Bisphosphonates such as risedronate and ibandronate are widely used to treat a variety of bone resorption diseases, preventing protein prenylation and disrupting osteoclast function[1]. Bisphosphonates also activate human γδ T cells (expressing the Vγ2Vδ2 T cell receptor), and these activated γδ T cells kill tumor cells[2, 3]. There has thus been interest in using bisphosphonates in cancer immunotherapy, with promising results against B-cell malignancies[4] and hormone refractory prostate cancer[5]. And in a very recent clinical trial, it was shown that zoledronate offered a significant anticancer benefit when added to hormone therapy, reducing the risk of cancer returning by 36%[6]. The bisphosphonates used in these trials are, however, extremely polar and are rapidly removed from the circulation by binding to bone. We reasoned that it might be possible to develop more lipophilic bisphosphonates[7] as γδ T cell stimulators that would have increased cell uptake properties, as well as decreased bone binding affinity[8]. Here, we report the discovery that novel, lipophilic, pyridinium bisphosphonates are ~250x more effective in γδ T cell activation than any other bisphosphonate drugs.
Current nitrogen-containing bisphosphonates are thought to act primarily by blocking farnesyl diphosphate (FPP) formation in the isoprene biosynthesis pathway (Figure 1), where they act as low nM FPP synthase (FPPS) inhibitors. Their stimulatory effects are thought to originate in the accumulation of isopentenyl diphosphate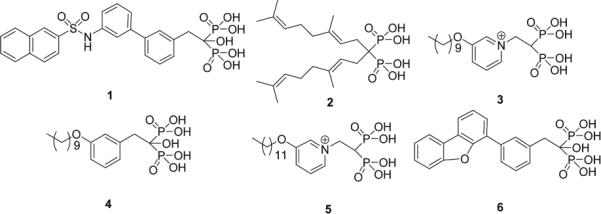 (IPP), a known “phosphoantigen” for γδ T cells[9], and their effects are blocked by statins[10, 11]. There are, however, four other targets in this pathway whose inhibition would also increase IPP levels: isopentenyl diphosphate/dimethylallyl diphosphate isomerase (IPPI); geranylgeranyl diphosphate synthase (GGPPS); decaprenyl diphosphate synthase (DPPS); and dehydrodolichyl diphosphate synthase (DeDPPS). Since four of these five enzymes produce long chain isoprenoids, we reasoned that they might be potently inhibited by more hydrophobic bisphosphonates which would also confer enhanced cell based activity. To test this idea, we determined the activity of the six lipophilic bisphosphonates[12] shown above, in γδ T cell activation. Several of these have been shown to have potent activity in tumor cell killing[12], but do they also activate γδ T cells?
(IPP), a known “phosphoantigen” for γδ T cells[9], and their effects are blocked by statins[10, 11]. There are, however, four other targets in this pathway whose inhibition would also increase IPP levels: isopentenyl diphosphate/dimethylallyl diphosphate isomerase (IPPI); geranylgeranyl diphosphate synthase (GGPPS); decaprenyl diphosphate synthase (DPPS); and dehydrodolichyl diphosphate synthase (DeDPPS). Since four of these five enzymes produce long chain isoprenoids, we reasoned that they might be potently inhibited by more hydrophobic bisphosphonates which would also confer enhanced cell based activity. To test this idea, we determined the activity of the six lipophilic bisphosphonates[12] shown above, in γδ T cell activation. Several of these have been shown to have potent activity in tumor cell killing[12], but do they also activate γδ T cells?
Figure 1.
