Abstract
The incidence of malignant melanoma is increasing faster than any other cancer. In cases of recurrent melanoma confined to the extremities, hyperthermic isolated limb perfusion and isolated limb infusion provide a way to isolate the extremity and deliver a dose of chemotherapy several orders of magnitude higher than would be tolerated systemically. Although complete response rates of up to 80% for hyperthermic isolated limb perfusion and 44% for isolated limb infusion have been observed, there is still room for improvement and standardization in these two procedures in an attempt to optimize response while minimizing toxicity. Currently, new chemotherapy agents and small-molecule inhibitors are being investigated as a means of overcoming chemoresistance and improving response rates. In patients with advanced cutaneous disease confined to the extremities, evaluation of these new therapies can be very informative, as tissue acquisition at multiple treatment time points is easy owing to the superficial and multifocal nature of the disease. Through studying the biomolecular and genetic alterations in tumor tissue in response to these new therapies, genetically customized treatment regimens in which tumor resistance and sensitivity is predicted and treatment strategy is optimized before treatment begins may soon be available. Progress in regional therapy will prove not only beneficial for patients with disease confined to an extremity, but may also provide insight into developing novel treatment strategies for patients with systemic disease for whom current disease management options are poor.
Keywords: ADH-1, bevacizumab, butathione sulfixime, dasatinib, hyperthermic isolated limb perfusion, IFN-γ, in-transit melanoma, isolated limb infusion, melanoma, melphalan, O6-benzylguanine, regional chemotherapy, satellitosis, sorafenib, temozolomide, TNF-α
In-transit melanoma of the extremities
The incidence of melanoma is increasing faster than any other cancer [1], and in 2008, approximately 62,480 people were newly diagnosed with melanoma in the USA alone [2]. This number makes up only 4% of the total number of dermatologic cancers, yet melanoma is responsible for 80% of the total mortality due to skin cancer [3]. Despite its relative rarity as a type of skin cancer, melanoma is the most lethal of skin malignancies because it often recurs following initial surgical resection, is disposed to metastasis and is highly resistant to conventional chemotherapy [4].
Approximately half of the newly diagnosed cases of melanoma are isolated to the extremities, and in 6–11% of these, lesions recur in a local–regional fashion, confined to the extremity and located somewhere between the site of the primary lesion and the regional lymph nodes [4–7]. These so-called ‘in-transit’ lesions most commonly afflict the lower extremities and can result in disabling effects by causing pain, bleeding, ulceration, infection and psychological distress. Treatment of recurrent regional melanoma is important, as at least half of these patients survive for at least 2 years without evidence of distant disease [8].
Regional treatment for extremity melanoma
Wide local excision is currently the standard of care for managing newly diagnosed cutaneous melanoma, as it has been for decades. Prior to the 1950s, however, if melanoma recurred after appropriate initial surgical therapy, wide local excision and, to some extent, systemic chemotherapy were the extent of treatment options. With the understanding that in-transit melanoma recurs in a local–regional pattern despite the surgeon’s best efforts to remove all visible tumor, Krementz and Creech developed a technique that would treat both the superficial disease affecting the extremity and the subclinical disease that would ultimately proliferate into new, visible lesions and could, theoretically, seed the rest of the limb and the body. This technique, termed isolated limb perfusion (ILP), consists of isolating and subsequently circulating within the affected limb a high-dose of chemotherapy. In so doing, the affected limb would be exposed to a dose of chemotherapy ten to 20-times higher than could be given systemically, while at the same time sparing the presumably unaffected remainder of the body [9].
The first ILP was performed at Charity Hospital in New Orleans (LA, USA) in 1958. The patient was a 76-year-old male who had developed extensive satellitosis following the excision of his primary lesion 2 years prior. As was common in the day, the patient was offered amputation but refused in favor of the ILP proposed by Krementz and Creech but never before attempted in humans. The chemotherapeutic agent proposed by the surgeons for the first ILP was melphalan based on contemporary evidence suggesting that the agent had significant in vivo anti-tumor activity in mice [10].
Following sufficient anesthetization and heparinization, the patient underwent surgical exposure and open cannulation of the external iliac artery and vein. An Esmarch tourniquet was placed at the most proximal aspect of the lower extremity, proximal to the cannulated vessels, to isolate the extremity from systemic circulation. Subsequently, the cannulas were connected to an extracorporeal high-flow oxygenating pump that circulated the oxygen-rich, chemotherapy-enriched perfusate for 23 min. At the conclusion of the perfusion the tourniquet was released, the vascular cannulas were removed and the vessels were surgically repaired. Subsequently, the patient experienced a complete response (CR; defined as disappearance of all lesions), which endured for at least 16 years, at which time he died of unrelated causes [10].
Although the outcomes of successive patients treated with ILP by Krementz and Creech were not nearly as successful (of the next 18 patients, two died and only one experienced a CR [11]), the initial success of the maiden procedure all but set in stone the surgical technique (Figure 1) and chemotherapy choice (melphalan) for ILP. A number of contributions have been made by other investigators over the years, including the use of hyperthermia to augment the cytotoxicity of melphalan by Cavaliere [12] and Stehlin [13] and the utilization of dactinomycin in combination with melphalan by Sanki [14], but the fundamentals of ILP have largely remained the same.
Figure 1. Hyperthermic isolated limb perfusion.
Following the achievement of a hyperthermic limb temperature and surgical exposure and open cannulation of the external iliac artery and vein, a chemotherapy-enriched (melphalan) perfusate is circulated through the affected limb. Acid–base status in the extremity is maintained through oxygenation of the perfusate.
ILP outcomes
Over the past 20 years, a number of investigators have reported their findings on the response rates observed in their patients treated with ILP, both hyperthermic (HILP) and normothermic. The CR rates ranged from 39 to 82% (Table 1) [14–22], a wide variation partially explained by variations in clinical assessment and procedural details [10]. Generally, single-center series have much higher CR rates compared with multicenter randomized trials, such as the American College of Surgeons Oncology Group (ACOSOG) Z0020 trial [22].
Table 1.
Response rates following isolated limb perfusion with melphalan in patients with melanoma.
| Study | Patients (n) | Conditions | CR (%) | PR (%) | Ref. |
|---|---|---|---|---|---|
| Minor (1985) | 18 | Hyperthermia | 82 | 18 | [15] |
| Storm (1985) | 26 | Hyperthermia | 50 | 31 | [16] |
| Kroon (1987) | 18 | Normothermia | 38 | 44 | [17] |
| Di Filippo (1989) | 69 | Hyperthermia | 39 | 43 | [18] |
| Kroon (1993) | 43 | Normothermia | 77 | 14 | [19] |
| Klasse (1994) | 120 | Normothermia | 54 | 25 | [20] |
| Aloia (2005) | 59 | Hyperthermia | 57 | 31 | [21] |
| Cornett* (2006) | 58 | Hyperthermia | 25 | 39 | [22] |
| Sanki (2007) | 120 | Hyperthermia | 69 | 15 | [14] |
Randomized, controlled trial.
CR: Complete response; ILP: Isolated limb perfusion; PR: Partial response.
The achievement of a CR following ILP has been shown to be a strong prognostic variable for long-term survival in a number of studies [14,23,24]. Unfortunately, there are no randomized, controlled trials that have demonstrated unequivocally that ILP prolongs survival.
