Summary
Allergic rhinitis (AR) is the fifth most common chronic disease, and the association between allergic disorders and anxiety is well-documented. To investigate how anxiety and stressors modulate skin prick test (SPT) responses and associated inflammatory responses, 28 men and women with AR were selected by clinical history and skin test responses. The participants were admitted twice to a hospital research unit for 4 hours in a crossover trial. Changes in SPT wheals were assessed before and after a standardized laboratory speech stressor, as well as again the following morning; skin responses assessed twice during a lab session without a stressor and again the following morning served as the contrast condition. Anxiety heightened the magnitude of allergen-induced wheals following the stressor. As anxiety increased, SPT wheal diameters increased after the stressor, compared to a slight decrease following the control task. Anxiety also substantially enhanced the effects of stress on late phase responses: even skin tests performed the day after the stressor reflected the continuing impact of the speech stressor among the more anxious participants. Greater anxiety was associated with more IL-6 production by Con A-stimulated leukocytes following the stressor compared to the control visit. The data suggest that stress and anxiety can enhance and prolong AR symptoms.
Keywords: Allergies, psychoneuroimmunology, stress, anxiety, allergic rhinitis, skin tests
Stressful events that range from commonplace daily hassles to chronic calamities can produce immune alterations that are consequential for health. Psychological stress can slow wound healing, diminish the strength of immune responses to vaccines, enhance susceptibility to infectious agents, and reactivate latent viruses (Glaser and Kiecolt-Glaser, 2005). Moreover, stressful events and the distress that they evoke can also substantially augment the production of proinflammatory cytokines that are associated with a spectrum of age-related diseases (Kiecolt-Glaser et al., 2003). In this study we examined how stress and anxiety influenced skin prick testing, a major diagnostic procedure and a clinically relevant surrogate for allergic symptomatology in allergic rhinitis (AR) (Bernstein and Storms, 1995; Brown et al., 1979).
AR is a heterogeneous disorder in which nasal inflammation is induced by IgE-mediated mast cell responses to specific allergens (Skoner, 2001). Stress and anxiety can augment humoral immunity at the expense of cell-mediated immunity (Marshall, 2004b), providing a mechanism through which stress can favor IgE production (Wu et al., 1991). Immunological changes associated with stress, particularly the shift from T helper 1 (TH1) to T helper 2 (TH2) can promote allergic responses in susceptible individuals; both cortisol and norepinephrine can promote the TH1 to TH2 shift (Marshall, 2004b).
These stress-related endocrine and immune alterations may have clinical consequences for a broad spectrum of allergic disorders including AR, the most common allergic disease, as well as allergic asthma, atopic dermatitis (AD), contact dermatitis, allergic urticaria, and food allergies. For example, both emotional distress and stressful events can magnify AD symptoms (Buske-Kirschbaum et al., 2001); in one naturalistic study, AD patients living in earthquake-damaged areas reported more AD symptoms than those who lived in nearby undamaged areas (Kodama et al., 1999). The stress of school examinations augmented allergic responses to inhaled allergens in asthmatic college students (Liu et al., 2002). In a prospective study, negative life events increased children's risk of an asthma attack, and chronic stressors further heightened that risk (Sandberg et al., 2000).
In accord with these data, Chen and Miller have argued that stress amplifies inflammatory responses to environmental triggers to exacerbate asthma (Chen and Miller, 2007); their model suggests that stressors appraised as threatening and unmanageable provoke negative emotional responses which in turn sensitize the TH2 pathway and augment responses to environmental triggers. Indeed, there is evidence that negative moods predicted poorer pulmonary function in asthmatics (Ritz and Steptoe, 2000). Furthermore, among patients with asthma, the presence of an anxiety disorder is a strong predictor of the number and frequency of respiratory symptoms, which in turn have been robustly associated with emergency room visits and hospitalizations (Richardson et al., 2006).
