Abstract
Arsenic is ubiquitously present in the environment; it is a known human carcinogen and paradoxically it is also a successful drug for the clinical remission of acute promyelocytic leukemia (APL). The cellular responses induced by arsenite treatment have been investigated for years; however, the precise mechanisms underlying its cytotoxicity and therapeutic activity remain unclear. Here we report the use of mass spectrometry together with stable isotope labeling by amino acids in cell culture (SILAC) for the comparative study of protein expression in HL-60 cells that were untreated or treated with a clinically relevant concentration of arsenite. Our results revealed that, among the 1067 proteins quantified in both forward and reverse SILAC measurements, 56 had significantly altered levels of expression induced by arsenite treatment. These included the up-regulation of core histones, neutrophil elastase, α-mannosidase as well as the down-regulation of fatty acid synthase and protein phosphatase 1α. We further demonstrated that the arsenite-induced growth inhibition of HL-60 cells could be rescued by treatment with palmitate, the final product of fatty acid synthase, supporting that arsenite exerts its cytotoxic effect, in part, via suppressing the expression of fatty acid synthase and inhibiting the endogenous production of fatty acid. The results from the present study offered important new knowledge for gaining insights into the molecular mechanisms of action of arsenite.
Introduction
Arsenic is ubiquitously present in the environment from both natural and anthropogenic sources, especially in groundwater 1. Arsenic contamination in groundwater has become a widespread public health problem in recent years, causing serious arsenic poisoning to a large population. Chronic arsenic exposure has been associated with increased incidence of various human diseases including atherosclerosis, diabetes, and cancers 2–4. Despite being a human carcinogen, arsenic trioxide (As2O3) has also been used successfully for the clinical remission of acute promyelocytic leukemia (APL) patients 5–7, including those who are resistant to all-trans retinoic acid 8; in 2001, FDA approved the use of arsenic trioxide for APL treatment.
The cellular responses toward arsenite treatment have been extensively studied over the years. Arsenite has been reported to induce the formation of micronuclei and sister chromatid exchanges 9, 10. It can bind to cysteine sulfhydryl groups in proteins 11, stimulate the formation of oxyradicals 12, inhibit DNA repair, and modulate DNA and histone methylation in mammalian cells 13. In addition, microarray technique revealed that over one hundred genes in human fibroblast cells were induced or repressed by arsenite treatment 14. However, microarray analysis does not provide information about the translational regulation of gene expression, which often exhibits a poor correlation with transcript levels owing to the different kinetics of protein translation and turnover 15.
Mass spectrometry (MS)-based proteomics allows for the identification and quantification of a large number of proteins in complex samples. Two-dimensional gel electrophoresis (2-DE) is a traditional technique for studying the effects of drug treatments on protein expression. In 2-DE, quantification is achieved by recording differences in the stained spot intensities of proteins derived from two states of cell populations or tissues 16. A number of proteomic studies underlying the effect of treatments with different anti-cancer drugs, such as cisplatin 17, 18, etoposide 19 and all-trans retinoic acid 20, have been performed by using mass spectrometry for protein identification and 2-DE for protein quantification. The combination 2-DE with mass spectrometry has also been used previously for assessing the arsenite- or arsenic trioxide-induced alterations in protein expression in rat lung epithelial cells 21, TK6 human lymphoblastoid cells 22, immortalized human keratinocytes 23, NB4 human promyelocytic leukemia cells 24, and U266 human multiple myeloma cells 25.
Other than 2-DE, several stable isotope labeling strategies, such as isotope-coded affinity tag 26, isobaric tags for relative and absolute quantitation 27 and stable isotope labeling by amino acids in cell culture (SILAC) 28, have been developed for MS-based analysis of differential protein expression. Among these isotope-labeling strategies, SILAC is a metabolic labeling method, which is simple, efficient, and can facilitate almost complete heavy isotope incorporation. SILAC is very suitable for the comparative study of protein expression in cells with and without drug treatments; accurate results could be obtained with minimal bias, allowing for relative quantification of small changes in protein abundance 28. In this context, Wiseman et al. 29 used SILAC together with LC-MS/MS and examined the arsenite-induced alterations in the subunit composition of the 26S human proteasome in HEK 293T cells; they found that arsenite treatment led to a 50-fold reduction in the proteasome-associated TRP32.
In the present study, we employed LC-MS/MS, together with SILAC, to assess quantitatively the perturbation of protein expression in cultured HL-60 human acute promyelocytic leukemia cells upon arsenite treatment. We were able to quantify a total of 1067 proteins in both forward and reverse SILAC measurements, among which 56 were significantly altered upon arsenite treatment. The MS quantification results for several target proteins were verified by Western blotting analysis. The identification of proteins perturbed by arsenite treatment sets a stage for understanding the biological pathways affected by arsenite treatment.
Materials and Methods
Materials
Heavy lysine and arginine ([13C6,15N2]-L-lysine and [13C6,15N4]-L-arginine) were purchased from Cambridge Isotope Laboratories (Andover, MA). All chemicals unless otherwise noted were from Sigma (St. Louis, MO).
The rabbit anti-histone H2A, H3, actin and mouse anti-histone H4 antibodies were purchased from Abcam (Cambridge, MA). The mouse anti-histone H2B was from MBL International (Woburn, MA). The rabbit anti-fatty acid synthase was from Cell Signaling (Danvers, MA). HRP-conjugated goat anti-rabbit and anti-mouse IgG secondary antibodies were obtained from Abcam and Santa Cruz Biotechnology (Santa Cruz, CA), respectively.
Cell Culture
HL-60 cells, obtained freshly from ATCC (Manassas, VA), were cultured in Iscove’s modified minimal essential medium (IMEM) supplemented with 10% fetal bovine serum (FBS, Invitrogen, Carlsbad, CA) and penicillin (100 IU/mL). Cells were maintained in a humidified atmosphere with 5% CO2 at 37°C, with medium renewal at every 2 or 3 days depending on cell density. For SILAC experiments, the IMEM medium without L-lysine or L-arginine was custom-prepared according to the ATCC formulation. The complete light and heavy IMEM media were prepared by the addition of light or heavy lysine and arginine, along with dialyzed FBS (Invitrogen), to the above lysine, arginine-depleted medium. The HL-60 cells were cultured in heavy IMEM medium for at least 5 cell doublings to achieve complete isotope incorporation.
