Abstract
The effect of vascular endothelial growth factor (VEGF) ligands and cediranib on tumor cell proliferation, migration, and invasion was determined. It has recently been suggested that autocrine signaling through the VEGF receptor (VEGFR) pathway may play a role in tumor cell survival, invasion, and migration. The purpose of the present study was to determine the expression of VEGFRs and VEGFR ligands in a panel of gastrointestinal carcinoma cells. Additionally, we evaluated the effects of VEGF autocrine signaling on tumor cell proliferation, migration, and invasion utilizing cediranib (AZD2171), a pan-VEGFR inhibitor. Five colorectal, three pancreatic, and two hepatocellular carcinoma cell lines were screened for VEGFR and VEGF expression by several methods. Expression of VEGFR-1 and VEGFR-3 was cell line–dependent, whereas VEGFR-2 was not detected. Secretion of VEGF-A was detected in the supernatants of all cell lines whereas VEGF-C secretion was detected in the Panc-1,MiaPaca2, and Hep1 cells only. Tumor cells showed increased migratory activity, but not proliferation, when stimulated with VEGFs. The pan-VEGFR inhibitor cediranib (100 nmol/L) inhibited tumor cell migration and invasion, with no effects on proliferation. Cediranib decreased VEGFR-1 and VEGFR-3 phosphorylation as well as activation of downstream effectors. VEGFR-1 and VEGFR-3 expression was detected in all the gastrointestinal carcinoma cells evaluated. Although activation of the VEGF pathway did not affect cell proliferation, our data indicate that this pathway seems to play a role in tumor cell migration and invasion in these cell lines. Therefore, inhibition of VEGFR by cediranib may represent a clinically relevant treatment option for gastrointestinal tumors.
Introduction
Vascular endothelial growth factor (VEGF) plays a critical role in angiogenesis, promoting endothelial cell proliferation, invasion, and migration that is required for neovascularization (1).Four ligands, VEGF-A, -B, -C, and -D, mediate their effects through VEGF receptor tyrosine kinases (VEGFR) VEGFR-1, -2, and -3. The angiogenic actions of VEGF in endothelial cells are mediated primarily through the binding and activation of VEGFR-2/KDR (1–5). Increased levels of VEGF expression have been reported in several human tumor types including colon, pancreatic, liver, ovarian, breast, and lung cancers (6–16). In patients with colorectal cancer, overexpression of VEGF by tumor cells and the increased serum levels of VEGF correlate with advanced disease and a poor prognosis (6, 12, 13, 15–19). Based on these observations, VEGF and its receptors have been identified as potential targets for novel cancer therapies. Towards this end, the first monoclonal antibody against VEGF-A, bevacizumab (20), was approved in 2004 for the treatment of metastatic colorectal cancer in combination with a chemotherapy regimen of irinotecan, 5-fluoruracil, and leucovorin. In phase III clinical trials, the addition of bevacizumab to this chemotherapy regimen significantly increased overall survival and progression-free survival in patients with advanced colorectal cancer (20).The success of bevacizumab resulted in the development of several new drugs targeting the VEGFR pathway, including small molecule inhibitors of VEGFR such as vandetanib (AZD6474; refs. 9, 21–26), sorafenib (BAY-43-9006; refs. 27–30), cediranib (31), and sunitinib (SU11248; refs. 29, 32–34), which are currently either Food and Drug Administration–approved or in advanced clinical development for the treatment of a broad spectrum of human cancers.
In addition to the well-established role of the VEGF-A/VEGFR-2 axis in the angiogenic cascade, the recent discovery of VEGF ligands and VEGFR-1 and VEGFR-3 expression in epithelial cancer cells suggests a further role for these VEGFR family members in other biological processes. For example, VEGFR-1 expression has been detected in human colorectal cancer and pancreatic cancer cell lines, and activation of this receptor by VEGF was associated with enhanced cell invasion, migration, and growth in soft agar, without effects on cell proliferation (35, 36). Additionally, intracellular VEGFR-3 has been detected in the cytoplasm of breast carcinoma cells as well as lymph node and distant metastases (37). Further evidence for a role of VEGFRs in cell migration was the detection of VEGF-C/VEGFR-3 expression in lung cancer patient samples and an active VEGF-C/VEGFR-3 autocrine pathway related to lung cancer cell migration in vitro and in vivo (38).
Based on this evidence, we hypothesized that a link exists between activation of the VEGF pathway and the invasive/metastatic phenotype of tumor cells. Validation of this hypothesis could potentially provide novel targets for cancer therapy. In the present study we determined the expression levels of VEGFR family members (VEGFR-1, -2, and -3) and VEGFR ligands (VEGF-A, -C, and -D) in a panel of gastrointestinal carcinoma cell lines. Additionally, we evaluated the effects of inhibition of VEGF autocrine signaling on tumor cell proliferation, migration, and invasion utilizing cediranib (AZD2171), a highly potent inhibitor of all three VEGF receptors.
Materials and Methods
Drugs
The VEGFR-1–, VEGFR-2–, and VEGFR-3–selective tyrosine kinase inhibitor cediranib (4-[(4-Fluoro-2-methyl-1H-indol-5-yl)oxy]-6-methoxy-7-[3-(pyrrolidin-1-yl)propoxy] quinazoline; AZD2171) was kindly provided by Astra-Zeneca. For all in vitro assays, AZD2171 was prepared initially as a 10 mmol/L stock solution in DMSO and diluted in the relevant assay media.
Cell Lines and Culture
Human colorectal cancer cell lines HCT116, HCT15 HT29, SW480, and SW620; human hepatocellular carcinoma cell lines Hep1 and HepG2; human pancreatic carcinoma cell lines BXPc3, MiaPaca2, and Panc-1; and the human lung cancer cell line A549 were obtained from the American Type Culture Collection and cultured according to its recommendations. Briefly, the colorectal cancer and the lung cancer cell lines were cultured in RPMI medium supplemented with 10% fetal bovine serum (FBS), 1% nonessential amino acids, and 1% penicillin/streptomycin. The pancreatic and the hepatocellular cancer cell lines were cultured in DMEM supplemented with 10% fetal bovine serum, 1% nonessential amino acids, and 1% penicillin/streptomycin. All cell lines were maintained at 37°C under an atmosphere containing 5% CO2. Human umbilical vein endothelial cells (HUVEC) were obtained from Lonza Inc. and cultured with EGM SingleQuots growth factor supplemented media (Lonza, Inc.) at 37°C in an incubator under an atmosphere containing 5% CO2 until passage 12. The cells were routinely screened for the presence of mycoplasma using an ELISA for mycoplasma-specific proteins (MycoAlert, Cambrex Bio Science).
Reverse Transcription-PCR
The cell lines were evaluated for VEGFR-1, -2, and -3 and for VEGF-A, -B, -C, and -D gene expression by quantitative reverse transcription-PCR (qRT-PCR). Cells were plated in 25-cm2 flasks and grown in standard conditions until 70% confluency was reached. Cells were harvested and total RNA was purified using the Qiagen RNeasy Plus Mini kit (Qiagen). Reverse transcription was done on 1 µg of total RNA using the Taqman Reverse Transcription Kit according the manufacturer's instructions (Applied Biosystems). Quantitative PCR was done on 100 ng of cDNA using Power SYBR Green detection chemistry (Applied Biosystems).
Immunoblotting
The cell lines were evaluated for VEGFR-1, VEGFR-2, and VEGFR-3 expression by Western blot analysis. Cells were plated and grown in 6-well dishes in cultured standard growth conditions until they reached 70% confluency. Protein extracts were obtained by lysing cells in radioimmuno-precipitation assay buffer (1× PBS, 1% Nonidet P-40, 0.1% SDS) containing a complete protease inhibitor cocktail (Boer-hinger Mannheim), 10 mg/mL phenylmethylsulfonylfluoride, and 100 mmol/L sodium orthovanadate. Total protein concentrations for each sample were determined using the BioRad Dc Protein Assay (BioRad, Inc.). Thirty micrograms of total protein were loaded onto a 4% to 20% gradient gel, electrophoresed, and transferred to Immobilon-P membranes (Millipore, Inc.). Membranes were blocked for 1 h at room temperature in TBS-Tween-20 (0.1%) with 5% bovine serum albumin (Fisher BioReagents). Primary antibody incubation was done overnight at 4°C with the appropriate antibody at the following dilutions: VEGFR-1/Flt-1 (Santa Cruz Biotechnology Inc.) rabbit polyclonal, 1:1,000; VEGFR-2/KDR (Cell Signaling) rabbit polyclonal, 1:1000; VEGFR-3 (Chemicon, Millipore Corporation) mouse monoclonal, 1:5,000. Blots were then washed 3 × 20 min in TBS-Tween, and incubated with the appropriate secondary antirabbit or antimouse IgG peroxidase-linked antibody at a 1:50,000 dilution (Jackson ImmunoResearch) for 1 h at room temperature. Blots were washed 3 × 20 min in TBS-Tween and developed using Immobilon Western Chemiluminescent HRP substrate kit (Millipore Corporation). In order to detect VEGFR and downstream effectors phosphorylation, cells were plated in 6-well dishes in standard media conditions for 24 h followed by incubation in serum-free media overnight with or without cediranib (AZD2171, 100 nmol/L). The following day, cells were stimulated with either FBS (10%), VEGF-A (10 ng/mL), VEGF-B (50 ng/mL), or VEGF-C (50 ng/mL; R&D Systems) for 10 min. Cell lysates were prepared as above and total protein was quantified. Due to lack of a phospho-specific VEGFR-3 antibody, cell lysates were incubated with a VEGFR-3 antibody (Chemicon) overnight at 4°C and immunoprecipitated with protein A for 3 h at 4°C.Immunoprecipitates were then immunoblotted with a phospho-Tyr antibody (Cell Signaling). Phospho-specific antibodies were used to detect p-VEGFR-1 (Upstate, Millipore Corporation; 1:1,000), p-AKT, and p-ERK (Cell Signaling; 1:1,000) by standard immunoblotting methods as described above.