In terms of toxicity, patients undergoing ILP and HILP may develop lymphedema (30–40%), compartment syndrome (10–15%) and long-term peripheral neuropathy (5–8%). Additionally, there is a 1–2% chance of severe limb toxicity necessitating amputation [25]. Erythema, desquamation, alopecia, onycholysis, skin color changes, peripheral neuropathy and pain are also common in the treated limb during the first few weeks of the procedure but are typically only transient [26].
Hyperthermic isolated limb perfusion has been studied in the adjuvant setting by a number of investigators. Although early analysis demonstrated a small long-term survival advantage and lower frequency of recurrence, a subsequent randomized, controlled trial of 852 patients randomized to either wide local excision (WLE) or WLE with HILP concluded that there was no difference in survival or time to development of distant metastases. Consequently, HILP is not recommended as an adjuvant treatment to primary therapy of newly diagnosed American Joint Committee on Cancer stage I and II melanoma and, by extrapolation, is generally not utilized adjuvantly in patients in whom recurrent or in-transit disease can easily be removed by surgical excision [5].
Other agents in addition to melphalan have been evaluated in the setting of HILP. These agents include dimethyltriazeno imidazole carboxamide, cisplatin, carboplatin and thiotepa. However, none of these agents have been evaluated extensively in formal Phase I or Phase II trials, and as such melphalan is still the drug of choice for HILP.
Strategies to improve the efficacy of HILP have most notably focused on TNF-α, which demonstrated promise in a Phase II trial conducted by Leinard et al. in which patients were treated with regionally-administered TNF-α, IFN-γ and melphalan via HILP. Although morbidity was significant, at a mean follow-up of 11 months, 89% of patients (n = 21) had a CR and 11% (n = 2) had a partial response (PR) following HILP with the combination of drugs [27]. These promising results have since been undermined by a recent randomized, controlled trial carried out by the ACOSOG, which failed to demonstrate improved efficacy of melphalan-based HILP in combination with TNF-α but which did show a higher risk of common terminology criteria for adverse events (CTCAE) grade IV adverse events (see Table 2 for an overview of CTCAE), including amputations in the group receiving both melphalan and TNF-α via HILP [22].
Table 2.
Common terminology criteria for adverse events: overview.
| Toxicity grade | Description |
|---|---|
| 1 | Mild adverse event, present but may be asymptomatic |
| 2 | Moderate adverse event, symptomatic but may not require intervention |
| 3 | Severe adverse event, may require intervention |
| 4 | Life-threatening or disabling adverse event |
| 5 | Death related to adverse event |
Adapted from [101].
Isolated limb infusion: an alternative to ILP
In 1996, John Thompson of the Sydney Melanoma unit (SMU) developed isolated limb infusion (ILI) as a low-morbidity, technically simple alternative to HILP. Instead of achieving vascular access to the affected limb by surgical exposure, arterial and venous catheters are placed by interventional radiology via the contralateral groin using a standard Seldinger technique and, in the case of the lower extremity ILI, threaded around the aortic and vena caval bifurcations into the femoral artery and vein of the affected lower extremity. Once the correct placement of the catheters is confirmed by x-ray, a pneumatic or Esmarch tourniquet is placed at the most proximal aspect of the affected limb in order to isolate it, just as in HILP. The chemotherapy-enriched infusate (most commonly 5–10 µg/l of melphalan and 50–100 µg/l of dactinomycin) is delivered via the arterial catheter by a pressure bag and intravenous fluid pump set. Circulation is driven manually via a syringe and high-flow, three-way stopcock (Figure 2). The procedure typically lasts between 2 and 3 h, with the infusion lasting only 20–30 min [26].
Figure 2. Schematic of hyperthermic isolated limb infusion.
Following placement of catheters percutaneously in the popliteal artery and vein, a chemotherapy-enriched (melphalan) solution is infused through the circulation by hand when limb temperatures reach 37.0°C. Since there is no oxygenator in the system the limb becomes hypoxic and acidotic.
Associated with ILI are many of the same toxicities observed with HILP, including transient tissue lymphedema, erythema, desquamation, alopecia, onycholysis, skin color changes, peripheral neuropathy and pain [26]. However, as in the case of HILP, these side effects typically resolve over a few months following ILI. Although the chance of limb loss is much lower than it is for HILP, it is not zero, and a number of institutions have treated patients whose ILI postoperative course required an amputation [25].
Still, the perceived regional and long-term toxicity associated with ILI is low. That ILI is used as a palliative treatment procedure for the control of in-transit extremity melanoma in patients with distant metastases is a testament to this perception. Kroon and colleagues at the SMU recently released a report of 37 patients with distant metastases on whom the group performed ILI for the purposes of disease palliation and regional disease control. These patients suffered from lesions that were ulcerated, necrotic, bleeding, severely painful, malodorous and/or expected to cause severe symptoms in the near future if left untreated. The group observed a limb salvage rate of 86% (all patients with a CR, PR or stable disease response) and an overall response (OR) rate of 76% despite the chemotherapy-refractory nature of stage IV melanoma [28].
Major differences between ILP & ILI
Although both ILP and ILI permit the isolated diseased limb to be treated with doses of chemotherapy several orders of magnitude higher than would be tolerated systemically, there are a number of important differences between the two procedures, which are summarized in Table 3. ILI is associated with less surgical morbidity and operating room time (2–3 h, compared with 4–6 h for HILP), permitting elderly and frail patients to undergo the procedure and allowing repeat procedures without prohibitively increasing complication rates, as occurs when repeat ILPs are undertaken [4]; ILI does not require a perfusion team or a heart– lung bypass machine, making it cheaper, less resource-intensive and logistically easier to perform compared with ILP; and because the blood is not oxygenated during ILI, the limb becomes acidotic and hypoxic, potentially augmenting the activity of melphalan [29,30]. HILP is not without its potential advantages as well. First, the flow rate during HILP is higher (150–1000 ml/min for ILP [31] vs 50–100 ml/min for ILI [32]) and lasts longer than with ILI (60-min perfusion vs 20–30-min infusion, respectively), potentially resulting in increased drug delivery to and increased drug uptake by the tumor (the theory that increased chemotherapy exposure results in increased tumor uptake has not, however, been supported in the literature [33]). Second, because of the higher flow rates possible with the perfusion technique, true limb hyperthermia (subcutaneous limb temperature > 41°C) may be achieved in the case of HILP but not in the case of ILI, in which subcutaneous limb temperature rarely exceeds 39°C. Hyperthermia is thought to augment melphalan cytotoxicity [12,13].
Table 3.