Arsenite Treatment and Cell Lysate Preparation
HL-60 cells, at a density of approximately 7.5×105 cells/mL, were collected by centrifugation at 300 g and at 4°C for 5 min, washed twice with ice-cold phosphate-buffered saline (PBS) to remove FBS, and resuspended in FBS-free heavy or light media. In forward SILAC experiment, the cells cultured in light medium were treated with 5 μM arsenite (Sigma) for 24 hrs, whereas the cells cultured in heavy medium were untreated. Reverse SILAC experiments were also performed where the cells cultured in the heavy and light medium were treated with arsenite and mock-treated, respectively (Figure 1). After 24 hrs, the light and heavy cells were collected by centrifugation at 300 g, and washed three times with ice-cold PBS.
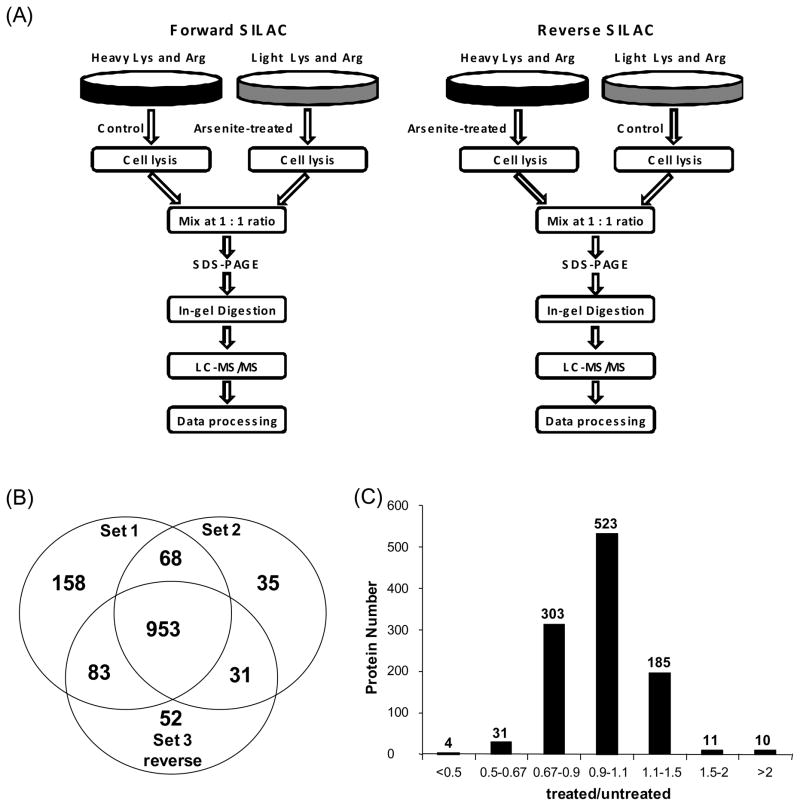
Figure 1.
Forward- and reverse-SILAC combined with LC-MS/MS for the comparative analysis of protein expression in HL-60 cells upon arsenite treatment (A). Shown in (B) and (C) are a summary of the number of proteins quantified from three independent SILAC experiments and the distribution of expression ratios (treated/untreated) for the proteins quantified, respectively. “Set 1” and “Set 2” results were obtained from forward, and “Set 3” results were from reverse SILAC experiments.
The cell pellets were resuspended in the CelLytic™ M cell lysis buffer (Sigma) for 30 min with occasional vortexing. Cell lysates were centrifuged at 12,000 g at 4°C for 30 min, and the resulting supernatants were collected. To the supernatant was subsequently added a protease inhibitor cocktail (Sigma), and the protein concentrations of the cell lysates were determined by using Quick Start Bradford Protein Assay kit (Bio-Rad, Hercules, CA).
SDS-PAGE Separation and In-gel Digestion
The light and heavy cell lysates were combined at 1:1 ratio (w/w), denatured by boiling in Laemmli loading buffer for 5 min and separated by a 12% SDS-PAGE with 4% stacking gel. The gel was stained with Coomassie blue; after destaining, the gel was cut into 20 bands, in-gel reduced with dithiothreitol and alkylated with iodoacetamide. The proteins were digested in-gel with trypsin (Promega, Madison, WI) for overnight, after which peptides were extracted from gels with 5% acetic acid in H2O and in CH3CN/H2O (1:1, v/v). The resulting peptide mixtures were dried and stored at −20°C for further analysis.
Western Blotting
For Western blotting analysis, lysates of the control and arsenite-treated HL-60 cells were prepared following the same procedures as described above. After SDS-PAGE separation, proteins were transferred to a nitrocellulose membrane under standard conditions. A basic transfer buffer containing 10 mM NaHCO3 and 3 mM Na2CO3 (pH 9.9) was used for immunoblotting histones. After protein transfer, the membranes were blocked with 5% non-fat milk in PBS-T buffer [PBS solution containing 0.1% (v/v) Tween-20, pH 7.5] for 7 hrs and incubated subsequently with primary antibodies for overnight at optimized dilution ratios. The membranes were washed five times (10 min each) with fresh changes of PBS-T at room temperature. After washing, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies at room temperature for 50 min. The membranes were washed thoroughly with PBS-T for five times (10 min each). The secondary antibody was detected by using ECL Advance Western Blotting Detection Kit (GE Healthcare) and visualized with HyBlot CL autoradiography film (Denville Scientific Inc., Metuchen, NJ).
Fatty Acid Synthase Inhibition and Cell Viability Assay
The palmitate-BSA complex was prepared following a previously published method 30. Bovine serum albumin (BSA, 5 g) was dissolved in 25 mL of 0.9% NaCl solution with pH being adjusted to 7.4 by NaOH at 5°C, while 4.016 mg sodium palmitate (Sigma) was dissolved in 15 mL of the same NaCl solution at 60°C. The BSA solution was then added to the hot palmitate solution, and the resulting BSA-palmitate mixture was stirred extensively and brought to a volume of 40 mL at a palmitate/BSA molar ratio of 1:5.
HL-60 cells were washed twice with ice-cold PBS to remove FBS, resuspended in FBS-free IMEM medium, and seeded in 6-well plates at a density of ~ 3×105 cells/mL. To the cultured cells were added cerulenin or arsenite solutions until their concentrations reached 2.5 μg/mL and 5 μM, respectively. The palmitate-BSA complex solution was added subsequently to the wells containing the control, cerulenin-, or arsenite-treated cells until palmitate concentration was 40 or 80 μM. After 24 or 48 hrs of treatment, cells were stained with trypan blue, and counted on a hemocytometer to measure cell viability.
