Abstract
Objectives
The objective of this prospective, single-site, two-year dietary intervention study was to evaluate the effects of moderate weight reduction and subsequent partial weight regain on cardiovascular structure and function.
Background
Obesity is associated with adverse cardiac and vascular structural and functional alterations.
Methods
Sixty obese subjects (age: 46±10 years, body mass index: 37±3 kg/m2) were evaluated during their participation in a weight loss study. Cardiac and vascular ultrasound studies were performed at baseline and at 3, 6, 12, and 24 months after start of intervention.
Results
Forty-seven subjects (78%) completed the entire two-year follow-up. Average weight loss was 7.3±4.0, 9.2±5.6, 7.8±6.6 and 3.8±7.9% at 3, 6, 12, and 24 months, respectively. Age- and sex- adjusted mixed linear models revealed that the follow-up time was significantly associated with decreases in weight (p<0.0001), left ventricular (LV) mass (p=0.001), and carotid intima-media thickness (p<0.0001); there was also significant improvement in LV diastolic (E’, p≤0.0001) and systolic (S’, p=.001) function. Partial weight regain diminished the maximal observed beneficial effects of weight loss, however cardiovascular parameters measured at two years still showed a net benefit compared with baseline.
Conclusions
Diet-induced moderate weight loss in obese subjects is associated with beneficial changes in cardiovascular structure and function. Subsequent weight regain is associated with partial loss of these beneficial effects.
Keywords: obesity, echocardiography, hypertrophy, carotid arteries, diastolic function
INTRODUCTION
Obesity is associated with a two-fold increase in the risk of developing heart failure.(1) Abnormalities in cardiovascular structure and function that have been documented in obesity include left ventricular hypertrophy (LVH), left ventricular (LV) enlargement, and systolic and diastolic dysfunction, all of which are independent risk factors for heart failure.(2-4) Weight reduction in obese subjects is associated with regression of these abnormalities.(2,5-8) However, most studies have been confined to class III obese subjects (body mass index [BMI] ≥40 kg/m2) who have experienced considerable weight loss (i.e., >20% total body weight) following bariatric surgery.
Most obese persons have class I (BMI: 30-34.9 kg/m2) and class II (BMI: 35-39.9 kg/m2) obesity.(9,10) The primary therapeutic approach in these patients is to decrease caloric intake and increase caloric expenditure by altering lifestyle behaviors. However, diet-induced weight loss is difficult to sustain, and many patients who lose weight by dieting regain their lost weight over time. The cardiovascular effects of weight regain in patients with class I and II obesity are not well known. The purpose of this study was to prospectively evaluate the effects of moderate diet-induced weight loss (5-10% of body weight) and subsequent weight regain on cardiovascular structure and function as assessed by cardiac and vascular ultrasound in patients with class I (BMI: 30-34.9 kg/m2) and II (BMI: 35-39.9 kg/m2) obesity over a 2-year period.
METHODS
Study population
The study population consisted of 60 obese adults prospectively enrolled in weight loss study at Washington University School of Medicine. All subjects underwent a medical history, physical examination, and cardiovascular ultrasound. Exclusion criteria: type 2 diabetes mellitus, taking weight-loss and/or lipid-lowering medications, pregnant, or lactating. The study was approved by the Human Research Protection Office at Washington University School of Medicine; written informed consent was obtained from all study participants.
Study design
Weight-loss intervention
Participants were randomly assigned to one of two reduced-calorie diets for two years: low-fat vs. low-carbohydrate diet. Low-carbohydrate diet subjects were instructed to limit carbohydrate intake and to eat foods rich in fat and protein. Low-fat diet subjects were instructed to limit fat intake to approximately 30% of total calories and reduce energy intake (women: 1200-1500 kcal/d; men: 1500-1800 kcal/d). All participants received: a) daily multivitamin supplement; b) comprehensive group behavioral treatment (weekly for 20 weeks, every other week for 20 weeks, and every other month for the remainder of the 2-year study); (11,12) and c) instructions for physical activity (principally walking), beginning at week 4 (4 sessions of 20 minutes each), progressing by week 19 to 4 sessions of 50 minutes each.
Body weight was measured at each treatment visit on calibrated scales while participants wore light clothing and no shoes. Height was measured by a stadiometer at baseline.
