range of defects in DNA. It has been proposed that MutS/Msh2-Msh6-DNA interaction is energetically favorable at sites that simply display increased local flexibility or bendability (17, 44); indeed, S. cerevisiae Msh2-Msh6 is trapped at multiple sites on λ DNA that does not contain any known MMR targets (10). Perhaps 10–100-fold higher stability of Msh2-Msh6 interactions with mismatches/IDLs/lesions over other sites limits promiscuous MMR to acceptably low levels. It remains possible that further selectivity for MMR targets is imposed after ATP binding to Msh2-Msh6 or by other proteins such as MutL/Mlh1-Pms1.
Materials and Methods
Reagents.
DNAs were purchased from Integrated DNA Technologies, Inc., and Midland Certified Reagent Company and labeled with TAMRA (Invitrogen) as described (24) (sequences in SI). S. cerevisiae Msh2-Msh6 proteins were purified from E. coli (19). Nucleotides were purchased from Sigma-Aldrich.
Msh2-Msh6-DNA Binding at Equilibrium.
Msh2-Msh6 binding to 2-Ap DNA was measured on FluoroMax-3 (Jobin-Yvon Horiba Group; Edison, NJ). DNA (0.025 μM) was titrated with Msh2-Msh6 (0–0.6 μM) in binding buffer (20 mM Tris-HCl, pH 8.0, 100 mM NaCl, 5 mM MgCl2), at 25 °C (λEX = 315 nm, λEM = 375 nm). Fluorescence anisotropy was measured with 0.01 μM TAMRA-labeled DNA (polarized λEX = 555 nm, λEM = 582 nm) (24).
Msh2-Msh6 DNA Binding Kinetics.
DNA binding kinetics were measured on a KinTek SF-2001 stopped-flow (KinTek Corporation; Austin, TX). Msh2-Msh6 (0.8 μM) was mixed with labeled DNA (0.12 μM) to measure on rates (λEX = 315 nm, λEM > 350 nm for 2-Ap fluorescence; polarized λEX = 555 nm, λEM > 570 nm for TAMRA anisotropy). Msh2-Msh6 (0.8 μM) was incubated with DNA (0.12 μM) for 10 min, then mixed with unlabeled G:T DNA (8 μM) for off rates (mixing ratio: 1∶1). In sequential mixing experiments Msh2-Msh6 (0.8 μM) was mixed with labeled DNA (0.12 μM) for 0.25 s followed by addition of unlabeled G:T DNA (10 μM) or buffer (mixing ratio: 1∶1∶1). Experiments with nucleotides were performed similarly except with 250 μM ATP, ATPγS or ADP added to Msh2-Msh6 and/or DNA (24).
Msh2-Msh6 ATPase Kinetics.
Pre–steady-state ATPase kinetics were measured by stopped-flow experiments using MDCC-labeled PBP (20 μM) to detect Pi release by Msh2-Msh6 (4 μM) on mixing with ATP (1 mM) (mixing ratio 1∶1) in the absence or presence of DNA (12 μM) (24).
Supplementary Material
Acknowledgments.
We thank Dr. Miho Sakato for insightful discussions. This work was supported by a grant from the National Science Foundation (MCB 0448379).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. E.E.A. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908302107/DCSupplemental.
References
- 1.Iyer RR, Pluciennik A, Burdett V, Modrich PL. DNA mismatch repair: Functions and mechanisms. Chem Rev. 2006;106(2):302–323. doi: 10.1021/cr0404794. [DOI] [PubMed] [Google Scholar]
- 2.Kunkel TA, Erie DA. DNA Mismatch Repair. Annu Rev Biochem. 2005;74:681–710. doi: 10.1146/annurev.biochem.74.082803.133243. [DOI] [PubMed] [Google Scholar]
- 3.Kadyrov FA, et al. Saccharomyces cerevisiae MutLalpha is a mismatch repair endonuclease. J Biol Chem. 2007;282(51):37181–37190. doi: 10.1074/jbc.M707617200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsieh P, Yamane K. DNA mismatch repair: Molecular mechanism, cancer, and ageing. Mech Ageing Dev. 2008;129(7-8):391–407. doi: 10.1016/j.mad.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiricny J. The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol. 2006;7(5):335–346. doi: 10.1038/nrm1907. [DOI] [PubMed] [Google Scholar]
- 6.Peltomaki P. Role of DNA mismatch repair defects in the pathogenesis of human cancer. J Clin Oncol. 2003;21(6):1174–1179. doi: 10.1200/JCO.2003.04.060. [DOI] [PubMed] [Google Scholar]
- 7.Lamers MH, et al. The crystal structure of DNA mismatch repair protein MutS binding to a G·T mismatch. Nature. 2000;407(6805):711–717. doi: 10.1038/35037523. [DOI] [PubMed] [Google Scholar]
- 8.Obmolova G, Ban C, Hsieh P, Yang W. Crystal structures of mismatch repair protein MutS and its complex with a substrate DNA. Nature. 2000;407(6805):703–710. doi: 10.1038/35037509. [DOI] [PubMed] [Google Scholar]