LC-MS/MS for Protein Identification and Quantification
Online LC-MS/MS analysis was performed on an Agilent 6510 Q-TOF system coupled with an Agilent HPLC-Chip Cube MS interface (Agilent Technologies, Santa Clara, CA). The sample injection, enrichment, desalting, and HPLC separation were carried out automatically on the Agilent HPLC Chip with an integrated trapping column (160 nL) and a separation column (Zorbax 300SB-C18, 75 μm×150 mm, 5 μm in particle size). The peptide mixture was first loaded onto the trapping column with a solvent mixture of 0.1% formic acid in CH3CN/H2O (2:98, v/v) at a flow rate of 4 μL/min, which was delivered by an Agilent 1200 capillary pump. The peptides were then separated with a 90-min linear gradient of 2–60% acetonitrile in 0.1% formic acid and at a flow rate of 300 nL/min, which was delivered by an Agilent 1200 Nano pump.
The Chip spray voltage (VCap) was set as 1950 V and varied depending on chip conditions. The temperature and flow rate of the drying gas were set at 325°C and 4 L/min, respectively. Nitrogen was used as the collision gas, and the collision energy followed an equation with a slope of 3 V/100 Da and an offset of 2.5 V. MS/MS experiments were carried out in the data-dependent scan mode with a maximum of five MS/MS scans following each MS scan. The m/z ranges for MS and MS/MS were 300–2000 and 60–2000, and the acquisition rates were 6 and 3 spectra/s, respectively.
Data Processing
Agilent MassHunter workstation software (Version B.01.03) was used to extract the MS and MS/MS data. The data were converted to m/z Data files with MassHunter Qualitative Analysis. Mascot Server 2.2 (Matrix Science, London, UK) was used for protein identification by searching the m/z Data files against the weekly-updated NCBI database. The maximum number of miss-cleavage for trypsin was set as one per peptide. Cysteine carbamidomethylation was set as a fixed modification. Methionine oxidation as well as lysine (+8 Da) and arginine (+10 Da) mass shifts introduced by heavy isotope labeling were considered as variable modifications. The mass tolerances for MS and MS/MS were 50 ppm and 0.6 Da, respectively. Peptides identified with individual scores at or above the Mascot-assigned homology score (p < 0.01 and individual peptide score > 30) were considered as specific peptide sequences. The false discovery rates (FDR) determined by decoy database search were less than 0.95%.
Protein quantification was carried out manually and was based on the average ratios of peptide pairs, which was obtained from the intensities of peaks of precursor ions of light and heavy peptides. For those proteins identified with less than 5 peptides, the abundance ratios for ions of all peptide pairs were used for the quantification; for those proteins identified with more than 5 peptides, five peptide pairs with the highest scores were chosen to determine the protein ratios. The ratio obtained for each individual protein was then normalized against the average ratio for all quantified proteins. This “multi-point” normalization strategy assumes that the ratios for the majority of proteins are not affected by the treatment, facilitating the use of the average ratio of all quantified proteins for re-scaling the data. This has been widely employed to eliminate the inaccuracy during sample mixing introduced by protein quantification with the Bradford assay 31, 32. The quantification was based on three independent SILAC and LC-MS/MS experiments, which included two forward and one reverse SILAC labeling, and the proteins reported here could be quantified in both forward and reverse SILAC experiments. Some peptides identified in only 1 or 2 trials of QTOF analysis could be quantified in all three trials, where the accurate mass of peptide ions, retention time, and the numbers of isotope-labeled lysine and/or arginine in the peptide were employed as criteria to locate the light/heavy peptide pairs for the quantification.
Results and Discussion
Arsenite Treatment, Protein Identification and Quantification
To gain insights into the molecular pathways perturbed by arsenite treatment, we employed SILAC combined with LC-MS/MS to assess the arsenite-induced differential expression of the whole proteome of HL-60 cells. In this context, clinical pharmacokinetic analyses indicate that the peak plasma arsenite concentration is in the low 33 to high 8, 34 micromolar range for APL patients treated with arsenite. We observed, based on trypan blue exclusion assay, a less than 5% cell death after a 24-hr treatment with 5 μM arsenite, whereas approximately 20% cells were dead if the cells were treated with 7.5 μM arsenite. Thus, we decided to employ 5 μM arsenite for the subsequent experiments to minimize the apoptosis-induced alteration in protein expression.
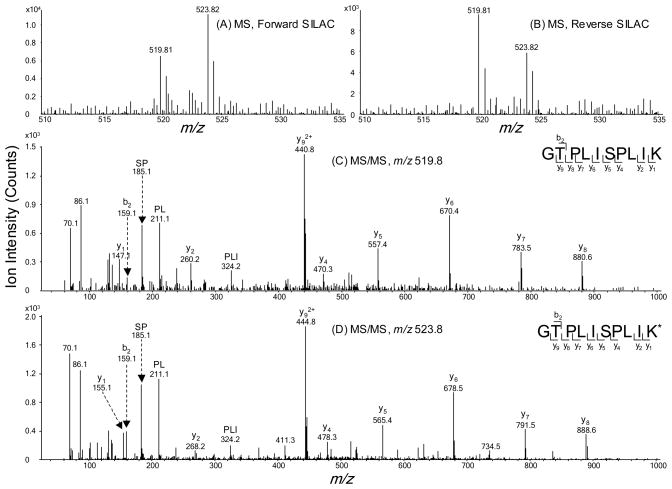
To obtain reliable results, we carried out the SILAC experiments in triplicate and both forward and reverse SILAC labeling was performed (Figure 1A, see also Materials and Methods). Figure 2 shows example results for the quantification of the peptide GTPLISPLIK from fatty acid synthase, which reveals clearly the down-regulation of this protein in both forward and reverse SILAC experiments (Figure 2A&B). The peptide sequence was confirmed by MS/MS analysis (Figure 2C&D). In addition, we employed serum-free medium in all SILAC-related cell culture experiments to avoid the interactions between arsenite and proteins in the FBS. After cell lysis, SDS-PAGE fractionation, in-gel digestion, LC-MS/MS analysis and database search, we were able to identify 1401 proteins, among which 1380 could be quantified.
Figure 2.
Example ESI-MS and MS/MS data revealed the arsenite-induced down-regulation of fatty acid synthase. Shown are the MS for the [M+2H]2+ ions of FAS peptide GTPLISPLIK and GTPLISPLIK* (‘K*’ represents the heavy lysine) from the forward (A) and reverse (B) SILAC samples. Depicted in (C) and (D) are the MS/MS for the [M+2H]2+ ions of GTPLISPLIK and GTPLISPLIK*, respectively.