Major differences between isolated limb perfusion and isolated limb infusion.
| Parameter | Isolated limb perfusion | Isolated limb infusion |
|---|---|---|
| Technical complexity | High | Low |
| Method of vascular access attainment |
Surgical exposure and open cannulation |
Percutaneous vascular catheter placement by interventional radiology |
| Infusion/perfusion time |
60 min | 30 min |
| Procedure duration | 3–6 h | 2–3 h |
| Specialized staff requirement |
Perfusionist and ancillary staff |
No perfusionist and fewer ancillary staff |
| Equipment requirement |
Heart–lung bypass machine | Three-way stopcock and extra 20 cc syringes |
| Anesthesia | General anesthesia required | Possible with regional anesthesia |
| Patient candidates | Frail, elderly, medically compromised are excluded |
Well tolerated even by poor surgical candidates |
| In patients with occlusive peripheral vascular disease |
Not possible | May be performed if superficial femoral vessels are occluded |
| Repeatability | Technically difficult with significantly higher complication rate |
Not difficult |
| Metastases as a contraindication |
Yes | No |
| Blood transfusion requirement |
Routine requirement in order to prime extracorporeal circuit |
Not required |
| Chances of systemic leakage |
Higher perfusion pressure increases likelihood |
Lower pressure system limits leakage |
| Blood oxygenation | Physiological O2 and pH maintained |
Progressive hypoxia and acidosis |
| Limb temperature | Hyperthermia (41–41.5°C [subcutaneous] may be achieved) |
Rarely does limb (subcutaneous) temperature rise to greater than 39°C |
ILI outcomes
Thompson and colleagues at the SMU published the first data on ILI outcomes. The group reported an OR rate of 85% with a CR and PR rate of 41 and 44%, respectively, for 128 ILIs performed on patients with extremity melanoma [32]. The group recently published data that included this initial experience of 128 ILIs as well as 57 subsequent ILIs. In 185 ILIs, the group found a CR rate of 38% and a PR rate of 46% [34]. The experience in the USA, however, has demonstrated slightly less efficacy, with the two major centers publishing on ILI – Duke University Hospital and Memorial Sloan–Kettering Cancer Center (MSKCC) – reporting CR and PR rates of 23–30% and 14–27%, respectively [35,36]. A recent review of 128 ILI procedures performed at eight institutions in the USA yielded a CR rate of 31%, a PR rate of 33% and no response in 36% of patients [25]. Higher disease stage is part of the reason for the disparity between the Australian and the American experience. When the SMU patients are stratified according to disease stage and tumor burden, response rates were similar [25]. Other factors that may explain this discrepancy include procedural modifications (e.g., differences in infusion and tourniquet time), variations in experience and the application of inconsistent response criteria [4]. A summary of ILI outcomes is given in Table 4 [25,32,34–38].
Table 4.
Response rates following isolated limb infusion in patients with melanoma.
| Study (year) | Drugs | Patients (n) | Response criteria | CR (%) | PR (%) | SD (%) | PD (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Mian et al. (2001) | Melph + dactino | 9* | Best response | 44 | 56 | 0 | 0 | [34] |
| Lindner et al. (2002) | Melph ± dactino | 128 | Best response | 41 | 44 | 12 | 4 | [29] |
| Bonenkamp et al. (2004) |
Fote + dacarb‡ | 13 | Best response | 31 | 61 | 0 | 8 | [35] |
| Brady et al. (2006) | Melph + dactino | 22§ | 3 months | 23 | 27 | 0 | 50 | [33] |
| Beasley et al. (2008) | Melph + dactino | 50 | 3 months | 30 | 14 | 10 | 46 | [32] |
| Kroon et al. (2008) | Melph + dactino | 185¶ | Best response | 38 | 46 | 10 | 6 | [31] |
| Beasley et al. (2009) | Melph ± dactino | 128 | 3 months | 31 | 33 | 7 | 29 | [23] |
Three patients had more than one isolated limb infusion.
Systemic administration.
One patient had advanced sarcoma.
Includes 128 patients reviewed in [29].
CR: Complete response; Dacarb: Dacarbazine; Dactino: Dactinomycin; Fote: Fotemustine; Melph: Melphalan; PD: Progressive disease; PR: Partial response; SD: Stable disease.
Patients who are nonresponsive or who progress either within the ILI perioperative period or after experiencing a CR or PR may be offered a second ILI. The SMU reported an OR rate in patients receiving a repeat ILI with melphalan and dactinomycin of 83% – not significantly different from the OR rate in patients treated with first-time ILIs. However, the CR rate was lower (23 vs 38%) and complications were more common (five grade IV toxicities vs one grade IV toxicity) in the group receiving a second infusion compared with the group receiving a single infusion [39].
For those patients who do experience a CR or PR, recurrence and progression are more common than not and no study has ever demonstrated that ILI (or ILP for that matter) improves survival in patients with in-transit extremity melanoma [34]. In the SMU study the median overall duration of response was 13 months, with a 22-month duration for patients who experienced a CR [34]. This compares with a 12-month median duration of CR observed at both MSKCC and Duke [35,36].
ILP versus ILI: outcomes
Unfortunately, there are no randomized, controlled trials that directly compare the efficacy and safety of perfusion and infusion. Comparisons of ILI with ILP between studies are problematic from the standpoint of matching stage and extent of disease, but some generalizations can be made. In the aforementioned summarized data, the response rates following ILI (23–41%) fall at the lower end of the response rates observed following ILP (25–82%) [32,34].
In terms of regional toxicity, there is some debate regarding whether or not ILI is associated with less toxicity than HILP. In a Duke study, 22% of patients undergoing ILI experienced a regional toxicity of grade 3 and above, while 44% of those patients undergoing HILP experienced a similar toxicity. However, in the study the melphalan dose was adjusted for ideal bodyweight (IBW) in 66% of the ILIs but in only 22% of HILPs, potentially skewing the toxicity results in favor of ILI [35]. The SMU has found comparable regional toxicity with HILP and ILI [34,40]. Long-term morbidity after ILI is less common and less severe. Notably, the catastrophic complication of limb loss related to the regional procedure occurs in 2% of HILP procedures versus 0.3% of ILI procedures [25].
