Abstract
The biologic role of thyroid-stimulating hormone (TSH; thyrotropin) as an activator (agonist) of the TSH receptor (TSHR) in the hypothalamic–pituitary–thyroid axis is well known and activation of TSHR by recombinant human TSH is used clinically in patients with thyroid cancer. TSHR ligands other than TSH could be used to probe TSHR biology in thyroidal and extrathyroidal tissues, and potentially be employed in patients. A number of different TSHR ligands have been reported, including TSH analogs, antibodies and small-molecule, drug-like compounds. In this review, we will provide an update on all these classes of TSHR agonists and antagonists but place emphasis on small-molecule ligands.
Keywords: small molecule, thyroid-stimulating hormone, thyrotropin, TSH analogs, TSHR antagonists, TSHR agonists, TSHR antibodies, TSH receptor, TSHR ligands
The biologic role of thyroid-stimulating hormone (TSH; thyrotropin) as an activator (agonist) of the TSH receptor (TSHR) in the hypothalamic–pituitary–thyroid axis is well known [1]. TSH, which is produced in thyrotrophs of the anterior pituitary gland, is secreted into the circulation in response to thyrotropin-releasing hormone stimulation. Circulating TSH activates TSHR, thereby stimulating the function of thyroid follicular cells (thyrocytes) leading, in particular, to increases in size and number of thyrocytes, and biosynthesis and secretion of thyroid hormones. The effects of TSHR activation on thyroid hormone biosynthesis include stimulation of iodide uptake by thyroid cells via the sodium–iodide symporter and synthesis of thyroglobulin, which is the precursor protein of thyroid hormones and is secreted in small amounts by thyroid cells [2]. Indeed, it is the effects on these two aspects of thyroid cell function by TSHR activation that underlies the usefulness of recombinant human TSH (rhTSH) in patients with thyroid cancer. For the last decade, rhTSH has been used in the follow-up of thyroid cancer patients to increase the sensitivity for detection of recurrent or metastatic thyroid cancer without the morbidity of hypothyroidism [3]. rhTSH stimulates uptake of iodide and release of thyroglobulin from differentiated thyroid cancer cells and thereby increases the sensitivity of measurements of radioiodine uptake or serum thyroglobulin levels to detect residual or recurrent cancer in these patients. In addition, rhTSH was recently approved by the US FDA for a supplemental indication to improve radioiodine ablation of thyroid remnants after surgical thyroidectomy in patients with thyroid cancer. rhTSH has also been shown to be useful in the treatment of nontoxic multinodular goiter [4], especially when radioiodine uptake is low, but this use has not yet been approved by the FDA.
Thyroid-stimulating hormone receptors are known to be expressed in multiple extrathyroidal tissues, including bone, brain, kidney, testis, fat and cells of the immune system [5] but the role of the TSHR in these tissues is not clear. Perhaps the most important of the potential effects of TSHR activation in extrathyroidal tissues is its proposed effects on bone metabolism [6–10]. This is based on the observations that osteoblasts and osteoclasts express TSHRs [6,7,9,11,12], bone cells in culture respond to TSH [6,7,12] and TSHR-deficient (TSHR−/−) mice exhibit a form of high bone turnover osteoporosis [9]. Furthermore, positive effects of TSH treatment on the skeleton have been reported in estrogen-deficient rodents [7,10]. These findings were interpreted as showing that TSH acts directly on bone cells and regulates skeletal remodeling. However, contradictory findings have been reported [11] and a definitive conclusion has not been drawn [5]. TSHR ligands might be used as probes to determine the effects of TSHR signaling in osteoblasts and osteoclasts, and in other extrathyroidal tissues so as to definitively determine the role of TSHRs in these tissues.
There are other potential uses for TSHR ligands. They could be used as imaging agents for thyroid cancer or to deliver drugs to cancer cells expressing TSHRs. TSHR antagonists could be used to treat hyperthyroidism caused by autoimmune antibodies in Graves’ disease or by germline mutations of the TSHR [13,14]. These antagonists could lead to improved treatment of Graves’ disease because they might not cause the untoward effects of current therapies. Antagonists could also be used as probes of extrathyroidal TSHR function. Graves’ ophthalmopathy (GO) represents the most frequent extrathyroidal manifestation of Graves’ disease, which is characterized by enlargement of the extraocular muscle bodies and expansion of the retro-orbital fat [15,16]. Orbital fibroblasts, which can differentiate into mature adipocytes, are suggested to be the major target cell of the autoimmune process in GO and have been shown to express functional TSHRs [17]. In fact, levels of TSHR are higher in orbital adipose tissue from patients with GO than in patients without GO [18]. Therefore, the TSHR in orbital adipose tissue might be a potential target for the development of pharmacologic therapeutic agents. TSHR inverse agonists, which are a subclass of antagonists that inhibit agonist-independent (or basal or constitutive) TSHR signaling, could be used to inhibit TSH-independent signaling in patients with recurrent or metastatic thyroid cancer who are receiving thyroid hormone to suppress TSH. Inhibition of agonist-independent signaling might benefit these patients because this signaling by TSHR may stimulate proliferation of the cancer cells. Lastly, TSHR ligands could be used as probes to delineate the molecular mechanism of TSHR activation, that is, the conformational changes that mediate conversion of inactive to active states of TSHR.
A number of different potential TSHR ligands have been reported, including TSH analogs, antibodies and small-molecule, drug-like compounds. In this review, we will provide an update on all these classes of TSHR agonists and antagonists but place the emphasis on small-molecule ligands.
TSH receptor
Thyroid-stimulating hormone receptor is a member of the class A subfamily of the ubiquitous family of cell-surface receptors known as seven transmembrane-spanning receptors (7TMRs) or G-protein-coupled receptors (GPCRs) (Figure 1) [1,19,20]. TSHR is highly conserved across species and in this review, TSHR will refer to the human receptor. TSH and TSHR antibodies bind to TSHR by interacting primarily, if not exclusively, with the large amino terminal ectodomain of TSHR. In contrast to other 7TMRs, including the other glycoprotein hormone receptors (receptors for luteinizing hormone [LH]/chorionic gonadotropin [CG] and follicle-stimulating hormone [FSH]), that are present on the surface of cells as single polypeptide chains, TSHR is cleaved within its ectodomain to generate a receptor composed of two subunits, A/α and B/β, that are held together by disulfide bridges [21]; a sequence of approximately 50 amino acid residues is excised and lost [22,23]. The biological role of receptor cleavage is still controversial [20,24]. The TSHR can activate members of all four G-protein families (Gs, Gq/11, Gi/o, G12/13) [25]. The best-characterized signaling cascades initiated by TSHR are activation of Gs, which activates adenylyl cyclase to increase production of the second messenger cAMP, and Gq, which activates phospholipase C and generates inositol 1,4,5-trisphosphate and 1,2-diacylglycerol. Increased intracellular cAMP is involved in iodide uptake, thyroid hormone secretion and cell proliferation [1]. Phospholipase C-dependent pathways are required for iodine organification and thyroid hormone secretion in response to TSH. In addition, this pathway seems to have an essential role in the adaptive growth of the thyroid gland [26]. Recently, it has been shown in primary human thyrocytes that TSH promotes a TSHR-dependent activation of the p44/42 MAPK pathway, which requires the activation of G13 [27].
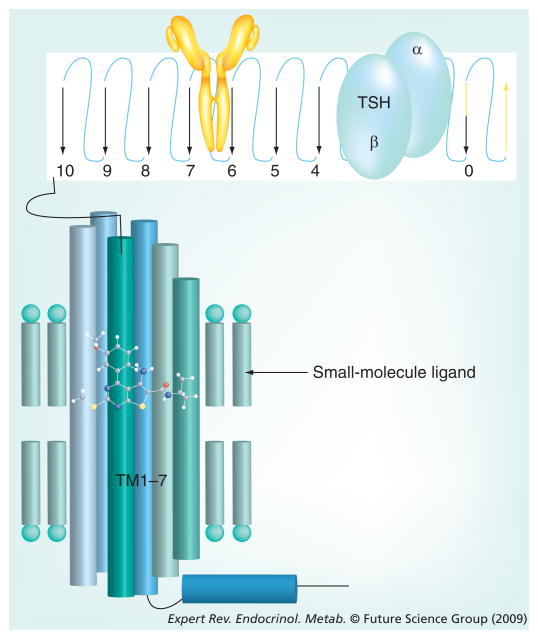
Figure 1. Primary sites of binding of TSH, anti-TSHR antibodies and small-molecule ligands.