Among the quantified proteins, 953 could be quantified in all three measurements, 182 could be quantified in two measurements, and another 245 could be quantified in only one measurement (Figure 1B). We include here only the quantification results for those proteins that could be quantified in all three experiments or in two experiments which include both the forward and reverse SILAC. Together, this gives quantifiable results for 1067 proteins (Table S1).
The distribution of the changes in protein expression levels induced by arsenite treatment is shown in Figure 1C. Among the quantified proteins, 56 display significant changes upon arsenite treatment (the ratio of treated/untreated was greater than 1.5 or less than 0.67), with 21 and 35 being up- and down-regulated, respectively. The quantification results for the proteins with significant changes are summarized in Table 1, and the detailed information about the quantified peptides and ratios for each measurement are listed in Table S2.
Table 1.
Proteins quantified with more than 1.5 fold changes, with GI numbers, protein names, average ratios and S.D. listed (Peptides used for the quantification of individual proteins are listed in Table S3).
| GI Number | Protein Name | Ratio (treated/untreated) |
|---|---|---|
| A. Histone and HMG proteins | ||
| 11321591 | HMG-2 | 1.65±0.26 |
| 968888 | HMG-1 | 1.85±0.07 |
| 223582 | histone H4 | 2.41±0.09 |
| 386772 | histone H3 | 2.80±0.42 |
| 356168 | histone H1b | 2.76±0.33 |
| 1568557 | histone H2B | 2.98±0.45 |
| 510990 | histone H2A | 4.21±0.54 |
| B. Translation-related proteins | ||
| 181969 | elongation factor 2 | 0.54±0.09 |
| 38202255 | threonyl-tRNA synthetase | 0.60±0.12 |
| 19353009 | Similar to elongation factor 2b | 0.62±0.06 |
| 4506707 | ribosomal protein S25 | 0.65±0.11 |
| C. hnRNPs | ||
| 386547 | d(TTAGGG)n-binding protein B39 | 0.50±0.22 |
| 119597533 | hnRNP U, isoform CRA_b | 0.59±0.01 |
| 55958547 | hnRNP K | 0.66±0.15 |
| D. Enzymes | ||
| 531202 | spermidine synthase | 0.57±0.18 |
| 41584442 | fatty acid synthase | 0.61±0.10 |
| 66392203 | NME1-NME2 protein | 0.64±0.18 |
| 1230564 | Gu protein | 0.64±0.14 |
| 2661039 | α-enolase | 0.65±0.07 |
| 190281 | protein phosphatase I α subunit | 0.66±0.02 |
| 4507789 | ubiquitin-conjugating enzyme E2L 3 isoform 1 | 0.67±0.06 |
| 5231228 | ribonuclease T2 precursor | 1.53±0.21 |
| 4758504 | hydroxysteroid (17-β) dehydrogenase 10 isoform 1 | 1.56±0.02 |
| 4503143 | cathepsin D | 1.65±0.08 |
| 186461558 | neutrophil elastase | 1.95±0.01 |
| 627372 | α-mannosidase precursor | 2.05±0.24 |
| 4504437 | heme oxygenase 1 | 2.32±0.78 |
| 12654715 | TXNDC5 protein | 3.14±0.35 |
| E. Others | ||
| 21755073 | unnamed protein product | 0.42±0.02 |
| 119587276 | hCG19802, isoform CRA_a | 0.47±0.20 |
| 35570 | unnamed protein product | 0.50±0.07 |
| 13477237 | ZNF607 protein | 0.52±0.05 |
| 194374111 | unnamed protein product | 0.57±0.11 |
| 35844 | unnamed protein product | 0.58±0.15 |
| 431422 | Ran/TC4 binding protein | 0.60±0.22 |
| 114645930 | PREDICTED: nucleosome assembly protein 1-like 1 isoform 9 | 0.60±0.02 |
| 2580550 | DEAD box, X isoform | 0.61±0.10 |
| 122168 | HLA class I histocompatibility antigen, B-58 α chain | 0.61±0.20 |
| 181486 | DNA-binding protein B | 0.63±0.07 |
| 193788267 | unnamed protein product | 0.63±0.08 |
| 801893 | leucine-rich PPR-motif containing protein | 0.63±0.10 |
| 5107666 | importin β | 0.63±0.20 |
| 1235727 | unnamed protein product | 0.64±0.10 |
| 40225729 | FUBP1 protein | 0.65±0.08 |
| 119571409 | hCG1643342, isoform CRA_a | 0.65±0.03 |
| 386777 | transplantation antigen | 0.66±0.05 |
| 1136741 | KIAA0002 | 0.66±0.20 |
| 23712 | myoblast antigen 24.1D5 | 0.66±0.08 |
| 13569879 | acidic (leucine-rich) nuclear phosphoprotein 32 family, member E isoform 1 | 0.67±0.08 |
| 4506773 | S100 calcium-binding protein A9 | 1.53±0.04 |
| 9955206 | Rho GDP-dissociation factor 2 | 1.55±0.19 |
| 8037945 | prothymosin α | 1.56±0.02 |
| 119239 | bone marrow proteoglycan | 1.71±0.08 |
| 28375485 | unnamed protein product | 1.81±0.22 |
| 4506191 | proteasome β10 subunit proprotein | 2.21±0.10 |
| 32111 | unnamed protein product | 4.90±0.96 |
Histone proteins are up-regulated upon arsenite treatment
Among the differentially expressed proteins, all four core histones and linker histone H1 were markedly up-regulated in arsenite-treated HL-60 cells (Table S3). In this respect, histone proteins bear many sites of post-translational modifications (PTMs), particularly on their N-terminal tails 35. Arsenite treatment may perturb the PTMs of histone proteins, which may give rise to inaccurate quantifications of their expression levels. Indeed the exposure of A549 human lung carcinoma cells to arsenite was found to increase H3K9 dimethylation and H3K4 trimethylation while decreasing H3K27 trimethylation 36. To avoid the inaccurate quantification of histones introduced by arsenite-induced change in PTMs, we chose to use those peptides that do not contain any known PTMs for the quantification 35. Consistent with what we found from SILAC and LC-MS/MS analysis, Western blotting results also revealed that all core histones were expressed at higher levels in arsenite-treated than in control HL-60 cells (Figure S1).
