Abstract
Protein transduction domains (PTDs) were recently demonstrated to increase the penetration of the model peptide P20 when the PTD and P20 were covalently attached. Here, we evaluated whether non-covalently linked PTDs were capable of increasing the skin penetration of P20. Two different PTDs were studied: YARA and WLR. Porcine ear skin mounted in a Franz diffusion cell was used to assess the penetration of P20 in the stratum corneum (SC) and viable skin (VS); VS consists of dermis and epidermis without SC. The transdermal delivery of P20 was also assessed. At 1 mM, YARA promoted a 2.33-fold increase in the retention of P20 in the SC but did not significantly increase the amount of P20 that reached VS. WLR significantly increased (2.88-fold) the penetration of P20 in VS. Compared to the non-attached form, the covalently linked WLR fragment was two times more effective in promoting the penetration of P20 into VS. None of the PTDs promoted transdermal delivery of P20 at 4 h post-application. It was concluded that selected non-covalently linked PTDs can be used as a penetration enhancer, but greater skin penetration efficiency can be achieved by covalently binding the PTD to the therapeutic agent.
Keywords: Protein transduction domains, Skin penetration, Topical delivery, Peptides
1. Introduction
Topical use of peptides has been increasingly studied due to their importance for the treatment of skin diseases, topical vaccination and for the improvement of skin properties (in the case of cosmetics) [1,2]. However, the outermost layer of the epidermis, the stratum corneum, hinders the skin penetration of compounds, especially hydrophilic peptides [2].
When topically applied, PTDs can deliver covalently attached therapeutic peptides into the skin with very small or no systemic absorption [2,3]. To date, most studies used PTDs covalently attached to the therapeutic peptide, which results in increased synthesis costs and limitations for the use of these carriers. Recently, Wang et al. [4] questioned the requirement of covalent linkage by showing that several arginine-rich intracellular delivery (AID) peptides not covalently attached to model proteins can promote the entry of the model protein into living cells in vitro and into mouse skin in vivo.
The present study was conducted to evaluate whether selected PTDs studied by our group facilitate the skin penetration of a model hydrophilic peptide (namely P20, a phosphopeptide analogue of HSP20) when the PTD and P20 are mixed in aqueous solution but are not covalently attached. We studied the effects of two different PTDs on the skin penetration of P20: YARA (YARAAARQARA) and WLR (WLRRIKAWLRRIKAWLRRIKA). YARA is an optimized version of the TAT transduction domain. When covalently linked to P20, YARA has been shown to penetrate into the skin with the linked peptide [2]. WLR is a proprietary transduction domain with a higher cationic composition developed in our laboratory that also presents the ability to penetrate cell membranes (Patent pending).
2. Materials and methods
2.1. Peptide synthesis and purification
YARA (YARAAARQARA, MW: 1203), WLR (WLRRIKAWLRRIKAWLRRIKA, MW: 2789), FITC-labeled P20 peptide (WLRRAS*APLPGLK, S* is a phosphorylated serine, MW: 1935) and FITC-labeled WLR–P20 (WLRRIKAWLRRAS*APLPGLK, MW: 2859) were synthesized using standard Fmoc solid phase peptide synthesis on an Apex 396 peptide synthesizer (Advanced ChemTech, Louisville, KY, USA). FITC was linked to a β-alanine residue added to the N-terminus of the peptide. The peptides were purified to greater than 90% by Fast Protein Liquid Chromatography (FPLC, Akta Explorer, Amersham Pharmacia Biotech, Piscataway, NJ, USA) using a reversed-phase column and identified by Matrix Assisted Laser Desorption-Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF-MS) using a Voyager-DE STR workstation (Applied Biosystems, Foster City, CA, USA).