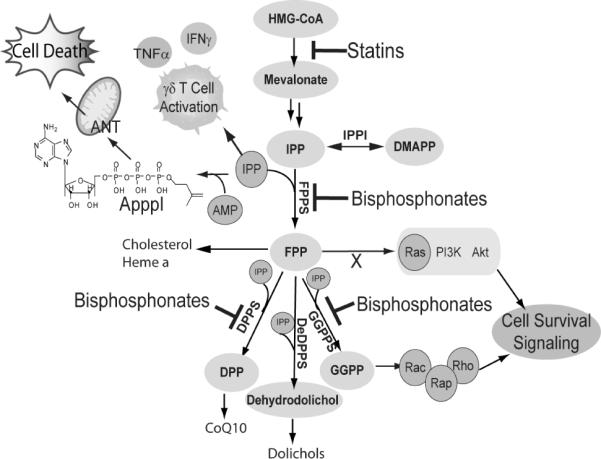
Schematic illustration of several pathways involved in bisphosphonate activity in γδ T cells and tumor cells. ANT = mitochondrial adenine nucleotide translocase.
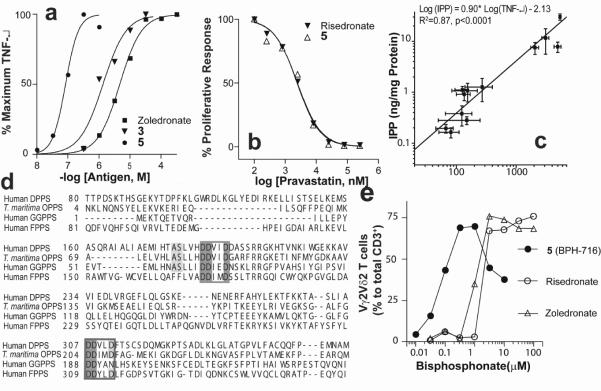
We first tested two specific inhibitors (1,2[13]) of GGPPS, which have IC50 (enzyme) values of 2.7, 1.0 μM. Neither had major effects on γδ T cell activation (TNF-α release) or proliferation. In a second experiment, we found that long n-alkyl containing bisphosphonates (3, 4) have IC50 values of 280, 590 nM against GGPPS. The pyridinium species (3) was a potent (800 nM) γδ T cell activator (Figure 2a), while the analog (4) lacking the positive charge feature had much less activity. A longer (C12) alkyl chain analog (5) of 3 had even greater activity, with an ED50 of 70 nM (Figure 2a) in γδ T cell activation. Only 3 and 5 were potent FPPS inhibitors (3, IC50=100 nM; 4, IC50=548μM; 5, IC50=3.8μM). The requirement of a positive charge feature for γδ T cell activation is of interest and is reminiscent of the requirement of a positive (imidazolium, ammonium, guanidinium, sulfonium) charge feature in bisphosphonates for FPPS inhibition[14, 15]. This feature is not required for inhibition of cis-prenyl transferases, such as undecaprenyl diphosphate synthase (UPPS), which has 37% identity and 55% similarity to human DeDPPS (and a BLAST e-value of 2 × 10−31 between the two sequences), and since potent UPPS inhibitors (e.g. 6) have no activity in γδ T cell activation, we conclude that γδ T cell activation by 3, 5 is unlikely to be due to inhibition of DeDPPS. Action in the isoprene biosynthesis pathway is clear since two statins (pravastatin and mevastatin) block γδ T cell activation by 3 with the same IC50 values as found for their blocking of γδ T cell activation by risedronate (Figure 2b and Supporting Information Figure S1). That is, the target is in the isoprenoid pathway downstream of HMG-CoA reductase. We find no activity of 3 or 5 against IPPI. However, in addition to FPPS, both 3 and 5 inhibit expressed human DPPS (Supporting Information Figure S2) with IC50 values of 585 nM (3) and 620 nM (5).
Figure 2.