Depiction of the amino-terminal ectodomain (top), which is on the extracellular surface of the cell, and the transmembrane helical domain (bottom), which lies within the cell-surface membrane, illustrates distinct binding sites for TSH (blue), anti-TSH receptor antibodies (yellow) and a small-molecule ligand. Simultaneous binding of these ligands to the same receptor has not been shown to occur. The placement of TSH and anti-TSH receptor antibodies is not meant to suggest interactions with these regions of the ectodomain.
TSH: Thyroid-stimulating hormone.
Furthermore, in contrast to other glycoprotein hormone receptors, TSHR exhibits constitutive signaling activity that can be inhibited by some antagonists acting as inverse agonists (see later). Indeed, it has been proposed that one aspect of the molecular mechanism of TSHR activation may be that the amino terminal ectodomain acts as a tethered inverse agonist whose inhibition is released when TSH binds [28,29]. However, the molecular details of TSHR activation, as with all 7TMRs, are still poorly delineated.
TSH & TSH analogs
TSH is a heterodimeric glycoprotein consisting of an α-chain that is common to the glycoprotein hormones – TSH, LH, CG and FSH, and a specific β-chain. TSH is produced in the thyrotrophs of the anterior pituitary gland and secreted into the peripheral circulation. TSH is the primary endogenous, physiologic stimulator of TSHR function. A second endogenous activator of TSHR function, thyrostimulin (or corticotroph-derived glycoprotein hormone), has been identified [30,31]. Thyrostimulin, like TSH, is a heterodimeric glycoprotein but is composed of different subunits – glycoprotein hormone α2 and glycoprotein hormone β5. In contrast to TSH, thyrostimulin may act primarily as a paracrine factor but its physiological role is not understood. At present, thyrostimulin has not been used to study TSHR biology.
Recombinant human TSH
Recombinant human TSH is produced in bioreactors using Chinese hamster ovary (CHO) cells stably expressing the genes for the α-subunit of the glycoprotein hormones and TSH-β [32]. Since CHO cells do not have the same ability to glycosylate proteins as TSH-producing cells of the anterior pituitary gland, rhTSH produced in CHO cells is comprised predominantly of TSH in which the three carbohydrate chains terminate in sialic acid residues without the penultimate N-acetylgalactosamine and terminal sulfate moieties. Nevertheless, the bioactivity of rhTSH is similar to that of human TSH [32,33]. As mentioned earlier, rhTSH (Thyrogen®, Genzyme) is used in clinical practice in patients with thyroid cancer and represents the first and only TSHR ligand successfully developed for this purpose approved by the FDA (for review see [34]). It is used as a probe of TSHR function in animal studies also, in particular in dogs. It has been shown that the TSH-stimulation test with rhTSH is a valuable diagnostic tool to assess thyroid function in selected dogs in which a diagnosis of hypothyroidism cannot be based on basal T4 and endogeneous TSH concentrations alone [35,36]. The effect of rhTSH has been well studied in mice [37], which allows the use of mouse models to study the effect of TSH in extrathyroidal tissue, for example, in bone. Recently, it has been shown that rhTSH when injected intermittently in rodents prevents bone loss, perhaps by inhibiting osteoclast-mediated resorption [10].
Biologically active single-chain TSH variants
The assembly of TSH-β and -α subunit is the rate limiting step in the production of functional heterodimeric TSH, and dissociation leads to hormone inactivation [38]. One issue regarding the clinical use of rhTSH is its rapid clearance from the circulation. Several studies were carried out to demonstrate that conversion of rhTSH to a single chain form could increase its biological half-life in the circulation, the stability and activity of the hormone that could be important for in vivo studies. It was thought that the single-chain approach could lead to the development of highly effective, long-acting TSH agonists or antagonists. Several groups showed that genetic fusion of the human TSH α- and β-subunits using the C-terminal peptide of the human CG β-subunit as a linker created unimolecular TSH whose receptor binding and bioactivity were comparable to native human TSH. The fused TSH had higher thermostability and a longer plasma half-life than native hTSH, suggesting that dimer dissociation may contribute to glycoprotein hormone inactivation in vivo [39,40].
TSH-β and TSH-α subunits contain one or two N-linked oligosaccharide chains, respectively, which play a critical role in the function of glycoprotein hormones [41]. Two N-linked carbohydrate-free single-chain hTSH variants [42] were shown to bind to TSHR with high affinity. However, instead of functioning as full agonists they had modest effects themselves and reduced TSH-induced thyroid hormone secretion by approximately 50% in vivo. These mutated single-chain polypeptides act, therefore, as partial agonists in mice and appear to bind to TSHR at a site similar to TSH [43].
Superagonist TSH analogs
In the 1990s, Szkudlinski, Weintraub and colleagues initiated a series of experiments in an attempt to produce TSH analogs with affinities, potencies and efficacies at TSHR greater than human TSH – ‘superagonists’ [19,44]. They reasoned that using site-specific mutagenesis of native human TSH they could develop superagonist TSH analogs. They based their mutagenesis strategies on homologies with other glycoprotein hormones, scanning mutagenesis of regions of TSH that were predicted to interact with TSHR and the known higher affinities of several non-human TSHs for TSHR compared with human TSH. They constructed a series of plasmids containing complementary DNAs encoding site-specific mutations in the α- or β-subunits of TSH, produced mutated TSH analogs by coexpressing these subunits in cells in culture and showed that a number of these analogs exhibited higher affinities, potencies and efficacies for TSHR than native TSH [45,46]. For example, an analog in which four lysine substitutions were made in the α-subunit and three arginine substitutions in the β-subunit produced a TSH analog with approximately 2500-fold increased affinity, 200-fold increased potency and 1.8-fold increased efficacy compared with native human TSH [46]. In preliminary experiments, superagonists were shown to stimulate thyroxine secretion in mice [44,46]. Importantly, these analogs were shown to be selective for TSHR in that they did not activate the LH/CG receptor (LHCGR) or FSH receptor (FSHR). Recently, one of these superagonist analogs was used as a probe to explore the interaction of TSH with the ‘hinge region’ of the TSHR amino terminal ectodomain [47]. Up to the present time, however, no definitive study has shown improved utility of single-chain TSH variants or these superagonists over native TSH as probes of TSHR function in intact animals and none has been approved for human use.
TSHR antibodies
The roles of antibodies targeting TSHR in patients with autoimmune thyroid disease have been known for many years (for review see [48]). Thyroid-stimulating antibodies (TSAbs) activate TSHR leading to hyperthyroidism in patients with Graves’ disease, whereas TSHR antibodies that inhibit TSHR activation (thyroid-blocking antibodies, [TBAbs]) are the cause of hypothyroidism in some patients. However, because of the complexity of the interactions between the polyclonal antibodies found in patients’ sera and their effects on TSHR function, (for reviews see [20,24,49]), it was necessary to develop monoclonal antibodies (mAbs) for use as probes or drug candidates [50–53]. A number of laboratories have generated anti-TSHR mAbs using plasma cells from different species, including mice [21,52–60], hamsters [50] and humans [61–63].
Inhibitory antibodies
Thyroid-stimulating antibodies that inhibit binding and signaling by TSH and TSAbs were developed using several different methods. Costagliola et al. induced generation of inhibitory mAbs by injecting mice with plasmid DNA constructs encoding the entire TSHR sequence [57], whereas Chen et al. used injection into mice of adenoviruses encoding the ‘A/α subunit’ of the amino terminal ectodomain of TSHR followed by booster injections with A/α-subunit [64]. Sanders et al. generated human mTBAbs using circulating white blood cells from the blood of a patient with autoimmune hypothyroidism [65]. These mTBAbs have been used to explore the molecular details of TSHR signaling in cells in culture but have not yet been used for other purposes.