Flow Cytometric Analysis of VEGFRs
Cells were grown in standard conditions until 70% confluence was reached, detached with 5 mmol/L EDTA/EGTA in Dulbecco's PBS (D-PBS) minus calcium and magnesium, pelleted, and washed with D-PBS, 0.5% bovine serum albumin. Cells were resuspended in 3 mL of 0.5% formaldehyde (Polyscience) and fixed for 10 min at 37°C. After fixing, cells were washed twice with 3 mL of ice cold D-PBS pelleted by centrifugation, and gently resuspended in 5 mL of cold 90% methanol D-PBS at 4°C for 30 min. Cells were again centrifuged and resuspended in 1mL of fluorescence activated cell sorting (FACS) buffer (D-PBS, 5% FBS) for 30 min at room temperature followed by centrifugation. Cell pellets were resuspended in 30 µL of FACS buffer and incubated with fluorochrome-labeled antibodies for VEGFR-1 and VEGFR-3 at a 1:5 dilution (R&D System), incubated for 30 min, then washed with 1 mL of flow buffer, centrifuged, and resuspended in 500 µL FACS buffer and analyzed by FACS at the University of Colorado Cancer Center Flow Cytometry Core Laboratory.
VEGF-A, -C, and -D ELISA
Secreted VEGFs in cell culture supernatants were measured by VEGF isotype-specific ELISA (R&D Systems). Briefly, cell lines were plated in 24-well plates at a density of 1 × 106 cells/well in standard growth conditions for 24 h. Media were then aspirated and replaced with serum-free media and supernatants were analyzed for secreted VEGF-A, -C, and -D after 24, 48, and 72 h in serum-free conditions. Of note, an ELISA that measures VEGF-B is not currently available.
Analysis of Cell Proliferation
Cell proliferation in response to activation of VEGF receptors in gastrointestinal cancer cell lines was measured by the sulforhodamide B (SRB) assay (39). HCT1 16, HT29, SW480, Hep1, or BXPC3 cells were plated in 96-well plates under normal growth conditions for 24 h, and then cultured in serum-free media for 16 h in the presence or in absence of cediranib (100 nmol/L). Cell media were then replaced with either serum-free media alone or serum-free media containing VEGF-A (10 ng/mL), VEGF-B (50 ng/mL), VEGF-C (50 ng/mL) or 10% FBS, in the presence or absence of cediranib. After 7 d, media were aspirated and cells were fixed with cold trichloracetic acid (10%) for 30 min at 4°C. Fixed cells were then washed with water and stained with 0.4% SRB (Fisher Sci.) for 30 min at room temperature, washed again with 1% acetic acid, followed by stain solubilization with 10 mmol/L tris at room temperature. The plate was then read on a plate reader (Biotek Synergy 2) set at an absorbance wavelength of 565 nm. Cell proliferation curves were derived from the raw absorbance data.
Assessment of Gastrointestinal Cancer Cell Motility and Migration
Cell migration was measured by wound healing assays (scratch) and a modified Boyden chamber assay. For the scratch assay, cell lines were seeded in 6-well plates under standard growth conditions. Cells were grown to confluency and a scratch was created by moving a sterile pipet tip along the bottom of the plate. The ability of the cells to move in to the scratch area was assessed 48 h after creation of the wound area. Cells were then fixed with 90% methanol,washed with D-PBS, and stained with crystal violet for 5 min. After a subsequent wash with water, the plates were photographed at 10× magnification on an inverted microscope. For VEGF ligand stimulation studies, cells were grown to confluency in 6-well plates under standard media conditions and then cultured in serum free media overnight with or without AZD2171 (100 nmol/L). Cells were then stimulated with FBS (10%), or VEGF-A (10 ng/mL), VEGF-B (50 ng/mL) or VEGF-C (50 ng/mL), scratched and after 48 h, fixed, stained, and photographed as described above.
Modified Boyden chamber assays were done using uncoated 8.0-μ transwell inserts placed in 24-well plates (Becton Dickinson Labware). Cells were resuspended in 250 µL of standard growth media at a density of 10 × 104 cells/well and plated in the upper chamber of the transwell inserts. The lower chamber of the wells contained 600 µL of standard growth media. After 24 h, the media in the upper transwell chamber were replaced with 0.1% FBS media, and the media in the bottom of the 24-well plate were replaced with fresh media containing 10% FBS. After 48 h the filters were fixed with 4% formaldehyde for 15 min (Polyscience), washed three times with D-PBS, and migrating cells were stained with 4', 6-diamidino-2-phenylindole for 30 min, washed with D-PBS 3 × 15 min, and photographed at 20× magnification on an inverted microscope. Five fields per well were counted manually and averaged. To measure VEGF ligand stimulatory effects and cediranib activity on cell migration 100,000 cells/well were resuspended in 250 µL of standard growth media and plated in the upper transwell chamber. The lower chamber of the wells contained 600 µL of standard growth media. After 24 h, the media in the upper and lower chambers of the trans-well insert were replaced with 0.1% FBS media and incubated overnight in the presence or absence of cediranib. Cell migration was then stimulated by replacing media in the lower chamber with media containing either 10% FBS or VEGF-A (10 ng/mL), VEGF-B (50 ng/mL), or VEGF-C (50 ng/mL) in absence or presence of cediranib (100 nmol/L). Cell migration was evaluated after 48 h as described above.
Cell Invasion Assay
The invasive potential of SW480 and Hep1 cells was assessed using a modified Boyden chamber assay, consisting of matrigel-coated 8-μ inserts (Becton Dickinson Labware), prepared according to the manufacturer's instructions. In this assay, cells must degrade the matrigel matrix in order to migrate through the 8-μ insert. For SW480 cells, 100,000 cells/well were resuspended in 250 µL of standard growth media and plated in the upper transwell chamber. The lower chamber of the wells contained 600 µL of standard growth media. After 24 h, the media in the upper and lower chambers of the transwell insert were replaced with media containing 0.1% FBS in the presence or absence of cediranib and incubated overnight. Cell invasion was then stimulated by replacing media in the lower chamber with media containing 10% FBS in the absence or presence of cediranib (100 nmol/L). After 72 h the filters were fixed with 4% formaldehyde for 15 min (Polyscience), washed three times with D-PBS, and migrating cells were stained with 4', 6-diamidino-2-phenylindole for 30 min, washed with D-PBS 3 × 15 min, and photographed at 20× magnification on an inverted microscope. Five fields per well were counted manually and averaged. Due to the highly invasive phenotype of Hep-1 cells, the number of cells plated was decreased to 25,000 cells/well and invasion was evaluated after 48 h.
Results
Expression Profile of VEGF Receptors in Gastrointestinal Tumor Cell Lines
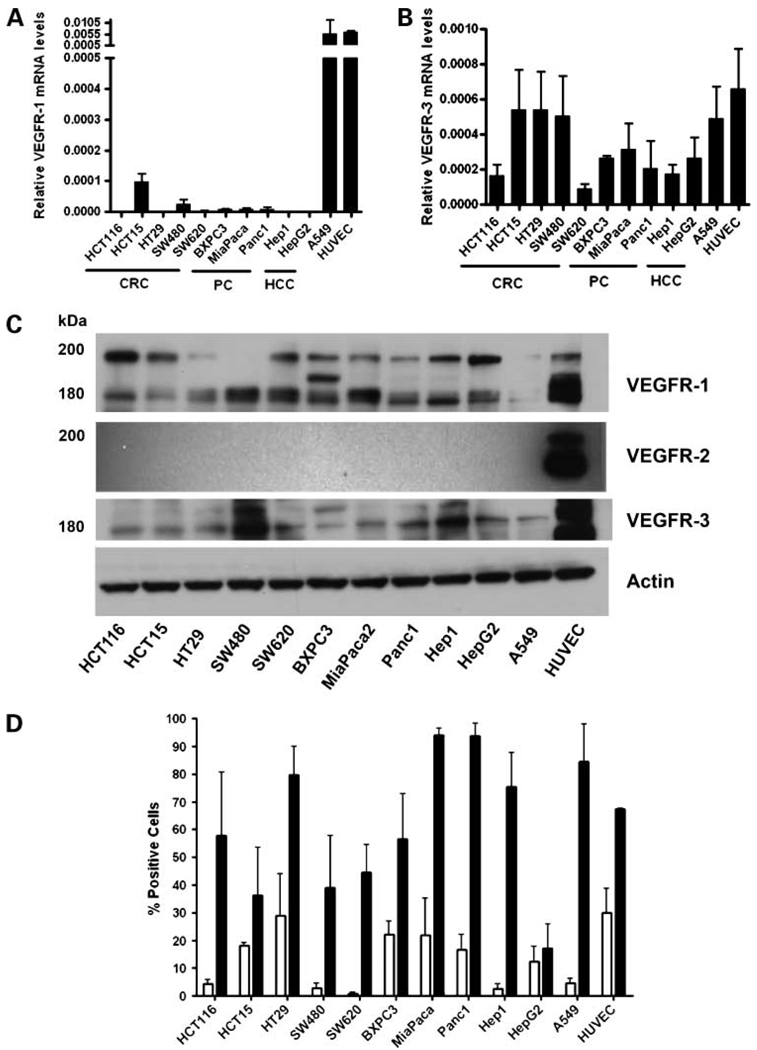
To determine the role of VEGF and VEGFR family members in gastrointestinal carcinoma cells, VEGFR-1/Flt-1, VEGFR-2/KDR, and VEGFR-3/Flt-4mRNA expression levels were evaluated for five colorectal, two hepatocellular, and three pancreatic cancer cell lines using qRT-PCR. The HUVEC cell line was used as a positive control for VEGFR-1,-2, and -3, and the lung cancer cell line A549 was used as positive control for VEGFR-3 expression (38). Expression of VEGFR-1 mRNA was detected at relatively low levels in the HCT15 and SW480 colorectal cancer cells, as well as the BXPC3, MiaPaca2, and Panc1 pancreatic cells, as compared with the A549 and the HUVEC cells. However, all other cell lines tested showed no detectable levels of VEGFR-1, even after 40 PCR cycles (Fig.1A). VEGFR-3 mRNA was detected in all cell lines, to varying degrees, with a trend towards higher expression in the HCT15, HT29, and SW480 (colorectal cancer) cell lines versus hepatocellular cancer and pancreatic cancer cell lines (Fig.1B). Notably , all cell lines, with the exception of HUVEC, were negative for VEGFR-2 expression (data not shown).