Although the mechanisms through which the histone proteins are up-regulated upon arsenite treatment remain unknown, many studies showed that arsenite could induce chromosome damage and modulate DNA methylation in mammalian cells 9, 13. The substantially increased expression of histones might be reflective of the considerable change in chromatin structure induced by arsenite treatment.
Modest down-regulation of heterogeneous nuclear ribonuclear proteins (hnRNPs) and proteins involved in translation
Aside from the considerable upregulation of histone proteins, arsenite treatment also led to a systematic down-regulation of hnRNPs and several important groups of proteins involved in translation (Table S4). In this context, hnRNPs, ribosomal proteins, translation initiation proteins, and translation elongation proteins were all modestly down-regulated upon arsenite treatment (Table S4); the average ratios (treated/untreated) for these four groups of proteins are 0.85, 0.88, 0.82 and 0.82, respectively. These results are in accordance with the previous findings 24, 37 and with the growth inhibition induced by arsentite treatment (vide infra).
Arsenite induced the down-regulation of fatty acid synthase (FAS)
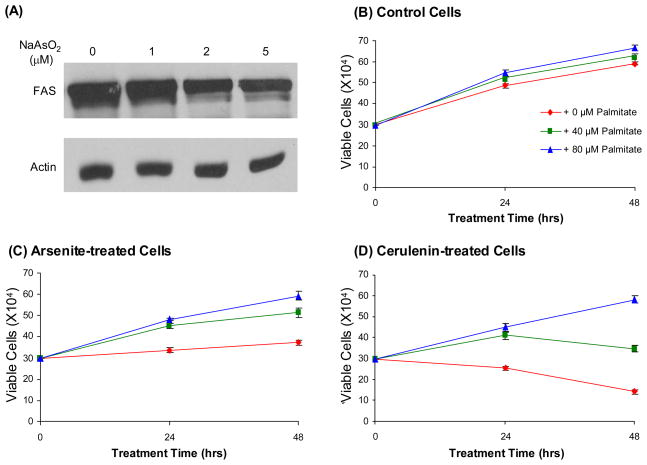
Our LC-MS/MS results showed that FAS was down-regulated by approximately 40% upon arsenite treatment. The LC-MS/MS quantification result was validated by Western blotting analysis, which revealed that arsenite treatment gives rise to the dose-dependant decrease in the expression of FAS (Figure 3A). FAS is the sole protein in the human genome capable of reductive synthesis of long-chain fatty acids from acetyl-coenzyme A (acetyl-CoA), malonyl-CoA and NADPH 38. It has been reported that FAS is highly expressed in human carcinomas 39. Inhibitors of FAS are selectively toxic to cell lines derived from human malignancies, supporting that cancer cells rely on endogenous fatty acid synthesis for survival and inhibition of FAS may afford an effective route for cancer treatment and prevention 39.
Figure 3.
Western blotting analysis of Fatty acid synthase (FAS) with lysates of untreated HL-60 cells (“control”) and HL-60 cells that are treated with 1 μM, 2 μM, or 5 μM of arsenite for 24 hrs (A), and actin was used as the loading control. The viabilities of HL-60 cells after 24 and 48 hrs of treatment with 0, 40, 80 μM palmitate alone (B), or together with 5 μM arsenite (C) or 2.5 μg/mL cerulenin (D).
We reason that the decreased expression of FAS may account partly for the cytotoxic effect of arsenite. If this is the case, the arsenite-induced growth inhibition of HL-60 cells should be rescued by palmitate, the end product of FAS. To test this, we assessed whether the proliferation of HL-60 cells is perturbed by arsenite treatment and how this perturbation is affected by externally added palmitate. It turned out that arsenite treatment inhibits the proliferation of HL-60 cells, and palmitate indeed protects, in a dose-dependent fashion, HL-60 cells from arsenite-induced growth inhibition (Figure 3B&C). It was previously observed that the cytotoxicity of cerulenin, a common FAS inhibitor 40, 41, could be abolished by co-administration with exogenous fatty acid 42. We also observed that the survival of HL-60 cells is compromised upon incubation with cerulenin, which can again be rescued by palmitate (Figure 3D). This result corroborates with the arsenite-induced down-regulation of FAS and underscores that arsenite may induce cytotoxic effect by inhibiting endogenous fatty acid synthesis through suppressing FAS expression. FAS expression has been found to be controlled by many pathways including Akt 43, and by tumor suppressors and oncogenes, which encompass p53, p63, p73, and H-ras 44, 45. Further study is needed to unravel the mechanisms involved in the arsenite-mediated down-regulation of FAS.
Arsenite induced the alteration in expression of other important enzymes
Arsenite treatment also gave rise to considerable changes in the expression levels of some other important enzymes, including neutrophil elastase, cathepsin D, α-enolase, α-mannosidase, etc. (Table 1). These proteins play pivotal roles in different cellular pathways, and we would like to discuss some of them in detail.
Neutrophil elastase (NE) is the key protease involved in the cleavage of PML-RARα fusion protein in mouse and human APL cells 46. The PML-RARα fusion protein generated by the t(15;17) translocation, which is associated with APL, initiated APL when expressed in the early myeloid compartment 46. Arsenite was shown to induce the degradation of the PML-RARα fusion protein in NB4-S1 cells, which was attributed to the destabilization of lysosome and the subsequent release of hydrolytic enzyme (i.e., cathepsin L) to the cytosol 47. We observed that NE was up-regulated by approximately 2 fold upon arsenite treatment. Viewing that neutrophil elastase is the dominant PML-RARα cleaving activity in human APL cells 46, our result suggests that arsenite treatment may result in the degradation of the PML-RARα protein through stimulating the expression of NE.
Cathepsin D (CatD) is a lysosomal aspartic protease found in neutrophils and monocytes 48. CatD is an important cell death mediator 49, and fibroblasts from CatD-deficient mice display more resistance toward etoposide- and adriamycin-induced apoptosis than fibroblasts from the wild-type littermates 50. Upon arsenite treatment, CatD was up-regulated by 60% (Table 1). The arsenite-induced up-regulation of CatD may also contribute, in part, to the cytotoxic effect of arsenite.