- 9.Warren JJ, et al. Structure of the human MutSalpha DNA lesion recognition complex. Mol Cell. 2007;26(4):579–592. doi: 10.1016/j.molcel.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Gorman J, et al. Dynamic basis for one-dimensional DNA scanning by the mismatch repair complex Msh2-Msh6. Mol Cell. 2007;28(3):359–370. doi: 10.1016/j.molcel.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y, Sass LE, Du C, Hsieh P, Erie DA. Determination of protein-DNA binding constants and specificities from statistical analyses of single molecules: MutS-DNA interactions. Nucleic Acids Res. 2005;33(13):4322–4334. doi: 10.1093/nar/gki708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang J, et al. Detection of high-affinity and sliding clamp modes for MSH2-MSH6 by single-molecule unzipping force analysis. Mol Cell. 2005;20(5):771–781. doi: 10.1016/j.molcel.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Marsischky GT, Kolodner RD. Biochemical characterization of the interaction between the Saccharomyces cerevisiae MSH2-MSH6 complex and mispaired bases in DNA. J Biol Chem. 1999;274(38):26668–26682. doi: 10.1074/jbc.274.38.26668. [DOI] [PubMed] [Google Scholar]
- 14.Gradia S, Acharya S, Fishel R. The role of mismatched nucleotides in activating the hMSH2-hMSH6 molecular switch. J Biol Chem. 2000;275(6):3922–3930. doi: 10.1074/jbc.275.6.3922. [DOI] [PubMed] [Google Scholar]
- 15.Gerland U, Moroz JD, Hwa T. Physical constraints and functional characteristics of transcription factor-DNA interaction. Proc Natl Acad Sci USA. 2002;99(19):12015–12020. doi: 10.1073/pnas.192693599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selmane T, Schofield MJ, Nayak S, Du C, Hsieh P. Formation of a DNA mismatch repair complex mediated by ATP. J Mol Biol. 2003;334(5):949–965. doi: 10.1016/j.jmb.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Mazurek A, Johnson CN, Germann MW, Fishel R. Sequence context effect for hMSH2-hMSH6 mismatch-dependent activation. Proc Natl Acad Sci USA. 2009;106(11):4177–4182. doi: 10.1073/pnas.0808572106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuck P, Minton AP. Analysis of mass transport-limited binding kinetics in evanescent wave biosensors. Anal Biochem. 1996;240(2):262–272. doi: 10.1006/abio.1996.0356. [DOI] [PubMed] [Google Scholar]
- 19.Antony E, Hingorani MM. Mismatch recognition-coupled stabilization of Msh2-Msh6 in an ATP-bound state at the initiation of DNA repair. Biochemistry. 2003;42(25):7682–7693. doi: 10.1021/bi034602h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antony E, Hingorani MM. Asymmetric ATP binding and hydrolysis activity of the Thermus aquaticus MutS dimer is key to modulation of its interactions with mismatched DNA. Biochemistry. 2004;43(41):13115–13128. doi: 10.1021/bi049010t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antony E, Khubchandani S, Chen S, Hingorani MM. Contribution of Msh2 and Msh6 subunits to the asymmetric ATPase and DNA mismatch binding activities of Saccharomyces cerevisiae Msh2-Msh6 mismatch repair protein. DNA Repair. 2006;5(2):153–162. doi: 10.1016/j.dnarep.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazur DJ, Mendillo ML, Kolodner RD. Inhibition of Msh6 ATPase activity by mispaired DNA induces a Msh2(ATP)-Msh6(ATP) state capable of hydrolysis-independent movement along DNA. Mol Cell. 2006;22(1):39–49. doi: 10.1016/j.molcel.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Studamire B, Quach T, Alani E. Saccharomyces cerevisiae Msh2p and Msh6p ATPase activities are both required during mismatch repair. Mol Cell Biol. 1998;18(12):7590–7601. doi: 10.1128/mcb.18.12.7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs-Palmer E, Hingorani MM. The effects of nucleotides on MutS-DNA binding kinetics clarify the role of MutS ATPase activity in mismatch repair. J Mol Biol. 2007;366(4):1087–1098. doi: 10.1016/j.jmb.2006.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marsischky GT, Filosi N, Kane MF, Kolodner R. Redundancy of Saccharomyces cerevisiae MSH3 and MSH6 in MSH2-dependent mismatch repair. Genes Dev. 1996;10(4):407–420. doi: 10.1101/gad.10.4.407. [DOI] [PubMed] [Google Scholar]
- 26.Nordlund TM, et al. Structure and dynamics of a fluorescent DNA oligomer containing the EcoRI recognition sequence: Fluorescence, molecular dynamics, and NMR studies. Biochemistry. 1989;28(23):9095–9103. doi: 10.1021/bi00449a021. [DOI] [PubMed] [Google Scholar]