The fact that negative emotions appear to promote allergic inflammatory responses is particularly important because of the well-documented association between allergic disorders and anxiety and depression (Cuffel et al., 1999; Goodwin, 2002; Hart et al., 1995; Katon et al., 2004). For example, in one study the odds of an anxiety disorder diagnosis were 1.41 times higher among AR patients than those without AR (Cuffel et al., 1999); a number of researchers have also reported appreciably higher levels of anxiety symptoms in allergic subjects than nonallergic controls (Katon et al., 2004; Stauder and Kovacs, 2003). Worry and rumination are key anxiety symptoms, and these kinds of perseverative cognitive patterns can promote and prolong stress-related emotional and physiological activation, both before and after stressors (Brosschot et al., 2006); moreover, anxious individuals evaluate stressors as more threatening and uncontrollable than less anxious individuals (Brosschot et al., 2006). Thus, both stress and anxiety could fuel allergic responses in AR.
Acute or early phase allergic responses typically occur within minutes of exposure and may include sneezing and itching. However, the early phase response often progresses to a late phase response 4-24 hours after initial exposure that can include nasal congestion, rhinorrhea/postnasal drainage, fatigue, irritability, depressive symptoms, and declines in cognitive functioning. These symptoms may persist for hours or days without the need for additional allergen exposure (Marshall and Colon, 1993; Skoner, 2001). More persistent allergen reactivity is clearly problematic both for patient symptoms as well as increasing risk for comorbidities such as acute sinusitis and asthma exacerbation. Accordingly, psychosocial influences on the late phase SPT were of particular interest in the present study.
To investigate how stressors and anxiety may modulate allergic symptoms, we utilized a clinically relevant surrogate (Bernstein and Storms, 1995; Brown et al., 1979) by examining changes in SPT responses before and after a lab stressor, as well as again the following morning. Skin responses assessed twice during a lab session without a stressor and again the following morning served as a contrast condition. By assessing both early and late phase responses, we were able to evaluate both the immediate and extended impact of the brief stressor on SPTs. Detailed endocrine and immune studies from both visits provided mechanistic information. We hypothesized that anxiety would interact with the stressor to enhance both early and late phase responses in more anxious subjects compared to those who were less anxious, as well as amplifying various markers of inflammation.
Method
Participants
Participants responded to ads seeking healthy individuals ages 18-40 who had a history of seasonal nasal allergies (hay fever). Exclusion criteria included active allergy immunotherapy, psychotropic medications, cardiovascular medications (statins, beta blockers, etc.), astemizole, smoking, asthma, excessive alcohol use, illnesses with immunological or endocrinological components other than allergy, or medications with obvious consequences for these systems or for allergies. We restricted recent use of a number of allergy medications in accord with recommendations designed to optimize skin testing results (Bernstein and Storms, 1995; Niemeijer et al., 1993). Subjects were asked to refrain from use of vitamin C supplements for at least 24 hours prior to all study sessions.
The screening visit included allergen skin tests and questionnaires. Subjects were selected for participation in the study based on both clinical history and skin test responses.
The average age of the sample of 10 men and 18 women was 24.73 (SD=4.35, range=18-33); 22 were white, 4 were African American, and 2 were Asian. Twenty-six had at least some college education. The Ohio State Biomedical Research Review Committee approved the project; all subjects gave written informed consent prior to participation.
Two General Clinical Research Center (GCRC) Visits
The two GCRC laboratory sessions were scheduled at least 2 weeks apart (mean=38.72 days, SD=45.74). Both visits followed the same timeline, differing only in the stressor/control condition randomization for that visit; participants returned for a 1.5-hour follow-up GCRC visit on the subsequent morning, and a 30 minute follow-up at 4:00 PM on both afternoons for assessment of late phase responses from the morning skin tests.
On arrival a heparin well was placed in one arm for subsequent serial blood draws. Participants then ate a standardized breakfast (after fasting since midnight) and completed questionnaires. Next they were told that the goal of the 30-min relaxation period was to become as relaxed as possible; they could choose to either listen to soothing classical music, or sit quietly in silence. After the relaxation period, participants provided blood and saliva samples, and a nurse conducted the first of the two skin test batteries. The baseline skin test was followed by the Trier Social Stress Test or a control task, a post-task skin test and blood and saliva sampling.