Expert commentary
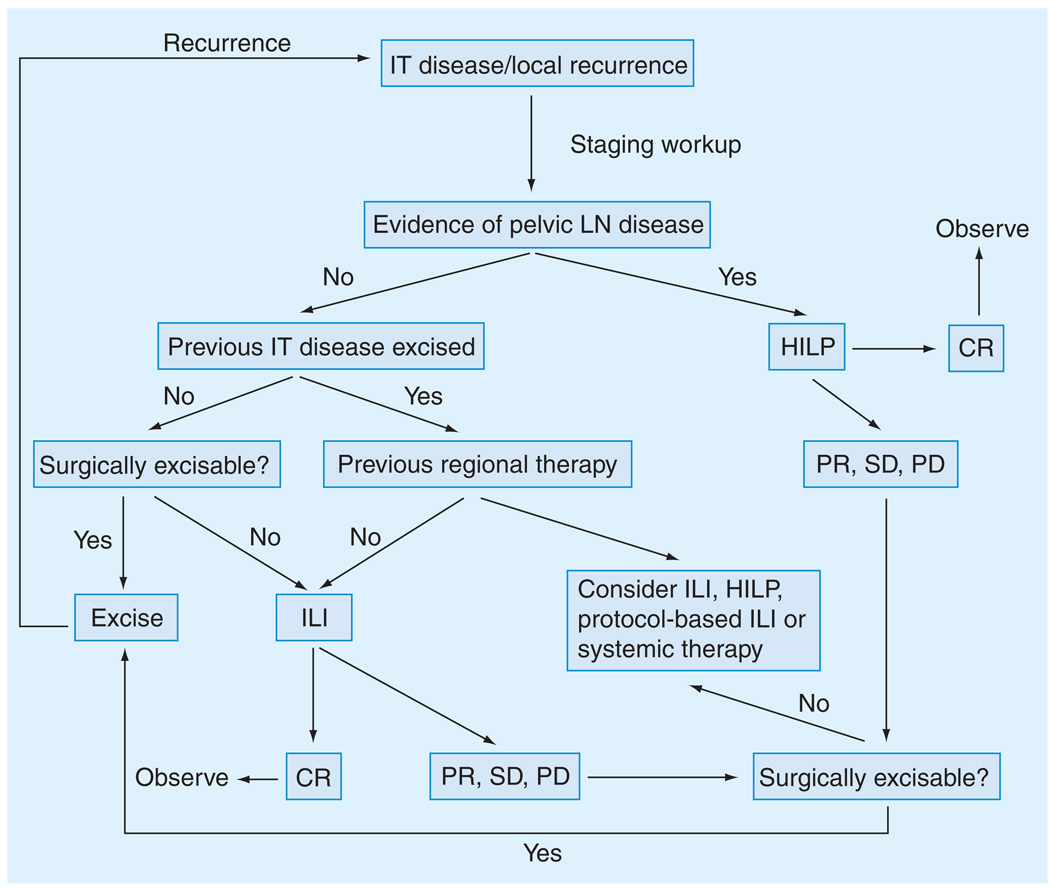
Although there seems to be the suggestion from the summarized data that ILP is superior to ILI in terms of clinical response, the possible benefit of increased response needs to be balanced with the potential for increased toxicity. Only a randomized, controlled trial comparing the two modalities could settle the debate. Given the data as currently outlined, we have developed the following algorithm for managing patients with upper or lower extremity in-transit disease utilizing WLE, ILI, HILP and/or systemic therapy (Figure 3). In patients with recurrent melanoma isolated to the extremity in which pelvic lymph node involvement is present, we advocate HILP initially as the pelvic vessels represent the highest level of isolation for the leg and usually cannot be completely redissected out (especially the pelvic vein) after a nodal dissection. For patients with axillary or inguinal lymph node disease we perform ILI and then do the nodal dissection after the ILI is finished and the heparin reversed. In the absence of lymph node involvement, we advocate repeat WLE if it is feasible (i.e., in cases of low disease burden) or ILI. The surgical morbidity of ILI is sufficiently low that repeat procedures can be performed should the patient again recur following the initial ILI. Should the initial ILI fail, we typically offer the patient enrollment in a clinical trial exploring novel therapy combinations (discussed later), which may help to overcome melphalan resistance. Additionally, we may offer repeat standard ILI or limb perfusion at that time. This allows us not only to offer patients more treatment choices, but also to test new chemosensitizing agents in a setting that is conducive to tissue acquisition (i.e., tissue biopsies) [25].
Figure 3. Algorithm for treatment of intratumoral extremity melanoma.
CR: Complete response; HILP: Hyperthermic isolated limb perfusion; ILI: Isolated limb infusion; IT: In-transit; LN: Lymph node; PD: Progressive disease; PR: Partial response; SD: Stable disease.
Reproduced from [25].
Optimization strategies: five-year view
Since the origination of ILP and ILI, investigators have been attempting to streamline and fine-tune the procedures by modifying any of the numerous variables at play in the procedure, such as perfusion/infusion time, tourniquet (i.e., ischemic) time, limb temperature and chemotherapy dosing (corrected for IBW vs limb volume-based only), to name just a few. More recently, optimization strategies have expanded to include the use of novel agents and new chemotherapies. The remainder of this review will discuss these strategies and the degree to which they have already improved clinical response and decreased toxicity or have the potential to do so in the future.
Procedural optimization
Although ILI seems to be less toxic than HILP, neither procedure is without morbidity. Optimization strategies aimed at lessening toxicity have focused on melphalan pharmacokinetics, specifically delivering a standard dose of melphalan that is high enough to maximize the therapeutic index of the drug without administering a quantity so large as to cause excessive toxicity without improving the response. Cheng and colleagues studied pharmacokinetic data derived from 14 standard HILPs in melanoma patients and found a fivefold difference in melphalan plasma concentration between patients despite using a similar dose estimation calculation. As may be expected, this large range of plasma concentrations resulted in varying degrees of toxicity, with five patients suffering from major toxicity (three CTCAE grade III and two grade IV toxicities). In studying the pharmacokinetic profiles of the HILP procedures in these patients and comparing them with the profiles from the other patients who did not experience severe adverse events, the group found that the ratio of estimated limb volume for treatment (Vesti) to melphalan volume distribution (Vss) was the best predictor of acute regional toxicity [41]. These findings suggest that toxicity from HILP may be associated with overestimation of melphalan dose based on limb volume. Additionally, the group found that increased toxicity was not associated with improved outcome, supporting the findings of a number of other studies [42,43]. Beasley and colleagues analyzed pharmacokinetic parameters from a subset of 21 patients undergoing ILI and found a wide distribution in mean plasma melphalan concentrations similar to those observed by Cheng et al. Just as Cheng and colleagues had observed, the Vesti–Vss ratio was highly associated with extremity toxicity. In the subsequent 40 patients undergoing ILI, the group corrected the melphalan dose for IBW by multiplying the Vesti (derived from circumferential measurements of the affected limb taken at 1.5-cm longitudinal intervals, encompassing the entire region to be infused from the most distal aspect to the most proximal aspect) by the ratio of IBW to absolute bodyweight. As a consequence, there was less variation in mean melphalan concentrations and less toxicity (10% ≥ grade III toxicity in patients with doses adjusted for IBW versus 33% of patients with doses not adjusted for IBW) observed in these patients. Importantly, there was no significant difference in tumor response between patients whose melphalan doses had been corrected for IBW and those patients whose dose had not been corrected for IBW [35]. McMahon and colleagues observed similar results, concluding that correcting for IBW in ILI lowers toxicity without negatively impacting response [44].
A recent multi-institutional review of 162 ILIs by Beasley and colleagues has substantiated the observation that correcting melphalan dosing for IBW decreases toxicity. In contrast to the smaller study, however, the larger review found a correlation between OR and melphalan dose not corrected for IBW. However, there was no difference in CR rate between patients treated with a melphalan dose corrected for IBW and patients treated with a dose not corrected for IBW [25]. At the current time, there is no uniform consensus on whether to correct melphalan dosing for IBW in either HILP or ILI.
Chemotherapy optimization
Melphalan has been the chemotherapeutic of choice for regional perfusion since it was first selected by Krementz and Creech for the first ILP over 50 years ago. It has subsequently been chosen by Thompson to be used in limb infusion. Although other agents have been studied in the context of regional therapy, melphalan continues to endure as the chemotherapeutic mainstay for both HILP and ILI. However, as mentioned previously, many patients treated with melphalan-based HILP or ILI either do not respond to melphalan-based regional therapy or recur following a clinical response, mandating the search for other therapies with which to treat in-transit malignant melanoma.
Temozolomide (Temodar®), a DNA-alkylating agent, is one of the most effective systemic, single-agent chemotherapeutic agents available for treating melanoma [45]. That the clinical response rate for systemic temozolomide is only 15–20% [45] speaks volumes about how resistant melanoma is to chemotherapy, at least at doses that can be safely administered systemically. However, its relative superiority in a systemic setting suggests that it has potential in the regional setting where it can be administered in much higher doses. Recently temozolomide has become available in an intravenous form. Preclinical data support temozolomide’s promise as a regional chemotherapeutic agent. Ueno and colleagues found that regionally infused temozolomide was much more effective than systemic temozolomide in an animal model of regionally advanced melanoma. Furthermore, the group compared regional temozolomide and regional melphalan in a rat model of extremity melanoma and found that temozolomide produced better responses at equitoxic doses [46]. Temozolomide has been shown to exhibit a strong synergistic cytotoxicity when combined with hyperthermia [45], making its suitability as a regional chemotherapeutic even more interesting. Currently, a Phase I trial of regionally administered temozolomide is being reviewed by the Institutional Review Board (IRB) at Duke, with plans to begin enrolling by the end of 2009.