Some 7TMR antagonists exhibit the property of inhibiting agonist-independent signaling and are referred to as inverse agonists [66]. Several mTBAbs have been shown to be inverse agonists. Chen et al. have generated a mouse mTBAb that is an inverse agonist and have used it to identify regions within the TSHR ectodomain that may be involved in constitutive signaling [64,67,68]. Sanders et al. [65] and Moriyama et al. [63] generated human mTBAbs with inverse agonist properties. As with mTBAbs without inverse agonist properties, these antibodies have been used to study some binding and signaling characteristics of TSHR in vitro but have not been used for other purposes. However, it has been suggested that antibodies that are inverse agonists may be used therapeutically to inhibit TSHR signaling in patients with thyroid cancer and in some patients with hyperthyroidism.
Stimulatory antibodies
Human [61,62], hamster [50] and murine [59] mTSAbs have been generated. Akamizu et al. [61] and Sanders et al. [62] generated human mTSAbs using lymphocytes obtained from patients with Graves’ disease. Ando et al. produced a mTSAb by injecting hamsters with an adenovirus expressing intact TSHR and boosting them with CHO cells expressing TSHR [50], and Gilbert et al. generated two mTSAbs in mice by injecting them with adenoviruses expressing the A/α-subunit of TSHR [59]. Animals in which mTSAbs were produced were studied to gain insight into TSAb-mediated hyperthyroidism. For example, Gilbert et al. found that mice in which mTSAbs were produced showed increased levels of thyroxine in their blood and naive mice given mTSAbs by passive transfer showed evidence of thyroid gland stimulation and thyrocyte necrosis [59]. However, none of these mice exhibited lymphocytic infiltration, a hallmark of Graves’ disease. mTSAbs have been used, like mTBAbs, to study aspects of TSHR binding and signaling and have been found to stimulate several pathways of signaling mediated by TSHR to different degrees [69,70]. However, a molecular mechanism(s) that underlies these differences has not been determined. No other uses of mTSAbs have been reported.
Small-molecule TSHR ligands
Small-molecule ligands are generally much more easily employed as probes and drugs compared with peptides or proteins. They are usually synthesized chemically, can be produced in large quantities at modest cost and can typically be administered orally because they are not degraded within, and can be absorbed from the gastrointestinal (GI) tract. For these reasons, a number of small-molecule agonists [71–75] and an antagonist [76] for LHCGR, and small-molecule agonists [77–83] and antagonists [82,84,85] for FSHR have recently been reported. Development of small-molecule ligands for TSHR has lagged behind those for other glycoprotein hormone receptors.
Development of small-molecule ligands is often initiated by one of the following approaches: candidate compounds; rational design; high-throughput screening; or a combination of these approaches. Lead compounds can then be modified to improve their affinities, potencies and efficacies.
Candidate approach to first small-molecule TSHR agonist
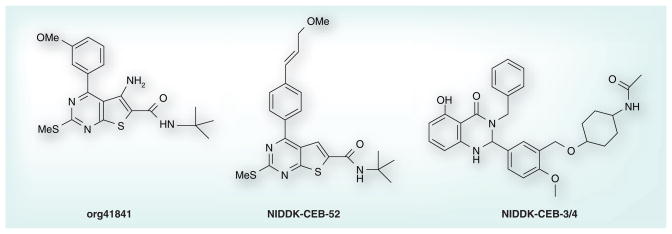
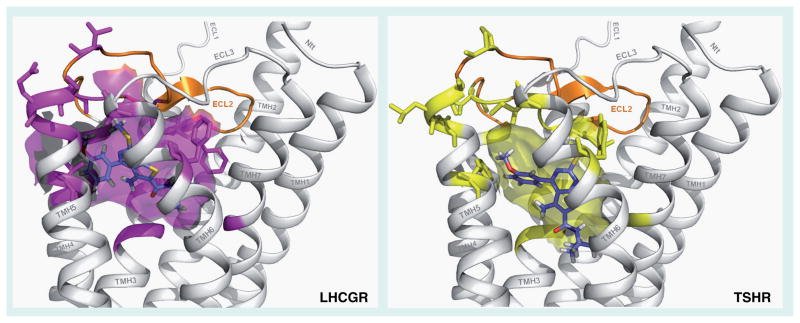
The thienopyrimidine org41841 (Figure 2) was the first reported small-molecule agonist for LHCGR [71]. Because many small-molecule ligands for class A 7TMRs bind within the receptors’ transmembrane helical domains (TMDs) [86] and since org41841 did not compete for LH binding to the LHCGR ectodomain, the authors suggested, but did not provide direct evidence, that org41841 binds to LHCGR within its TMD [71]. Owing to the high homology within the TMDs of TSHR and LHCGR, we predicted that org41841 might also bind to TSHR within its TMD. We began exploring this hypothesis by docking org41841 into 3D homology models of the TMDs of LHCGR and TSHR and by determining whether org41841 could activate TSHR. Computer-based docking to the homology models identified a putative binding pocket for org41841 in a cleft between transmembrane helices 3, 4, 5, 6 and 7 close to extracellular loop 2 in both receptors (Figure 3) and, indeed, org41841 was shown to be a partial agonist of TSHR [87]. At LHCGR, org41841 was a partial agonist eliciting a response that was 34% of that stimulated by a maximally effective concentration of LH with a potency of 220 nM whereas at TSHR, org41841 elicited a response that was 23% of that of TSH with a potency of 7700 nM. Thus, org41841 exhibited a lower potency at TSHR compared with LHCGR and behaved as a partial agonist at both receptors. A prediction based on these data was that replacement of amino acid residues within the binding pocket of TSHR with homologous residues present in LHCGR would improve activity of org41841 at the mutated TSHR compared with native TSHR. We performed a series of experiments in which homologous residues in the TSHR binding pocket were changed to the residues present in the LHCGR pocket. We found that several of these mutated receptors responded to org41841 with increased potency or efficacy, or both compared with TSHR. For example, org41841 behaved as a full agonist (99% of the response of TSH at native TSHR) with 2.9-fold higher potency than at TSHR at a mutant TSHR in which nine amino acid residues within the TMD and extracellular loop 2 were substituted by residues present in LHCGR. These data allowed us to tentatively conclude that org41841 activated TSHR by binding to its TMD. The low potency and efficacy of org41841 at TSHR limit its usefulness as a probe of TSHR function. Thus, we described org41841 as a small-molecule agonist for TSHR and provided strong evidence that it binds within the TMDs of TSHR and LHCGR [87]. This publication represented the first report of a small molecule ligand for TSHR and provided the first direct evidence that small-molecule ligands of glycoprotein hormone receptors may bind within the receptor’s TMD.
Figure 2.
Org41841, NIDDK-CEB-52 and NIDDK-CEB-3/4.
Figure 3. Comparison of the docking of the partial agonist org41841 (blue) in homology models of the transmembrane helical bundles of LHCGR and TSHR.
The transmembrane pocket is located within the extracellular half of the transmembrane helical bundle between transmembrane helices (TMHs) 3, 4, 5, 6 and 7 close to extracellular loop 2 (ECL2). The cleft is covered by ECL2 (orange) and the three junctions of TMH4/ECL2, ECL2/TMH5 and TMH6/ECL3. The binding cavity in LHCGR is in magenta and in TSHR in yellow. The side chains of the differing residues that line and cover the binding cleft are generally less bulky in LHCGR than in TSHR, which may explain the preferential binding of org41841 to LHCGR.
These models were constructed by Gunnar Kleinau and Gerd Krause, Leibniz-Institute of Molecular Pharmacology, 13125 Berlin, Germany. LHCGR: LH/CG receptor; TSHR: Thyroid-stimulating hormone receptor.
