SUMMARY
Broad-band resonance excitation via a tailored waveform in a high pressure collision cell (Q2) on a hybrid quadrupole/time-of-flight (QqTOF) tandem mass spectrometer has been implemented for cation transmission mode electron transfer ion/ion reactions of tryptic polypeptides. The frequency components in the broadband waveform were defined to excite the first generation intact electron transfer products for relatively large tryptic peptides. The optimum amplitude of the arbitrary waveform applied has been determined empirically to be 3.0 Vp-p, which is effective for relatively high mass-to-charge (m/z) ratio precursor ions with little elimination of sequence information for low m/z ions. The application of broadband activation during the transmission mode ion/ion reaction obviates frequency and amplitude tuning normally associated with ion trap collision induced dissociation (CID). This approach has been demonstrated with triply and doubly charged tryptic peptides with and without post-translational modifications. Enhanced structural information was achieved by production of a larger number of informative c- and z-type fragments using the tailored waveform on unmodified and modified (phosphorylated and glycosylated) peptides when the first generation intact electron transfer products fell into the defined frequency range. This approach can be applied to a wide range of tryptic peptide ions, making it attractive as a rapid and general approach for ETD LC-MS/MS of tryptic peptides in a QqTOF instrument.
Keywords: ETD, intact ET product, broadband excitation, QqTOF
INTRODUCTION
Dissociation chemistry of gaseous protein/peptide ions in mass spectrometry (MS) plays an important role for the characterization and identification of proteins and peptides. One of the commonly used strategies to characterize proteins is referred to as “bottom-up” proteomics.1,2 Tandem mass spectrometry of enzymatically produced polypeptide ions is a widespread bottom-up approach that relies on obtaining peptide sequence information, which is heavily dependent on the dissociation method applied. The most widely used dissociation method is collision induced dissociation (CID),3 which involves energetic collisions of ions with a gas, generating b- and y-type fragment ions from cleavages of amide bonds along the peptide backbone. Generally, CID is very useful in deriving primary structure information of peptide/protein ions. However, there is no single dissociation method that has proven to be capable of providing all of the desired structural information for the ions of interest. For this reason, dissociation methods that are complementary to CID are of interest.
Electron capture dissociation (ECD),4, 5 an ion-electron reaction that is most generally implemented in Fourier-transform ion cyclotron resonance (FT-ICR) mass spectrometers, is complementary to CID. Compared to CID, ECD is less sequence dependent and has been shown to preserve post-translational modifications (PTMs), enabling the localization of modification sites.6–8 An analogue of ECD, electron transfer dissociation (ETD),9, 10 which has been effected in electrodynamic ion traps, is the dissociation induced by electron transfer (ET) between the ions of opposite polarity. ETD fragmentation behavior has been noted to be similar to that observed in ECD, including preservation of PTMs.11, 12 In both ECD and ETD processes, the cleavage of the N-Cα bond along the peptide/protein backbone is the major fragmentation channel, giving rise to sequence informative c- and z-type fragment ions.
In the most commonly executed bottom-up tandem MS approach, the protein is initially subjected to enzymatic digestion using trypsin, which specifically cleaves proteins at the C-terminal sides of lysine and arginine residues. The resulting tryptic peptides, which are mostly doubly charged upon electrospray ionization (ESI), are subjected to dissociation. It has been noted that the structural information derived from ETD of doubly protonated polypeptide ions13, 14 or ions of relatively high mass-to-charge (m/z) ratio with charge state ≥ 3 (above m/z 850)15 is often limited. In addition to the electron transfer induced c- and z-type fragment ions, proton transfer and intact electron transfer products are also observed. Minimizing the proton transfer channel and converting the intact electron transfer products to ETD fragment ions are two different ways to enhance the ETD performance of doubly charged peptide ions. For a given peptide ion, the extent of proton transfer is determined by the identity of the electron transfer reagent anion, none of which has thus far been shown to react exclusively via electron transfer.16 Converting the intact electron transfer products, sometimes abbreviated as ET, no D products, into sequence informative c- and z-type ions is another way to improve the ETD performance of relatively low charge state polypeptide ions. Elevated bath gas temperature was demonstrated to provide more sequence coverage, but an increase in ETD efficiency is not consistently observed compared to room temperature experiments.13 By applying the post ion/ion beam-type collisional activation method implemented on a hybrid triple quadrupole/linear ion trap instrument (all the ions were accelerated axially from the linear ion trap (LIT), where the ion/ion reaction was effected, to an adjacent LIT for mass analysis), both ETD yield and sequence coverage were improved significantly by choosing an optimized acceleration potential.17 Ion trap CID of intact electron transfer products on 3D18 and 2D14, 19 ion trap instruments has been proven to be useful in improving both the ETD yields and the structural information derived from ETD with minimal contribution from fragment ions from dissociation of proton transfer products. Electron transfer ion/ion reactions in all the methods mentioned above are performed in a mutual trapping mode, in which the positive and negative ions are stored simultaneously for a period of time. Recently, ion trap CID of intact electron transfer product ions was effected during the cation transmission mode electron transfer ion/ion reaction (passing the cations through while the anions are stored) on a quadrupole/time-of flight (QqTOF) instrument, which enhanced utility of electron transfer ion/ion reactions for relatively low charge state peptide and protein ions.20 The transmission mode approach has yielded comparable performance to the mutual storage mode for a number of ion/ion reaction scenarios without the need for a discrete mutual ion storage step or the need to apply radio-frequency voltages to the trapping plates of the LIT.21, 22 However, frequency and amplitude tuning is still required for conventional single-frequency ion trap collisional activation of ET, no D products.
