Abstract
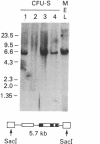
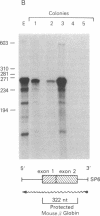
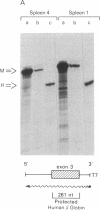
Efficient transfer of the beta-globin gene into primitive hematopoietic progenitors was achieved with consistent and significant expression in the progeny of those cells. Retroviral vectors containing the intact genomic human beta-globin gene and the neomycin (G418)-resistance (neoR) gene were constructed. These gave titers of 10(6) or more neoR colony-forming units/ml when packaged in psi 2 cells. Mouse bone marrow cells were infected by coculture with producer cells and injected into lethally irradiated animals. Several parameters were varied to enhance infection frequency of colony-forming units, spleen (CFU-S); overall 41% of 116 foci studied contained an intact proviral genome. The human beta-globin gene was expressed in 31 of 35 CFU-S-derived spleen colonies that contained the intact vector genome at levels ranging from 1% to 5% of that of the mouse beta-globin genes. Infected bone marrow cells were also injected into genetically anemic W/Wv recipients without prior irradiation. Human beta-globin chains were detected in circulating erythrocytes by immunofluorescent staining with a specific monoclonal antibody. All animals injected with donor cells that had been cultured in G418 (1 mg/ml) for 48 hr after retroviral infection had circulating erythrocytes containing human beta-globin chains between 3 and 8 weeks after transplantation.
Full text
PDF
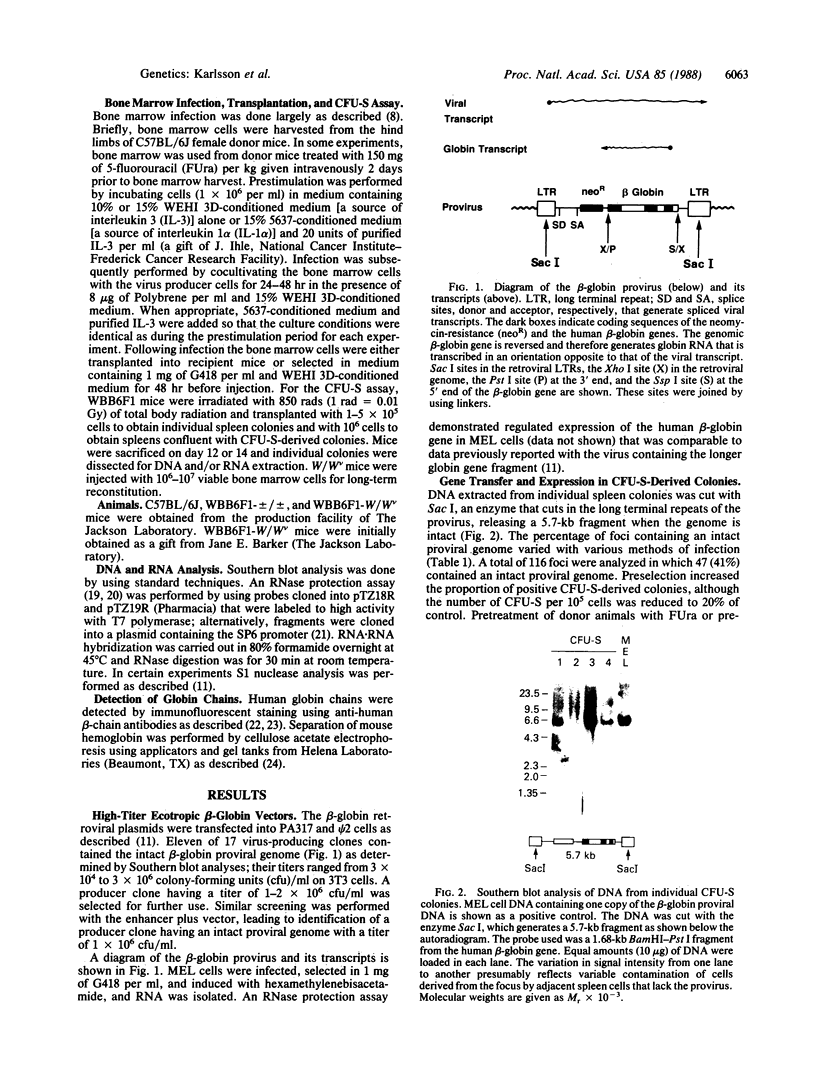

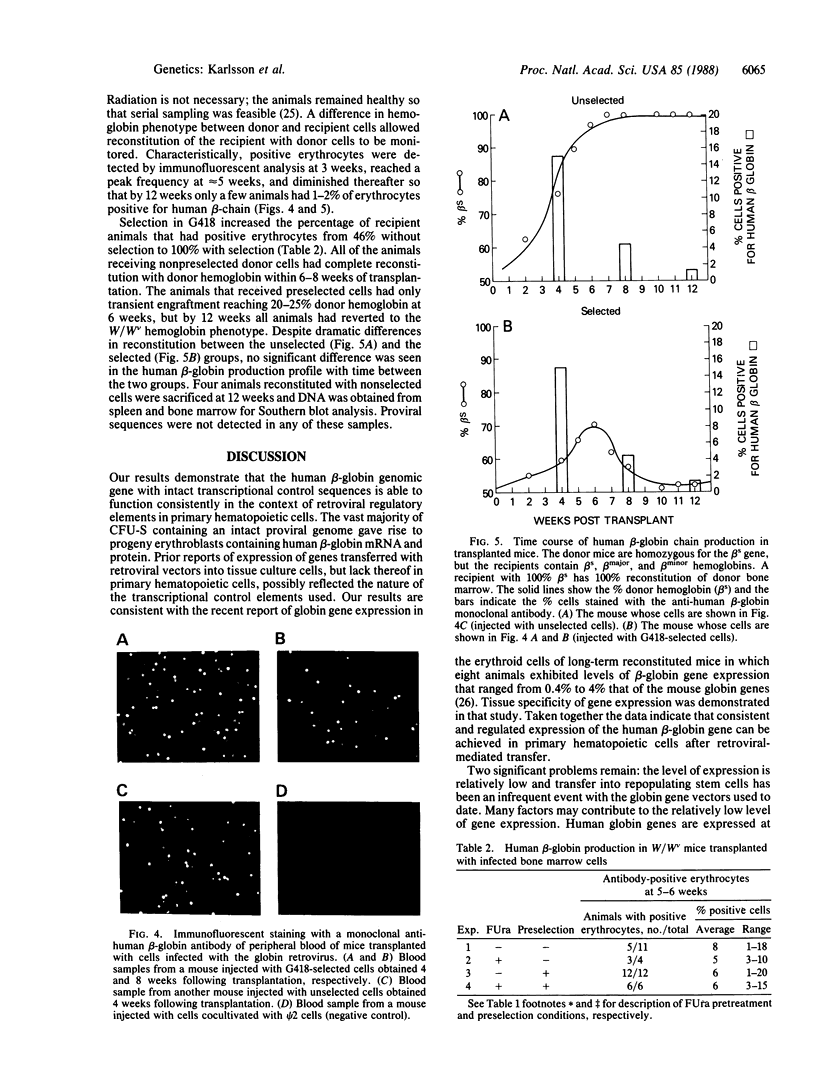

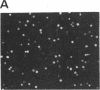
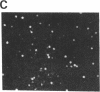
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnou N. P., Karlsson S., Moulton A. D., Keller G., Nienhuis A. W. Promoter sequences required for function of the human gamma globin gene in erythroid cells. EMBO J. 1986 Jan;5(1):121–126. doi: 10.1002/j.1460-2075.1986.tb04185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson W. F. Prospects for human gene therapy. Science. 1984 Oct 26;226(4673):401–409. doi: 10.1126/science.6093246. [DOI] [PubMed] [Google Scholar]
- Behringer R. R., Hammer R. E., Brinster R. L., Palmiter R. D., Townes T. M. Two 3' sequences direct adult erythroid-specific expression of human beta-globin genes in transgenic mice. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7056–7060. doi: 10.1073/pnas.84.20.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone R. D., Weber-Benarous A., Baorto D., Mulligan R. C. Regulated expression of a complete human beta-globin gene encoded by a transmissible retrovirus vector. Mol Cell Biol. 1987 Feb;7(2):887–897. doi: 10.1128/mcb.7.2.