2.2. Skin penetration experiments
FITC-labeled P20 was dissolved in phosphate-buffered saline (PBS) at a final concentration of 0.1 mM. Either YARA or WLR was added to the FITC-labeled P20 solution to a final concentration of 0.1 or 1 mM. The solutions (70 μl) were applied to the surface of freshly excised, full-thickness porcine ear skin mounted in a Franz diffusion cell (diffusion area of 1 cm2; Laboratory Glass Apparatus, Inc., Berkeley, CA, USA). A solution of FITC-labeled P20 (0.1 mM) in PBS containing no PTD was used as the control formulation. The receptor cell compartment was filled with phosphate buffer (3 ml, 100 mM, pH 7.2) kept at 37 °C with constant magnetic stirring. After applying the formulations for 4 h, skin surfaces were thoroughly washed with distilled water, and the retention of FITC-labeled P20 in the stratum corneum (SC) and viable skin (VS, meaning dermis and epidermis without SC) was assessed. The skin penetration experiment was conducted for 4 h because, in a previous study, maximal penetration of PTDs at this time point was observed [2]. Skin samples were subjected to tape stripping for quantification of FITC-labeled P20 in the SC using 15 pieces of adhesive tape. The pieces of tape were immersed in 3 ml of a water:methanol solution (1:1 v/v) and stirred in a vortex for 2 min. The resulting solution was filtered through a 450-nm membrane. FITC-labeled P20 in the remaining VS was determined by homogenizing VS in 2 ml of a water:methanol solution (1:1 v/v) using a tissue homogenizer, followed by bath sonication for 15 min and filtration through a 450-nm membrane. The receptor phase (3 ml) was lyophilized and suspended in 0.3 ml of water to evaluate the transdermal delivery of P20. In a separate experiment, a solution of FITC-labeled WLR–P20 (covalently linked, 0.1 mM) was used to verify whether the attachment of P20 to WLR improves its skin penetration.
FITC-labeled P20 was quantified by fluorimetry using a Gemini SpectraMax microplate reader (Molecular Devices, Sunnyvale, CA, USA) with excitation at 495 nm and emission at 518 nm. The method was linear within the concentration range studied (0.05–2.0 μM). To evaluate the recovery of FITC-labeled P20 from the skin in the extraction procedure, tissue sections (1 cm2) were spiked with 20 μl of a 0.5 mM solution of FITC-labeled P20 in a water:methanol solution (1:1 v/v). After 20 min, the skin sections were homogenized as described above. The recovery of the peptide was ~85%, and this recovery percentage was accounted for in the quantification of P20.
2.3. Fluorescence microscopy
To visualize the skin penetration of FITC-labeled P20, fluorescence microscopy was performed. At the end of the penetration experiment (4 h), skin was frozen using iso-pentane at −30 °C, embedded in Tissue-Tek® OCT compound (Pelco International, Redding, CA, USA), and sectioned (8 μm) using a cryostat microtome (Leica, Wetzlar, Germany). The tissue sections were visualized without any additional staining or treatment through a 20× objective by fluorescence microscopy (Carl Zeiss, Thornwood, NY, USA). Tissue auto-fluorescence was assessed using skin sections treated with PBS.
2.4. Statistical analysis
The results were reported as means ± SD. Data were statistically analyzed by non-parametric Kruskal–Wallis test followed by Dunn post-test. The level of significance was set as p < 0.05.