Figure 1.
VEGFR family expression by qRT-PCR, immunoblotting, and flow cytometry. A, B, total RNA was extracted from colorectal (CRC) cell lines HCT-116, HCT15, HT29, SW480, and SW620; pancreatic cancer (PC) cell lines BXPC3, MiaPaca2, and Panc-1; hepatocellular carcinoma (HCC) cell lines Hep-1 and HepG2; lung cancer cell line A549; and human umbilical cord endothelial cell line (HUVEC), and qRT-PCR was done to assess expression of VEGFR-1 (A) or VEGFR-3 (B). C, the same cell lines were analyzed for VEGFR protein expression by immunoblotting. Thirty micrograms of total protein from each cell line were fractionated through SDS-PAGE, transferred to polyvinylidene difluoride membranes, and incubated with the appropriate antibodies as described in Materials and Methods. The experiment was done in triplicate. Actin protein was used as a protein loading control. D, FACS analysis for VEGFR-1 or VEGFR-3 expression. Results are expressed as percent of cells positive for the specific VEGFR antibody versus isotype control antibody. HUVECs were used as a positive control. White bars, mean ± SE of three independent experiments for VEGFR-1; black bars, mean ± SE of three independent experiments for VEGFR-3.
Next, we evaluated VEGFR protein levels in the gastrointestinal cancer cell lines by Western blot analysis. As previously reported, the membrane-bound form of VEGFR-1 exists primarily as a 180-kDa protein with glycoslyated forms at approximately 200 and 220 kDa (40). Whereas VEGFR-1 mRNA expression was only detected in a few cell lines by qRT-PCR, immunoblot analysis showed that VEGFR-1 protein is expressed in most of the cell lines examined (Fig.1C). This apparent discrepancy could be the result of a high turnover rate or a high translational efficiency for VEGFR-1 mRNA. Expression of VEGFR-3 protein was detected by immunoblotting in all cell lines screened (Fig.1C). Among the colorectal and pancreatic cancer cell lines, VEGFR-3 expression levels were relatively equal, with the SW480 and the Hep1 showing the highest amount of VEGFR-3. Consistent with the qRT-PCR results, immunoblotting of whole cell extracts with a specific VEGFR-2 antibody indicated no VEGFR-2 expression in any of cell lines analyzed with the exception of HUVEC (Fig.1C).
To further verify VEGFR-1 and VEGFR-3 expression levels in the panel of gastrointestinal cancer cell lines, a separate analysis was done using FACS. As shown in Fig.1D, all of the colorectal cancer cell lines express VEGFR-1 to varying degrees, with HT29 cells expressing the highest levels of this receptor. Similarly, the pancreatic cancer cell lines showed varying levels of VEGFR-1, with the BxPc3 and MiaPaca2 lines having the highest levels. However, the two hepatocellular cancer cell lines showed very low levels of VEGFR-1 compared with the colorectal cancer and pancreatic cancer cell lines. With regard to VEGFR-3 levels, FACS analysis indicated relatively strong expression of this receptor isotype, as compared with VEGFR-1, in all of the cell lines examined, with the exception of the HepG2 hepatocellular cancer cell line (Fig.1D). Of note, we were unable to measure substantial amounts of VEGFR-1 protein by Western blotting or FACS analysis in A549 cells, despite a strong signal for this receptor as measured by qRT-PCR (Fig.1A, C, D). These results may indicate low translational efficiency of VEGFR-1 mRNA or high protein turnover rate in these cells.
Secretion of VEGF Ligands by Gastrointestinal Tumor Cell Lines
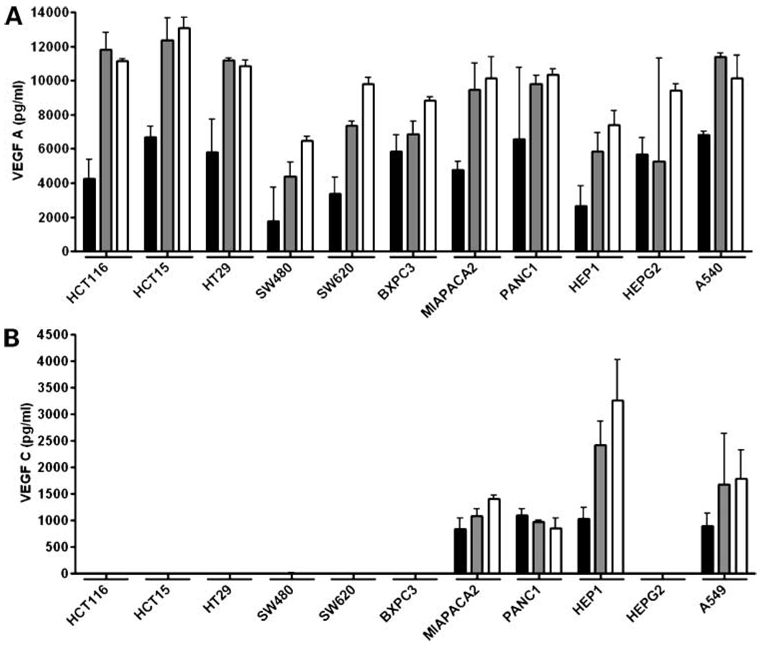
We next analyzed secretion of VEGF ligands by the panel of gastrointestinal cancer cell lines into cell culture media using ELISA specific for VEGF-A, -C, and -D. To ensure that the VEGF measured was produced exclusively by the cell lines, they were plated in normal growth media which was were changed to serum-free media after 24 hours. Subsequently, VEGF ELISAs were done on cell culture supernatants 24, 48, and 72 hours after incubation in serum-free media. The results of the ELISA for VEGF-A showed that this ligand was secreted at high levels by all cell lines examined at the 24-hour time point, with an approximate additive increase in the amount secreted at 48 and 72 hours (Fig.2A). By contrast, VEGF-C was secreted only by the MiaPaca2, Panc1, Hep1, and A549 cell lines, and at much lower levels than VEGF-A, with no measurable levels of this ligand secreted by any of the colorectal cancer cell lines or the BXPC3 and HepG2 cell lines (Fig.2B). Of note, our results show that VEGF-C was secreted only by hepatocellular and pancreatic cancer cell lines of the mesenchymal phenotype and not by those of the epithelial phenotype, as determined previously by e-cadherin or vimentin expression (data not shown). Finally, we were unable to detect VEGF-D secretion in any of the cell lines at any time point. Taken together, these expression data suggest that the VEGF pathway is present and potentially functional in gastrointestinal cancer cell lines.
Figure 2.
VEGF-A and VEGF-C secretion by ELISA. A, B, VEGF ligand levels as measured in cell culture supernatants by ELISA 24, 48, and 72 h after culture in serum-free media. Bars, mean ± SE of three independent experiments. Columns, 24 h (black),48 h(grey), and 72 h postserum starvation.