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio M. J., Kantoff P., Schafer M. P., Anderson W. F., Safer B. Compensatory increase in levels of beta minor globin in murine beta-thalassemia is under translational control. J Biol Chem. 1986 Dec 5;261(34):16126–16132. [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Dzierzak E. A., Papayannopoulou T., Mulligan R. C. Lineage-specific expression of a human beta-globin gene in murine bone marrow transplant recipients reconstituted with retrovirus-transduced stem cells. Nature. 1988 Jan 7;331(6151):35–41. doi: 10.1038/331035a0. [DOI] [PubMed] [Google Scholar]
- Forrester W. C., Takegawa S., Papayannopoulou T., Stamatoyannopoulos G., Groudine M. Evidence for a locus activation region: the formation of developmentally stable hypersensitive sites in globin-expressing hybrids. Nucleic Acids Res. 1987 Dec 23;15(24):10159–10177. doi: 10.1093/nar/15.24.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J. N., Pickett E. B., Heinz R. Platelet membrane microparticles in blood bank fresh frozen plasma and cryoprecipitate. Blood. 1986 Jul;68(1):307–309. [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Ikebuchi K., Wong G. G., Clark S. C., Ihle J. N., Hirai Y., Ogawa M. Interleukin 6 enhancement of interleukin 3-dependent proliferation of multipotential hemopoietic progenitors. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9035–9039. doi: 10.1073/pnas.84.24.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantoff P. W., Kohn D. B., Mitsuya H., Armentano D., Sieberg M., Zwiebel J. A., Eglitis M. A., McLachlin J. R., Wiginton D. A., Hutton J. J. Correction of adenosine deaminase deficiency in cultured human T and B cells by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6563–6567. doi: 10.1073/pnas.83.17.6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson S., Papayannopoulou T., Schweiger S. G., Stamatoyannopoulos G., Nienhuis A. W. Retroviral-mediated transfer of genomic globin genes leads to regulated production of RNA and protein. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2411–2415. doi: 10.1073/pnas.84.8.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson S., Van Doren K., Schweiger S. G., Nienhuis A. W., Gluzman Y. Stable gene transfer and tissue-specific expression of a human globin gene using adenoviral vectors. EMBO J. 1986 Sep;5(9):2377–2385. doi: 10.1002/j.1460-2075.1986.tb04507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Kollias G., Hurst J., deBoer E., Grosveld F. The human beta-globin gene contains a downstream developmental specific enhancer. Nucleic Acids Res. 1987 Jul 24;15(14):5739–5747. doi: 10.1093/nar/15.14.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemischka I. R., Raulet D. H., Mulligan R. C. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986 Jun 20;45(6):917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- Lim B., Williams D. A., Orkin S. H. Retrovirus-mediated gene transfer of human adenosine deaminase: expression of functional enzyme in murine hematopoietic stem cells in vivo. Mol Cell Biol. 1987 Oct;7(10):3459–3465. doi: 10.1128/mcb.7.10.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magram J., Chada K., Costantini F. Developmental regulation of a cloned adult beta-globin gene in transgenic mice. Nature. 1985 May 23;315(6017):338–340. doi: 10.1038/315338a0. [DOI] [PubMed] [Google Scholar]
- McIvor R. S., Johnson M. J., Miller A. D., Pitts S., Williams S. R., Valerio D., Martin D. W., Jr, Verma I. M. Human purine nucleoside phosphorylase and adenosine deaminase: gene transfer into cultured cells and murine hematopoietic stem cells by using recombinant amphotropic retroviruses. Mol Cell Biol. 1987 Feb;7(2):838–846. doi: 10.1128/mcb.7.2.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T. H., Lindsley D., Kurachi S., Lewison K., Hemenway T., Melis M., Anagnou N. P., Najfeld V. Adult and fetal human globin genes are expressed following chromosomal transfer into MEL cells. Proc Natl Acad Sci U S A. 1985 Feb;82(3):780–784. doi: 10.1073/pnas.82.3.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell E. S. Hereditary anemias of the mouse: a review for geneticists. Adv Genet. 1979;20:357–459. [PubMed] [Google Scholar]
- Stamatoyannopoulos G., Farquhar M., Lindsley D., Brice M., Papayannopoulou T., Nute P. E. Monoclonal antibodies specific for globin chains. Blood. 1983 Mar;61(3):530–539. [PubMed] [Google Scholar]
- Whitney J. B., 3rd Simplified typing of mouse hemoglobin (Hbb) phenotypes using cystamine. Biochem Genet. 1978 Aug;16(7-8):667–672. doi: 10.1007/BF00484723. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Orkin S. H., Mulligan R. C. Retrovirus-mediated transfer of human adenosine deaminase gene sequences into cells in culture and into murine hematopoietic cells in vivo. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2566–2570. doi: 10.1073/pnas.83.8.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. A., Orkin S. H. Somatic gene therapy. Current status and future prospects. J Clin Invest. 1986 Apr;77(4):1053–1056. doi: 10.1172/JCI112403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing M. C., Nienhuis A. W., Anderson W. F. Selective activation of human beta-but not gamma-globin gene in human fibroblast x mouse erythroleukaemia cell hybrids. Nature. 1979 Feb 15;277(5697):534–538. doi: 10.1038/277534a0. [DOI] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]