Small-molecule TSHR antagonist
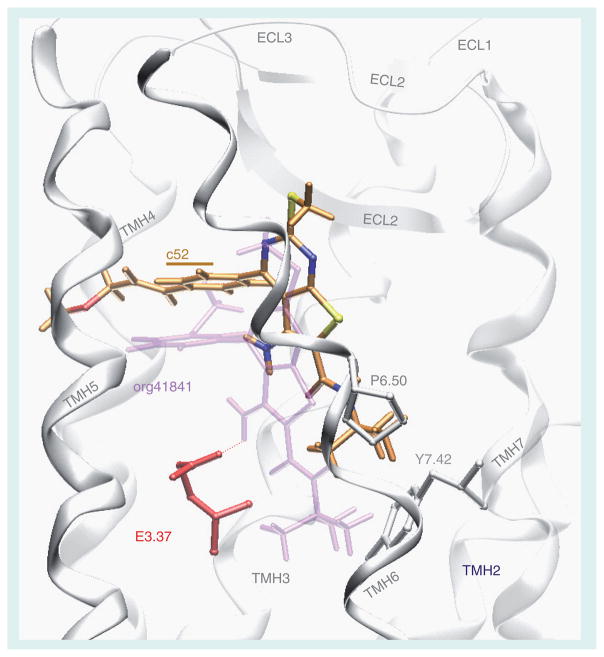
We used molecular modeling, chemical modification of org41841 and functional experiments to identify additional small-molecule ligands for TSHR. We consider this to be rational design because the initial modifications of org41841 are based on predictions from the model of the org41841-TSHR complex and the org41841 analogs were then tested in signaling assays. Several iterations of this process can be performed in attempts to optimize the properties of the analogs. Prior to using this rational approach, we tested a number of modifications at different sites on org41841, including several modifications of the t-butyl moiety of org41841, but these analogs either exhibited no activity or were partial agonists [88]. Based on the model of the org41841–TSHR complex, we predicted that elongated analogs would sit differently in the binding pocket of TSHR than org41841. We enlarged the molecule by adding a propylene-methyl-ether group at the para-position of the aromatic moiety of the 3-methoxyphenyl group to elongate the longitudinal axis. This analog was named NIDDK/CEB-52 (compound 52) (Figure 2) [89]. The model of the compound 52/TSHR complex predicts that compound 52 binds in the same pocket as org41841 (Figure 4). However, the presence of the propylene-methyl-ether substituent causes compound 52 to dock closer to extracellular loop 2 compared with org41841 and to interact with TSHR higher in the binding cleft than does org41841. Compound 52 points towards the proline in transmembrane helix 6 but is not predicted to cause movement of helix 6 at the intracellular portion during binding, and impairment of TSHR activation was expected. In addition, the model predicted that compound 52 would not be an effective agonist because its amino group does not interact with the highly conserved, negatively charged Asp in helix 3 that was found to be essential for TSHR activation by org41841 [87,88].
Figure 4. Comparison of docking modes of the antagonist NIDDK-CEB-52 (C atoms orange) and the partial agonist org41841 (light purple) in a model of the transmembrane helical bundle of thyroid-stimulating hormone receptor (TSHR).
The binding pocket for NIDDK-CEB-52 is within the extracellular half of the transmembrane helical bundle between helices 3, 5, 6, and 7 (white) and close to extracellular loop 2 (white). The model predicts that NIDDK-CEB-52 would not be an agonist because its amino group does not interact with the negatively charged glutamic acid in helix 3 (E3.37) that is essential for TSHR activation by org41841. In addition, in contrast to org41841, the model predicts that the t-butyl group of NIDDK-CEB-52 sits higher in the binding cleft and points toward a proline residue in helix 6 (P6.50). Therefore, movement of helix 6 during TSHR activation is not enforced by NIDDK-CEB-52. This structural constraint may cause an antagonistic effect on TSHR signaling. Our experimental findings are consistent with the model of the binding pocket.
Reproduced from [90] © US Federal Government.
Compound 52 was shown to be an antagonist for TSHR with no agonist activity. Compound 52 inhibited TSH-stimulated cAMP production by 71% at 30 μM with an IC50 of 4.2 μM. Compound 52 is a selective antagonist towards TSHR, having weak partial agonist activity at LHCGR and no activity at FSHR. We also showed that compound 52 could act as a TSH antagonist in a more physiologically relevant system. In human thyrocytes in primary culture, compound 52 inhibited TSH-induced increases in thyroperoxidase mRNA. Perhaps most relevant from a clinical viewpoint, compound 52 inhibits activation of TSHR by TSAbs. For example, compound 52 inhibited TSAb-induced increases in thyroperoxidase mRNA in human thyrocytes.
We concluded that compound 52 is proof of the principle that small-molecule TSHR antagonists can inhibit activation by TSH and TSAbs. It will serve as a lead for the development of more potent and efficacious analogs that could be used in animal studies and for the development of drugs that could be used to treat hyperthyroid patients, including patients with Graves’ disease.
Small-molecule TSHR agonist
As none of the analogs designed from the org41841 scaffold showed improved agonist activity, we used quantitative high-throughput screening to identify additional small-molecule TSHR agonists. This method allows in-depth analysis of the primary screening results and provides information regarding potency, efficacy and structure–activity relationships [90,91]. We used a target cell line that expresses cyclic nucleotide-gated cation channels and TSHRs, and screened more than 73,000 compounds [92]. Analysis of data from the primary and secondary screens yielded 49 small-molecule TSHR agonists with various potencies and efficacies. We selected the eight most potent compounds and re-tested these for efficacy and selectivity. Compound NIDDK-CEB-3 was chosen for further study as it is a full agonist at TSHR, exhibited a moderately high potency (EC50 of 660 nM) and was selective for TSHR with no detectable agonist activity at LHCGR or FSHR. We synthesized and tested over 100 analogs of NIDDK-CEB-3 [93]. Figure 2 shows the structure of the most potent analog NIDDK-CEB-3/4 (compound 3/4), which is a full agonist at TSHR with an EC50 of 40 nM; it has no activity at FSHR or LHCGR.
Based on our findings with org41841 and compound 52, we predicted that compound 3/4 would also bind to the TMD of TSHR. We docked compound 3/4 into the model of TSHR. The model of the compound 3/4–TSHR complex predicted that compound 3/4 binds to the same cavity in the TMD as org41841 and compound 52; however, the complex exhibits different interactions between compound 3/4 and residues within TSHR than with the other ligands (Figure 5). We performed experiments with mutant TSHRs to provide evidence in support of this binding cavity. We showed that compound 3/4 could activate a TSHR mutant in which the large amino-terminal ectodomain was deleted; this mutant TSHR could not be activated by TSH [28]. And, we showed that a mutant TSHR in which an Asn residue in helix 5, which was predicted by the model to interact with compound 3/4, was substituted by Ala, responded to TSH but not to compound 3/4. These findings provided very strong evidence in support of the idea that compound 3/4 binds in a pocket within the TMD of TSHR.
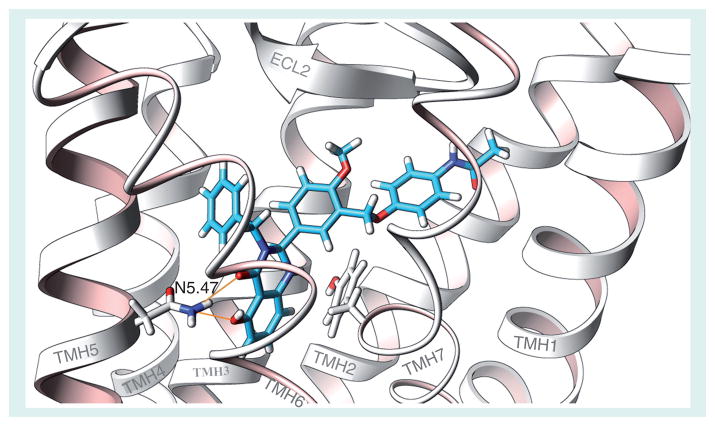
Figure 5. Docking of the full agonist NIDDK-CEB-3/4 in a model of the transmembrane helical bundle of thyroid-stimulating hormone receptor (TSHR).
The model predicts that NIDDK-CEB-3/4 binds in the same pocket as org41841 and NIDDK-CEB-52 but interacts with different receptor residues; NIDDK-CEB-3/4 interacts with an Asn in transmembrane helix 5 (N5.47) that NIDDK-CEB-52 does not contact. To test the hypothesis that an interaction with N5.47 is necessary for activation of TSHR by NIDDK-CEB-3/4, we constructed a site-specific mutant of TSHR in which N5.47 was substituted by Ala (N5.47A). The N5.47A mutant is activated by TSH with a potency and efficacy similar to TSHR but NIDDK-CEB-3/4 has almost no activity at N5.47A. These findings are consistent with the model.
Reproduced from [93] © US Federal Government.