3. Results and discussion
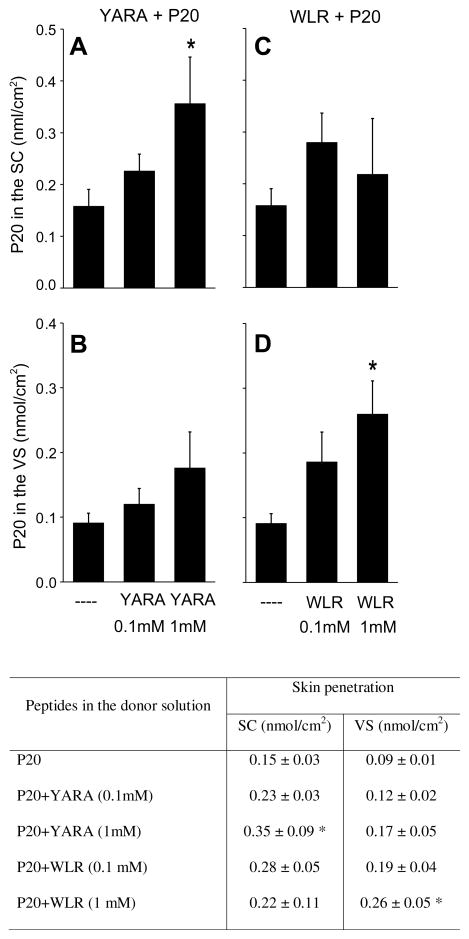
Since P20 is a hydrophilic peptide and has a relatively high molecular weight, its skin penetration is expected to be small [5]. At 4 h post-application, only 0.15 ± 0.03 and 0.09 ± 0.01 nmol/cm2 of the peptide were detected in SC and VS, respectively (Fig. 1), which is equivalent to 2.2% and 1.3% of the applied dose, respectively. Even at the higher concentration (1 mM), non-attached YARA had no significant effect on P20 penetration into VS, even though it promoted a 2.33-fold increase (p < 0.05) in the retention of P20 in the SC (Fig. 1A and B). Only when covalently attached does YARA significantly increase the penetration of P20 in VS (~5-fold increase), and it does so at a concentration of 0.1 mM [2]. Whether in the non-attached (present study) or attached [2] form, YARA did not promote transdermal delivery of P20 after 4 h of application.
Fig. 1.
In vitro penetration of FITC-labeled P20 in the stratum corneum (SC) (A and C) and viable skin (VS) (B and D) after 4 h. This figure also displays a table containing the numeric data plotted in the graph. The skin was treated with FITC-labeled P20 (0.1 mM, control solution), in the presence or absence of YARA (0.1 or 1 mM) or WLR (0.1 or 1 mM). The results were reported as means ± SD; the number of replicates was 5–6 per experimental group. *p < 0.05 compared to the control solution.
Non-attached WLR at 1 mM (but not at 0.1 mM) promoted a significant (2.88-fold) increase in the penetration of P20 in VS. No transdermal delivery or increased retention in the SC was observed. The ability of WLR to significantly increase the penetration of P20 in VS was further explored by fluorescence microscopy (Fig. 2). Pictures of PBS-treated skin sections (used as controls) are shown in Fig. 2A (halogen light) and B (auto-fluorescence). As expected [6], the skin treated with PBS presented a very weak auto-fluorescence. Treatment of the skin with FITC-labeled P20 resulted in fluorescent staining of SC with only a very weak fluorescence observed in the epidermis (Fig. 2C). When the skin was treated with WLR at 1 mM and FITC-labeled P20, we observed strong fluorescence in the SC and epidermis, but little to no fluorescence in the dermis (Fig. 2D). This result was consistent with the lack of an effect of WLR on the transdermal delivery of P20.
Fig. 2.
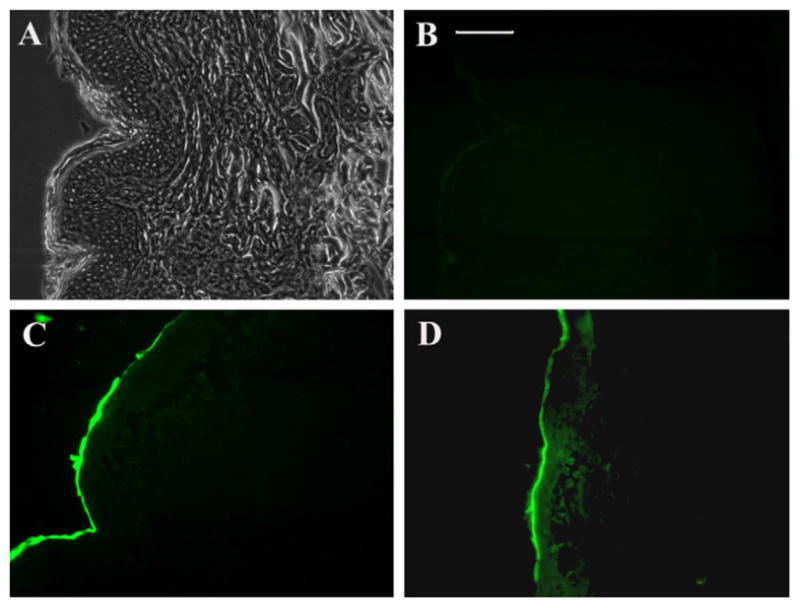
Fluorescence microscopy of porcine ear skin sections 4 h after application of PBS or FITC-labeled P20 solutions. (A) PBS-treated skin section observed under halogen light, (B) PBS-treated skin section observed under fluorescent light to show auto-fluorescence, (C) skin sections treated with FITC-labeled P20, (D) skin sections treated with FITC-labeled P20 and WLR (1 mM). Sections were visualized using FITC filter through a 20× objective. Scale bar, 100 μm.