Vγ2Vδ2 T cell stimulation by lipophilic bisphosphonates. (a) γδ T cell stimulation by bisphosphonates evaluated by TNF-α secretion in the presence of CP.EBV(Epstein-Barr Virus) B cells. (b) Inhibition of bisphosphonate-induced γδ T cell proliferative responses by the HMG-CoA reductase inhibitor, pravastatin. The IC50 values are both 2.4 μM. Mevastatin results are in Supporting Information Figure S1. (c) Correlation between IPP levels in CP.EBV cells treated with different concentrations of 1, 3, 4, 5, or zoledronate(determined by following supporting ref 5) and TNF-α release by γδ T cells(determined by following supporting ref 6). (d) Partial sequence alignment between human FPPS and DPPS. (e) Response of blood Vγ2Vδ2 T cells to risedronate, zoledronate and 5 presented by monocytes.
These results indicate that 3 and 5 can inhibit both FPPS and DPPS, which is expected to result in accumulation of the phosphoantigen, IPP. In fact, TNF-α release is directly proportional to IPP levels in the target cells, as shown in Figure 2c (and Supporting Information Table S1) with an R2=0.87 (p<0.0001). Interestingly, the bisphosphonate zoledronate also inhibits DPPS (IC50 = 5.5 μM), but the long alkyl pyridiniums are more potent. In retrospect, the ability of the cationic bisphosphonates to inhibit FPPS as well as DPPS should not be unexpected, since both enzymes contain the two highly conserved “DDXXD” repeats found in most trans-prenyl synthases (including e.g. hexaprenyl diphosphate synthase and octaprenyl diphosphate synthase)[16]. This is illustrated graphically in the partial sequence alignment between human FPPS and human DPPS (the catalytic subunit 1) in Figure 2d. In FPPS, there are two Phe residues that block chain elongation (or the binding of long chain bisphosphonates), but these residues are Ala, Ser in DPPS, permitting stronger binding of 3 and 5. And as expected, lipophilic bisphosphonates such as 1, 4 that are poor FPPS and DPPS inhibitors (FPPS: 1, 126 μM; 4, 0.5 mM; DPPS: 1, 45μM; 4, 24 μM) have essentially no activity in TNF-α release. We thus conclude that these lipophilic bisphosphonates can target both FPPS and DPPS, resulting in elevated IPP levels (and hence, potent γδ T cell activation), due to their more hydrophobic nature.
Intravenous bisphosphonate stimulation of Vγ2Vδ2 T cells in patients for cancer immunotherapy is thought to involve a similar accumulation of IPP, in monocytes[17–19]. To investigate the effects of the lipophilic bisphosphonates on monocytes, we therefore tested the ability of monocytes in PBMC (Peripheral Blood Mononuclear Cell) to stimulate Vγ2Vδ2 T cells in vitro by determining Vγ2Vδ2 T cell expansion. Pulsing of 5 into monocytes present in PBMC stimulated a major expansion of the Vγ2Vδ2 T cell subset with a 12.5-fold lower EC50 compared to the most potent non-lipophilic bisphosphonate, zoledronate (the EC50 was 80 nM for 5, versus 1.0 μM for zoledronate, Figure 2e). Thus, 5 also strongly stimulates Vγ2Vδ2 T cells ex vivo, when monocytes are used as presenting cells.
Overall, these results are of broad general interest since they show that lipophilic, pyridinium bisphosphonates are far more active in γδ T cell activation than are the drugs used in several clinical trials[4, 5]. And since such compounds bind only weakly to bone[12] and inhibit GGPPS, they also have direct activity against tumor cell growth and invasiveness[12], opening up the possibility of new and improved routes to combined cancer chemotherapy and immunotherapy, using lipophilic bisphosphonates.
Experimental Section
Experimental details of human IPPI, FPPS, GGPPS and DPPS inhibition, γδ T cell activation and determination of IPP levels in cells can be found in the Supporting Information.
Supplementary Material
Acknowledgments
We thank K. Kavanagh and U. Oppermann for providing the human FPPS expression system; Johan Wouters for providing the human IPPI expression system; H. Sagami for providing the human GGPPS expression system, and M. Kawamukai for providing the human DPPS expression system. This work was supported by the United States Public Health Service (NIH grants GM065307, GM073216, CA113874, AR 045504 and AI057160). Y.Z. was supported by a Postdoctoral Fellowship from the American Heart Association, Midwest Affiliate.