The aforementioned data were obtained using a model cell system overexpressing TSHRs. We then showed that compound 3/4 activated TSHR in a more physiologically relevant cell system, human thyrocytes in primary cultures. We showed that compound 3/4, as with TSH, increases expression of several thyrocyte genes, including thyroglobulin, thyroperoxidase, sodium-iodide symporter and deiodinase type 2. These results showed that compound 3/4 is an effective agonist in human thyrocytes that express TSHR at physiological levels. We next showed that compound 3/4, as with TSH, was effective in mice, increasing serum thyroxine after intraperitoneal injection. More importantly for its clinical potential, compound 3/4 elevated serum thyroxine and stimulated radioiodide uptake by the mouse thyroid gland after its absorption from the GI tract following administration by esophageal gavage. By contrast, TSH is not effective when administered via the GI tract.
We concluded that compound 3/4 is proof of the principle that small-molecule TSHR agonists can be used in in vitro and in vivo systems. These data show that compound 3/4 can be used as a probe of the molecular mechanism of TSHR activation and to study TSHR function in cells in culture and in an animal model, and may be a drug candidate to be used in patients with thyroid cancer.
Future directions
It is evident that with the exception of rhTSH, which is approved for clinical use in patients with thyroid cancer and has been used as a probe for TSHR function in in vitro and in vivo studies, the TSHR ligands reviewed in this article are at early stages of development and have only been employed in initial studies that were directed at demonstrating the activities of the ligands rather than at probing TSHR biology. All of these ligands require further development before they will be applicable as probes of TSHR function in vivo and perhaps as therapeutic agents in humans. For example, all ligands will have to undergo studies of absorption, distribution, metabolism and excretion, and toxicology for use in animals. For TSH analogs and mAbs, biosynthetic schemes will have to be developed to produce large amounts of highly purified biomolecules; and for small-molecule ligands, new analogs with improved potencies and efficacies will have to be developed and their specificities for TSHR confirmed. We are encouraged because all of these steps are feasible. They will occupy pharmacologists and physiologists for at the least the next several years.
Expert commentary
A number of different TSHR activators (agonists) and inhibitors (antagonists) have been reported, including TSH analogs, antibodies and small-molecule, drug-like compounds. The authors believe that small-molecule compounds, which are easily synthesized, readily controlled for quality and can be absorbed from the GI tract after oral administration are potentially the most useful of these ligands. By contrast, biomolecules, such as TSH analogs and TSHR antibodies, must be synthesized in cells in tissue culture and purified from culture mediums, are difficult to control for quality and must be administered parenterally. We think that the availability of small-molecule ligands for TSHR will enhance the ability of scientists to delineate the physiology of TSHR in extra-thyroidal tissues, in which a role for TSHR is currently unclear, and broaden the clinical utility to include patient populations around the world that do not currently have access to rhTSH.
Five-year view
We think that all types of TSHR activators (agonists) and inhibitors (antagonists) – TSH analogs, antibodies and small-molecule, drug-like compounds – will be improved with regard to the properties that would enhance their abilities to serve as probes of TSHR biology in animal models and as drugs in humans. We predict that with sufficient support from the pharmaceutical industry or its surrogates, small-molecule TSHR agonists could be in clinical trials to be used in diagnosis and treatment of patients with thyroid cancer and perhaps with non-thyroidal diseases, such as osteoporosis, and that small-molecule antagonists could be entered in trials to be used in the treatment of patients with hyperthyroidism.
Key issues
The biologic role of thyroid-stimulating hormone (TSH; thyrotropin) as an activator (agonist) of the TSH receptor (TSHR) in the hypothalamic–pituitary–thyroid axis is well known but its role in extrathyroidal tissues that express TSHR is not clear.
A number of different TSHR ligands have been reported, including TSH analogs, antibodies and small-molecule, drug-like compounds.
TSHR ligands could be used as probes of TSHR biology in extrathyroidal tissues, for example, in bone, and as diagnostic agents and drugs in patients.
Recombinant human TSH is currently used clinically in patients with thyroid cancer.
Single-chain and ‘superagonist’ TSH analogs have been synthesized and are being studied.
Monoclonal anti-TSHR antibodies that are agonists or antagonists have been generated and are being studied.
Small-molecule, drug-like TSHR agonists and antagonists are the most recent TSHR ligands to be developed and may hold special promise as biologic probes and therapeutics.
We predict that with sufficient support from the pharmaceutical industry or its surrogates, small-molecule TSHR ligands could be in clinical trials within the next 5 years.
Acknowledgments
The authors thank all of their collaborators who have contributed to the TSHR small-molecule research.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
The authors’ research was supported by the Intramural Research Program, NIDDK, NIH (1 Z01 DK011006 CEB and 1 Z01 DK 047044 CEB). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Susanne Neumann, Email: susannen@intra.niddk.nih.gov, Clinical Endocrinology Branch, NIDDK, NIH, 50 South Drive, Bethesda, MD 20892-28029, USA, Tel.: +1 301 451 6324, Fax: +1 301 480 4214.
Bruce M Raaka, Email: raaka@intra.niddk.nih.gov, Clinical Endocrinology Branch, NIDDK, NIH, 50 South Drive, Bethesda, MD 20892-28029, USA, Tel.: +1 301 451 6307, Fax: +1 301 480 4214.
Marvin C Gershengorn, Email: marving@intra.niddk.nih.gov, Clinical Endocrinology Branch, NIDDK, NIH, 50 South Drive, Bethesda, MD 20892-28029, USA, Tel.: +1 301 451 6305, Fax: +1 301 480 4214.
References
- 1.Vassart G, Dumont JE. The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev. 1992;13:596–611. doi: 10.1210/edrv-13-3-596. [DOI] [PubMed] [Google Scholar]
- 2.Dohan O, Carrasco N. Thyroidal iodide transport and thyroid cancer. Cancer Treat Res. 2004;122:221–236. doi: 10.1007/1-4020-8107-3_13. [DOI] [PubMed] [Google Scholar]
- 3.Duntas LH, Cooper DS. Review on the occasion of a decade of recombinant human TSH: prospects and novel uses. Thyroid. 2008;18(5):509–516. doi: 10.1089/thy.2007.0331. [DOI] [PubMed] [Google Scholar]
- 4.Albino CC, Mesa CO, Jr, Olandoski M, et al. Recombinant human thyrotropin as adjuvant in the treatment of multinodular goiters with radioiodine. J Clin Endocrinol Metab. 2005;90(5):2775–2780. doi: 10.1210/jc.2004-0458. [DOI] [PubMed] [Google Scholar]
- 5.Bassett JH, Williams GR. Critical role of the hypothalamic–pituitary–thyroid axis in bone. Bone. 2008;43(3):418–426. doi: 10.1016/j.bone.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Abe E, Marians RC, Yu W, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115(2):151–162. doi: 10.1016/s0092-8674(03)00771-2. [DOI] [PubMed] [Google Scholar]