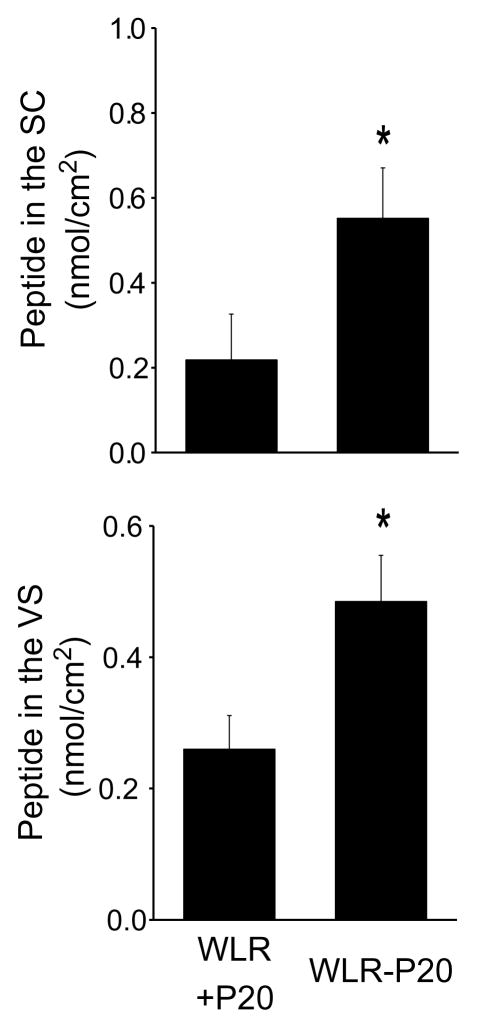
Having demonstrated that non-attached WLR at 1 mM significantly increased the penetration of P20 in VS compared to the control solution, we next evaluated the skin penetration of a WLR fragment covalently attached to P20 (Fig. 3). The fragment was selected so that the molecular mass of the WLR–P20 conjugate was similar to that of non-attached WLR. The WLR–P20 conjugate used is able to penetrate rat astrocyte cells in vitro (patent pending). Interestingly, the attached WLR fragment was two times more effective than non-attached WLR in promoting the penetration of P20 into VS. This difference might be underestimated considering that the WLR–P20 conjugate was used at a concentration ten times smaller than the non-covalently linked WLR.
Fig. 3.
In vitro peptide penetration in the stratum corneum (SC) and viable skin (VS) after 4 h. The skin was treated with 0.1 mM FITC-labeled P20 mixed with 1 mM WLR (WLR+P20, also represented in Fig. 1) or 0.1 mM FITC-labeled WLR–P20. The results were reported as means ± SD; the number of replicates was 6 per experimental group. *p < 0.05 compared to WLR+P20.
The ability of PTDs to penetrate the skin and carry conjugated peptides is demonstrated in the literature [2,3]. However, the exact mechanism by which PTDs can promote the penetration of a peptide into the skin and other tissues is still unclear [3,4]. Although in this study we did not investigate the mechanisms by which covalently attached or non-attached PTDs can promote the penetration of peptides into viable layers of the skin, some speculation can be made. Micropinocytosis was described as an important mechanism for the penetration of PTDs across cell membranes [7]. This mechanism, however, is unlikely to be involved in the penetration of non-attached PTDs into the skin, because the outermost layer of the skin is composed of non-viable cells. Two mechanisms might explain the skin penetration-enhancing effect of non-attached PTDs. First, it is well known that several PTDs are able to interact with lipids [8], and this interaction might destabilize the SC and make it more permeable. Second, PTDs such as poly-arginine increase the permeability of mucosal tight junctions [9]. The presence of tight junctions in the skin has already been demonstrated [10], and the disruption of these structures by PTDs might be important for successful penetration of peptides into the viable layers of skin.