Cardiovascular evaluations
Cardiovascular assessments were performed at baseline and at 3, 6, 12, and 24 months after starting a hypocaloric diet therapy program. Fasting serum lipoproteins and glucose were collected after a 12-hour fast at baseline and each follow-up visit; fasting insulin was drawn at baseline. Heart rate and blood pressure were assessed using automated instruments (Dinamap, GE Healthcare, United Kingdom) after 5 minutes of quiet sitting with the average of two readings taken 1 minute apart reported. Metabolic syndrome was diagnosed according to the amended National Cholesterol Education Program’s (NCEP) Adult Treatment Panel III guidelines except a BMI ≥ 30 kg/m2 satisfied the criteria for increased waist circumference (13).
Complete two-dimensional, M-mode, and Doppler echocardiograms and carotid artery ultrasound were performed by use of commercially-available ultrasound equipment (Sequoia-C256, Acuson-Siemens, Mountain View, CA). Two-dimensional directed echocardiographic measurements included the LV ejection fraction (LVEF) calculated using the biplane method of discs (modified Simpson’s method). LV mass was measured by the M-mode-derived cubed method and indexed to height2.7 (LVM/Ht2.7).(14) LV geometric patterns were determined as previously described.(15) Tissue Doppler imaging (TDI)-derived myocardial systolic (S’) and early diastolic (E’) tissue velocities were obtained from the apical four-chamber view (septal and lateral velocities averaged and reported as a global measurement).(14,16-18) All measurements were performed in accordance to published guidelines and represent the average of three consecutive cardiac cycles obtained by a single observer blinded to all clinical parameters.(19)
Carotid artery intima-media thickness (CIMT) was measured by a single vascular sonographer from B-mode images of both carotid arteries expressed as the average of the far-walls of the right and left common carotid arteries; each site represents the average of three separate measurements.(20) The intra-class correlation coefficient for repeated measures of the CIMT is 0.91 and for echocardiographic measurements ranges from 0.85-0.90 at our laboratory.
Statistical analysis
SAS software (v, 9.2, SAS Institute, Cary, North Carolina) was used for all statistical analyses. -square analysis and Student’s T-tests were performed to compare baseline values. Mixed linear models with repeated measures and pairwise contrasts were performed with a covariance structure including the fixed-effect parameters of the duration of dietary intervention (i.e. follow-up time interval) for all models. All regression models included age, sex, and dietary group as potential covariates. Continuous variables are presented as the mean ± one standard deviation except in graphics where the standard error is shown. A p value < 0.05 after adjustment for multiple testing by the False Discovery Rate was considered statistically significant. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
I. Baseline characteristics
The study population consisted of 60 obese subjects; all subjects had normal LV systolic function (i.e., LVEF>50%). There were no differences between subjects who consumed a low-carbohydrate or low-fat diet in terms of baseline characteristics (age, sex, racial composition, systolic and diastolic blood pressures [SBP and DBP, respectively], serum lipids and triglycerides, glucose, insulin, homeostasis model assessment insulin resistance or percent with metabolic syndrome). Mean age was 46±10 years, 43 (72%) female, 15 (25%) African American, BMI 37±3 kg/m2, insulin 13.7±9.9 μU/mL, and homeostasis model assessment insulin resistance of 3.1±2.5. Additional baseline characteristics of the combined group are shown in Table 1.
Table 1.
Serial changes in weight and hemodynamics, plasma lipids and glucose during the study.
| Baseline | 3 Months | 6 Months | 12 Months | 24 Months | |
|---|---|---|---|---|---|
| Weight, kg | 104±16 | 96±14* | 94±14* | 94±13* | 99±15‡ |
| Heart Rate, bpm | 73±11 | 68±10* | 67±9† | 70±8 | 70 9 |
| SBP, mmHg | 125±13 | 116±13* | 120±12† | 120±10* | 121±13‡ |
| DBP, mmHg | 76±9 | 73±8† | 72±8* | 74±8‡ | 73±8† |
| Triglycerides, mmol/L | 1.30±0.67 | 1.11±0.49† | 1.05±0.47* | 1.12±0.56† | 1.11±0.45‡ |
| Total-C, mmol/L | 4.81±0.78 | 5.07±0.96† | 4.68±0.83† | 4.71±0.85‡ | 4.99±0.85 |
| LDL-C, mmol/L | 3.10±0.67 | 3.34±0.85† | 3.05±0.75 | 2.95±0.67† | 3.16±0.67 |
| HDL-C, mmol/L | 1.16±0.31 | 1.27±0.34* | 1.22±0.3† | 1.24±0.28† | 1.34±0.31* |
| Glucose, mmol/L | 5.00±0.50 | 4.94±0.56 | 5.00±0.61 | 5.05±0.50 | NA |
| Metabolic Syndrome, n (%) | 26 (43) | 14 (26) | 17 (32) | 12 (27) | NA |
P-value reflects the overall adjusted model significance.