Each of these two 4-hour laboratory appointments thus included two skin tests using alternate arms, as well as a third skin test the following morning. We provided a boxed lunch for participants after their 4-hour and 1½ -hour sessions.
The Trier Social Stress Test (TSST) and the Control Task
The TSST, a well-validated laboratory stressor, provokes reliable changes in cardiovascular function, cortisol, catecholamines, and self-reported stress (Kirschbaum et al., 1993). Subjects make a speech and perform mental arithmetic in front of an “audience” panel of 2-3 individuals. For the speech, the participants were told to imagine that they had applied for a position and had been invited to an interview by the selection committee; they had 10 minutes to prepare a speech about why they would be best for the job, and 5 minutes to deliver the speech, followed by a 5 minute serial subtraction task.
After providing a saliva sample, the participant was escorted to another experimental room where they saw a microphone stand and video camera, and the audience panel. They were told that at least one member of the panel was trained in behavioral observation, and would rate their speech's content and style. Participants were also told that they would watch the videotape of their speech, without anyone else present, to review the performance upon which the committee would base their evaluations (Heffner et al., 2002).
The participant was taken back to the study room and responded to two stress appraisal questions (Heffner et al., 2002; Tomaka et al., 1993), described below. The participant then had 10 minutes to prepare for the speech; they could make written notes during this phase, but notes could not be used for the speech.
Viewing of the videotape occurred immediately after the serial subtraction and was considered part of the stress period. This self-observation procedure provided a way to further enhance the threatening aspects of the stressor by augmenting evaluation apprehension (Heffner et al., 2002), and also extended the stressor period by 10 minutes. After participants had watched their speech and math videotape, they provided another saliva sample, and a nurse performed a skin test. Ten minutes later, the participant provided another saliva sample.
Participants were debriefed at the end of the next day's follow-up appointment. They were told that the committee was composed of project research assistants who were not actually evaluating them, and no one had expertise in behavioral observation.
The control task
The control task served as the contrast condition for the TSST, with the order of each counterbalanced across subjects. Participants silently read a magazine section for 10 minutes before reading the same material out loud while being audiotaped. Afterwards they listened to an audiotape, without anyone else present, of someone else reading the material. They were told that they were being asked to read aloud and listen to the audiotape to control for the effects of speaking and listening related to other experimental tasks; we emphasized that their performance was not being evaluated.
Psychological Data
State-Trait Anxiety Scale
The Spielberger State-Trait Anxiety Scale (STAI) is widely used, has excellent norms, and is strong in terms of both reliability and validity (Spielberger et al., 1970). The 20-item state anxiety measure, asks about how one feels at the moment, with adjectives such as calm tense, at ease, and upset rated on a scale from 1-4. It was administered at the beginning of each 4-hour session, before the interaction tasks were introduced; the correlation between the two was r=.85, p<.0001, and thus we used the mean of the two administrations as the summary anxiety measure for each subject.
Stress appraisals
Prior to the preparation phase, the participant responded on 7-point Likert-type scales to two stress appraisal questions (Heffner et al., 2002; Tomaka et al., 1993): “How threatened are you by the upcoming task?” and “How able are you to cope with the task?” Stress appraisals were operationalized as the ratio of perceived threat (primary appraisal) to coping (secondary appraisal) following the convention used in other studies (Heffner et al., 2002; Tomaka et al., 1993).
Helplessness/control
Following the TSST or control task, subjects rated their feelings of control and helplessness during these periods on a scale from 1-7 (Breier et al., 1987b): “How much control did you feel you had during the tasks?” and “To what degree did you experience feelings of helplessness during the tasks?” These ratings were combined to provide a summary measure, with higher scores reflecting less control and greater helplessness.