In this study, activation of intact electron transfer products using a broadband waveform with an optimized amplitude applied to one pair of quadrupole rods was effected in the high pressure collision cell (Q2) of a QqTOF tandem mass spectrometer during the cation transmission/ETD reagent anion storage mode ion/ion reaction. The approach is demonstrated by activating the intact electron transfer products formed in cation transmission mode ion/ion reactions of a series of tryptic peptide cations with and without PTMs (phosphorylation and glycosylation) in reaction with azobenzene radical anions in the Q2 LIT. This method obviates a discrete mutual storage reaction step and does not require frequency and amplitude tuning for ion trap CID. Therefore, this activation approach is expected to be particularly suitable for tryptic peptide analyses via ETD MS/MS coupled with liquid chromatography (LC).
RESULTS AND DISCUSSION
Optimization of the Tailored Broadband Waveform
The frequency bands of the tailored broadband waveform were defined as follows: (1) the mass range excited with relatively high amplitude was set to m/z 950–1900 in order to activate the first generation intact ET products within this particular mass-to-charge range because the majority of tryptic peptide ions contains 9–17 amino acid residues (approximate average residue mass = 112 Da) with a charge state of +2 being dominant upon ESI. Additionally, when one or two trypsin missed cleavages are taken into account, the sizes of the resulting peptides increase considerably, usually giving rise to dominant high charge states of +3 upon ESI, with their first generation intact ET products (doubly charged) also falling into this mass range; and (2) the mass range activated with relatively lower amplitude was set to m/z 475–950 in order to enhance ETD performance of the precursor ions (the minority of tryptic peptides containing 5–8 amino acid residues) with their first generation intact ET products falling into this region. Lower amplitude is applied to minimize the ejection of directly produced ETD fragments (see below), which are usually formed with precursor ions of low mass-to-charge ratio.13, 17 This particular broadband waveform, detailed in Table 1, is specifically designed to enhance ETD performance of relatively high m/z ions for the majority of tryptic peptides, which is demonstrated in this study to be suitable for activating the first generation ET, no D products efficiently without losing the structural information derived from normal ETD. The first frequency band, 40–80 kHz, corresponds to m/z 950–1900 when the low-mass-cut-off (LMCO) of Q2 LIT in the reaction/activation step is set to m/z 170 and the frequency spacing of this band is set to 0.5 kHz. The spacing of the second frequency band, 80.5–120.5 kHz, corresponding to the m/z range 475–950 with the same LMCO, is set to 1 kHz. Collisional activation performance during the ion/ion reaction was found to be insensitive to frequency spacing when it was 1 kHz or less (data not shown).
Table 1.
Defined frequency bands for a tailored broadband waveform.
| Band | Freq first (kHz) | Freq last (kHz) | Freq inc (kHz) | Phase | Amplitude |
|---|---|---|---|---|---|
| 1 | 40 | 80 | 0.5 | quadratic | 1.0 |
| 2 | 80.5 | 120.5 | 1.0 | quadratic | 0.3 |
In addition to examining the effect of frequency spacing, different amplitude ratios of the two frequency bands (1/0, 1/0.2, 1/0.3, 1/0.4, 1/0.5 and 1/1) in this particular broadband waveform were investigated. When the relative amplitude for the second frequency band is low (e.g., ratios of 1/0 and 1/0.2), no enhancement for the ETD performance of +2 peptide ions falling within the second category was observed with the output of 3.0 Vp-p or 3.5 Vp-p. With increased amplitude for the second frequency band (i.e., ratios 1/0.3, 1/0.4, 1/0.5 and 1/1) under 3.0 Vp-p or 3.5 Vp-p output, improvements in the extent of sequence information from activation of ET, no D +2 peptide ions falling into the second category was found to be the same. However, because the precursor ions for the majority of the trypsin digested peptides fell into the m/z 475–950 range, higher amplitudes applied to this region significantly decreased the ion/ion reaction rate via a phenomenon referred to as ion parking,23, 24 which reduced the reaction efficiency. Increased amplitude for the low mass region also produced more CID fragments from the precursor ion. Therefore, the relative amplitude ratio of the two bands, 1/0.3 (shown in Table 1), and a waveform generator output of 3.0 Vp-p (see below) was determined in this study to be the most effective ratio for the activation of ET, no D products, resulting in informative c-/z-type species, with minimal CID fragment ions from the precursor ion or proton transfer products.