VEGFR-1 and VEGFR-3 in Gastrointestinal Tumor Cell Lines Are Coupled to Intracellular Signaling Pathways
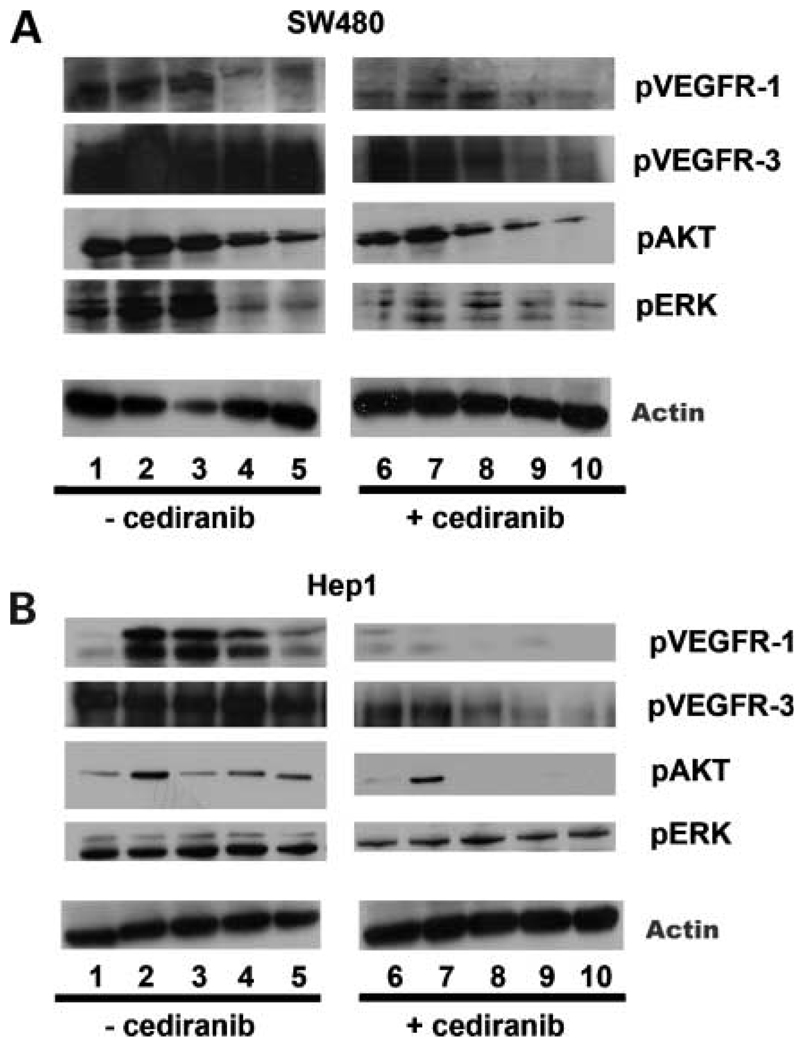
To determine if VEGFR-1 and VEGFR-3 activation results in changes in intracellular signaling molecules in gastrointestinal tumor cells, we measured known downstream effectors of activated VEGFRs in the SW480 (colorectal cancer) and Hep1 (hepatocellular cancer) cell lines, which were selected based on their high degree of motility. In order to assess the role of individual VEGF ligands on these signaling pathways, cells were plated in standard media conditions for 24 hours and then switched to serum-free media for an additional 16 hours in the presence or absence of cediranib (AZD2171), a small molecule pan-VEGFR inhibitor. Cells were then exposed to either 10% FBS or VEGF-A, -B, or -C and analyzed by Western blot for phospho-VEGFR-1 (pVEGFR-1), pAkt, and pERK levels, and by Western blot and immunoprecipitation for pVEGFR-3 levels. As shown in Fig.3A and B, VEGFR-1 is activated by FBS and VEGF-A in both SW480 and Hep1 cell lines. In Hep1 cells, however, VEGFR-1 activation was induced by VEGF-B and, to a lesser degree, VEGF-C. As previously reported (41), we consistently observed high levels of pVEGFR-3 in both SW480 and Hep1 cells, regardless of whether they are cultured in serum-free conditions or with addition of specific VEGFs (Fig.3A and B). The addition of the pan-VEGFR inhibitor cediranib effectively reversed the phosphorylation of VEGFR-1 and VEGFR-3 in the Hep1 cells as well as the phosphorylation of the downstream signaling molecules AKT and ERK, indicating that these receptors may be functionally linked to signaling pathways that are involved in tumor cell proliferation, and motility.
Figure 3.
Ligand-induced activation VEGFR-1/-3 activation and downstream AKT and ERK activation by immunoblotting. A, SW480 cells cultured in serum-free media were pretreated with or without cediranib (100 nmol/L), were left untreated (SF; lanes 1, 6), or were stimulated with 10% FBS (lanes 2, 7), 10 ng VEGF-A (lanes 3, 8), 50 ng VEGF-B (lanes 4, 9), or 50 ng VEGF-C (lanes 5, 10) for 10 min. B, Hep-1 hepatocellular carcinoma cells treated as in A. Immunoblotting was done on 50 µg of total cell lysate per well and probed with the appropriate phospho-specific (p) antibody. Experiments were done in triplicate.
VEGFR Inhibition by Cediranib Does Not Affect Gastrointestinal Tumor Cell Proliferation
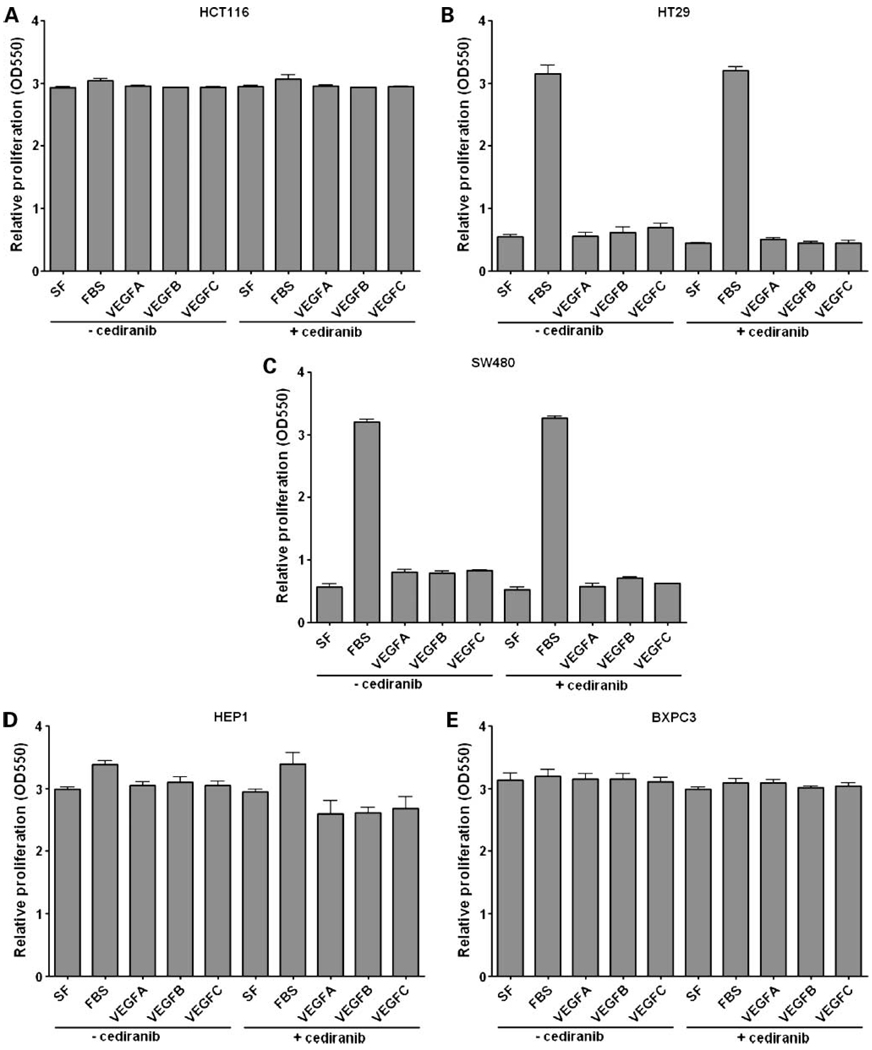
To determine the biological significance of VEGF receptors and VEGF ligands in gastrointestinal cancer cell lines, we initially investigated their potential role in proliferation in vitro. To accomplish this, HCT116, HT29, SW480, Hep1, and BXPC3 cells were serum-starved for 16 hours and then stimulated with FBS, VEGF-A, VEGF-B, or VEGF-C for 48 hours or 7 days, in the presence or absence of cediranib, and analyzed for proliferation by the SRB assay. The results, depicted in Fig.4, indicate that addition of VEGF ligands does not stimulate gastrointestinal cancer cell proliferation, and like-wise, blocking VEGFR activity with cediranib also had no inhibitory effect on cell proliferation. Although none of the cell lines showed a proliferative phenotype with VEGF ligands, it is interesting to note the differential effects of FBS on the cell lines, indicating that the HCT116, Hep1, and BXPc3 cells seem to have autocrine production of growth factors that mimic the effects of serum (Fig.4).
Figure 4.
Effects of VEGF-A, -B, or -C stimulation on gastrointestinal cancer cell proliferation. HCT116 (A), HT29 (B), SW480 (C), Hep-1 (D), and BXPC3 (E) cells were plated in 96-well plates under standard growth conditions for 24 h. Cells were then cultured in serum-free conditions and pretreated with or without cediranib (100 nmol/L) for 16 h followed by stimulation with FBS (10%), VEGF-A (10 ng), VEGF-B (50 ng), or VEGF-C (50 ng) for 7 d. Proliferation was assessed by the SRB assay. Bars, mean ± SE of three independent experiments.