Reducing chemoresistance with targeted therapies
Melphalan induces cytotoxicity through alkylation of DNA bases resulting in DNA crosslinkage. Resistance to melphalan occurs by enhanced repair of these DNA linkages, decreased uptake into the cell, and inactivation of the drug once inside the cell. The latter resistance mechanism is thought to be the most important, with glutathione (GSH) playing a central role. GSH has been shown to form GSH S-alkylating-agent conjugates, in so doing preventing melphalan from interacting with DNA and leading to detoxification and dechlorination [47]. This process is known to be augmented by the enzyme glutathione S-transferase, which catalyzes the conjugation of GSH to a number of xenobiotics, including melphalan [48]. Resistance to chemotherapy has been correlated with elevations in GSH levels [49,50] and high levels of GSH S-transferase activity [51].
Butathione sulfixime (BSO) is a small-molecule inhibitor of γ-glutamylcysteine-synthetase, the rate-limiting enzyme responsible for GSH synthesis, which has been implicated in melphalan resistance in melanoma [49,52]. Systemic BSO therapy was found to augment the effects of regional melphalan without increasing toxicity in an in-transit melanoma animal model [53]. Currently, a Phase I trial at Duke using a 3-day infusion of BSO around the time of melphalan ILI is enrolling patients who have failed a previous melphalan infusion/perfusion. Biopsies taken before and after the therapy will be used to determine the degree to which glutathione is depleted and correlate this finding with clinical response.
There is also interest in investigating whether temozolomide tumorigenicity could be augmented by tempering the major temozolomide resistance pathway in melanoma. This pathway is maintained by O6-alkylguanine-DNA alkyltransferase, which is responsible for DNA repair [54]. O6-benzylguanine is an inhibitor of O6-alkylguanine-DNA alkyltransferase, and has been shown to improve tumor response following ILI with temozolomide in an animal model of extremity melanoma [55]. Although these results are promising, O6-benzylguanine will not be able to be tested in human trials until intravenous temozolomide has been evaluated in the regional setting.
Two other potential agents that have shown promise in preclinical models to improve tumor response to melphalan are bevacizumab (Avastin®) and ADH-1 (Exherin™), both of which are thought to modulate chemotherapy delivery to the tumor. Bevacizumab is a monoclonal antibody specific for human VEGF that inhibits the downstream, proangiogenic pathways responsible for formation of new blood vessels [56,57]. The compound is theorized to initiate a process of ‘vascular normalization’, a transient ‘pruning’ of malignant blood vessels and bolstering of wild-type vessels, such that tumor perfusion is actually increased [58]. This hypothesis has been supported by the work of Dickson and colleagues demonstrating reduced tumor interstitial fluid pressure and vascular permeability, leading to increased topotecan penetration in spite of decreased microvessel density in neuroblastoma xenografts. Interestingly, while tumor chemotherapy perfusion was increased above baseline within 24 h and 3 days of bevacizumab administration, it actually decreased at 7 days, lending credence to the understanding that vascular normalization is transient [59].
Bevacizumab has been shown to enhance the cytotoxic effects of chemotherapy in the setting of colon, lung and breast cancer and in a number of CNS tumors when administered systemically during or prior to the administration of systemic chemotherapeutic agents. Tyler and colleagues showed that the combination of systemic Avastin and regional melphalan (rat extremity melanoma ILI model) significantly improved tumor responses [Tyler D et al., Unpublished Data]. Currently a proposal to carry out a Phase I trial of intravenous bevacizumab in combination with ILI is being reviewed by the Duke IRB.
The recent work of Beasley et al. has demonstrated that ADH-1 also has promise as a chemosensitizing agent. ADH-1 is a cyclic pentapeptide that disrupts the binding interactions between N-cadherin molecules. The wild-type cadherin, E-cadherin, is responsible for cell-to-cell adhesion. Malignant transformation leading to melanoma is thought to be associated with a switching from E-cadherin to N-cadherin, a change that probably modifies cell-to-cell interactions but also alters intracellular signaling pathways leading to uncontrolled proliferation, survival and angiogenesis. Thus, targeting the mutated cadherin may, in theory, antagonize N-cadherin-containing malignant cells and reduce proliferation and survival [60]. The results from a Phase I dose-escalation trial in which systemic ADH-1 was used in combination with regional infusion of melphalan were recently reported by Beasley et al. The group observed a 50% CR rate and a 12.5% PR rate in 16 patients in the absence of increased toxicity [61]. Interestingly, the group observed that ADH-1 treatment increased melphalan-induced DNA adduct formation, suggesting that ADH-1 improved tumoral chemotherapy penetration similar to what has been observed with bevacizumab. What seems different, however, is the mechanism by which this occurs, as bevacizumab seems to normalize vasculature so that more chemotherapy reaches the tumor via blood vessels, while ADH-1 instigates a degree of vessel ‘leakiness’ that may allow intravascular chemotherapy to seep into surrounding tumor [Unpublished Data]. The Phase II trial of systemic ADH-1 in combination with regional melphalan has completed accrual and the data analysis for outcomes and correlative studies is currently in progress.
Reducing tumor apoptotic threshold with targeted therapy
Approximately 60% of melanoma tumors contain an activating mutation of B-raf kinase, leading to increased proliferation and survival [62]. Sorafenib (Nexavar®) is an oral, multikinase inhibitor with antagonistic activity against B-raf, C-raf and a variety of other kinases. We studied the compound in combination with melphalan in vitro in order to determine whether melphalan resistance could be augmented using the targeted therapy. We found that the addition of sorafenib markedly improved cytotoxicity in a number of our cell lines above melphalan alone. In these studies sorafenib markedly increased the degree of apoptosis in tumor cells when combined with melphalan compared with either agent alone. Preclinical data using a rat model of advanced extremity melanoma also demonstrated improved tumor responses in animals receiving systemic sorafenib plus regional melphalan. In these preclinical studies, the mechanism of increased tumor response was different than was seen with bevacizumab or ADH-1. Sorafenib pretreatment led to marked increases in tumor apoptosis without increasing the amount of drug or DNA adduct formation in the tumor. Using these data as rationale, a Phase I trial of systemic sorafenib in combination with regionally administered melphalan in patients with in-transit extremity melanoma has been initiated and the enrollment was just recently completed. Analysis of this study is currently underway.
The src family of kinases provides another potential target. In approximately 85% of melanoma cell lines, Stat3, a downstream target of src kinase, is activated, leading to proliferation, survival and the upregulation of VEGF, resulting in increased tumor angiogenesis [63,64]. Niu and colleagues have demonstrated that inhibition of src kinase blocks melanoma cell proliferation in vivo [64]. Currently, preclinical studies are exploring whether or not targeting src with the src inhibitor dasatinib (Sprycel®) can also alter tumor apoptotic threshold.
Finally, evaluation of compounds specifically targeting apoptotic proteins, such as Bcl-2, is also underway. The results of these studies will help to determine other novel therapeutic regional trials over the next 5 years.