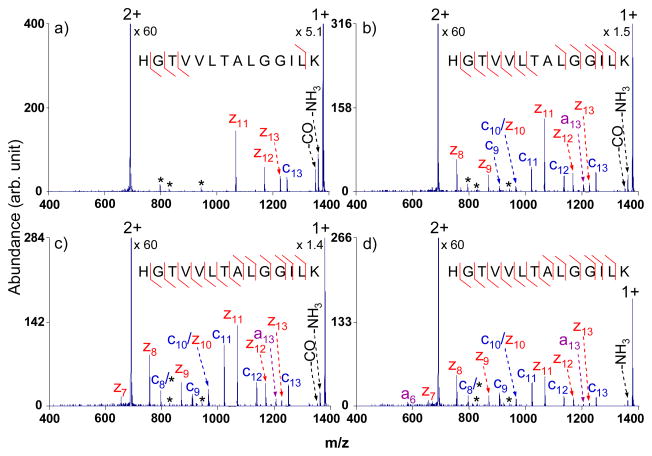
For the majority of the tryptic peptides, with their first generation intact electron transfer product ions falling into the m/z 950–1900 range, the optimal amplitude was found to be 3.0 Vp-p, as illustrated in Figure 1. The sequence coverage for doubly protonated HGTVVLTALGGILK, defined as the percentage of possible N-Cα cleavages, was as follows: 31% with no activation (Figure 1a), 85% with an output of 2.8 Vp-p (Figure 1b), 100% with an output of 3.0 Vp-p (Figure 1c), and 100% with an output of 3.2 Vp-p (Figure 1d). Even though full sequence coverage was obtained with both 3.0 Vp-p and 3.2 Vp-p outputs, the abundances of the fragments was lower with the higher amplitude (Figure 1d), presumably due to ion ejection by the waveform and/or CID of the first generation products. Similar experiments performed on other tryptic peptides with and without PTMs (phosphorylation and glycosylation) also showed that the 3.0 Vp-p amplitude is suitable for activation during the transmission mode ETD process using the waveform defined in Table 1. This output corresponds to 213 mVp-p per frequency component over the 40–80 kHz range and 64 mVp-p per frequency component over the 80.5–120.5 kHz range (see Equation (1)).
Figure 1.
Electron transfer ion/ion reactions of doubly charged tryptic peptide HGTVVLTALGGILK from myoglobin with azobenzene radical anions (a) with no activation; (b) with multi-frequency activation, an amplitude of 2.8 Vp-p; (c) with multi-frequency activation, an amplitude of 3.0 Vp-p; (d) with multi-frequency activation, an amplitude of 3.2 Vp-p. (The peaks labeled with asterisks are present during the isolation.)
Transmission Mode Electron Transfer with Broad-band Activation Applied to Unmodified Tryptic Peptides
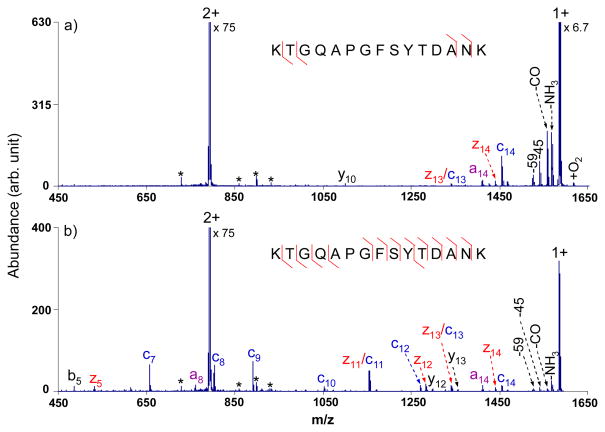
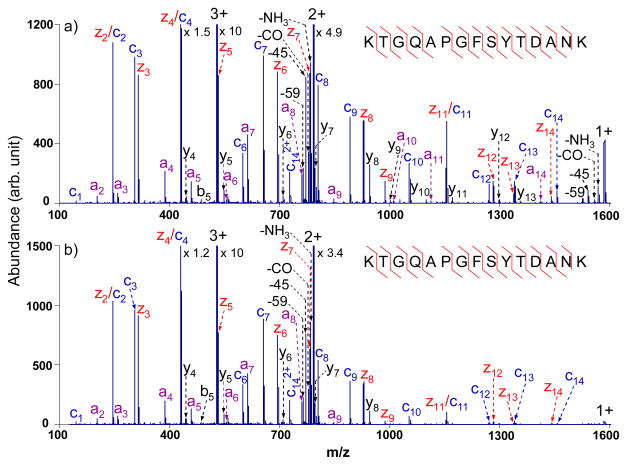
Since most of the tryptic peptides are doubly charged upon ESI, with their singly charged intact ET products falling within the low frequency band, the results shown in Figure 1 are relevant to the most common scenario. Figure 2, which shows results for doubly protonated KTGQAPGFSYTDANK, provides another example of the use of the tailored multi-frequency waveform with an output of 3.0 Vp-p. Broadband activation provides 86% of the sequence coverage (Figure 2b) compared to only 29% derived from normal ETD (Figure 2a), with few fragments formed from CID of the proton transfer product. Comparing Figures 2a and 2b, it is noted that the abundances of the peaks observed in the normal ETD spectrum of m/z 950 and greater dropped a minor amount upon activation with the 3.0 Vp-p waveform but are still sufficiently abundant to be used for sequencing. The product ion a8 is a radical species, which is believed to be generated from a minor cleavage channel in ETD. Furthermore, even-electron fragment ions y12 and y13 observed in Figure 2b, could arise either from CID of the proton transfer product or from a minor cleavage channel in ETD.
Figure 2.