In conclusion, the approach presented here is innovative because it demonstrates that some PTDs can be used as penetration enhancers when the PTD and the drug are not covalently linked. However, in spite of the obvious advantages of using PTDs as penetration enhancers in a non-attached manner, the PTDs studied here promote a higher penetration of P20 when the PTD and P20 are covalently attached.
Acknowledgments
We thank Dr. Alexandre A. Steiner (Albany College of Pharmacy, Albany, NY, USA) for critical comments on the manuscript. This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” (CAPES, Brazil) and Conselho Nacional de Pesquisa (CNPq, Brazil) L.B. Lopes was the recipient of a CAPES fellowship.
Footnotes
Publisher's Disclaimer: This article was published in an Elsevier journal. The attached copy is furnished to the author for non-commercial research and education use, including for instruction at the author’s institution, sharing with colleagues and providing to institution administration.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit:
References
- 1.Partidos CD, Beignon AS, Mawas F, Belliard G, Briand JP, Muller S. Immunity under the skin: potential application for topical delivery of vaccines. Vaccine. 2003;21:776–780. doi: 10.1016/s0264-410x(02)00597-2. [DOI] [PubMed] [Google Scholar]
- 2.Lopes LB, Brophy CM, Furnish E, Flynn CR, Sparks O, Komalavilas P, Joshi L, Panitch A, Bentley MVLB. Comparative study of the skin penetration of protein transduction domains and a conjugated peptide. Pharm Res. 2005;22:750–757. doi: 10.1007/s11095-005-2591-x. [DOI] [PubMed] [Google Scholar]
- 3.Rothbard JB, Garlington S, Lin Q, Kirschberg T, Kreider E, Mcgrane PL, Wender PA, Khavari PA. Conjugation of arginine oligomers to cyclosporin A facilitates topical delivery and inhibition of inflammation. Nat Med. 2000;6:1253–1257. doi: 10.1038/81359. [DOI] [PubMed] [Google Scholar]
- 4.Wang AH, Chen CP, Chan MH, Chang M, Hou YW, Chen HH, Hsu HR, Liu K, Lee HJ. Arginine-rich intracellular delivery peptides noncovalently transport protein into living cells. Biochem Biol Res Commun. 2006;346:758–767. doi: 10.1016/j.bbrc.2006.05.205. [DOI] [PubMed] [Google Scholar]
- 5.Bos JD, Meinardi MMHM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9:165–169. doi: 10.1034/j.1600-0625.2000.009003165.x. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez-Román R, Naik A, Kalia YN, Fessi H, Guy RH. Visualization of skin penetration using confocal laser scanning microscopy. Eur J Pharm Biopharm. 2004;58:301–316. doi: 10.1016/j.ejpb.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 7.Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion protein after lipid raft macropinocytosis. Nat Med. 2004;3:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- 8.Thoren PE, Persson D, Esbjorner EK, Goksor M, Lincoln P, Norden B. Membrane binding and translocation of cell penetrating peptides. Biochemistry. 2004;43:3471–3489. doi: 10.1021/bi0360049. [DOI] [PubMed] [Google Scholar]
- 9.Ohtake K, Maeno T, Ueda H, Natsume H, Morimoto Y. Poly-L-arginine predominantly increases the paracellular permeability of hydrophilic macromolecules across rabbit nasal epithelium in vitro. Pharm Res. 2003;20:153–160. doi: 10.1023/a:1022485816755. [DOI] [PubMed] [Google Scholar]
- 10.Morita K, Myachi Y. Tight junctions in the skin. J Dermatol Sci. 2003;31:81–89. doi: 10.1016/s0923-1811(03)00038-0. [DOI] [PubMed] [Google Scholar]