- 7.Sampath TK, Simic P, Sendak R, et al. Thyroid-stimulating hormone restores bone volume, microarchitecture, and strength in aged ovariectomized rats. J Bone Miner Res. 2007;22(6):849–859. doi: 10.1359/jbmr.070302. [DOI] [PubMed] [Google Scholar]
- 8.Marians RC, Ng L, Blair HC, Unger P, Graves PN, Davies TF. Defining thyrotropin-dependent and -independent steps of thyroid hormone synthesis by using thyrotropin receptor-null mice. Proc Natl Acad Sci USA. 2002;99(24):15776–15781. doi: 10.1073/pnas.242322099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hase H, Ando T, Eldeiry L, et al. TNFα mediates the skeletal effects of thyroid-stimulating hormone. Proc Natl Acad Sci USA. 2006;103(34):12849–12854. doi: 10.1073/pnas.0600427103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun L, Vukicevic S, Baliram R, et al. Intermittent recombinant TSH injections prevent ovariectomy-induced bone loss. Proc Natl Acad Sci USA. 2008;105(11):4289–4294. doi: 10.1073/pnas.0712395105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bassett JH, Williams AJ, Murphy E, et al. A lack of thyroid hormones rather than excess thyrotropin causes abnormal skeletal development in hypothyroidism. Mol Endocrinol. 2008;22(2):501–512. doi: 10.1210/me.2007-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inoue M, Tawata M, Yokomori N, Endo T, Onaya T. Expression of thyrotropin receptor on clonal osteoblast-like rat osteosarcoma cells. Thyroid. 1998;8(11):1059–1064. doi: 10.1089/thy.1998.8.1059. [DOI] [PubMed] [Google Scholar]
- 13.Davies TF, Ando T, Lin RY, Tomer Y, Latif R. Thyrotropin receptor-associated diseases: from adenomata to Graves disease. J Clin Invest. 2005;115(8):1972–1983. doi: 10.1172/JCI26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duprez L, Parma J, Van Sande J, et al. TSH receptor mutations and thyroid disease. Trends Endocrinol Metab. 1998;9(4):133–140. doi: 10.1016/s1043-2760(98)00036-8. [DOI] [PubMed] [Google Scholar]
- 15.Garrity JA, Bahn RS. Pathogenesis of graves ophthalmopathy: implications for prediction, prevention, and treatment. Am J Ophthalmol. 2006;142(1):147–153. doi: 10.1016/j.ajo.2006.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiersinga WM, Prummel MF. Graves’ ophthalmopathy: a rational approach to treatment. Trends Endocrinol Metab. 2002;13(7):280–287. doi: 10.1016/s1043-2760(02)00622-7. [DOI] [PubMed] [Google Scholar]
- 17.Valyasevi RW, Erickson DZ, Harteneck DA, et al. Differentiation of human orbital preadipocyte fibroblasts induces expression of functional thyrotropin receptor. J Clin Endocrinol Metab. 1999;84(7):2557–2562. doi: 10.1210/jcem.84.7.5838. [DOI] [PubMed] [Google Scholar]
- 18.Bahn RS, Dutton CM, Natt N, Joba W, Spitzweg C, Heufelder AE. Thyrotropin receptor expression in Graves’ orbital adipose/connective tissues: potential autoantigen in Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1998;83(3):998–1002. doi: 10.1210/jcem.83.3.4676. [DOI] [PubMed] [Google Scholar]
- 19.Szkudlinski MW, Fremont V, Ronin C, Weintraub BD. Thyroid-stimulating hormone and thyroid-stimulating hormone receptor structure–function relationships. Physiol Rev. 2002;82(2):473–502. doi: 10.1152/physrev.00031.2001. [DOI] [PubMed] [Google Scholar]
- 20.Latif R, Morshed SA, Zaidi M, Davies TF. The thyroid-stimulating hormone receptor: impact of thyroid-stimulating hormone and thyroid-stimulating hormone receptor antibodies on multimerization, cleavage, and signaling. Endocrinol Metab Clin North Am. 2009;38(2):319–341. doi: 10.1016/j.ecl.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Loosfelt H, Pichon C, Jolivet A, et al. Two-subunit structure of the human thyrotropin receptor. Proc Natl Acad Sci USA. 1992;89:3765–3769. doi: 10.1073/pnas.89.9.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chazenbalk GD, Tanaka K, Nagayama Y, et al. Evidence that the thyrotropin receptor ectodomain contains not one, but two, cleavage sites. Endocrinology. 1997;138(7):2893–2899. doi: 10.1210/endo.138.7.5259. [DOI] [PubMed] [Google Scholar]
- 23.de Bernard S, Misrahi M, Huet JC, et al. Sequential cleavage and excision of a segment of the thyrotropin receptor ectodomain. J Biol Chem. 1999;274(1):101–107. doi: 10.1074/jbc.274.1.101. [DOI] [PubMed] [Google Scholar]
- 24.Rapoport B, McLachlan SM. The thyrotropin receptor in Graves’ disease. Thyroid. 2007;17(10):911–922. doi: 10.1089/thy.2007.0170. [DOI] [PubMed] [Google Scholar]
- 25.Laugwitz KL, Allgeier A, Offermanns S, et al. The human thyrotropin receptor: a heptahelical receptor capable of stimulating members of all four G protein families. Proc Natl Acad Sci USA. 1996;93(1):116–120. doi: 10.1073/pnas.93.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kero J, Ahmed K, Wettschureck N, et al. Thyrocyte-specific Gq/G11 deficiency impairs thyroid function and prevents goiter development. J Clin Invest. 2007;117(9):2399–2407. doi: 10.1172/JCI30380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buch TR, Biebermann H, Kalwa H, et al. G13-dependent activation of MAPK by thyrotropin. J Biol Chem. 2008;283(29):20330–20341. doi: 10.1074/jbc.M800211200. [DOI] [PubMed] [Google Scholar]
- 28.Vlaeminck-Guillem V, Ho SC, Rodien P, Vassart G, Costagliola S. Activation of the cAMP pathway by the TSH receptor involves switching of the ectodomain from a tethered inverse agonist to an agonist. Mol Endocrinol. 2002;16(4):736–746. doi: 10.1210/mend.16.4.0816. [DOI] [PubMed] [Google Scholar]
- 29.Zhang M, Tong KPT, Fremont V, et al. The extracellular domain suppresses constitutive activity of the transmembrane domain of the human TSH receptor: implications for hormone-receptor interaction and antagonist design. Endocrinology. 2000;141(9):3514–3517. doi: 10.1210/endo.141.9.7790. [DOI] [PubMed] [Google Scholar]
- 30.Nakabayashi K, Matsumi H, Bhalla A, et al. Thyrostimulin, a heterodimer of two new human glycoprotein hormone subunits, activates the thyroid-stimulating hormone receptor. J Clin Invest. 2002;109(11):1445–1452. doi: 10.1172/JCI14340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okada SL, Ellsworth JL, Durnam DM, et al. A glycoprotein hormone expressed in corticotrophs exhibits unique binding properties on thyroid-stimulating hormone receptor. Mol Endocrinol. 2006;20(2):414–425. doi: 10.1210/me.2005-0270. [DOI] [PubMed] [Google Scholar]
- 32.Cole ES, Lee K, Lauziere K, et al. Recombinant human thyroid stimulating hormone: development of a biotechnology product for detection of metastatic lesions of thyroid carcinoma. Biotechnology. 1993;11(9):1014–1024. doi: 10.1038/nbt0993-1014. [DOI] [PubMed] [Google Scholar]
- 33.Szkudlinski MW, Thotakura NR, Bucci I, et al. Purification and characterization of recombinant human thyrotropin (TSH) isoforms produced by Chinese hamster ovary cells: the role of sialylation and sulfation in TSH bioactivity. Endocrinology. 1993;133(4):1490–1503. doi: 10.1210/endo.133.4.8404588. [DOI] [PubMed] [Google Scholar]
- 34.Szkudlinski MW. Recombinant human thyrotropins of the twenty-first century. Expert Opin Pharmacother. 2004;5(12):2435–2440. doi: 10.1517/14656566.5.12.2435. [DOI] [PubMed] [Google Scholar]
- 35.Boretti FS, Sieber-Ruckstuhl NS, Favrot C, Lutz H, Hofmann-Lehmann R, Reusch CE. Evaluation of recombinant human thyroid-stimulating hormone to test thyroid function in dogs suspected of having hypothyroidism. Am J Vet Res. 2006;67(12):2012–2016. doi: 10.2460/ajvr.67.12.2012. [DOI] [PubMed] [Google Scholar]
- 36.Daminet S, Fifle L, Paradis M, Duchateau L, Moreau M. Use of recombinant human thyroid-stimulating hormone for thyrotropin stimulation test in healthy, hypothyroid and euthyroid sick dogs. Can Vet J. 2007;48(12):1273–1279. [PMC free article] [PubMed] [Google Scholar]