ETD MS/MS spectra of doubly charged tryptic peptide KTGQAPGFSYTDANK from cytochrome c with azobenzene radical anions (a) without activation; (b) with activation via the optimized broadband waveform with an output of 3.0 Vp-p. (The peaks labeled with asterisks are present after isolation in the absence of ion/ion reaction and application of the waveform.)
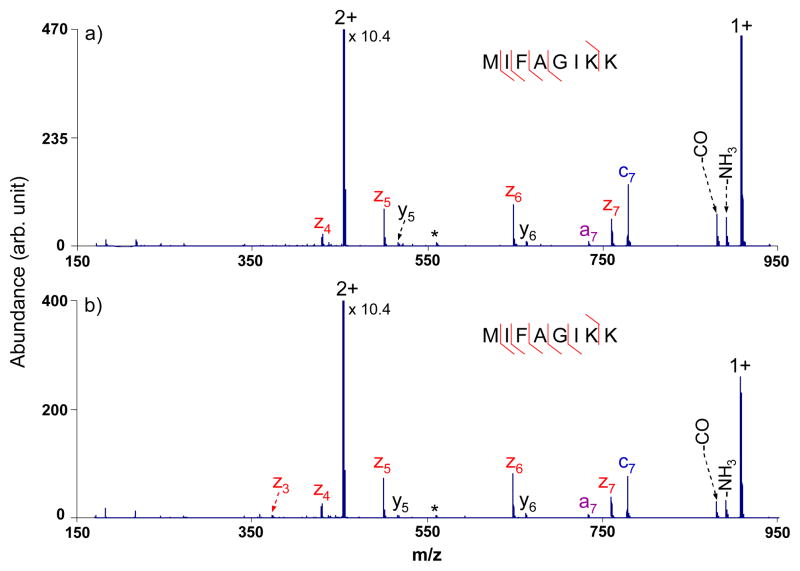
Figure 3 shows an example of data typical for the minority of doubly protonated tryptic peptides that generate first generation intact electron transfer product ions that fall into the higher frequency band of the waveform (m/z 475–950). Eighty-six percent sequence coverage was obtained from ETD of the doubly charged peptide MIFAGIKK under broad-band activation with a 3.0 Vp-p output in Figure 3b, compared to 71% coverage in the absence of supplementary activation (Figure 3a). Sequence coverage tends to be high for peptides of this type without supplementary activation. Improvements from use of the waveform, therefore, can only be limited. Nevertheless, improved performance is generally observed in cases in which sequence coverage is not already 100%, provided the amplitude of the waveform is sufficiently low to avoid ejecting the product ions formed directly from ETD.
Figure 3.
ETD MS/MS spectra of doubly charged tryptic peptide MIFAGIKK from cytochrome c with azobenzene radical anions (a) without activation; (b) with activation via the optimized broadband waveform with an output of 3.0 Vp-p. (The peaks labeled with asterisks are present during the isolation.)
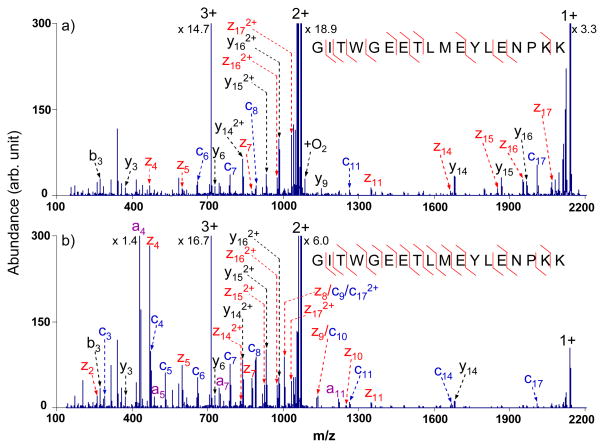
Though most of the tryptic peptides are doubly charged upon ESI, a significant fraction of tryptic peptides are of higher charge.25 For triply charged tryptic peptides, one of two scenarios typically prevails: (1) the first generation intact electron transfer product, which is doubly charged, falls within the m/z 950–1900 range; and (2) the doubly charged intact electron transfer product falls within m/z 475–950 while its singly charged intact electron transfer product, formed via sequential electron transfer, falls within m/z 950–1900. An example that applies to the first scenario is shown in Figure 4. The sequence coverage for the peptide GITWGEETLMEYLENPKK from bovine cytochrome c increased from 65% (13 out of 34 possible c- and z-type fragments) to 88% (23 out of 34 possible c- and z-type fragments) upon multi-frequency collisional activation during the ion/ion reaction. Compared to the ETD spectrum obtained without the waveform in Figure 4a, a few radical a-type species, a4, a5, a7 and a11, were observed in the activated ETD spectrum, as shown in Figure 4b, which arose from minor ETD cleavage channels. The a4 product ion, due to the Cα-CO cleavage between tryptophan and glycine residues, was observed to be the most abundant fragment generated upon activation. Upon activation, it was also noted that the abundances and the number of fragments in the low m/z range increased significantly but the abundances and the number of the peaks in the high m/z range decreased because of the simultaneous activation of the fragments generated from the normal ETD process. The information gained, however, more than compensated for that lost, which is consistent with the results from other triply charged tryptic peptides for which the first scenario applies (data not shown). The abundance of +1 species decreased roughly six-fold, which can result from dissociation of the +2 ET, no D product as well as reduction the ion/ion reaction rate of the +2 species due to the ion acceleration. Therefore, by applying broadband activation to precursor ions with their first generation ET products falling into the defined m/z range, more sequence information can be produced and sequential charge reduction reactions can be limited to some extent.