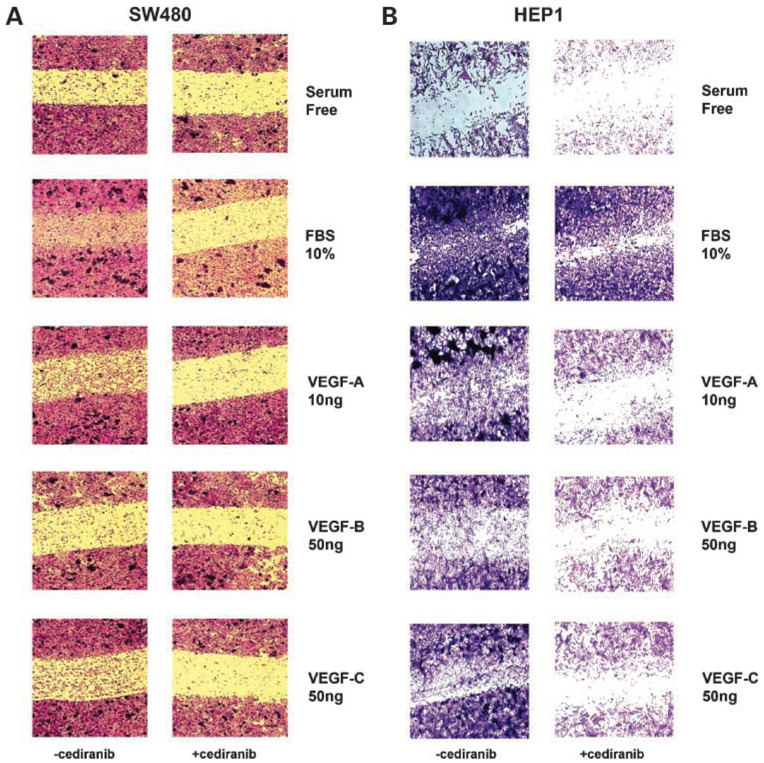
Activation of the VEGF/VEGFR Pathway Enhances the Migratory Phenotype of Gastrointestinal Cancer Cell Lines
Initially, we assessed the migratory potential of gastrointestinal cancer cells by carrying out scratch motility assays and modified Boyden chamber assays on the panel of colorectal, hepatocellular, and pancreatic cancer cell lines. We found that all of the cell lines tested were capable of migration in vitro, to varying degrees (data not shown). To specifically measure the effects of VEGFR activation on migratory behavior, we did scratch motility assays on selected cell lines, SW480 and Hep1, based on their highly motile phenotype. As shown in Fig.5A and B, both SW480 and Hep1 cells cultured in media with 10% FBS showed increased motility into the scratch area when compared with cells cultured in serum-free media. To determine the role of specific VEGF ligands on migration, SW480 and Hep1 cells cultured under serum-free conditions were treated with VEGF-A, -B, or -C for 48 hours. SW480 cells showed increased motility when stimulated with VEGF-A and VEGF-C, but stimulation with VEGF-B did not result in any change in motility when compared with serum-free conditions (Fig.5A). By contrast, Hep1 cells responded relatively equally to all three VEGF ligands, with increased migration into the wound area after stimulation with VEGF-A, -B, or -C (Fig.5B). To further verify that the effects of VEGF ligands on motility were mediated through VEGFRs, SW480 and Hep1 cells were treated for 48 hours in the presence of cediranib at the dose of 100 nmol/L. As depicted in Fig.5A and B, treatment with cediranib effectively blocked motility of SW480 cells in response to 10% FBS as well as to stimulation with VEGF-A, -B, or -C. Treatment of Hep1 cells with cediranib was only partially effective in blocking FBS-stimulated migration, but cediranib completely inhibited VEGF-A–, VEGF-B–, or VEGF-C– stimulated migration.
Figure 5.
Effects of VEGF ligands and cediranib on the migratory phenotype of SW480 and Hep-1 cells. SW480 (A) or Hep1 (B) cells were evaluated for their migratory phenotype in standard growth conditions by the scratch assay. Cells were grown to 80% confluency and a cleared scratch area was created prior to stimulation with FBS, VEGF-A, VEGF-B, or VEGF-C in the presence or absence of cediranib. Cell migration was measured by the ability of cells to migrate into the scratch area after 48 h. Cells were then fixed, stained, and photographed as described in Materials and Methods. Images are representative of three independent experiments.
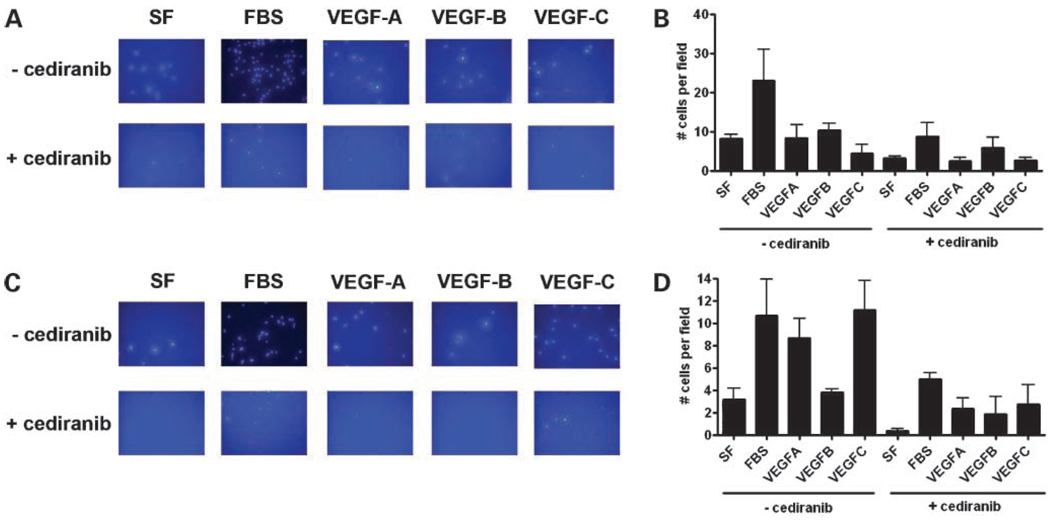
In order to quantify the effects of VEGFs and cediranib on cell migration, we next carried out motility assays using a modified Boyden chamber. As shown in Fig.6A and B, SW480 cells stimulated with 10% FBS resulted in a 5-fold increase in motility compared with cells in serum-free media. Although there was no significant increase in the motility of SW480 cells stimulated with VEGF-A, -B, or -C, treatment with 100 nmol/L cediranib for 48 hours resulted in decreased motility not only in the cells stimulated with FBS, but also in cells stimulated with VEGF-A, -B, or -C. Interestingly, we also observed a decrease in motility in the cells cultured in serum-free conditions in the presence of cediranib, suggesting the presence of an autocrine feedback loop that is inhibited by cediranib. As shown in Fig.6C and D, the motility of Hep1 cells was increased approximately 2.5-fold by stimulation with 10% FBS and to a similar degree by stimulation with VEGF-A and VEGF-C. However , VEGF-B had no apparent effect on Hep1 motility. Treatment of Hep1 cells with cediranib resulted in an almost complete inhibition of migration of cells cultured in serum-free conditions and an approximate 50% reduction when stimulated with FBS or VEGF-A. Of note, we observed a 70% reduction in motility in cells treated with VEGF-C and cediranib as compared with VEGF-C alone (Fig.6C and D).
Figure 6.
Quantitative analysis of SW480 and Hep-1 migration. The modified Boyden chamber was used to quantify the effects of VEGF ligands and cediranib on SW480 (A, B) and Hep-1 cells (C, D). A total of 100,000 cells were plated in the upper transwell chamber and treated with either FBS (10%) or VEGF-A (10 ng/mL), VEGF-B (50 ng/mL) or VEGF-C (50ng/mL) in the presence or absence of cediranib for 48 h. Transwell filters were fixed and stained as described in Materials and Methods. Migration was assessed by counting the number of stained cells visible on the lower surface of the transwell filter. Data represent mean cell counts of five separate fields per well ± SE of three independent experiments. Representative fields for each condition are shown in A and C.
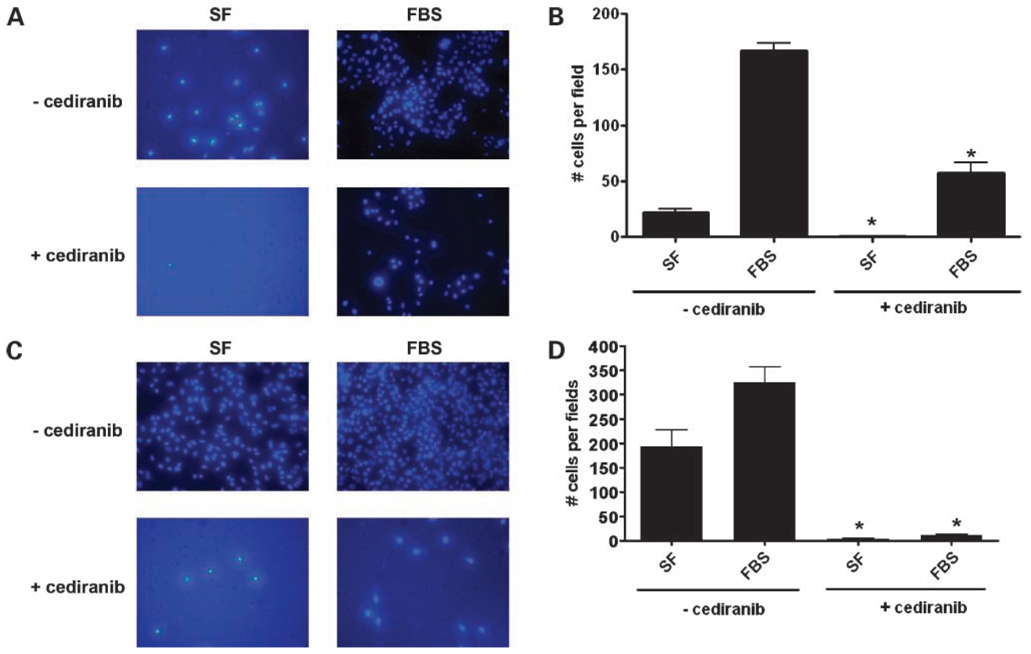
Cediranib Decreases Gastrointestinal Tumor Cell Invasiveness
To evaluate the role of VEGFRs in gastrointestinal tumor cell invasiveness, we used cediranib to block VEGFR activity and then evaluated the invasive potential of SW480 cells treated with and without this drug using matrigel-coated modified Boyden chambers. As depicted in Fig.7A to D, the SW480 and the Hep1 cell lines showed a baseline invasive phenotype that was significantly increased by stimulation with 10% FBS. Treatment with cediranib (100 nmol/L) resulted in a significant decrease in the number of cells that were capable of invading the matrigel barrier and migrating through the pores of the filter separating the two chambers. (Fig.7A–D).