Tailored therapy for melanoma
Current attempts to more accurately direct or personalize regional therapy revolve around developing gene signatures of drug resistance. Our group performed gene-array mapping on tumor samples form 28 patients treated with ILI (Figure 4) [Tyler D et al., Unpublished Data]. We identified 100 genes which were correlated with tumor response to melphalan-based ILI. Although this ‘melphalan signature’ is in the process of being validated, it represents a promising approach to tailoring therapy to melanoma. A gene signature for temozolomide sensitivity/resistance has already been validated [65].
Figure 4. Gene expression map of 100 genes correlated with response to melphalan-based isolated limb infusion.
Normalized gene expression is color coded, where red represents genes that are more highly expressed and green represents genes expressed at a lower level. A total of 100 genes were identified that showed significantly different expression patterns across 28 patients (52 lesions) based on response. Lesions from the same patient were clustered together in the analysis.
CR: Complete response; PD: Progressive disease; PR: Partial response; SD: Stable disease.
Conclusion
Both HILP and ILP are considerably more effective in controlling regional melanoma isolated to the extremity than systemic therapy. Importantly, the optimization of regional infusion benefits, not just for the 6–11% of patients whose extremity melanoma manifests in an in-transit pattern, but also patients with stage IV metastatic melanoma, by providing a powerful means of evaluating new therapies that may help us to better understand how to develop rational systemic treatment strategies. As we become more familiar with the underlying biomolecular and genetic mechanisms of melanoma chemoresistance and chemotherapy cytotoxicity, we will further refine our ability to personalize treatment strategies to the particular chemoresistance/chemosensitivity profile of each individual’s variety of melanoma
Key issues
Regional treatment strategies, such as melphalan-based hyperthermic isolated limp perfusion (HILP) and isolated limb infusion(ILI), are effective ways to control in-transit malignant melanoma.
While HILP appears to be somewhat more effective than ILI, ILI appears to be associated with less major morbidity.
Novel treatment approaches to advancing regional therapy involve standardizing procedural variables, exploring new chemotherapeutic agents such as temozolomide, understanding how targeted agents can best be used to optimize drug delivery or alter tumor apoptotic threshold and developing gene signatures of drug resistance that can be used to personalize treatment.
Footnotes
Financial & competing interests disclosure
Douglas Tyler receives grant support from Adherex Technologies. Douglas Tyler is also a coinventor on a patent entitled ‘Cancer treatment methods using cadherin antagonists in combination with anticancer agents’. The patent application number is 60/848,624 and it was filed on 27 September 2006. Douglas Tyler’s rights to this patent have been signed over to the US Government. Douglas Tyler has material transfer agreements with Bayer, Schering and Genta pharmaceuticals. Adherex Technologies funded the Phase I and II clinical trials of systemic ADH-1 and regional melphalan. Bayer provided drug only (sorafenib) for the Phase I trial of systemic sorafenib and regional melphalan. This paper was supported in part by Duke University’s CTSA grant TL1RR024126 from NCRR/NIH. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Andrew Coleman, Duke University School of Medicine, Durham, NC 27710, USA, Tel.: +1 309 912 3009, Fax: +1 919 684 6044, apc10@duke.edu.
Christina K Augustine, Box 3118 Medical Center, Duke University Medical Center, Durham, NC 27710, USA, Tel.: +1 919 286 0411 ext. 5191, Fax: +1 919 684 6044, christi.augustine@duke.edu.
Georgia Beasley, Department of Surgery, Duke University Medical Center, Durham, NC 27710, USA, Tel.: +1 919 684 6858, Fax: +1 919 684 6044, schwe009@mc.duke.edu.
Gretchen Sanders, Department of Surgery, Duke University Medical Center, Durham, NC 27710, USA, Tel.: +1 919 684 8132, Fax: +1 919 684 6044, gretchen.sanders@duke.edu.
Douglas Tyler, Chief, Section of Surgical Oncology, Vice-Chairman, Department of Surgery, Box 3118, Duke University Medical Center, Durham, NC 27710, USA, Tel.: +1 919 684 6858, Fax: +1 919 684 6044, doug.tyler@duke.edu.
References
Papers of special note have been highlighted as:
• of interest
- 1.Diepgen TL, Mahler V. The epidemiology of skin cancer. Br. J. Dermatol. 2002;146 Suppl. 61:1–6. doi: 10.1046/j.1365-2133.146.s61.2.x. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts and Figures 2008. GA, USA: American Cancer Society; 2008. [Google Scholar]
- 3.Miller AJ, Mihm MC., Jr Melanoma. N. Engl. J. Med. 2006;355(1):51–65. doi: 10.1056/NEJMra052166. [DOI] [PubMed] [Google Scholar]
- 4.Balch CM, Houghton AN, Soper AJ, Soong S, Thompson JF. Isolated Limb Infusion. MO, USA: Quality Medical Publishing; 2008. [Google Scholar]
- 5.Koops HS, Vaglini M, Suciu S, et al. Prophylactic isolated limb perfusion for localized, high-risk limb melanoma: results of a multicenter randomized Phase III trial: European Organization for Research and Treatment of Cancer Malignant Melanoma cooperative group protocol 18832, the World Health Organization Melanoma Program trial 15, and the North American Perfusion Group Southwest Oncology group-8593. J. Clin. Oncol. 1998;16(9):2906–2912. doi: 10.1200/JCO.1998.16.9.2906. [DOI] [PubMed] [Google Scholar]
- 6.Pawlik TM, Ross MI, Johnson MM, et al. Predictors and natural history of in-transit melanoma after sentinel lymphadenectomy. Ann. Surg. Oncol. 2005;12(8):587–596. doi: 10.1245/ASO.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 7.Kretschmer L, Beckmann I, Thoms KM, Mitteldorf C, Bertsch HP, Neumann C. Factors predicting the risk of in-transit recurrence after sentinel lymphonodectomy in patients with cutaneous malignant melanoma. Ann. Surg. Oncol. 2006;13(8):1105–1112. doi: 10.1245/ASO.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Balch CM, Houghton AN, Sober A, Soong S. Recurrent Regional Metastases and Their Management. MO, USA: Quality Medical Publishing; 1998. [Google Scholar]
- 9.Creech O, Jr, Krementz ET, Ryan RF, Winblad JN. Chemotherapy of cancer: regional perfusion utilizing an extracorporeal circuit. Ann. Surg. 1958;148(4):616–632. doi: 10.1097/00000658-195810000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ariyan CE, Brady MS. History of regional chemotherapy for cancer of the extremities. Int. J. Hyperthermia. 2008;24(3):185–192. doi: 10.1080/02656730701785102. [DOI] [PubMed] [Google Scholar]