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- [1].Russell RG, et al. Annu. N. Y. Acad. Sci. 2007;1117:209. doi: 10.1196/annals.1402.089. [DOI] [PubMed] [Google Scholar]; see Supporting Information
- [2].Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Blood. 2000;96:384. [PubMed] [Google Scholar]
- [3].Caccamo N, Meraviglia S, Cicero G, Gulotta G, Moschella F, Cordova A, Gulotta E, Salerno A, Dieli F. Curr. Med. Chem. 2008;15:1147. doi: 10.2174/092986708784310468. [DOI] [PubMed] [Google Scholar]
- [4].Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, Tony HP. Blood. 2003;102:200. doi: 10.1182/blood-2002-12-3665. [DOI] [PubMed] [Google Scholar]
- [5].Dieli F, et al. Cancer Res. 2007;67:7450. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]; see Supporting Information
- [6].Gnant M, et al. N. Engl. J. Med. 2009;360:679. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]; see Supporting Information
- [7].Wiemer AJ, Yu JS, Shull LW, Barney RJ, Wasko BM, Lamb KM, Hohl RJ, Wiemer DF. Bioorg. Med. Chem. 2008;16:3652. doi: 10.1016/j.bmc.2008.02.016. [DOI] [PubMed] [Google Scholar]
- [8].Hirabayashi H, Sawamoto T, Fujisaki J, Tokunaga Y, Kimura S, Hata T. Pharm. Res. 2001;18:646. doi: 10.1023/a:1011033326980. [DOI] [PubMed] [Google Scholar]
- [9].Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Nature. 1995;375:155. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- [10].Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. J. Exp. Med. 2003;197:163. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Thompson K, Rogers MJ. J. Bone Miner. Res. 2004;19:278. doi: 10.1359/JBMR.0301230. [DOI] [PubMed] [Google Scholar]
- [12].Zhang Y, et al. J. Am. Chem. Soc. 2009;131:5153. doi: 10.1021/ja808285e. [DOI] [PMC free article] [PubMed] [Google Scholar]; see Supporting Information
- [13].Wiemer AJ, Tong H, Swanson KM, Hohl RJ. Biochem. Biophys. Res. Commun. 2006 doi: 10.1016/j.bbrc.2006.12.094. [DOI] [PubMed] [Google Scholar]
- [14].Widler L, et al. J. Med. Chem. 2002;45:3721. doi: 10.1021/jm020819i. [DOI] [PubMed] [Google Scholar]; see Supporting Information
- [15].Zhang Y, Hudock MP, Krysiak K, Cao R, Bergan K, Yin F, Leon A, Oldfield E. J. Med. Chem. 2007;50:6067. doi: 10.1021/jm700991k. [DOI] [PubMed] [Google Scholar]
- [16].Guo RT, et al. Proc. Natl. Acad. Sci. U S A. 2007;104:10022. doi: 10.1073/pnas.0702254104. [DOI] [PMC free article] [PubMed] [Google Scholar]; see Supporting Information
- [17].Miyagawa F, Tanaka Y, Yamashita S, Minato N. J. Immunol. 2001;166:5508. doi: 10.4049/jimmunol.166.9.5508. [DOI] [PubMed] [Google Scholar]
- [18].Roelofs AJ, Jauhiainen M, Mönkkönen H, Rogers MJ, Mönkkönen J, Thompson K. Br. J. Haematol. 2009;144:245. doi: 10.1111/j.1365-2141.2008.07435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Eberl M, Roberts GW, Meuter S, Williams JD, Topley N, Moser B. PLoS Pathog. 2009;5:e1000308. doi: 10.1371/journal.ppat.1000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.