Aside from the enzymes discussed above, there are many other important proteins that are significantly up- or down-regulated by arsenite in HL-60 cells (Table 1). For instance, we found that prothymosin α was substantially up-regulated upon arsenite treatment. This protein is known to compete with transcription factor Nrf2 for binding to the same domain on Keap1, thereby releasing Nrf2 from the Nrf2-Keap1 inhibitory complex 51. The liberation of Nrf2 can result in the up-regulation of genes involved in defense against oxidative stress and electrophilic attack by binding to the anti-oxidant response elements in promoters of these genes 51–53. Consistent with this notion, we indeed observed that arsenite treatment led to substantial up-regulation of heme oxygenase 1 (Table 1), a Nrf2 target gene 53, and arsenite is known to induce the formation of reactive oxygen species 12.
Conclusions
Arsenite is an established human carcinogen 54; on the other hand, it is also a clinically successful anticancer drug for APL treatment 7. In this study, we employed SILAC, together with LC-MS/MS, and assessed quantitatively the perturbation of protein expression in HL-60 leukemic cells induced by arsenite treatment. Our results revealed that the treatment led to the up- or down-regulation of many important proteins, including histones, fatty acid synthase, neutrophil elastase, cathepsin D, etc. In addition, most ribosomal proteins, translation initiation factors, translation elongation factors, and hnRNPs were modestly down-regulated upon the treatment.
We also confirmed the up- and down-regulation of several proteins by Western blotting analysis. Among the proteins whose expressions are perturbed by arsenite, the down-regulation of fatty acid synthase is of particular importance. In this context, normal adult cells acquire fatty acids mainly from dietary sources and rarely rely on de novo fatty acid synthesis because nutritional fatty acids inhibit strongly the expression of the genes involved in fatty acid synthesis 55. Cancer cells, however, are no longer sensitive to this nutritional signal and instead depend on endogenous fatty acid synthesis to provide vital structural lipids that are required for survival and proliferation 42. Therefore, fatty acid synthase is substantially up-regulated in many types of tumors and inhibition of this enzyme has been suggested for cancer treatment 39. Our cell survival data support that the arsenite-induced growth inhibition of HL-60 cells can be rescued by palmitate, the final product of fatty acid synthase. This finding parallels the growth inhibition induced by cerulenin, which is a specific noncompetitive inhibitor of the β-ketoacyl transferase activity of FAS 40, 41. Thus, our result underscored that the inhibition of endogenous fatty acid synthase may constitute a novel mechanism for arsenite-induced cytotoxic effect.
Further studies about the implications of arsenite-induced perturbation of other proteins may also lead to the discovery of additional molecular pathways that are altered by arsenite and contribute to the arsenite-induced cytotoxicity. The pharmacoproteomic profiling could constitute a valuable tool for the identification of drug-responsive biomarkers for arsenite treatment and establish a molecular basis for developing novel and more effective therapeutic approaches for the treatment of APL and other human cancers.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (R01 CA 116522).
Abbreviations
- APL
acute promyelocytic leukemia
- SILAC
stable isotope labeling by amino acids in cell culture
- MS
Mass spectrometry
- 2-DE
two-dimensional gel electrophoresis
- acetyl-CoA
acetyl-coenzyme A
- FAS
fatty acid synthase
- CatD
cathepsin D
- hnRNPs
heterogeneous nuclear ribonuclear proteins
Footnotes
Supporting Information Available: Tables for quantification results and Western blot data for histone proteins. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.National Research Council. Arsenic in Drinking Water. National Academy Press; Washington DC: 1999. 2001 update. [Google Scholar]
- 2.Abernathy CO, Liu YP, Longfellow D, Aposhian HV, Beck B, Fowler B, Goyer R, Menzer R, Rossman T, Thompson C, Waalkes M. Arsenic. Health effects, mechanisms of actions, and research issues. Environ Health Perspect. 1999;107:593–597. doi: 10.1289/ehp.99107593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morales KH, Ryan L, Kuo TL, Wu MM, Chen CJ. Risk of internal cancers from arsenic in drinking water. Environ Health Perspect. 2000;108:655–661. doi: 10.1289/ehp.00108655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantor KP, Lubin JH. Arsenic, internal cancers, and issues in inference from studies of low-level exposures in human populations. Toxicol Appl Pharmacol. 2007;222:252–7. doi: 10.1016/j.taap.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J, Cai X, Han ZG, Ni JH, Shi GY, Jia PM, Liu MM, He KL, Niu C, Ma J, Zhang P, Zhang TD, Paul P, Naoe T, Kitamura K, Miller W, Waxman S, Wang ZY, deThe H, Chen SJ, Chen Z. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL).1. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997;89:3345–3353. [PubMed] [Google Scholar]
- 6.Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ, Si GY, Jin XL, Tang W, Li XS, Xong SM, Shen ZX, Sun GL, Ma J, Zhang P, Zhang TD, Gazin C, Naoe T, Chen SJ, Wang ZY, Chen Z. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood. 1996;88:1052–1061. [PubMed] [Google Scholar]
- 7.Zhu J, Chen Z, Lallemand-Breitenbach V, de The H. How acute promyelocytic leukaemia revived arsenic. Nat Rev Cancer. 2002;2:705–13. doi: 10.1038/nrc887. [DOI] [PubMed] [Google Scholar]
- 8.Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, Chen Y, Zhou L, Fang ZW, Wang YT, Ma J, Zhang P, Zhang TD, Chen SJ, Chen Z, Wang ZY. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354–60. [PubMed] [Google Scholar]