- 11.Creech O, Jr, Krementz ET, Ryan RF, Reemtsma K, Winblad JN. Experiences with isolation-perfusion technics in the treatment of cancer. Ann. Surg. 1959;149(5):627–640. doi: 10.1097/00000658-195905000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavaliere R, Ciocatto EC, Giovanella BC, et al. Selective heat sensitivity of cancer cells. Biochemical and clinical studies. Cancer. 1967;20(9):1351–1381. doi: 10.1002/1097-0142(196709)20:9<1351::aid-cncr2820200902>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 13.Stehlin JS., Jr Hyperthermic perfusion with chemotherapy for cancers of the extremities. Surg. Gynecol. Obstet. 1969;129(2):305–308. [PubMed] [Google Scholar]
- 14.Sanki A, Kam PC, Thompson JF. Long-term results of hyperthermic, isolated limb perfusion for melanoma: a reflection of tumor biology. Ann. Surg. 2007;245(4):591–596. doi: 10.1097/01.sla.0000251746.02764.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minor DR, Allen RE, Alberts D, Peng YM, Tardelli G, Hutchinson J. A clinical and pharmacokinetic study of isolated limb perfusion with heat and melphalan for melanoma. Cancer. 1985;55(11):2638–2644. doi: 10.1002/1097-0142(19850601)55:11<2638::aid-cncr2820551118>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 16.Storm FK, Morton DL. Value of therapeutic hyperthermic limb perfusion in advanced recurrent melanoma of the lower extremity. Am. J. Surg. 1985;150(1):32–35. doi: 10.1016/0002-9610(85)90006-6. [DOI] [PubMed] [Google Scholar]
- 17.Kroon BB, Van Geel AN, Benckhuijsen C, Wieberdink J. Normothermic isolation perfusion with melphalan for advanced melanoma of the limbs. Anticancer Res. 1987;7(3 Pt B):441–442. [PubMed] [Google Scholar]
- 18.Di Filippo F, Calabro A, Giannarelli D, et al. Prognostic variables in recurrent limb melanoma treated with hyperthermic antiblastic perfusion. Cancer. 1989;63(12):2551–2561. doi: 10.1002/1097-0142(19890615)63:12<2551::aid-cncr2820631233>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 19.Kroon BB, Klaase JM, van Geel BN, Eggermont AM, Franklin HR, van Dongen JA. Results of a double perfusion schedule with melphalan in patients with melanoma of the lower limb. Eur. J. Cancer. 1993;29A(3):325–328. doi: 10.1016/0959-8049(93)90377-r. [DOI] [PubMed] [Google Scholar]
- 20.Klaase JM, Kroon BB, van Geel AN, et al. Limb recurrence-free interval and survival in patients with recurrent melanoma of the extremities treated with normothermic isolated perfusion. J. Am. Coll. Surg. 1994;178(6):564–572. [PubMed] [Google Scholar]
- 21.Aloia TA, Grubbs E, Onaitis M, et al. Predictors of outcome after hyperthermic isolated limb perfusion: role of tumor response. Arch. Surg. 2005;140(11):1115–1120. doi: 10.1001/archsurg.140.11.1115. [DOI] [PubMed] [Google Scholar]
- 22. Cornett WR, McCall LM, Petersen RP, et al. Randomized multicenter trial of hyperthermic isolated limb perfusion with melphalan alone compared with melphalan plus tumor necrosis factor: American College of Surgeons Oncology Group trial Z0020. J. Clin. Oncol. 2006;24(25):4196–4201. doi: 10.1200/JCO.2005.05.5152. • Multicenter randomized, controlled trial elucidating the efficacy and safety of hyperthermic isolated limb perfusion with melphalan with or without the addition of TNF-α.
- 23.Noorda EM, Vrouenraets BC, Nieweg OE, van Geel BN, Eggermont AM, Kroon BB. Isolated limb perfusion for unresectable melanoma of the extremities. Arch. Surg. 2004;139(11):1237–1242. doi: 10.1001/archsurg.139.11.1237. [DOI] [PubMed] [Google Scholar]
- 24.Bryant PJ, Balderson GA, Mead P, Egerton WS. Hyperthermic isolated limb perfusion for malignant melanoma: response and survival. World J. Surg. 1995;19(3):363–368. doi: 10.1007/BF00299159. [DOI] [PubMed] [Google Scholar]
- 25.Beasley GM, Caudle A, Petersen RP, et al. A multi-institutional experience of isolated limb infusion: defining response and toxicity in the US. J. Am. Coll. Surg. 2009;208(5):706–715. doi: 10.1016/j.jamcollsurg.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 26.Thompson JF, Kam PC, Waugh RC, Harman CR. Isolated limb infusion with cytotoxic agents: a simple alternative to isolated limb perfusion. Semin. Surg. Oncol. 1998;14(3):238–247. doi: 10.1002/(sici)1098-2388(199804/05)14:3<238::aid-ssu8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 27.Lienard D, Ewalenko P, Delmotte JJ, Renard N, Lejeune FJ. High-dose recombinant tumor necrosis factor α in combination with interferon γ and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J. Clin. Oncol. 1992;10(1):52–60. doi: 10.1200/JCO.1992.10.1.52. [DOI] [PubMed] [Google Scholar]
- 28.Kroon HM, Lin DY, Kam PC, Thompson JF. Isolated limb infusion as palliative treatment for advanced limb disease in patients with AJCC stage IV melanoma. Ann. Surg. Oncol. 2009;16(5):1193–1201. doi: 10.1245/s10434-009-0326-7. [DOI] [PubMed] [Google Scholar]
- 29.Siemann DW, Chapman M, Beikirch A. Effects of oxygenation and pH on tumor cell response to alkylating chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1991;20(2):287–289. doi: 10.1016/0360-3016(91)90106-e. [DOI] [PubMed] [Google Scholar]
- 30.van de Merwe SA, van den Berg AP, Kroon BB, van den Berge AW, Klaase JM, van der Zee J. Modification of human tumour and normal tissue pH during hyperthermic and normothermic antiblastic regional isolation perfusion for malignant melanoma: a pilot study. Int. J. Hyperthermia. 1993;9(2):205–217. doi: 10.3109/02656739309022535. [DOI] [PubMed] [Google Scholar]
- 31.Thompson JF, Morton DL, Kroon BB. Isolated Limb Perfusion for Melanoma: Technical Aspects. London, UK: Martin Dunitz; 2004. [Google Scholar]
- 32.Lindner P, Doubrovsky A, Kam PC, Thompson JF. Prognostic factors after isolated limb infusion with cytotoxic agents for melanoma. Ann. Surg. Oncol. 2002;9(2):127–136. doi: 10.1007/BF02557363. [DOI] [PubMed] [Google Scholar]
- 33.Parsons PG, Carter FB, Morrison L, Regius MS. Mechanism of melphalan resistance developed in vitro in human melanoma cells. Cancer Res. 1981;41(4):1525–1534. [PubMed] [Google Scholar]
- 34. Kroon HM, Moncrieff M, Kam PC, Thompson JF. Outcomes following isolated limb infusion for melanoma. A 14-year experience. Ann. Surg. Oncol. 2008;15(11):3003–3013. doi: 10.1245/s10434-008-9954-6. • Outlines the 14-year experience of isolated limb infusion outcomes at the Sydney Melanoma Unit (Australia).
- 35. Beasley GM, Petersen RP, Yoo J, et al. Isolated limb infusion for in-transit malignant melanoma of the extremity: a well-tolerated but less effective alternative to hyperthermic isolated limb perfusion. Ann. Surg. Oncol. 2008;15(8):2195–2205. doi: 10.1245/s10434-008-9988-9. • Comparison of response and toxicity outcomes from isolated limb infusion and hyperthermic isolated limb perfusion from the Duke University Medical Center (NC, USA).