- 37.Colzani RM, Alex S, Fang SL, Braverman LE, Emerson CH. The effect of recombinant human thyrotropin (rhTSH) on thyroid function in mice and rats. Thyroid. 1998;8(9):797–801. doi: 10.1089/thy.1998.8.797. [DOI] [PubMed] [Google Scholar]
- 38.Szkudlinski MW, Grossmann M, Weintraub BD. Structure–function studies of human TSH – new advances in design of glycoprotein hormone analogs. Trends Endocrinol Metab. 1996;7(8):277–286. doi: 10.1016/s1043-2760(96)00129-4. [DOI] [PubMed] [Google Scholar]
- 39.Grossmann M, Wong R, Szkudlinski MW, Weintraub BD. Human thyroid-stimulating hormone (hTSH) subunit gene fusion produces hTSH with increased stability and serum half-life and compensates for mutagenesis-induced defects in subunit association. J Biol Chem. 1997;272(34):21312–21316. doi: 10.1074/jbc.272.34.21312. [DOI] [PubMed] [Google Scholar]
- 40.Fares FA, Yamabe S, Ben Menahem D, Pixley M, Hsueh AJ, Boime I. Conversion of thyrotropin heterodimer to a biologically active single-chain. Endocrinology. 1998;139(5):2459–2464. doi: 10.1210/endo.139.5.6021. [DOI] [PubMed] [Google Scholar]
- 41.Pierce JG, Parsons TF. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- 42.Fares FA, Levi F, Reznick AZ, Kraiem Z. Engineering a potential antagonist of human thyrotropin and thyroid-stimulating antibody. J Biol Chem. 2001;276(7):4543–4548. doi: 10.1074/jbc.M008093200. [DOI] [PubMed] [Google Scholar]
- 43.Azzam N, Bar-Shalom R, Kraiem Z, Fares F. Human thyrotropin (TSH) variants designed by site-directed mutagenesis block TSH activity in vitro and in vivo. Endocrinology. 2005;146(6):2845–2850. doi: 10.1210/en.2005-0012. [DOI] [PubMed] [Google Scholar]
- 44.Szkudlinski MW, Teh NG, Grossmann M, Tropea JE, Weintraub BD. Engineering human glycoprotein hormone superactive analogues. Bio Technol. 1996;14(10):1257–1263. doi: 10.1038/nbt1096-1257. [DOI] [PubMed] [Google Scholar]
- 45.Grossmann M, Leitolf H, Weintraub BD, Szkudlinski MW. A rational design strategy for protein hormone superagonists. Nat Biotechnol. 1998;16(9):871–875. doi: 10.1038/nbt0998-871. [DOI] [PubMed] [Google Scholar]
- 46.Leitolf H, Tong KP, Grossmann M, Weintraub BD, Szkudlinski MW. Bioengineering of human thyrotropin superactive analogs by site-directed ‘lysine-scanning’ mutagenesis. Cooperative effects between peripheral loops. J Biol Chem. 2000;275(35):27457–27465. doi: 10.1074/jbc.M003707200. [DOI] [PubMed] [Google Scholar]
- 47.Mueller S, Kleinau G, Szkudlinski MW, Jaeschke H, Krause G, Paschke R. The superagonistic activity of bovine TSH and the human TR1401 TSH analog is determined by specific amino acids in the hinge region of the human TSH-receptor. J Biol Chem. 2009;284(24):16317–16324. doi: 10.1074/jbc.M109.005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schott M, Scherbaum WA, Morgenthaler NG. Thyrotropin receptor autoantibodies in Graves’ disease. Trends Endocrinol Metab. 2005;16(5):243–248. doi: 10.1016/j.tem.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 49.Rees SB, McLachlan SM, Furmaniak J. Autoantibodies to the thyrotropin receptor. Endocr Rev. 1988;9(1):106–121. doi: 10.1210/edrv-9-1-106. [DOI] [PubMed] [Google Scholar]
- 50.Ando T, Latif R, Pritsker A, Moran T, Nagayama Y, Davies TF. A monoclonal thyroid-stimulating antibody. J Clin Invest. 2002;110(11):1667–1674. doi: 10.1172/JCI16991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanders J, Allen F, Jeffreys J, et al. Characteristics of a monoclonal antibody to the thyrotropin receptor that acts as a powerful thyroid-stimulating autoantibody antagonist. Thyroid. 2005;15(7):672–682. doi: 10.1089/thy.2005.15.672. [DOI] [PubMed] [Google Scholar]
- 52.Sanders J, Jeffreys J, Depraetere H, et al. Thyroid-stimulating monoclonal antibodies. Thyroid. 2002;12(12):1043–1050. doi: 10.1089/105072502321085135. [DOI] [PubMed] [Google Scholar]
- 53.Costagliola S, Franssen JD, Bonomi M, et al. Generation of a mouse monoclonal TSH receptor antibody with stimulating activity. Biochem Biophys Res Commun. 2002;299(5):891–896. doi: 10.1016/s0006-291x(02)02762-6. [DOI] [PubMed] [Google Scholar]
- 54.Huang GC, Page MJ, Nicholson LB, Collison KS, McGregor AM, Banga JP. The thyrotrophin hormone receptor of Graves’ disease: overexpression of the extracellular domain in insect cells using recombinant baculovirus, immunoaffinity purification and analysis of autoantibody binding. J Mol Endocrinol. 1993;10(2):127–142. doi: 10.1677/jme.0.0100127. [DOI] [PubMed] [Google Scholar]
- 55.Johnstone AP, Cridland JC, DaCosta CR, Harfst E, Shepherd PS. Monoclonal antibodies that recognize the native human thyrotropin receptor. Mol Cell Endocrinol. 1994;105(2):R1–R9. doi: 10.1016/0303-7207(94)90175-9. [DOI] [PubMed] [Google Scholar]
- 56.Seetharamaiah GS, Wagle NM, Morris JC, Prabhakar BS. Generation and characterization of monoclonal antibodies to the human thyrotropin (TSH) receptor: antibodies can bind to discrete conformational or linear epitopes and block TSH binding. Endocrinology. 1995;136(7):2817–2824. doi: 10.1210/endo.136.7.7540542. [DOI] [PubMed] [Google Scholar]
- 57.Costagliola S, Rodien P, Many MC, Ludgate M, Vassart G. Genetic immunization against the human thyrotropin receptor causes thyroiditis and allows production of monoclonal antibodies recognizing the native receptor. J Immunol. 1998;160(3):1458–1465. [PubMed] [Google Scholar]
- 58.Oda Y, Sanders J, Evans M, et al. Epitope analysis of the human thyrotropin (TSH) receptor using monoclonal antibodies. Thyroid. 2000;10(12):1051–1059. doi: 10.1089/thy.2000.10.1051. [DOI] [PubMed] [Google Scholar]
- 59.Gilbert JA, Gianoukakis AG, Salehi S, et al. Monoclonal pathogenic antibodies to the thyroid-stimulating hormone receptor in Graves’ disease with potent thyroid-stimulating activity but differential blocking activity activate multiple signaling pathways. J Immunol. 2006;176(8):5084–5092. doi: 10.4049/jimmunol.176.8.5084. [DOI] [PubMed] [Google Scholar]
- 60.Costagliola S, Bonomi M, Morgenthaler NG, et al. Delineation of the discontinuous-conformational epitope of a monoclonal antibody displaying full in vitro and in vivo thyrotropin activity. Mol Endocrinol. 2004;18(12):3020–3034. doi: 10.1210/me.2004-0231. [DOI] [PubMed] [Google Scholar]
- 61.Akamizu T, Moriyama K, Miura M, Saijo M, Matsuda F, Nakao K. Characterization of recombinant monoclonal antithyrotropin receptor antibodies (TSHRAbs) derived from lymphocytes of patients with Graves’ disease: epitope and binding study of two stimulatory TSHRAbs. Endocrinology. 1999;140(4):1594–1601. doi: 10.1210/endo.140.4.6664. [DOI] [PubMed] [Google Scholar]
- 62.Sanders J, Evans M, Premawardhana LD, et al. Human monoclonal thyroid stimulating autoantibody. Lancet. 2003;362(9378):126–128. doi: 10.1016/s0140-6736(03)13866-4. [DOI] [PubMed] [Google Scholar]
- 63.Moriyama K, Okuda J, Saijo M, et al. Recombinant monoclonal thyrotropin-stimulation blocking antibody (TSBAb) established from peripheral lymphocytes of a hypothyroid patient with primary myxedema. J Endocrinol Invest. 2003;26(11):1076–1080. doi: 10.1007/BF03345253. [DOI] [PubMed] [Google Scholar]
- 64.Chen CR, McLachlan SM, Rapoport B. Suppression of thyrotropin receptor constitutive activity by a monoclonal antibody with inverse agonist activity. Endocrinology. 2007;148(5):2375–2382. doi: 10.1210/en.2006-1754. [DOI] [PubMed] [Google Scholar]
- 65.Sanders J, Evans M, Betterle C, et al. A human monoclonal autoantibody to the thyrotropin receptor with thyroid-stimulating blocking activity. Thyroid. 2008;18(7):735–746. doi: 10.1089/thy.2007.0327. [DOI] [PubMed] [Google Scholar]