- 9.Lerda D. Sister-chromatid exchange (Sce) among individuals chronically exposed to arsenic in drinking-water. Mutat Res. 1994;312:111–120. doi: 10.1016/0165-1161(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 10.Gonsebatt ME, Vega L, Salazar AM, Montero R, Guzman P, Blas J, DelRazo LM, GarciaVargas G, Albores A, Cebrian ME, Kelsh M, OstroskyWegman P. Cytogenetic effects in human exposure to arsenic. Mutat Res. 1997;386:219–228. doi: 10.1016/s1383-5742(97)00009-4. [DOI] [PubMed] [Google Scholar]
- 11.Yan H, Wang N, Weinfeld M, Cullen WR, Le XC. Identification of arsenic-binding proteins in human cells by affinity chromatography and mass spectrometry. Analytical chemistry. 2009;81:4144–52. doi: 10.1021/ac900352k. [DOI] [PubMed] [Google Scholar]
- 12.Liu SX, Athar M, Lippai I, Waldren C, Hei TK. Induction of oxyradicals by arsenic: implication for mechanism of genotoxicity. Proc Natl Acad Sci USA. 2001;98:1643–8. doi: 10.1073/pnas.031482998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitchin KT. Recent advances in arsenic carcinogenesis: Modes of action, animal model systems, and methylated arsenic metabolites. Toxicol Appl Pharmacol. 2001;172:249–261. doi: 10.1006/taap.2001.9157. [DOI] [PubMed] [Google Scholar]
- 14.Yih LH, Peck K, Lee TC. Changes in gene expression profiles of human fibroblasts in response to sodium arsenite treatment. Carcinogenesis. 2002;23:867–876. doi: 10.1093/carcin/23.5.867. [DOI] [PubMed] [Google Scholar]
- 15.Wilkins MR, Sanchez JC, Williams KL, Hochstrasser DF. Current challenges and future applications for protein maps and post-translational vector maps in proteome projects. Electrophoresis. 1996;17:830–838. doi: 10.1002/elps.1150170504. [DOI] [PubMed] [Google Scholar]
- 16.Klose J, Kobalz U. 2-Dimensional Electrophoresis of Proteins - an Updated Protocol and Implications for a Functional-Analysis of the Genome. Electrophoresis. 1995;16:1034–1059. doi: 10.1002/elps.11501601175. [DOI] [PubMed] [Google Scholar]
- 17.Castagna A, Antonioli P, Astner H, Hamdan M, Righetti SC, Perego P, Zunino F, Righetti PG. A proteomic approach to cisplatin resistance in the cervix squamous cell carcinoma cell line A431. Proteomics. 2004;4:3246–3267. doi: 10.1002/pmic.200400835. [DOI] [PubMed] [Google Scholar]
- 18.Sinha P, Poland J, Kohl S, Schnolzer M, Helmbach H, Hutter G, Lage H, Schadendorf D. Study of the development of chemoresistance in melanoma cell lines using proteome analysis. Electrophoresis. 2003;24:2386–2404. doi: 10.1002/elps.200305456. [DOI] [PubMed] [Google Scholar]
- 19.Urbani A, Poland J, Bernardini S, Bellincampi L, Biroccio A, Schnolzer M, Sinha P, Federici G. A proteomic investigation into etoposide chemo-resistance of neuroblastoma cell lines. Proteomics. 2005;5:796–804. doi: 10.1002/pmic.200401147. [DOI] [PubMed] [Google Scholar]
- 20.Bertagnolo V, Grassilli S, Bavelloni A, Brugnoli F, Piazzi M, Candiano G, Petretto A, Benedusi M, Capitani S. Vav1 modulates protein expression during ATRA-induced maturation of APL-derived promyelocytes: A proteomic-based analysis. J Proteome Res. 2008;7:3729–3736. doi: 10.1021/pr7008719. [DOI] [PubMed] [Google Scholar]
- 21.Lau AT, He QY, Chiu JF. A proteome analysis of the arsenite response in cultured lung cells: evidence for in vitro oxidative stress-induced apoptosis. Biochem J. 2004;382:641–50. doi: 10.1042/BJ20040224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tapio S, Danescu-Mayer J, Asmuss M, Posch A, Gomolka M, Hornhardt S. Combined effects of gamma radiation and arsenite on the proteome of human TK6 lymphoblastoid cells. Mutat Res. 2005;581:141–52. doi: 10.1016/j.mrgentox.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 23.Berglund SR, Santana AR, Li D, Rice RH, Rocke DM, Goldberg Z. Proteomic analysis of low dose arsenic and ionizing radiation exposure on keratinocytes. Proteomics. 2009;9:1925–38. doi: 10.1002/pmic.200800118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng PZ, Wang KK, Zhang QY, Huang QH, Du YZ, Zhang QH, Xiao DK, Shen SH, Imbeaud S, Eveno E, Zhao CJ, Chen YL, Fan HY, Waxman S, Auffray C, Jin G, Chen SJ, Chen Z, Zhang J. Systems analysis of transcriptome and proteome in retinoic acid/arsenic trioxide-induced cell differentiation/apoptosis of promyelocytic leukemia. Proc Natl Acad Sci USA. 2005;102:7653–8. doi: 10.1073/pnas.0502825102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ge F, Lu XP, Zeng HL, He QY, Xiong S, Jin L. Proteomic and functional analyses reveal a dual molecular mechanism underlying arsenic-induced apoptosis in human multiple myeloma cells. J Proteome Res. 2009;8:3006–19. doi: 10.1021/pr9001004. [DOI] [PubMed] [Google Scholar]
- 26.Gygi SP, Rist B, Gerber SA, Turecek F, Gelb MH, Aebersold R. Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat Biotechnol. 1999;17:994–999. doi: 10.1038/13690. [DOI] [PubMed] [Google Scholar]
- 27.Ross PL, Huang YLN, Marchese JN, Williamson B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, Purkayastha S, Juhasz P, Martin S, Bartlet-Jones M, He F, Jacobson A, Pappin DJ. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics. 2004;3:1154–1169. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- 28.Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, Mann M. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics. 2002;1:376–386. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- 29.Wiseman RL, Chin KT, Haynes CM, Stanhill A, Xu CF, Roguev A, Krogan NJ, Neubert TA, Ron D. Thioredoxin-related Protein 32 is an arsenite-regulated Thiol Reductase of the proteasome 19 S particle. J Biol Chem. 2009;284:15233–45. doi: 10.1074/jbc.M109.002121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Harken DR, Dixon CW, Heimberg M. Hepatic lipid metabolism in experimental diabetes. V. The effect of concentration of oleate on metabolism of triglycerides and on ketogenesis. J Biol Chem. 1969;244:2278–85. [PubMed] [Google Scholar]