Figure 4.
ETD MS/MS spectra of triply charged tryptic peptide GITWGEETLMEYLENPKK from bovine cytochrome c with azobenzene radical anions with (a) no activation; (b) activation using the optimized broadband waveform with an amplitude of 3.0 Vp-p.
For the second scenario mentioned above, high (frequently full) sequence coverage is usually obtained. In this scenario, there is no need for a supplementary waveform. However, an approach that seeks to minimize tuning from one analyte ion to the next would apply the waveform to all precursor ions. In this case, it is important that the supplementary waveform avoid significantly compromising the information that can be derived directly via ETD. Figure 5 shows ETD results for the triply charged peptide KTGQAPGFSYTDANK from bovine cytochrome c without (Figure 5a) and with (Figure 5b) multi-frequency activation. The same sequence coverage was derived from both spectra, which indicates no loss of the sequence information under activation using an output of 3.0 Vp-p. Other peptides studied in the second category gave similar results (data not shown), showing that almost identical sequence information was obtained without or with activation during electron transfer ion/ion reactions for peptide ions that fall into this category.
Figure 5.
ETD MS/MS spectra of triply charged tryptic peptide KTGQAPGFSYTDANK from bovine cytochrome c with azobenzene radical anions (a) without activation; (b) with activation using the optimized broadband waveform with an amplitude of 3.0 Vp-p.
Transmission Mode Electron Transfer with Broad-band Activation Applied to Modified Tryptic Peptides
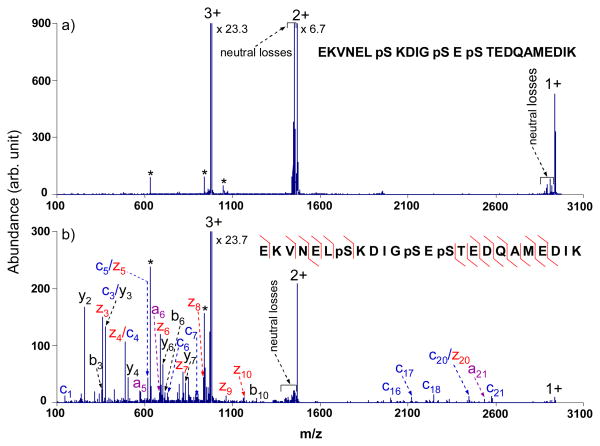
Collisional activation using the broadband waveform with a 3.0 Vp-p output was performed to examine the effect on tryptic peptides with phosphorylation. ETD and ECD are particularly useful for determining the sites of labile post-translational modifications because, unlike CID, cleavage of backbone bonds is favored over cleavage of the modification. The use of a supplementary collisional activation technique, however, could compromise the use of ETD for modified peptides. We therefore applied the waveform described above for several modified peptide ions. Figure 6 shows transmission mode ETD spectra of triply protonated EKVNELpSKDIGpSEpSTEDQAMEDIK from α-casein with no activation (Figure 6a) and with activation (Figure 6b) using the waveform. For this particular peptide, no sequence information is obtained without multi-frequency activation. However, under activation conditions, 61% of the sequence is reflected in the c- and z-type ions, with one phosphorylation site identified to be the seventh residue from the N-terminus from the mass difference between the c6 and c7 species. Despite significantly improved sequence coverage upon radial activation, the other two phosphorylations sites cannot be localized to specific residues. Besides the c- and z-type ions produced, some a-, b- and y-type species were also formed, which were either from the minor ETD channels or from CID of even-electron species. The loss of phosphoricacid (H3PO4), commonly seen in CID spectra of phosphopeptides, however, is not observed in the activated ETD spectrum shown in Figure 6b. The 3.0 Vp-p output on the waveform generator is high enough to dissociate the electron transfer survivor ions, but not high enough to cleave the covalent bonds of the even-electron species to a significant extent. Similar results were obtained for another triply charged singly phosphorylated tryptic peptide (YKVPQLEIVPNpSAEER), for which 87% peptide sequence coverage upon broadband activation of 3.0 Vp-p was noted compared to 73% without activation, with no indication of H3PO4 loss from the precursor or product ions.
Figure 6.
ETD tandem mass spectra of triply charged tryptic phosphopeptide EKVNELpSKDIGpSEpSTEDQAMEDIK from α-casein with azobenzene radical anions (a) without activation; (b) with activation using the optimized broadband waveform with an amplitude of 3.0 Vp-p. (The peaks labeled with asterisks are present during the isolation.)