- 27.Law SM, Eritja R, Goodman MF, Breslauer KJ. Spectroscopic and calorimetric characterizations of DNA duplexes containing 2-aminopurine. Biochemistry. 1996;35(38):12329–12337. doi: 10.1021/bi9614545. [DOI] [PubMed] [Google Scholar]
- 28.Lycksell PO, et al. Base pair opening dynamics of a 2-aminopurine substituted Eco RI restriction sequence and its unsubstituted counterpart in oligonucleotides. Nucleic Acids Res. 1987;15(21):9011–9025. doi: 10.1093/nar/15.21.9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clayton LK, Goodman MF, Branscomb EW, Galas DJ. Error induction and correction by mutant and wild type T4 DNA polymerases. Kinetic error discrimination mechanisms. J Biol Chem. 1979;254(6):1902–1912. [PubMed] [Google Scholar]
- 30.Jean JM, Hall KB. 2-Aminopurine electronic structure and fluorescence properties in DNA. Biochemistry. 2002;41(44):13152–13161. doi: 10.1021/bi020308y. [DOI] [PubMed] [Google Scholar]
- 31.Nag N, Rao BJ, Krishnamoorthy G. Altered dynamics of DNA bases adjacent to a mismatch: A cue for mismatch recognition by MutS. J Mol Biol. 2007;374(1):39–53. doi: 10.1016/j.jmb.2007.08.065. [DOI] [PubMed] [Google Scholar]
- 32.Gradia S, Acharya S, Fishel R. The human mismatch recognition complex hMSH2-hMSH6 functions as a novel molecular switch. Cell. 1997;91(7):995–1005. doi: 10.1016/s0092-8674(00)80490-0. [DOI] [PubMed] [Google Scholar]
- 33.Junop MS, Obmolova G, Rausch K, Hsieh P, Yang W. Composite active site of an ABC ATPase: MutS uses ATP to verify mismatch recognition and authorize DNA repair. Mol Cell. 2001;7(1):1–12. doi: 10.1016/s1097-2765(01)00149-6. [DOI] [PubMed] [Google Scholar]
- 34.Gradia S, et al. hMSH2-hMSH6 forms a hydrolysis-independent sliding clamp on mismatched DNA. Mol Cell. 1999;3(2):255–261. doi: 10.1016/s1097-2765(00)80316-0. [DOI] [PubMed] [Google Scholar]
- 35.Mendillo ML, Mazur DJ, Kolodner RD. Analysis of the interaction between the Saccharomyces cerevisiae MSH2-MSH6 and MLH1-PMS1 complexes with DNA using a reversible DNA end-blocking system. J Biol Chem. 2005;280(23):22245–22257. doi: 10.1074/jbc.M407545200. [DOI] [PubMed] [Google Scholar]
- 36.Lebbink JH, et al. Dual role of MutS glutamate 38 in DNA mismatch discrimination and in the authorization of repair. Embo J. 2006;25(2):409–419. doi: 10.1038/sj.emboj.7600936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glickman BW, Radman M. Escherichia coli mutator mutants deficient in methylation-instructed DNA mismatch correction. Proc Natl Acad Sci USA. 1980;77(2):1063–1067. doi: 10.1073/pnas.77.2.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burdett V, Baitinger C, Viswanathan M, Lovett ST, Modrich P. In vivo requirement for RecJ, ExoVII, ExoI, and ExoX in methyl-directed mismatch repair. Proc Natl Acad Sci USA. 2001;98(12):6765–6770. doi: 10.1073/pnas.121183298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith BT, Grossman AD, Walker GC. Visualization of mismatch repair in bacterial cells. Mol Cell. 2001;8(6):1197–1206. doi: 10.1016/s1097-2765(01)00402-6. [DOI] [PubMed] [Google Scholar]
- 40.Hess MT, Gupta RD, Kolodner RD. Dominant Saccharomyces cerevisiae msh6 mutations cause increased mispair binding and decreased dissociation from mispairs by Msh2-Msh6 in the presence of ATP. J Biol Chem. 2002;277(28):25545–25553. doi: 10.1074/jbc.M202282200. [DOI] [PubMed] [Google Scholar]
- 41.Schofield MJ, et al. The Phe-X-Glu DNA binding motif of MutS. The role of hydrogen bonding in mismatch recognition. J Biol Chem. 2001;276(49):45505–45508. doi: 10.1074/jbc.C100449200. [DOI] [PubMed] [Google Scholar]
- 42.Junop MS, Yang W, Funchain P, Clendenin W, Miller JH. In vitro and in vivo studies of MutS, MutL and MutH mutants: Correlation of mismatch repair and DNA recombination. DNA Repair. 2003;2(4):387–405. doi: 10.1016/s1568-7864(02)00245-8. [DOI] [PubMed] [Google Scholar]
- 43.Holmes SF, Scarpinato KD, McCulloch SD, Schaaper RM, Kunkel TA. Specialized mismatch repair function of Glu339 in the Phe-X-Glu motif of yeast Msh6. DNA Repair. 2007;6(3):293–303. doi: 10.1016/j.dnarep.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Isaacs RJ, Spielmann HP. A model for initial DNA lesion recognition by NER and MMR based on local conformational flexibility. DNA Repair. 2004;3(5):455–464. doi: 10.1016/j.dnarep.2004.01.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.