Adjusted p≤0.001
p≤.01
p≤.05 for the difference compared with baseline.
C, cholesterol; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NA, not available; NS, not significant; SBP, systolic blood pressure.
II. Body weight response to diet intervention
The entire 24-month follow-up was completed by 47 subjects (78%). There were no differences between the two diet groups in terms of weight loss or any of the primary endpoints, namely diet-induced changes in cardiac structure/function and CIMT; therefore, the data for the two groups were combined (Table 1 and Figure). For the entire group, average weight loss was 7.8±4.9, 9.9±6.9, 8.4±7.6, and 4.1±8.8 kg at 3-, 6-, 12- and 24-months, respectively, representing 7.3±4.0, 9.2±5.6, 7.8±6.6 and 3.8±7.9% decrease from baseline body weight. Maximal weight loss occurred at 6-months. Although weight regain occurred at 12- and 24-months, mean body weight remained lower than baseline values. Both dietary intervention groups exhibited significant differences between baseline and some follow-up intervals in weight, heart rate, SBP, DBP, and lipid levels. Where dietary group assignment resulted in group differences, the low-carbohydrate diet showed differences in triglyceride and HDL levels whereas only HDL was significantly different in the high-carbohydrate group. Neither group demonstrated significant changes in blood glucose or in the percentage of subjects with metabolic syndrome during the follow-up interval (Table 1).
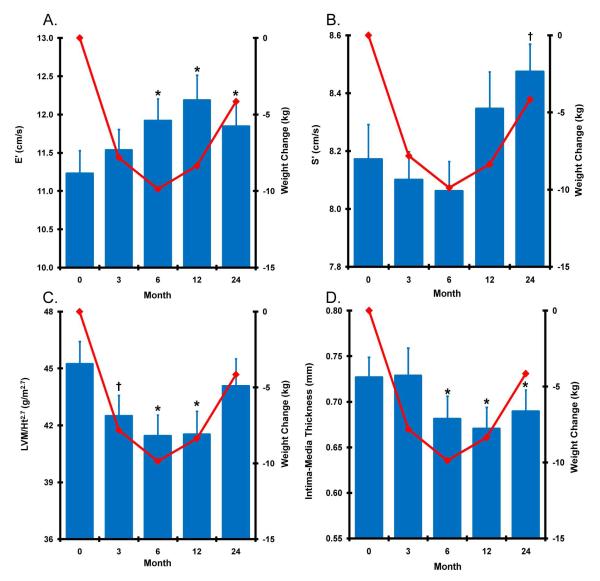
Figure. Cardiovascular structure-function response to weight loss.
Mean (±SE) change in E’ (Panel A), S’ (Panel B), LVM/Ht2.7 (Panel B), and CIMT (Panel C) in the combined cohort (blue bars) with weight change (red line) shown for reference. The combined group had 60 subjects at baseline and 3 months, 56 subjects at 6 months, and 47 subjects at 12 and 24 months. There was no significant difference between diet groups for any of these phenotypes. *Adjusted p≤0.005, † adjusted p≤0.05 vs. baseline.
III. Cardiovascular structure-function response to weight loss
Compared with baseline measures, there was a significant increase (improvement) in the E’ (an index of LV diastolic relaxation) at 6, 12, and 24 months; a significant decrease (improvement) in LVM/Ht2.7 at 3, 6 and 12 months; and a significant decrease (improvement) in CIMT at 6, 12, and 24 months (Figure). Age- and sex-adjusted mixed linear models revealed that follow-up time was significantly associated with decreases in weight (F[.05,4,58] = 42.51, p<.0001), E’ (F[.05,4,58] = 8.71, p ≤ 0.0001), LV mass (F[.05,4,58] = 5.27, p = 0.004), and carotid intima-media thickness (F[.05,4,58] = 10.18, p < 0.0001).There was a modest increase (improvement) in S’ (an index of LV contractile performance) at 24 months (F[.05,4,58] = 5.21, p = 0.001). Further post-hoc analyses divided the cohort into two equal groups by the median of S’ at baseline. In the baseline S’ < 8.0 cm/s group, a significant improvement in S’ was noted at 3, 6, 12 and 24 months compared with baseline (F[.05,4,28] = 16.75, p ≤ 0.0001); there were no significant differences over time in the baseline S’ ≥ 8.0 cm/s group.