Figure 7.
Effects of cediranib on SW480 and Hep1 cell invasion. A matrigel-coated invasion chamber assay was used to evaluate the role of VEGFRs on SW480 (A, B) and Hep1 (C, D) cell invasion. SW480 (100,000 cells/well) and Hep1 (25,000 cells/well) cell lines were seeded onto the upper chamber of the transwells for 24 h in standard media conditions. Serum-free media were then replaced in the upper chamber with or without cediranib (100 nmol/L) for a subsequent 16 h. The lower chambers were filled with either serum-free media (SF) or media containing 10% FBS in the presence or absence of cediranib, and invasion was assessed after 72 h for the SW480 and after 48 h for the Hep 1 cells by counting the number of stained cells visible on the lower surface of the transwell filter, as described in Material and Methods. Data represent mean cell counts of five separate fields per well ± SE of three independent experiments. Representative fields for each condition are shown in A and C (*, P < 0.05 SF, FBS without cediranib versus SF, FBS with cediranib).
Discussion
Recent evidence suggests that VEGF receptors are expressed on tumor epithelial cells in a wide variety of cancer cell lines as well as tumor samples (35, 38, 41–54). However, these findings remain controversial, with some groups failing to detect VEGFR expression on tumor epithelium, and furthermore, the functional significance of this expression with respect to tumor cell biology remains unclear (55–57). The current study was conducted in order to assess VEGFR expression and function in a panel of gastrointestinal tumor cell lines and to determine their functional significance. Our results showed varying levels of expression of VEGFR-1 and VEGFR-3 in colorectal, pancreatic, and hepatocellular carcinoma cell lines. VEGFR stimulation with VEGF ligands showed that these receptors were functionally linked to intracellular signaling pathways, resulting in alterations in tumor cell motility and invasion. Additionally, we determined that treatment with cediranib, a small molecule VEGFR tyrosine kinase inhibitor that inhibits all three VEGFRs, could reverse VEGFR-A– and VEGF-C–stimulated cell motility and invasion. These results suggest that VEGFR expression in tumor epithelial cells is biologically relevant and that cediranib represents a potential dual-compartmental therapy for gastrointestinal cancers by inhibiting tumor cell motility and invasiveness in addition to its previously demonstrated antiangiogenic and antilymphangiogenic effects (31, 58, 59).
The VEGF/VEGFR signaling pathway plays a critical role in angiogenesis, promoting endothelial cell proliferation, invasion, and migration that is required for neovascularization (1). Recent reports suggest, however, that VEGFR expression is not restricted to endothelial cells. Ellis et al. first reported the expression of VEGFR-1 in several cancer cell lines and suggested that VEGFR-1 activation by VEGF-A and VEGF-B increased the levels of the transcription factor, Twist, which in turn induced epithelial to mesenchymal transition in pancreatic cell lines (60). Additionally, VEGFR-3 and VEGF-C expression has been reported in lung cancer lines as well as human lung tumor samples, where VEGFR-3 expression levels were shown to be positively correlated with clinical metastasis and poor prognosis in lung cancer patients (37, 61, 62). Despite this evidence for tumor cell expression of VEGFRs, these findings remain controversial and are in conflict with other reports that have failed to corroborate VEGFR-3 expression in a variety of cancer cell lines and further concluded that tumor expression of VEGFRs was restricted to blood vessels within the tumor (55, 57). Our laboratory has previously detected VEGFR-1 and VEGFR-3 expression in HT29 and HCT116 colorectal cancer cell lines by flow cytometry (23). In the present study, we employed a variety of methodologies to further characterize VEGFR and VEGF expression and function in a panel of nine gastrointestinal cancer cell lines, including colorectal, pancreatic, and hepatocellular cancer cells. Utilizing RT-PCR, flow cytometry, and Western blotting, we found that VEGFR-1 and VEGFR-3 were expressed in all of the cell lines examined, to varying degrees, but we were unable to detect VEGFR-2 expression in any of the cell lines tested. With respect to VEGF ligand expression, all cell lines secreted high levels of VEGF-A, but only three of the cell lines tested secreted VEGF-C. Interestingly, VEGF-C expression was restricted to cells exhibiting the mesenchymal phenotype, but the functional significance of this finding has yet to be determined.
Cediranib (AZD2171) is a small molecule tyrosine kinase inhibitor that has highly selective activity against VEGFR-1,-2, and -3 (31). Because we found that our panel of gastrointestinal cancer cell lines express VEGFR-1 and VEGFR-3, we hypothesized that cediranib would have inhibitory effects on VEGFR signaling pathways and thereby allow insights into VEGFR-regulated biological activity. Furthermore, the specificity of cediranib for VEGFRs serves as an additional means to confirm the presence of VEGFRs and show that they are functionally linked to biologically relevant signaling pathways in gastrointestinal cancer cells. Although the intracellular signaling pathways activated by VEGFRs in endothelial cells is well characterized, much less is known about signaling in epithelial cancer cells. In lung cancer cell lines, VEGFR-3 stimulation resulted in activation of p38 MAPK but not ERK1/2, and inhibition of p38 in these cells resulted in a decrease in VEGF-C– dependent cell invasion. In lung cancer cell lines, VEGFR-3 stimulation was shown to activate p38 MAPK but not ERK1/2 (38). In this study, we found that cediranib inhibited VEGFR-1 and VEGFR-3 phosphorylation as well as phosphorylation of downstream effector molecules, AKT and ERK. With respect to biological activity, we found that addition of either VEGF-A or -B or -C to gastrointestinal cancer cells cultured in serum-free conditions had no effect on cell proliferation. This observation was further confirmed by our finding that treatment with cediranib, at a clinically relevant dose, also failed to have any effects on gastrointestinal cancer cell proliferation. These results are in agreement with another report that showed VEGF-A and VEGF-B did not increase proliferation in colorectal cancer cell lines (35) and, taken together, suggest that VEGF/VEGFR signaling is not directly involved in gastrointestinal tumor cell proliferation.
The relationship between VEGF stimulation of VEGFRs and endothelial and lymphatic cell migration is well established (63–65). More recently, VEGFR activation has been associated with increased migration of colorectal, pancreatic, and lung cancer cells (35, 36, 38, 60, 66, 67). Consistent with these findings, our results indicate that VEGFR-1 and VEGFR-3 activation results in increased gastrointestinal cancer cell motility as measured by two separate motility assays. SW480 and Hep1 cells repopulated the scratch wound zone in response to stimulation with VEGF-A, -B, or -C, and this effect was blocked by treatment with cediranib. Similar results were seen using the modified Boyden chamber assay, which has the advantages of being more quantitative and effectively distinguishes between motility and proliferation. Although SW480 cells increased cell migration only when stimulated with FBS in this assay, we again observed significant inhibition of motility when cells were also treated with cediranib. However, because FBS is the migration stimulus, we cannot rule out the possibility that cediranib inhibits growth factor pathways other than VEGFRs.
The ability of tumor cells to invade the surrounding extra-cellular matrix is a critical step in the metastatic process and is strongly correlated with drug resistance and poor prognosis in several cancer types (68–70). Using a modified Boyden chamber assay, where matrigel-coated filters represent a synthetic extracellular matrix, we showed that SW480 cells were capable of invading the matrigel in response to serum stimulation. Treatment of these cells with cediranib resulted in significantly fewer numbers of cells that were capable of invading this barrier, suggesting that in addition to their role in gastrointestinal tumor cell migration VEGFR-1 and VEGFR-3 may also play an important role in tumor cell invasion of the extracellular matrix. Again, it remains possible that cediranib acts on other growth factor receptors in addition to VEGFRs to exert its inhibitory effects on tumor cell migration in this assay.
In summary, our results show that VEGFR-1 and VEGFR-3 are expressed in a variety of gastrointestinal cancer cell lines. Furthermore, these receptors seem to be linked to functional signaling pathways in these cells, which regulate cell motility and invasion. As cediranib treatment reversed VEGF-stimulated motility and invasion of gastrointestinal cancer cells in vitro, we conclude that targeting VEGFRs present on gastrointestinal tumors may represent an effective therapeutic treatment for highly invasive gastrointestinal cancers. Further investigation using in vivo preclinical models is needed to confirm this hypothesis.
Acknowledgments
We thank Dr. Colin Weekes for critical review of the manuscript and Blair Britt for his technical expertise with photomicroscopy and quantification of the migration and invasion assays. We also acknowledge the assistance of the University of Colorado Cancer Center Flow Cytometry Core.
Grant support: Fellowship Grant to M.P. Morelli from Astra-Zeneca Research Agreement. Grants CA106349 and CA043934 to S.G. Eckhardt.