- 36.Brady MS, Brown K, Patel A, Fisher C, Marx W. A Phase II trial of isolated limb infusion with melphalan and dactinomycin for regional melanoma and soft tissue sarcoma of the extremity. Ann. Surg. Oncol. 2006;13(8):1123–1129. doi: 10.1245/ASO.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Mian R, Henderson MA, Speakman D, Finkelde D, Ainslie J, McKenzie A. Isolated limb infusion for melanoma: a simple alternative to isolated limb perfusion. Can. J. Surg. 2001;44(3):189–192. [PMC free article] [PubMed] [Google Scholar]
- 38.Bonenkamp JJ, Thompson JF, de Wilt JH, Doubrovsky A, de Faria Lima R, Kam PC. Isolated limb infusion with fotemustine after dacarbazine chemosensitisation for inoperable loco-regional melanoma recurrence. Eur. J. Surg. Oncol. 2004;30(10):1107–1112. doi: 10.1016/j.ejso.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 39.Kroon HM, Lin DY, Kam PC, Thompson JF. Efficacy of repeat isolated limb infusion with melphalan and actinomycin D for recurrent melanoma. Cancer. 2009;115(9):1932–1940. doi: 10.1002/cncr.24220. [DOI] [PubMed] [Google Scholar]
- 40.Thompson JF, Hunt JA, Shannon KF, Kam PC. Frequency and duration of remission after isolated limb perfusion for melanoma. Arch. Surg. 1997;132(8):903–907. doi: 10.1001/archsurg.1997.01430320105017. [DOI] [PubMed] [Google Scholar]
- 41.Cheng TY, Grubbs E, Abdul-Wahab O, et al. Marked variability of melphalan plasma drug levels during regional hyperthermic isolated limb perfusion. Am. J. Surg. 2003;186(5):460–467. doi: 10.1016/j.amjsurg.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 42.Vrouenraets BC, Hart GA, Eggermont AM, et al. Relation between limb toxicity and treatment outcomes after isolated limb perfusion for recurrent melanoma. J. Am. Coll. Surg. 1999;188(5):522–530. doi: 10.1016/s1072-7515(99)00018-6. [DOI] [PubMed] [Google Scholar]
- 43.Vrouenraets BC, Klaase JM, Nieweg OE, Kroon BB. Toxicity and morbidity of isolated limb perfusion. Semin. Surg. Oncol. 1998;14(3):224–231. doi: 10.1002/(sici)1098-2388(199804/05)14:3<224::aid-ssu6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 44.McMahon N, Cheng TY, Beasley GM, et al. Optimizing melphalan pharmacokinetics in regional melanoma therapy: does correcting for ideal body weight alter regional response or toxicity? Ann. Surg. Oncol. 2009;16(4):953–961. doi: 10.1245/s10434-008-0288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ko SH, Ueno T, Yoshimoto Y, et al. Optimizing a novel regional chemotherapeutic agent against melanoma: hyperthermia-induced enhancement of temozolomide cytotoxicity. Clin. Cancer Res. 2006;12(1):289–297. doi: 10.1158/1078-0432.CCR-05-0210. [DOI] [PubMed] [Google Scholar]
- 46.Ueno T, Ko SH, Grubbs E, Pruitt SK, Friedman HS, Tyler DS. Temozolomide is a novel regional infusion agent for the treatment of advanced extremity melanoma. Am. J. Surg. 2004;188(5):532–537. doi: 10.1016/j.amjsurg.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 47.Grubbs EG, Abdel-Wahab O, Cheng TY, et al. In-transit melanoma: the role of alkylating-agent resistance in regional therapy. J. Am. Coll. Surg. 2004;199(3):419–427. doi: 10.1016/j.jamcollsurg.2004.05.271. [DOI] [PubMed] [Google Scholar]
- 48.Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol. 1995;30(6):445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 49.Green JA, Vistica DT, Young RC, Hamilton TC, Rogan AM, Ozols RF. Potentiation of melphalan cytotoxicity in human ovarian cancer cell lines by glutathione depletion. Cancer Res. 1984;44(11):5427–5431. [PubMed] [Google Scholar]
- 50.Suzukake K, Petro BJ, Vistica DT. Reduction in glutathione content of L-PAM resistant L1210 cells confers drug sensitivity. Biochem. Pharmacol. 1982;31(1):121–124. doi: 10.1016/0006-2952(82)90249-0. [DOI] [PubMed] [Google Scholar]
- 51.Robson CN, Lewis AD, Wolf CR, et al. Reduced levels of drug-induced DNA cross-linking in nitrogen mustard-resistant Chinese hamster ovary cells expressing elevated glutathione S-transferase activity. Cancer Res. 1987;47(22):6022–6027. [PubMed] [Google Scholar]
- 52.Suzukake K, Vistica BP, Vistica DT. Dechlorination of l-phenylalanine mustard by sensitive and resistant tumor cells and its relationship to intracellular glutathione content. Biochem. Pharmacol. 1983;32(1):165–167. doi: 10.1016/0006-2952(83)90671-8. [DOI] [PubMed] [Google Scholar]
- 53.Grubbs EG, Ueno T, Abdel-Wahab O, et al. Modulation of resistance to regional chemotherapy in the extremity melanoma model. Surgery. 2004;136(2):210–218. doi: 10.1016/j.surg.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 54.Ueno T, Ko SH, Grubbs E, et al. Modulation of chemotherapy resistance in regional therapy: a novel therapeutic approach to advanced extremity melanoma using intra-arterial temozolomide in combination with systemic O6-benzylguanine. Mol. Cancer Ther. 2006;5(3):732–738. doi: 10.1158/1535-7163.MCT-05-0098. [DOI] [PubMed] [Google Scholar]
- 55.Yoshimoto Y, Augustine CK, Yoo JS, et al. Defining regional infusion treatment strategies for extremity melanoma: comparative analysis of melphalan and temozolomide as regional chemotherapeutic agents. Mol. Cancer Ther. 2007;6(5):1492–1500. doi: 10.1158/1535-7163.MCT-06-0718. [DOI] [PubMed] [Google Scholar]
- 56.Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362(6423):841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 57.Presta LG, Chen H, O’Connor SJ, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57(20):4593–4599. [PubMed] [Google Scholar]
- 58.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat. Med. 2001;7(9):987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 59.Dickson PV, Hamner JB, Sims TL, et al. Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin. Cancer Res. 2007;13(13):3942–3950. doi: 10.1158/1078-0432.CCR-07-0278. [DOI] [PubMed] [Google Scholar]
- 60.Augustine CK, Yoshimoto Y, Gupta M, et al. Targeting N-cadherin enhances antitumor activity of cytotoxic therapies in melanoma treatment. Cancer Res. 2008;68(10):3777–3784. doi: 10.1158/0008-5472.CAN-07-5949. [DOI] [PubMed] [Google Scholar]
- 61.Beasley GM, McMahon N, Sanders G, et al. A Phase I study of systemic ADH-1 in combination with melphalan via isolated limb infusion in patients with locally advanced in-transit malignant melanoma. Cancer. 2009;115(20):4766–4774. doi: 10.1002/cncr.24509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 63.Niu G, Bowman T, Huang M, et al. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene. 2002;21(46):7001–7010. doi: 10.1038/sj.onc.1205859. [DOI] [PubMed] [Google Scholar]
- 64.Niu G, Wright KL, Huang M, et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21(13):2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 65.Augustine CK, Yoo JS, Potti A, et al. Genomic and molecular profiling predicts response to temozolomide in melanoma. Clin. Cancer Res. 2009;15(2):502–510. doi: 10.1158/1078-0432.CCR-08-1916. [DOI] [PubMed] [Google Scholar]
Website
- 101.Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events, Version 3.0. http://ctep.cancer.gov.