IV. Changes in LV geometric patterns
At baseline, a majority (52%) exhibited normal geometry (Table 2); whereas at 3 months, the proportion with normal geometry and eccentric LVH both increased and the proportion with concentric remodeling and concentric LVH decreased. Further analyses show that the changes in LV geometry are related to the percentage of subjects with increased relative wall thickness (adjusted p-value = 0.03); these findings are consistent with decreased LVM/Ht2.7 at 3, 6, and 12 months.
Table 2.
LV Geometry and RWT at Baseline and Follow-up.
| LV Geometry, n (%) |
Relative Wall Thickness <0.45, n (%) |
||||
|---|---|---|---|---|---|
| Normal | Concentric Remodeling |
Concentric LVH |
Eccentric LVH |
||
| Baseline | 31 (52) | 13 (22) | 12 (20) | 4 (7) | 25 (42) |
| 3 Months | 35 (58) | 4 (7) | 7 (12) | 14 (23) | 11 (18) |
| 6 Months | 35 (63) | 4 (7) | 6 (11) | 11 (20) | 10 (18) |
| 12 Months | 29 (62) | 7 (15) | 4 (9) | 7 (15) | 11 (23) |
| 24 Months | 26 (55) | 9 (19) | 6 (13) | 6 (13) | 15 (32) |
DISCUSSION
The results of this two-year study demonstrate that a weight loss intervention was associated with beneficial changes in cardiovascular structure and function, manifested by decreased LV mass, improved diastolic and systolic function, and decreased vascular hypertrophy. Whereas maximal weight loss occurred at 6 months, the maximal cardiovascular benefits “lagged” behind the maximal weight loss by 3- to 12-months for all measured variables. Subsequent weight regain, which occurred by 6-12 months, was paralleled by worsening of LV mass, diastolic function, and vascular hypertrophy.
Abnormalities in cardiovascular structure-function in obesity have been well-characterized and include increased blood pressures, increased LV volumes, LV hypertrophy, increased wall stress, and systolic and/or diastolic dysfunction.(2-8) Data from previous studies have shown that obese subjects undergoing a combined exercise-hypocaloric weight management program experience modest weight loss that is associated with lower blood pressure and with beneficial changes in cardiac structure (i.e., decreased relative wall thickness and LVH) at 6 and 12 months.(6,7) However, the current study extends these finding by showing that changes in LV structure and function are not only sustained at 24 months after the start of the intervention but also also associated with improvement in LV diastolic function and CIMT. It is reasonable to assume that improvement of these adverse cardiovascular imaging biomarkers with weight loss intervention would result in a risk reduction compared with those who remain obese.(21,22) Studies conducted in morbidly obese individuals have shown that profound weight reduction after bariatric surgery (i.e., >20% loss from initial body weight) results in regression of LV hypertrophy and improved systolic function.(2,4,22-24).
The present study shows that obese subjects exhibit mild alterations in cardiovascular structure and function. There are major implications of the present study. First, the modest observed weight loss (i.e., ~10% of initial body weight) represents a realistic and attainable goal for most obese individual. Second, improvement in cardiovascular structure-function parameters occur relatively early (i.e., at 3-12 months) during modest weight loss and most persist for the entire 24-month follow-up period, thus implying that obesity-related cardiovascular structural-functional abnormalities are reversible. Third, partial weight regain diminished the maximal observed beneficial effects of weight loss in the cardiovascular system. Further studies are necessary to better define the mechanisms responsible for the improvement in cardiac and vascular structure and function observed in this study. Possible mechanisms include an improvement in blood pressure reduction, insulin resistance, alterations in myocardial substrate metabolism, and/or reduction in inflammatory cytokines.(18,25-31) The results of the present study are interpreted in the context of a recent, large, two-year trial that found that the magnitude of weight loss was related to overall caloric intake, independent of the different diet macronutrient composition.(32) Thus, taken together, the findings of these two studies suggest that the beneficial effects on the cardiovascular system are related to the weight loss independent of the diet.