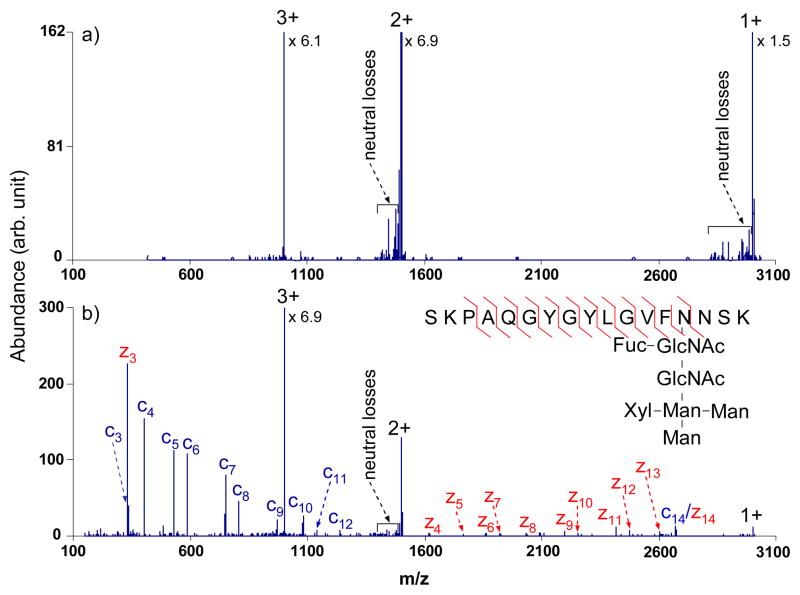
A tryptic peptide with one N-linked glycosylation site (see inset of Figure 7b) was interrogated by broadband activation using the tailored waveform with the output of 3.0 Vp-p. This N-linked glycopeptide, SKPAQGYGYLGVFgNNSK from Erythrina cristagalli lectin, has been extensively studied before by ECD and infrared multiphoton dissociation (IRMPD) in an FT-ICR instrument and ETD and CID in a linear ion trap, with ECD and ETD providing peptide sequence and the IRMPD and CID providing information about the glycan structure.11, 12, 26 Although no sequence information was generated from electron transfer to the triply charged glycopeptide (Figure 7a), the glycosylation site was localized to be the fourth amino acid from the C-terminus via the m/z difference between the fragments z3 and z4 upon activation. The use of the broad-band waveform in conjunction with the transmission mode ion/ion reaction resulted in the generation of fragments from 75% of the possible N-Cα bond cleavages (Figure 7b). Furthermore, with the broadband activation on, essentially no evidence for cleavages of the glycosidic bonds was observed, indicating minimal contribution from CID at this waveform amplitude. However, some relatively abundant peaks due to cleavages of glycosidic bonds was noted when the amplitude increased to 3.1 Vp-p (data not shown), which further demonstrated that the optimum amplitude is 3.0 Vp-p for the phosphorylated and glycosylated peptides examined here.
Figure 7.
ETD tandem mass spectra of triply charged tryptic glycopeptide SKPAQGYGYLGVFgNNSK from Erythrina cristagalli lectin with azobenzene radical anions (a) without activation; (b) with activation using the optimized broadband waveform with an amplitude of 3.0 Vp-p.
EXPERIMENTAL SECTION
Materials
Horse skeletal muscle myoglobin, bovine cytochrome c, bovine α-casein, lectin from Erythrina cristagalli (Cockspur Coral Tree), TPCK-treated trypsin, ammonium bicarbonate and azobenzene were purchased from Sigma-Aldrich (St. Louis, MO). Methanol, acetonitrile and glacial acetic acid were obtained from Mallinckrodt (Phillipsburg, NJ). Trifluoroacetic acid (TFA) was purchased from Pierce (Rockford, IL). All materials were used without further purification.
Trypsin Digestion
Myoglobin and cytochrome c (~1 mg) were dissolved in 0.5 mL of aqueous 200 mM ammonium bicarbonate, respectively. TPCK-treated trypsin (20 μL of a 1 mg/mL aqueous solution) was added to both of the protein solutions to effect the digestion. Each protein solution was incubated at 38°C in a water bath for ~12 h followed by reverse-phase HPLC separation (Agilent 1100, Palo Alto, CA) using an Aquapore RP-300 (7 μm particle size, 100 × 4.6 mm i.d.) column (Perkin-Elmer, Wellesley, MA) operated at a flow rate of 1 mL/min. A linear 60 min. gradient from 0 to 100% buffer B was used, where buffer A was an aqueous solution with 0.1% (v) TFA, and buffer B solution contained 59.5/39.6/0.09 (v/v/v) acetonitrile/water/TFA. The fractions were dried in vacuum and then dissolved in 200 μL of 49.5/49.5/1 (v/v/v) methanol/water/acetic acid for positive electrospray. Erythrina cristagalli lectin and α-casein were also trypsin digested, and then separated by reverse-phase HPLC using a similar procedure as outlined above for myoglobin and cytochrome c. The only difference was the digestion time, ~24 h for alpha-casein and ~4 h for Erythrina cristagalli lectin.
Broadband Waveform
The tailored broadband waveforms were built by defining frequency bands (starting frequency; ending frequency; frequency spacing; phase and relative amplitude) using the SxWave program, provided by MDS Sciex. One example is shown in Table 1. The Louris and Taylor scheme was used for quadratic phasing in the SxWave program.27 After optimization and normalization, the waveforms were then downloaded from the computer to an Agilent 33220A waveform generator (Agilent, Santa Clara, CA) via a 2.0 USB cable. The home built arbitrary waveform was applied to one pair of the Q2 quadrupole rods through the waveform generator to effect excitation of ions in the radial direction in Q2 LIT.
