Abstract
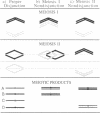
A mutation at the RED1 locus was identified in a search for sporulation-proficient, meiotic-lethal yeast mutants. The few viable spores produced in the red1-1 mutant are highly aneuploid, suggesting that the spore lethality results from a high frequency of chromosome nondisjunction. Disomic spores produced by the red1-1 mutant contain nonsister chromatids and the red1-1 spore inviability phenotype is alleviated in red1-1 spo13 double mutants; these results indicate that nondisjunction occurs at the first meiotic division. The red1-1 mutant is recombination-proficient. The RED1 gene was cloned by complementation of the meiotic lethal phenotype; strains carrying a disruption of the gene are mitotically viable. We propose that the RED1 gene product is involved in meiosis I chromosome disjunction, perhaps by maintaining the connections between homologous chromosomes through metaphase I.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker B. S., Carpenter A. T., Esposito M. S., Esposito R. E., Sandler L. The genetic control of meiosis. Annu Rev Genet. 1976;10:53–134. doi: 10.1146/annurev.ge.10.120176.000413. [DOI] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Dawes I. W., Hardie I. D. Selective killing of vegetative cells in sporulated yeast cultures by exposure to diethyl ether. Mol Gen Genet. 1974;131(4):281–289. doi: 10.1007/BF00264859. [DOI] [PubMed] [Google Scholar]
- Gethmann R. C. The genetic analysis of a chromosome-specific meiotic mutant that permits a premature separation of sister chromatids in Drosophila melanogaster. Genetics. 1984 May;107(1):65–77. doi: 10.1093/genetics/107.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein L. S. Mechanisms of chromosome orientation revealed by two meiotic mutants in Drosophila melanogaster. Chromosoma. 1980;78(1):79–111. doi: 10.1007/BF00291909. [DOI] [PubMed] [Google Scholar]
- Klapholz S., Esposito R. E. A new mapping method employing a meiotic rec-mutant of yeast. Genetics. 1982 Mar;100(3):387–412. doi: 10.1093/genetics/100.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapholz S., Esposito R. E. Recombination and chromosome segregation during the single division meiosis in SPO12-1 and SPO13-1 diploids. Genetics. 1980 Nov;96(3):589–611. doi: 10.1093/genetics/96.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapholz S., Waddell C. S., Esposito R. E. The role of the SPO11 gene in meiotic recombination in yeast. Genetics. 1985 Jun;110(2):187–216. doi: 10.1093/genetics/110.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambie E. J., Roeder G. S. A yeast centromere acts in cis to inhibit meiotic gene conversion of adjacent sequences. Cell. 1988 Mar 25;52(6):863–873. doi: 10.1016/0092-8674(88)90428-x. [DOI] [PubMed] [Google Scholar]
- Lin H. P., Church K. Meiosis in Drosophila melanogaster, III. The effect of orientation disruptor (ord) on gonial mitotic and the meiotic divisions in males. Genetics. 1982 Dec;102(4):751–770. doi: 10.1093/genetics/102.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone R. E., Esposito R. E. Recombinationless meiosis in Saccharomyces cerevisiae. Mol Cell Biol. 1981 Oct;1(10):891–901. doi: 10.1128/mcb.1.10.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W., Szostak J. W. Chromosome segregation in mitosis and meiosis. Annu Rev Cell Biol. 1985;1:289–315. doi: 10.1146/annurev.cb.01.110185.001445. [DOI] [PubMed] [Google Scholar]
- Nicklas R. B. Chromosome distribution: experiments on cell hybrids and in vitro. Philos Trans R Soc Lond B Biol Sci. 1977 Mar 21;277(955):267–276. doi: 10.1098/rstb.1977.0017. [DOI] [PubMed] [Google Scholar]
- Perkins D. D. Biochemical Mutants in the Smut Fungus Ustilago Maydis. Genetics. 1949 Sep;34(5):607–626. doi: 10.1093/genetics/34.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockmill B., Fogel S. DIS1: a yeast gene required for proper meiotic chromosome disjunction. Genetics. 1988 Jun;119(2):261–272. doi: 10.1093/genetics/119.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. D., Novick P., Thomas J. H., Botstein D., Fink G. R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60(2-3):237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- Seifert H. S., Chen E. Y., So M., Heffron F. Shuttle mutagenesis: a method of transposon mutagenesis for Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Feb;83(3):735–739. doi: 10.1073/pnas.83.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathern J. N., Herskowitz I. Asymmetry and directionality in production of new cell types during clonal growth: the switching pattern of homothallic yeast. Cell. 1979 Jun;17(2):371–381. doi: 10.1016/0092-8674(79)90163-6. [DOI] [PubMed] [Google Scholar]
- Wang H. T., Frackman S., Kowalisyn J., Esposito R. E., Elder R. Developmental regulation of SPO13, a gene required for separation of homologous chromosomes at meiosis I. Mol Cell Biol. 1987 Apr;7(4):1425–1435. doi: 10.1128/mcb.7.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]