Limitations of the study
Although subjects were encouraged to increase their physical activity, a structured, monitored exercise program was not part of the study; it is thus possible that increased physical activity contributed to the beneficial effects observed.
CONCLUSIONS
Moderate weight loss leads to early improvement of cardiovascular structural-functional abnormalities. Although partial weight regain diminished the maximal observed beneficial effects of weight loss, cardiovascular parameters measured at two years still showed a net benefit compared with baseline. Whether the salutary effects of weight loss on cardiovascular structure-function observed in this study translate into improved clinical outcomes requires further investigation.
Acknowledgments
The authors would like to thank Joann R. Reagan, RN, RVT, Sharon Heuerman, RN, and Karen Spence, MS for their assistance in the performance of this study.
This study was supported in part by NIH grants K12RR023249, KL2RR024994, and UL1RR024992 (LdlF), AT 1103 (SK), Robert Wood Johnson Foundation (Princeton, NJ) grant #048875 (LdlF); and a grant from the Barnes-Jewish Hospital Foundation (St. Louis, MO).
Glossary
Abbreviations list
- BMI
body mass index
- CIMT
carotid intima-media thickness
- DBP
diastolic blood pressure
- E’
early diastolic myocardial velocity
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- LVH
left ventricular hypertrophy
- LVM/Ht2.7
left ventricular mass indexed to height 2.7
- SBP
systolic blood pressure
- TDI
tissue Doppler imaging
Footnotes
Disclosures of industry relationships Alan D. Waggoner, MHS, RDCS, is a consultant for St. Jude Medical and Boston Scientific; however such relationships present no conflicts related to the present work. Gary Foster, PhD, is on the scientific advisory board for ConAgra Foods, and serves on the scientific advisory board and has received research grants from NutriSystem. Holly Wyatt, MD serves on scientific advisory boards for Arena, Wellspring, and Pfizer. Victor G. Davila-Roman, MD, FACC, FASE, is a consultant for St. Jude Medical, AGA Medical, Arbor Surgical Technologies, Inc., Boston Scientific, CoreValve Inc., Medtronic, and AtriCure, Inc; however such relationships present no conflicts related to the present work. The remaining authors have no industry relationships to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov: “The Safety and Effectiveness of Low and High Carbohydrate Diets”, NCT00079547, http://clinicaltrials.gov/ct2/show/NCT00079547
REFERENCES
- 1.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–13. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 2.Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001;321:225–36. doi: 10.1097/00000441-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Peterson LR, Waggoner AD, Schechtman KB, Meyer T, Gropler RJ, Barzilai B, Davila-Roman VG. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol. 2004;43:1399–404. doi: 10.1016/j.jacc.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 4.Wong CY, O’Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation. 2004;110:3081–7. doi: 10.1161/01.CIR.0000147184.13872.0F. [DOI] [PubMed] [Google Scholar]
- 5.Alaud-din A, Meterissian S, Lisbona R, MacLean LD, Forse RA. Assessment of cardiac function in patients who were morbidly obese. Surgery. 1990;108:809–18. [PubMed] [Google Scholar]
- 6.Himeno E, Nishino K, Nakashima Y, Kuroiwa A, Ikeda M. Weight reduction regresses left ventricular mass regardless of blood pressure level in obese subjects. Am Heart J. 1996;131:313–9. doi: 10.1016/s0002-8703(96)90360-9. [DOI] [PubMed] [Google Scholar]
- 7.Hinderliter A, Sherwood A, Gullette EC, Babyak M, Waugh R, Georgiades A, Blumenthal JA. Reduction of left ventricular hypertrophy after exercise and weight loss in overweight patients with mild hypertension. Arch Intern Med. 2002;162:1333–9. doi: 10.1001/archinte.162.12.1333. [DOI] [PubMed] [Google Scholar]
- 8.Klein S, Burke LE, Bray GA, Blair S, Allison DB, Pi-Sunyer X, Hong Y, Eckel RH. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2004;110:2952–67. doi: 10.1161/01.CIR.0000145546.97738.1E. [DOI] [PubMed] [Google Scholar]