The relationship between the amplitude for a specific frequency component (Vc) and the output of the waveform generator (V) is shown in the following equation:
| (1) |
where Ac is the zero-to-peak amplitude of the component of interest, which can be acquired from the SxWave software after optimization and normalization of the arbitrary waveform, and Ais the dynamic range of the composite waveform, which can also be obtained from the software, and was 0.9999988 for this particular waveform. For this example waveform, a value of Ac of 0.07101 was applied to each component in the 40–80 kHz range and 0.0213 was applied for each component in the 80.5–120.5 kHz range.
Mass Spectrometry
All experiments were performed on a QqTOF tandem mass spectrometer (QSTAR XL, Applied Biosystems/MDS Sciex, Concord, ON, Canada), which was modified for ion/ion reactions.28, 29 A home-built pulsed dual nano-ESI/atmosphere pressure chemical ionization (APCI) source,30 was coupled directly to the interface of the QSTAR mass spectrometer to generate multiply charged peptide cations and azobenzene radical anions, respectively. Cation transmission mode electron transfer ion/ion reactions (passing the analyte cations while the reagent radical anions are trapped) was performed in the Q2 LIT with a LINAC function,31, 32 an axial electric field used to transmit ions efficiently through the Q2 cell. All the parameters, including lens voltages, quadrupole rod offsets, and amplitude and direction of the LINAC, were adjusted to achieve optimal transmission mode ETD performance. The experimental sequence was controlled by a research version of software provided by MDS Sciex, Daetalyst 3.14. The pressure in the Q2 LIT was optimized at ~5 mTorr with nitrogen as the bath gas.
A typical procedure for broadband excitation during the cation transmission mode electron transfer ion/ion reaction consists of the following steps: (1) pulsing the high voltage (−2 kV) applied to the APCI needle and injecting the azobenzene radical anions, selected by Q1 in the mass-resolving mode, into the Q2 LIT where ions were trapped by DC (200 ms); (2) switching off the high voltage on the APCI needle while the anions were cooled in Q2 for 30 ms; (3) switching on the high voltage (+1.0–1.5 kV) applied to the nano-ESI emitter, and passing the Q1 isolated analyte ions through the Q2 LIT to effect electron transfer ion/ion reaction and acquisition of product ions by reflectron TOF mass analysis (150 ms); (4) dumping any remaining ions that might be present in Q0-Q2 (50 ms). The injection q value (Mathieu dimensionless parameter) for the azobenzene reagent anions into Q2 was ~0.39 and the DC trapping voltages applied to IQ2 and IQ3, the containment lenses of Q2, were 1 V repulsive relative to the Q2 DC offset. The broadband excitation was triggered on in step (3), the time period for the transmission mode electron transfer ion/ion reaction. The TOF mass spectra shown herein were typically the averages of 50–200 individual scans. The spectral averaging is the same for both normal and activated ETD spectra shown.
CONCLUSIONS
Radial broadband excitation via a waveform built specifically for tryptic peptides has been implemented during the cation transmission mode ETD process in the high pressure Q2 LIT of a QqTOF instrument. For the particular waveform employed in this study, the optimum output of the waveform generator was determined to be 3.0 Vp-p. This voltage is high enough to fragment ions that survive a single electron transfer to produce more c- and z-type sequencing fragments and low enough to avoid significantly affecting the normal ETD performance. Not surprisingly, little additional information is provided via use of the waveform when extensive direct ETD of the precursor ion takes place. However, higher sequence coverage is obtained for unmodified peptides when their first generation intact ET products are activated. For phosphorylated and glycosylated peptides, the 3.0 Vp-p amplitude is effective in generating c/z-type fragments while retaining the labile modifications. An attractive feature of the use of the broadband approach in a transmission mode experiment on the QqTOF platform is that electron transfer, collisional activation of the ET survivor ions, and mass analysis take place more or less in parallel. Furthermore, a single waveform is effective for a wide variety of tryptic peptide ions, which obviates frequency and amplitude tuning steps that would otherwise be required with a single frequency waveform approach. This simple excitation approach via a tailored waveform in conjunction with transmission mode electron transfer is expected to be particularly attractive for tryptic peptide analysis via on-line LC-MS/MS using ETD.
Acknowledgments
This research was sponsored by the National Institute of General Medical Sciences under Grant GM 45372 and MDS Sciex, an Industrial Associate of the Department of Chemistry.