Footnotes
Disclosure of Potential Conflicts of Interest
A. Ryan and J.M. Jurgensmeier are employees of AstraZeneca, Inc. S. Gail Eckhardt has received research funding from AstraZeneca Inc. RECENTIN is a trademark of the AstraZeneca group of companies.
References
- 1.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 2.Zetter BR. Angiogenesis and tumor metastasis. Ann Rev Med. 1998;49:407–424. doi: 10.1146/annurev.med.49.1.407. [DOI] [PubMed] [Google Scholar]
- 3.Wey JS, Stoeltzing O, Ellis LM. Vascular endothelial growth factor receptors: expression and function in solid tumors. Clin Adv Hematol Oncol. 2004;2:37–45. [PubMed] [Google Scholar]
- 4.McMahon G. VEGF receptor signaling in tumor angiogenesis. Oncologist. 2000;5 Suppl 1:3–10. doi: 10.1634/theoncologist.5-suppl_1-3. [DOI] [PubMed] [Google Scholar]
- 5.Ellis LM, Takahashi Y, Liu W, Shaheen RM. Vascular endothelial growth factor in human colon cancer: biology and therapeutic implications. The oncologist. 2000;5 Suppl 1:11–15. doi: 10.1634/theoncologist.5-suppl_1-11. [DOI] [PubMed] [Google Scholar]
- 6.Linderholm B, Tavelin B, Grankvist K, Henriksson R. Vascular endothelial growth factor is of high prognostic value in node-negative breast carcinoma. J Clin Oncol. 1998;16:3121–3128. doi: 10.1200/JCO.1998.16.9.3121. [DOI] [PubMed] [Google Scholar]
- 7.Linderholm B, Grankvist K, Wilking N, Johansson M, Tavelin B, Henriksson R. Correlation of vascular endothelial growth factor content with recurrences, survival, and first relapse site in primary node-positive breast carcinoma after adjuvant treatment. J Clin Oncol. 2000;18:1423–1431. doi: 10.1200/JCO.2000.18.7.1423. [DOI] [PubMed] [Google Scholar]
- 8.Lee JC, Chow NH, Wang ST, Huang SM. Prognostic value of vascular endothelial growth factor expression in colorectal cancer patients. Eur J Cancer. 2000;36:748–753. doi: 10.1016/s0959-8049(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 9.Herbst RS, Onn A, Sandler A. Angiogenesis and lung cancer: prognostic and therapeutic implications. J Clin Oncol. 2005;23:3243–3256. doi: 10.1200/JCO.2005.18.853. [DOI] [PubMed] [Google Scholar]
- 10.O'Byrne KJ, Goddard J, Giatromanolaki A, Koukourakis MI. Vascular endothelial growth factor expression in non-small cell lung cancer. Methods Mol Med. 2003;74:357–373. doi: 10.1385/1-59259-323-2:357. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Wang L, Zhang W, et al. Correlation of serum VEGF levels with clinical stage, therapy efficacy, tumor metastasis and patient survival in ovarian cancer. Anticancer Res. 2004;24:1973–1979. [PubMed] [Google Scholar]
- 12.Marion-Audibert AM, Barel C, Gouysse G, et al. Low microvessel density is an unfavorable histoprognostic factor in pancreatic endocrine tumors. Gastroenterology. 2003;125:1094–1104. doi: 10.1016/s0016-5085(03)01198-3. [DOI] [PubMed] [Google Scholar]
- 13.Ugurel S, Rappl G, Tilgen W, Reinhold U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol. 2001;19:577–583. doi: 10.1200/JCO.2001.19.2.577. [DOI] [PubMed] [Google Scholar]
- 14.Schoell WM, Pieber D, Reich O, et al. Tumor angiogenesis as a prognostic factor in ovarian carcinoma: quantification of endothelial immuno-reactivity by image analysis. Cancer. 1997;80:2257–2262. [PubMed] [Google Scholar]
- 15.Yuan A, Yu CJ, Kuo SH, et al. Vascular endothelial growth factor 189 mRNA isoform expression specifically correlates with tumor angiogenesis, patient survival, and postoperative relapse in non-small-cell lung cancer. J Clin Oncol. 2001;19:432–441. doi: 10.1200/JCO.2001.19.2.432. [DOI] [PubMed] [Google Scholar]
- 16.Galizia G, Lieto E, Ferraraccio F, et al. Determination of molecular marker expression can predict clinical outcome in colon carcinomas. Clin Cancer Res. 2004;10:3490–3499. doi: 10.1158/1078-0432.CCR-0960-03. [DOI] [PubMed] [Google Scholar]
- 17.Galizia G, Lieto E, Ferraraccio F, et al. Prognostic significance of epidermal growth factor receptor expression in colon cancer patients under-going curative surgery. Ann Surg Oncol. 2006;13:823–835. doi: 10.1245/ASO.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 18.De Vita F, Orditura M, Lieto E, et al. Elevated perioperative serum vascular endothelial growth factor levels in patients with colon carcinoma. Cancer. 2004;100:270–278. doi: 10.1002/cncr.11911. [DOI] [PubMed] [Google Scholar]
- 19.Lurje G, Zhang W, Schultheis AM, et al. Polymorphisms in VEGF and IL-8 predict tumor recurrence in stage III colon cancer. Ann Oncol. 2008;19:1734–1741. doi: 10.1093/annonc/mdn368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 21.Heymach JV, Johnson BE, Prager D, et al. Randomized, placebo-controlled phase II study of vandetanib plus docetaxel in previously treated non small-cell lung cancer. J Clin Oncol. 2007;25:4270–4277. doi: 10.1200/JCO.2006.10.5122. [DOI] [PubMed] [Google Scholar]
- 22.Wedge SR, Ogilvie DJ, Dukes M, et al. ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res. 2002;62:4645–4655. [PubMed] [Google Scholar]
- 23.Troiani T, Lockerbie O, Morrow M, Ciardiello F, Eckhardt SG. Sequence-dependent inhibition of human colon cancer cell growth and of prosurvival pathways by oxaliplatin in combination with ZD6474 (Zactima), an inhibitor of VEGFR and EGFR tyrosine kinases. Mol Cancer Ther. 2006;5:1883–1894. doi: 10.1158/1535-7163.MCT-06-0055. [DOI] [PubMed] [Google Scholar]
- 24.Troiani T, Serkova NJ, Gustafson DL, et al. Investigation of two dosing schedules of vandetanib (ZD6474), an inhibitor of vascular endothelial growth factor receptor and epidermal growth factor receptor signaling, in combination with irinotecan in a human colon cancer xenograft model. Clin Cancer Res. 2007;13:6450–6458. doi: 10.1158/1078-0432.CCR-07-1094. [DOI] [PubMed] [Google Scholar]
- 25.Tuccillo C, Romano M, Troiani T, et al. Antitumor activity of ZD6474, a vascular endothelial growth factor-2 and epidermal growth factor receptor small molecule tyrosine kinase inhibitor, in combination with SC-236, a cyclooxygenase-2 inhibitor. Clin Cancer Res. 2005;11:1268–1276. [PubMed] [Google Scholar]
- 26.Miller KD, Trigo JM, Wheeler C, et al. A multicenter phase II trial of ZD6474, a vascular endothelial growth factor receptor-2 and epidermal growth factor receptor tyrosine kinase inhibitor, in patients with previously treated metastatic breast cancer. Clin Cancer Res. 2005;11:3369–3376. doi: 10.1158/1078-0432.CCR-04-1923. [DOI] [PubMed] [Google Scholar]
- 27.Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–4300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 28.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 29.Gridelli C, Maione P, Del Gaizo F, et al. Sorafenib and sunitinib in the treatment of advanced non-small cell lung cancer. Oncologist. 2007;12:191–200. doi: 10.1634/theoncologist.12-2-191. [DOI] [PubMed] [Google Scholar]
- 30.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 31.Wedge SR, Kendrew J, Hennequin LF, et al. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65:4389–4400. doi: 10.1158/0008-5472.CAN-04-4409. [DOI] [PubMed] [Google Scholar]
- 32.Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007;25:884–896. doi: 10.1200/JCO.2006.06.3602. [DOI] [PubMed] [Google Scholar]
- 33.Saltz LB, Rosen LS, Marshall JL, et al. Phase II trial of sunitinib in patients with metastatic colorectal cancer after failure of standard therapy. J Clin Oncol. 2007;25:4793–4799. doi: 10.1200/JCO.2007.12.8637. [DOI] [PubMed] [Google Scholar]
- 34.Socinski MA, Novello S, Brahmer JR, et al. Multicenter, phase II trial of sunitinib in previously treated, advanced non-small-cell lung cancer. J Clin Oncol. 2008;26:650–656. doi: 10.1200/JCO.2007.13.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan F, Wey JS, McCarty MF, et al. Expression and function of vascular endothelial growth factor receptor-1 on human colorectal cancer cells. Oncogene. 2005;24:2647–53. doi: 10.1038/sj.onc.1208246. [DOI] [PubMed] [Google Scholar]