- 66.Bond RA, IJzerman AP. Recent developments in constitutive receptor activity and inverse agonism, and their potential for GPCR drug discovery. Trends Pharmacol Sci. 2006;27(2):92–96. doi: 10.1016/j.tips.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 67.Chen CR, McLachlan SM, Rapoport B. Identification of key amino acid residues in a thyrotropin receptor monoclonal antibody epitope provides insight into its inverse agonist and antagonist properties. Endocrinology. 2008;149(7):3427–3434. doi: 10.1210/en.2008-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen CR, McLachlan SM, Rapoport B. A monoclonal antibody with TSH receptor inverse agonist and TSH antagonist activities binds to the receptor hinge region as well as to the leucine-rich domain. Endocrinology. 2009;150(7):3401–3408. doi: 10.1210/en.2008-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Latif R, Morshed SA, Zaidi M, Davies TF. The thyroid-stimulating hormone receptor: impact of thyroid-stimulating hormone and thyroid-stimulating hormone receptor antibodies on multimerization, cleavage, and signaling. Endocrinol Metab Clin North Am. 2009;38(2):319–341. viii. doi: 10.1016/j.ecl.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 70.Michalek K, Morshed SA, Latif R, Davies TF. TSH receptor autoantibodies. Autoimmun Rev. 2009 doi: 10.1016/j. autrev.2009.03.012. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Straten NC, Schoonus-Gerritsma GG, van Someren RG, et al. The first orally active low molecular weight agonists for the LH receptor: thienopyr(im)idines with therapeutic potential for ovulation induction. Chembiochem. 2002;3(10):1023–1026. doi: 10.1002/1439-7633(20021004)3:10<1023::AID-CBIC1023>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 72.Heitman LH, Oosterom J, Bonger KM, Timmers CM, Wiegerinck PH, IJzerman AP. [3H]Org 43553, the first low-molecular-weight agonistic and allosteric radioligand for the human luteinizing hormone receptor. Mol Pharmacol. 2008;73(2):518–524. doi: 10.1124/mol.107.039875. [DOI] [PubMed] [Google Scholar]
- 73.Jorand-Lebrun C, Brondyk B, Lin J, et al. Identification, synthesis, and biological evaluation of novel pyrazoles as low molecular weight luteinizing hormone receptor agonists. Bioorg Med Chem Lett. 2007;17(7):2080–2085. doi: 10.1016/j.bmcl.2006.12.062. [DOI] [PubMed] [Google Scholar]
- 74.Van Koppen CJ, Zaman GJ, Timmers CM, et al. A signaling-selective, nanomolar potent allosteric low molecular weight agonist for the human luteinizing hormone receptor. Naunyn Schmiedebergs Arch Pharmacol. 2008;378(5):503–514. doi: 10.1007/s00210-008-0318-3. [DOI] [PubMed] [Google Scholar]
- 75.Bonger KM, van den Berg RJ, Knijnenburg AD, et al. Discovery of selective luteinizing hormone receptor agonists using the bivalent ligand method. ChemMedChem. 2009;4(7):1189–1195. doi: 10.1002/cmdc.200900058. [DOI] [PubMed] [Google Scholar]
- 76.Heitman LH, Narlawar R, de Vries H, et al. Substituted terphenyl compounds as the first class of low molecular weight allosteric inhibitors of the luteinizing hormone receptor. J Med Chem. 2009;52(7):2036–2042. doi: 10.1021/jm801561h. [DOI] [PubMed] [Google Scholar]
- 77.Guo T, Adang AE, Dolle RE, et al. Small molecule biaryl FSH receptor agonists. Part 1: lead discovery via encoded combinatorial synthesis. Bioorg Med Chem Lett. 2004;14(7):1713–1716. doi: 10.1016/j.bmcl.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 78.Guo T, Adang AE, Dong G, et al. Small molecule biaryl FSH receptor agonists. Part 2: lead optimization via parallel synthesis. Bioorg Med Chem Lett. 2004;14(7):1717–1720. doi: 10.1016/j.bmcl.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 79.Yanofsky SD, Shen ES, Holden F, et al. Allosteric activation of the follicle-stimulating hormone (FSH) receptor by selective, nonpeptide agonists. J Biol Chem. 2006;281(19):13226–13233. doi: 10.1074/jbc.M600601200. [DOI] [PubMed] [Google Scholar]
- 80.MacLean D, Holden F, Davis AM, et al. Agonists of the follicle stimulating hormone receptor from an encoded thiazolidinone library. J Comb Chem. 2004;6(2):196–206. doi: 10.1021/cc0300154. [DOI] [PubMed] [Google Scholar]
- 81.Wrobel J, Jetter J, Kao W, et al. 5-Alkylated thiazolidinones as follicle-stimulating hormone (FSH) receptor agonists. Bioorg Med Chem. 2006;14(16):5729–5741. doi: 10.1016/j.bmc.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 82.Pelletier JC, Rogers J, Wrobel J, Perez MC, Shen ES. Preparation of highly substituted γ-lactam follicle stimulating hormone receptor agonists. Bioorg Med Chem. 2005;13(21):5986–5995. doi: 10.1016/j.bmc.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 83.van Straten NC, van Berkel TH, Demont DR, et al. Identification of substituted 6-amino-4-phenyltetrahydroquinoline derivatives: potent antagonists for the follicle-stimulating hormone receptor. J Med Chem. 2005;48(6):1697–1700. doi: 10.1021/jm049676l. [DOI] [PubMed] [Google Scholar]
- 84.Wrobel J, Green D, Jetter J, et al. Synthesis of (bis)sulfonic acid, (bis)benzamides as follicle-stimulating hormone (FSH) antagonists. Bioorg Med Chem. 2002;10(3):639–656. doi: 10.1016/s0968-0896(01)00324-8. [DOI] [PubMed] [Google Scholar]
- 85.Arey BJ, Deecher DC, Shen ES, et al. Identification and characterization of a selective, nonpeptide follicle-stimulating hormone receptor antagonist. Endocrinology. 2002;143(10):3822–3829. doi: 10.1210/en.2002-220372. [DOI] [PubMed] [Google Scholar]
- 86.van Straten NC, van Berkel TH, Demont DR, et al. Identification of substituted 6-amino-4-phenyltetrahydroquinoline derivatives: potent antagonists for the follicle-stimulating hormone receptor. J Med Chem. 2005;48(6):1697–1700. doi: 10.1021/jm049676l. [DOI] [PubMed] [Google Scholar]
- 87.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3(9):639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 88.Jaschke H, Neumann S, Moore S, et al. A low molecular weight agonist signals by binding to the transmembrane domain of thyroid-stimulating hormone receptor (TSHR) and luteinizing hormone/chorionic gonadotropin receptor (LHCGR) J Biol Chem. 2006;281(15):9841–9844. doi: 10.1074/jbc.C600014200. [DOI] [PubMed] [Google Scholar]
- 89.Moore S, Jaeschke H, Kleinau G, et al. Evaluation of small-molecule modulators of the luteinizing hormone/choriogonadotropin and thyroid stimulating hormone receptors: structure–activity relationships and selective binding patterns. J Med Chem. 2006;49(13):3888–3896. doi: 10.1021/jm060247s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Neumann S, Kleinau G, Costanzi S, et al. A low-molecular-weight antagonist for the human thyrotropin receptor with therapeutic potential for hyperthyroidism. Endocrinology. 2008;149(12):5945–5950. doi: 10.1210/en.2008-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Inglese J, Auld DS, Jadhav A, et al. Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc Natl Acad Sci USA. 2006;103(31):11473–11478. doi: 10.1073/pnas.0604348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Titus S, Neumann S, Zheng W, et al. Quantitative high-throughput screening using a live-cell cAMP assay identifies small-molecule agonists of the TSH receptor. J Biomol Screen. 2008;13(2):120–127. doi: 10.1177/1087057107313786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Neumann S, Huang W, Titus S, et al. Small-molecule agonists for the thyrotropin receptor stimulate thyroid function in human thyrocytes and mice. Proc Natl Acad Sci USA. 2009;106(30):12471–12476. doi: 10.1073/pnas.0904506106. [DOI] [PMC free article] [PubMed] [Google Scholar]