- 9.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 10.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 11.Foster GD, Makris AP, Bailer BA. Behavioral treatment of obesity. Am J Clin Nutr. 2005;82:230S–5S. doi: 10.1093/ajcn/82.1.230S. [DOI] [PubMed] [Google Scholar]
- 12.Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007;132:2226–38. doi: 10.1053/j.gastro.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 13.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr., Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: Executive summary. Circulation. 2005;112:e285–90. [Google Scholar]
- 14.de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, Alderman MH. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–60. doi: 10.1016/0735-1097(92)90385-z. [DOI] [PubMed] [Google Scholar]
- 15.Ganau A, Devereux RB, Roman MJ, de Simone G, Pickering TG, Saba PS, Vargiu P, Simongini I, Laragh JH. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550. doi: 10.1016/0735-1097(92)90617-v. [DOI] [PubMed] [Google Scholar]
- 16.Alam M, Wardell J, Andersson E, Samad BA, Nordlander R. Characteristics of mitral and tricuspid annular velocities determined by pulsed wave Doppler tissue imaging in healthy subjects. J Am Soc Echocardiogr. 1999;12:618–28. doi: 10.1053/je.1999.v12.a99246. [DOI] [PubMed] [Google Scholar]
- 17.Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, Lee MM, Park YB, Choi YS, Seo JD, Lee YW. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. 1997;30:474–80. doi: 10.1016/s0735-1097(97)88335-0. [DOI] [PubMed] [Google Scholar]
- 18.de las Fuentes L, Waggoner AD, Brown AL, Davila-Roman VG. Plasma triglyceride level is an independent predictor of altered left ventricular relaxation. J Am Soc Echocardiogr. 2005;18:1285–91. doi: 10.1016/j.echo.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 20.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–6. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 22.Krumholz HM, Larson M, Levy D. Prognosis of left ventricular geometric patterns in the Framingham Heart Study. J Am Coll Cardiol. 1995;25:879–84. doi: 10.1016/0735-1097(94)00473-4. [DOI] [PubMed] [Google Scholar]
- 23.Karason K, Wallentin I, Larsson B, Sjostrom L. Effects of obesity and weight loss on cardiac function and valvular performance. Obes Res. 1998;6:422–9. doi: 10.1002/j.1550-8528.1998.tb00374.x. [DOI] [PubMed] [Google Scholar]
- 24.Lauer MS, Anderson KM, Kannel WB, Levy D. The impact of obesity on left ventricular mass and geometry. The Framingham Heart Study. JAMA. 1991;266:231–6. [PubMed] [Google Scholar]
- 25.Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, Dence C, Klein S, Marsala J, Meyer T, Gropler RJ. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109:2191–6. doi: 10.1161/01.CIR.0000127959.28627.F8. [DOI] [PubMed] [Google Scholar]
- 26.Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–9. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aasum E, Hafstad AD, Severson DL, Larsen TS. Age-dependent changes in metabolism, contractile function, and ischemic sensitivity in hearts from db/db mice. Diabetes. 2003;52:434–41. doi: 10.2337/diabetes.52.2.434. [DOI] [PubMed] [Google Scholar]
- 28.Engeli S, Bohnke J, Gorzelniak K, Janke J, Schling P, Bader M, Luft FC, Sharma AM. Weight loss and the renin-angiotensin-aldosterone system. Hypertension. 2005;45:356–62. doi: 10.1161/01.HYP.0000154361.47683.d3. [DOI] [PubMed] [Google Scholar]
- 29.Ho JT, Keogh JB, Bornstein SR, Ehrhart-Bornstein M, Lewis JG, Clifton PM, Torpy DJ. Moderate weight loss reduces renin and aldosterone but does not influence basal or stimulated pituitary-adrenal axis function. Horm Metab Res. 2007;39:694–9. doi: 10.1055/s-2007-985354. [DOI] [PubMed] [Google Scholar]
- 30.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. Jama. 2003;289:1799–804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 31.Mavri A, Stegnar M, Sentocnik JT, Videcnik V. Impact of weight reduction on early carotid atherosclerosis in obese premenopausal women. Obes Res. 2001;9:511–6. doi: 10.1038/oby.2001.67. [DOI] [PubMed] [Google Scholar]
- 32.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, McManus K, Champagne CM, Bishop LM, Laranjo N, Leboff MS, Rood JC, de Jonge L, Greenway FL, Loria CM, Obarzanek E, Williamson DA. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–73. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]