- 36.Lesslie DP, Summy JM, Parikh NU, et al. Vascular endothelial growth factor receptor-1 mediates migration of human colorectal carcinoma cells by activation of Src family kinases. Br J Cancer. 2006;94:1710–1717. doi: 10.1038/sj.bjc.6603143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Longatto Filho A, Martins A, Costa SM, Schmitt FC. VEGFR-3 expression in breast cancer tissue is not restricted to lymphatic vessels. Pathol Res Practice. 2005;201:93–99. doi: 10.1016/j.prp.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Su JL, Yang PC, Shih JY, et al. The VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells. Cancer Cell. 2006;9:209–223. doi: 10.1016/j.ccr.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 39.Papazisis KT, Geromichalos GD, Dimitriadis KA, Kortsaris AH. Optimization of the sulforhodamine B colorimetric assay. J Immunol Methods. 1997;208:151–158. doi: 10.1016/s0022-1759(97)00137-3. [DOI] [PubMed] [Google Scholar]
- 40.Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001;114:853–865. doi: 10.1242/jcs.114.5.853. [DOI] [PubMed] [Google Scholar]
- 41.Arinaga M, Noguchi T, Takeno S, Chujo M, Miura T, Uchida Y. Clinical significance of vascular endothelial growth factor C and vascular endothelial growth factor receptor 3 in patients with nonsmall cell lung carcinoma. Cancer. 2003;97:457–464. doi: 10.1002/cncr.11073. [DOI] [PubMed] [Google Scholar]
- 42.Bianco R, Rosa R, Damiano V, et al. Vascular endothelial growth factor receptor-1 contributes to resistance to anti-epidermal growth factor receptor drugs in human cancer cells. Clin Cancer Res. 2008;14:5069–5080. doi: 10.1158/1078-0432.CCR-07-4905. [DOI] [PubMed] [Google Scholar]
- 43.Bando H, Weich HA, Horiguchi S, Funata N, Ogawa T, Toi M. The association between vascular endothelial growth factor-C, its corresponding receptor, VEGFR-3, and prognosis in primary breast cancer: a study with 193 cases. Oncol Rep. 2006;15:653–659. [PubMed] [Google Scholar]
- 44.Bando H, Brokelmann M, Toi M, et al. Immunodetection and quantification of vascular endothelial growth factor receptor-3 in human malignant tumor tissues. Int J Cancer. 2004;111:184–191. doi: 10.1002/ijc.20211. [DOI] [PubMed] [Google Scholar]
- 45.Lee TH, Seng S, Sekine M, et al. Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through internally expressed VEGFR1/FLT1. PLoS Med. 2007;4:e186. doi: 10.1371/journal.pmed.0040186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu Y, Zhong Z, Huber J, et al. Anti-vascular endothelial growth factor receptor-1 antagonist antibody as a therapeutic agent for cancer. Clin Cancer Res. 2006;12:6573–6584. doi: 10.1158/1078-0432.CCR-06-0831. [DOI] [PubMed] [Google Scholar]
- 47.Garces CA, Kurenova EV, Golubovskaya VM, Cance WG. Vascular endothelial growth factor receptor-3 and focal adhesion kinase bind and suppress apoptosis in breast cancer cells. Cancer Res. 2006;66:1446–1454. doi: 10.1158/0008-5472.CAN-05-1661. [DOI] [PubMed] [Google Scholar]
- 48.Liu XE, Sun XD, Wu JM. Expression and significance of VEGF-C and FLT-4 in gastric cancer. World J Gastroenterol. 2004;10:352–355. doi: 10.3748/wjg.v10.i3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gray MJ, Van Buren G, Dallas NA, et al. Therapeutic targeting of neuropilin-2 on colorectal carcinoma cells implanted in the murine liver. J Natl Cancer Inst. 2008;100:109–120. doi: 10.1093/jnci/djm279. [DOI] [PubMed] [Google Scholar]
- 50.Juttner S, Wissmann C, Jons T, et al. Vascular endothelial growth factor-D and its receptor VEGFR-3: two novel independent prognostic markers in gastric adenocarcinoma. J Clin Oncol. 2006;24:228–240. doi: 10.1200/JCO.2004.00.3467. [DOI] [PubMed] [Google Scholar]
- 51.Mimori K, Fukagawa T, Kosaka Y, et al. Hematogenous metastasis in gastric cancer requires isolated tumor cells and expression of vascular endothelial growth factor receptor-1. Clin Cancer Res. 2008;14:2609–2616. doi: 10.1158/1078-0432.CCR-07-4354. [DOI] [PubMed] [Google Scholar]
- 52.Gockel I, Moehler M, Frerichs K, et al. Co-expression of receptor tyrosine kinases in esophageal adenocarcinoma and squamous cell cancer. Oncol Rep. 2008;20:845–850. [PubMed] [Google Scholar]
- 53.Moehler M, Frings C, Mueller A, et al. VEGF-D expression correlates with colorectal cancer aggressiveness and is downregulated by cetuximab. World J Gastroenterol. 2008;14:4156–4167. doi: 10.3748/wjg.14.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kodama M, Kitadai Y, Tanaka M, et al. Vascular endothelial growth factor c stimulates progression of human gastric cancer via both autocrine and paracrine mechanisms. Clin Cancer Res. 2008;14:7205–7214. doi: 10.1158/1078-0432.CCR-08-0818. [DOI] [PubMed] [Google Scholar]
- 55.Laakkonen P, Waltari M, Holopainen T, et al. Vascular endothelial growth factor receptor 3 is involved in tumor angiogenesis and growth. Cancer Res. 2007;67:593–599. doi: 10.1158/0008-5472.CAN-06-3567. [DOI] [PubMed] [Google Scholar]
- 56.Padera TP, Kuo AH, Hoshida T, et al. Differential response of primary tumor versus lymphatic metastasis to VEGFR-2 and VEGFR-3 kinase inhibitors cediranib and vandetanib. Mol Cancer Ther. 2008;7:2272–2279. doi: 10.1158/1535-7163.MCT-08-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petrova TV, Bono P, Holnthoner W, et al. VEGFR-3 expression is restricted to blood and lymphatic vessels in solid tumors. Cancer Cell. 2008;13:554–556. doi: 10.1016/j.ccr.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 58.Heckman CA, Holopainen T, Wirzenius M, et al. The tyrosine kinase inhibitor cediranib blocks ligand-induced vascular endothelial growth factor receptor-3 activity and lymphangiogenesis. Cancer Res. 2008;68:4754–4762. doi: 10.1158/0008-5472.CAN-07-5809. [DOI] [PubMed] [Google Scholar]
- 59.Smith NR, James NH, Oakley I, et al. Acute pharmacodynamic and antivascular effects of the vascular endothelial growth factor signaling inhibitor AZD2171 in Calu-6 human lung tumor xenografts. Mol Cancer Ther. 2007;6:2198–2208. doi: 10.1158/1535-7163.MCT-07-0142. [DOI] [PubMed] [Google Scholar]
- 60.Yang AD, Camp ER, Fan F, et al. Vascular endothelial growth factor receptor-1 activation mediates epithelial to mesenchymal transition in human pancreatic carcinoma cells. Cancer Res. 2006;66:46–51. doi: 10.1158/0008-5472.CAN-05-3086. [DOI] [PubMed] [Google Scholar]
- 61.Li J, Hong M, Pan T. Clinical significance of VEGF-C and VEGFR-3 expression in non-small cell lung cancer. Journal of Huazhong University of Science and Technology Medical sciences = Hua zhong ke ji da xue xue bao Yi xue Ying De wen ban = Huazhong keji daxue xuebao. 2006;26:587–590. doi: 10.1007/s11596-006-0528-1. [DOI] [PubMed] [Google Scholar]
- 62.Takizawa H, Kondo K, Fujino H, et al. The balance of VEGF-C and VEGFR-3 mRNA is a predictor of lymph node metastasis in non-small cell lung cancer. Br J Cancer. 2006;95:75–79. doi: 10.1038/sj.bjc.6603209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1:219–227. doi: 10.1016/s1535-6108(02)00051-x. [DOI] [PubMed] [Google Scholar]
- 64.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocrine Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 65.Yu L, Wu X, Cheng Z, et al. Interaction between bevacizumab and murine VEGF-A: a reassessment. Invest Ophthalmol Visual Sci. 2008;49:522–527. doi: 10.1167/iovs.07-1175. [DOI] [PubMed] [Google Scholar]
- 66.Wey JS, Fan F, Gray MJ, et al. Vascular endothelial growth factor receptor-1 promotes migration and invasion in pancreatic carcinoma cell lines. Cancer. 2005;104:427–438. doi: 10.1002/cncr.21145. [DOI] [PubMed] [Google Scholar]
- 67.Abdelrahim M, Baker CH, Abbruzzese JL, et al. Regulation of vascular endothelial growth factor receptor-1 expression by specificity proteins 1, 3, and 4 in pancreatic cancer cells. Cancer Res. 2007;67:3286–3294. doi: 10.1158/0008-5472.CAN-06-3831. [DOI] [PubMed] [Google Scholar]
- 68.Chen CN, Chang CC, Su TE, et al. Identification of calreticulin as a prognosis marker and angiogenic regulator in human gastric cancer. Anna Surg Oncol. 2008;16:524–533. doi: 10.1245/s10434-008-0243-1. [DOI] [PubMed] [Google Scholar]
- 69.Han H, Silverman JF, Santucci TS, et al. Vascular endothelial growth factor expression in stage I non-small cell lung cancer correlates with neoangiogenesis and a poor prognosis. Ann Surg Oncol. 2001;8:72–79. doi: 10.1007/s10434-001-0072-y. [DOI] [PubMed] [Google Scholar]
- 70.Liao X, Siu MK, Au CW, et al. Aberrant activation of hedgehog signaling pathway in ovarian cancers: effect on prognosis, cell invasion and differentiation. Carcinogenesis. 2008;30:131–140. doi: 10.1093/carcin/bgn230. [DOI] [PMC free article] [PubMed] [Google Scholar]