References
- 1.Hunt DF, Yates JR, Shabanowitz J, Winston S, Hauer CR. Proc Natl Acad Sci USA. 1986;83:6233–6237. doi: 10.1073/pnas.83.17.6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reid GE, McLuckey SA. J Mass Spectrom. 2002;37:663–675. doi: 10.1002/jms.346. [DOI] [PubMed] [Google Scholar]
- 3.Wells JM, McLuckey SA. Method Enzymol. 2005;402:148–185. doi: 10.1016/S0076-6879(05)02005-7. [DOI] [PubMed] [Google Scholar]
- 4.Zubarev RA, Kelleher NL, McLafferty FW. J Am Chem Soc. 1998;120:3265–3266. [Google Scholar]
- 5.Zubarev RA, Horn DM, Fridriksson EK, Kelleher NL, Kruger NA, Lewis MA, Carpenter BK, McLafferty FW. Anal Chem. 2000;72:563–573. doi: 10.1021/ac990811p. [DOI] [PubMed] [Google Scholar]
- 6.Mirgorodskaya E, Roepstorff P, Zubarev RA. Anal Chem. 1999;71:4431–4436. doi: 10.1021/ac990578v. [DOI] [PubMed] [Google Scholar]
- 7.Hakansson K, Cooper HJ, Emmett MR, Costello CE, Marshall AG, Nilsson CL. Anal Chem. 2001;73:4530–4536. doi: 10.1021/ac0103470. [DOI] [PubMed] [Google Scholar]
- 8.Shi SD, Hemling ME, Carr SA, Horn DM, Lindh I, McLafferty FW. Anal Chem. 2001;73:19–22. doi: 10.1021/ac000703z. [DOI] [PubMed] [Google Scholar]
- 9.Syka JEP, Coon JJ, Schroeder MJ, Shabanowitz J, Hunt DF. Proc Nat Acad Sci USA. 2004;101:9528–9533. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coon JJ, Syka JEP, Shabanowitz J, Hunt DF. Int J Mass Spectrom. 2004;236:33–42. [Google Scholar]
- 11.Hogan JM, Pitteri SJ, Chrisman PA, McLuckey SA. J Proteome Res. 2005;4:628–632. doi: 10.1021/pr049770q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han H, Xia Y, Yang M, McLuckey SA. Anal Chem. 2008;80:3492–3497. doi: 10.1021/ac7022734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pitteri SJ, Chrisman PA, McLuckey SA. Anal Chem. 2005;77:5662–5669. doi: 10.1021/ac050666h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swaney DL, McAlister GC, Wirtala M, Schwartz JC, Syka JEP, Coon JJ. Anal Chem. 2007;79:477–485. doi: 10.1021/ac061457f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Good DM, Wirtala M, McAlister GC, Coon JJ. Mol Cell Proteomics. 2007;6:1942–1951. doi: 10.1074/mcp.M700073-MCP200. [DOI] [PubMed] [Google Scholar]
- 16.Gunawardena HP, He M, Chrisman PA, Pitteri SJ, Hogan JM, Hodges BDM, McLuckey SA. J Am Chem Soc. 2005;127:12627–12639. doi: 10.1021/ja0526057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han H, Xia Y, McLuckey SA. Rapid Commun Mass Spectrom. 2007;21:1567–1573. doi: 10.1002/rcm.2994. [DOI] [PubMed] [Google Scholar]
- 18.Pitteri SJ, Chrisman PA, Hogan JM, McLuckey SA. Anal Chem. 2005;77:1831–1839. doi: 10.1021/ac0483872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu SL, Huehmer AFR, Hao Z, Karger BL. J Proteome Res. 2007;6:4230–4244. doi: 10.1021/pr070313u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia Y, Han H, McLuckey SA. Anal Chem. 2008;80:1111–1117. doi: 10.1021/ac702188q. [DOI] [PubMed] [Google Scholar]
- 21.Liang X, McLuckey SA. J Am Soc Mass Spectrom. 2007;18:882–890. doi: 10.1016/j.jasms.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang X, Hager JW, McLuckey SA. Anal Chem. 2007;79:3363–3370. doi: 10.1021/ac062295q. [DOI] [PubMed] [Google Scholar]
- 23.McLuckey SA, Reid GE, Wells JM. Anal Chem. 2002;74:336–346. doi: 10.1021/ac0109671. [DOI] [PubMed] [Google Scholar]
- 24.Chrisman PA, Pitteri SJ, McLuckey SA. Anal Chem. 2005;77:3411–3414. doi: 10.1021/ac0503613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang YY, Triscari JM, Tseng GC, Pasa-Tolic L, Lipton MS, Smith RD, Wysocki VH. Anal Chem. 2005;77:5800–5813. doi: 10.1021/ac0480949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hakansson K, Cooper HJ, Hudgins RR, Nilsson CL. Curr Org Chem. 2003;7:1503–1525. [Google Scholar]
- 27.Louris JN, Taylor DM. 5,324,939. U.S. Patent. 1994 June 28;
- 28.Shevchenko A, Chernushevich I, Ens W, Standing KG, Thomson B, Wilm M, Mann M. Rapid Commun Mass Spectrom. 1997;11:1015–1024. doi: 10.1002/(SICI)1097-0231(19970615)11:9<1015::AID-RCM958>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 29.Xia Y, Chrisman PA, Erickson DE, Liu J, Liang X, Londry FA, Yang MJ, McLuckey SA. Anal Chem. 2006;78:4146–4154. doi: 10.1021/ac0606296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang X, Xia Y, McLuckey SA. Anal Chem. 2006;78:3208–3212. doi: 10.1021/ac052288m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loboda A, Krutchinsky A, Loboda O, McNabb J, Spicer V, Ens W, Standing K. Eur J Mass Spectrom. 2000;6:531–536. [Google Scholar]
- 32.Thomson BA, Jolliffe CL. 5,847,386. U. S. Patent. 1998 Dec 8;