- 31.Romijn EP, Christis C, Wieffer M, Gouw JW, Fullaondo A, van der Sluijs P, Braakman I, Heck AJR. Expression clustering reveals detailed coexpression patterns of functionally related proteins during B cell differentiation - A proteomic study using a combination of one-dimensional gel electrophoresis, LC-MS/MS, and stable isotope labeling by amino acids in cell culture (SILAC) Mol Cell Proteomics. 2005;4:1297–1310. doi: 10.1074/mcp.M500123-MCP200. [DOI] [PubMed] [Google Scholar]
- 32.Uitto PM, Lance BK, Wood GR, Sherman J, Baker MS, Molloy MP. Comparing SILAC and two-dimensional gel electrophoresis image analysis for profiling urokinase plasminogen activator signaling in ovarian cancer cells. J Proteome Res. 2007;6:2105–2112. doi: 10.1021/pr060638v. [DOI] [PubMed] [Google Scholar]
- 33.Zhang TD, Chen GQ, Wang ZG, Wang ZY, Chen SJ, Chen Z. Arsenic trioxide, a therapeutic agent for APL. Oncogene. 2001;20:7146–53. doi: 10.1038/sj.onc.1204762. [DOI] [PubMed] [Google Scholar]
- 34.Douer D, Tallman MS. Arsenic trioxide: new clinical experience with an old medication in hematologic malignancies. J Clin Oncol. 2005;23:2396–410. doi: 10.1200/JCO.2005.10.217. [DOI] [PubMed] [Google Scholar]
- 35.Lennartsson A, Ekwall K. Histone modification patterns and epigenetic codes. Biochim Biophys Acta. 2009;1790:863–8. doi: 10.1016/j.bbagen.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Zhou X, Sun H, Ellen TP, Chen HB, Costa M. Arsenite alters global histone H3 methylation. Carcinogenesis. 2008;29:1831–1836. doi: 10.1093/carcin/bgn063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Othumpangat S, Kashon M, Joseph P. Sodium arsenite-induced inhibition of eukaryotic translation initiation factor 4E (eIF4E) results in cytotoxicity and cell death. Mol Cell Biochem. 2005;279:123–131. doi: 10.1007/s11010-005-8284-2. [DOI] [PubMed] [Google Scholar]
- 38.Wakil SJ. Fatty-acid synthase, a proficient multifunctional enzyme. Biochemistry. 1989;28:4523–4530. doi: 10.1021/bi00437a001. [DOI] [PubMed] [Google Scholar]
- 39.Kuhajda FP. Fatty acid synthase and cancer: New application of an old pathway. Cancer Res. 2006;66:5977–5980. doi: 10.1158/0008-5472.CAN-05-4673. [DOI] [PubMed] [Google Scholar]
- 40.Funabashi H, Kawaguchi A, Tomoda H, Omura S, Okuda S, Iwasaki S. Binding site of cerulenin in fatty acid synthetase. J Biochem. 1989;105:751–5. doi: 10.1093/oxfordjournals.jbchem.a122739. [DOI] [PubMed] [Google Scholar]
- 41.Omura S. The antibiotic cerulenin, a novel tool for biochemistry as an inhibitor of fatty acid synthesis. Bacteriol Rev. 1976;40:681–97. doi: 10.1128/br.40.3.681-697.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pizer ES, Wood FD, Pasternack GR, Kuhajda FP. Fatty acid synthase (FAS): A target for cytotoxic antimetabolites in HL60 promyelocytic leukemia cells. Cancer Research. 1996;56:745–751. [PubMed] [Google Scholar]
- 43.Furuta E, Pai SK, Zhan R, Bandyopadhyay S, Watabe M, Mo YY, Hirota S, Hosobe S, Tsukada T, Miura K, Kamada S, Saito K, Iiizumi M, Liu W, Ericsson J, Watabe K. Fatty acid synthase gene is up-regulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Res. 2008;68:1003–11. doi: 10.1158/0008-5472.CAN-07-2489. [DOI] [PubMed] [Google Scholar]
- 44.Yang YA, Han WF, Morin PJ, Chrest FJ, Pizer ES. Activation of fatty acid synthesis during neoplastic transformation: role of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Exp Cell Res. 2002;279:80–90. doi: 10.1006/excr.2002.5600. [DOI] [PubMed] [Google Scholar]
- 45.D’Erchia AM, Tullo A, Lefkimmiatis K, Saccone C, Sbisa E. The fatty acid synthase gene is a conserved p53 family target from worm to human. Cell Cycle. 2006;5:750–8. doi: 10.4161/cc.5.7.2622. [DOI] [PubMed] [Google Scholar]
- 46.Lane AA, Ley TJ. Neutrophil elastase cleaves PML-RAR alpha and is important for the development of acute promyelocytic leukemia in mice. Cell. 2003;115:305–318. doi: 10.1016/s0092-8674(03)00852-3. [DOI] [PubMed] [Google Scholar]
- 47.Kitareewan S, Roebuck BD, Demidenko E, Sloboda RD, Dmitrovsky E. Lysosomes and trivalent arsenic treatment in acute promyelocytic leukemia. J Natl Cancer Instit. 2007;99:41–52. doi: 10.1093/jnci/djk004. [DOI] [PubMed] [Google Scholar]
- 48.Benes P, Vetvicka V, Fusek M. Cathepsin D--many functions of one aspartic protease. Crit Rev Oncol Hematol. 2008;68:12–28. doi: 10.1016/j.critrevonc.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaattela M, Cande C, Kroemer G. Lysosomes and mitochondria in the commitment to apoptosis: a potential role for cathepsin D and AIF. Cell Death Differ. 2004;11:135–6. doi: 10.1038/sj.cdd.4401333. [DOI] [PubMed] [Google Scholar]
- 50.Wu GS, Saftig P, Peters C, El-Deiry WS. Potential role for cathepsin D in p53-dependent tumor suppression and chemosensitivity. Oncogene. 1998;16:2177–83. doi: 10.1038/sj.onc.1201755. [DOI] [PubMed] [Google Scholar]
- 51.Karapetian RN, Evstafieva AG, Abaeva IS, Chichkova NV, Filonov GS, Rubtsov YP, Sukhacheva EA, Melnikov SV, Schneider U, Wanker EE, Vartapetian AB. Nuclear oncoprotein prothymosin α is a partner of Keap1: implications for expression of oxidative stress-protecting genes. Mol Cell Biol. 2005;25:1089–99. doi: 10.1128/MCB.25.3.1089-1099.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayes JD, McMahon M. Molecular basis for the contribution of the antioxidant responsive element to cancer chemoprevention. Cancer Lett. 2001;174:103–13. doi: 10.1016/s0304-3835(01)00695-4. [DOI] [PubMed] [Google Scholar]
- 53.Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem. 2000;275:16023–9. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- 54.Snow ET, Sykora P, Durham TR, Klein CB. Arsenic, mode of action at biologically plausible low doses: what are the implications for low dose cancer risk? Toxicol Appl Pharmacol. 2005;207:557–64. doi: 10.1016/j.taap.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 55.Sul HS, Wang D. Nutritional and hormonal regulation of enzymes in fat synthesis: studies of fatty acid synthase and mitochondrial glycerol-3-phosphate acyltransferase gene transcription. Annu Rev Nutr. 1998;18:331–51. doi: 10.1146/annurev.nutr.18.1.331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.