Impact of Events Scale (IES)
The IES (Horowitz et al., 1979) assesses experiences of avoidance and intrusion following a stressful experience. The avoidance items reflect attempts to suppress thoughts about the experience, and the intrusion items address unintended thoughts. Participants completed the IES when they returned the morning after each 4-hour visit, with instructions to rate their thoughts about their recent visit, providing an assessment of perseverative worry associated with a stressor.
Positive and Negative Affect Schedule (PANAS)
The PANAS includes two 10-item mood scales (Watson et al., 1988). The positive and negative scales are largely uncorrelated, and show good convergent and discriminant validity when related to state mood scales and other variables (Watson et al., 1988). We used the PANAS to assess changes in affective responses to the visits over time; in each session the first PANAS was administered after the subject had eaten breakfast and had the heparin well inserted, the second followed the stressor, the third was near the end of the session, after the final cortisol sample, and the fourth occurred on the following morning, when the participant returned to the GCRC for their third skin prick test.
Pittsburgh Sleep Quality Index (PSQI)
The PSQI, a self-rated questionnaire, assesses sleep quality and disturbances over a one-month interval (Buysse et al., 1989). It has good diagnostic sensitivity and specificity in distinguishing good and poor sleepers.
Assessments of Allergic Status and Responsiveness: Skin Tests
Skin prick tests (SPT) are widely used clinically (Bernstein and Storms, 1995; Brown et al., 1979). We selected individuals who met SPT criteria for sensitivity to house dust mite, Dermatophagoides pteronyssinus (Der P 1), because we wanted one common allergen across subjects and we had Der P 1-specific assays. We also assessed allergic status to other common allergens: ragweed, North American dust mite (Dermatophagoides farinae), mold mix, weed mix, tree mix, grass mix, cat dander, and sagebrush.
The battery was performed on the volar aspect of both forearms (alternating between tests) using the DermaPIK (Corder and Wilson, 1995). Histamine served as the positive control to verify the skin's ability to respond to inflammatory mediators (Bernstein and Storms, 1995), and glycerinated saline was the negative control (Turkeltaub, 2000). The prick tests were applied using a DermaPIK and read 20 minutes later by measuring the largest diameter of the wheal (in mm). A wheal ≥ 3 mm than the concurrent saline control provided evidence of an allergen-specific IgE response (Bernstein and Storms, 1995); all SPT data are expressed as the difference between the allergen response and the concurrent saline control. The nurses who performed the tests and read results 20 minutes after application were blind to the subject's assigned condition for the day.
Late phase reactions were assessed at 4:00 PM when subjects returned to the GCRC, as well as when participants returned the next morning for follow-up (Bernstein and Storms, 1995). Discrete measurement was not possible for many late phase reactions, because the subcutaneous swelling from one or more allergens blended with others, creating a large swollen area across multiple allergen sites; accordingly, we assessed the presence or absence of any late phase responses.
Endocrine Data
Both cortisol and norepinephrine can promote the TH1 to TH2 shift that promotes allergic responses in susceptible individuals (Marshall and Roy, 2007; Straub et al., 2001), and thus were assessed in this study. Saliva was collected for cortisol assay using a salivette (Sarstedt, Newton, North Carolina), an untreated sterile cotton roll placed in the subject's mouth for ∼2 minutes to ensure saturation, at 8 time-points during the protocol: following the relaxation period, after the initial skin test assessment, at the end of the TSST or control task preparation period, at the end of the TSST or control task, at 10- and 20-minutes post-task, after the post-task skin test assessment (approximately 30-minutes post-task), and prior to departure (approximately 1 hour post-task). , Each subject's saliva samples were frozen after collection and analyzed within the same assay using the Cortisol Coat-A-Count RIA (Siemens Medical Solutions Diagnostics, Los Angeles); the intra-assay coefficient of variation is 4.3%, the inter-assay coefficient of variation is 5.2%, and sensitivity is .025 ug/dl.
Plasma catecholamines were sampled 6 times during the protocol: following the relaxation period, immediately after the task instructions, at the end of the TSST or control task preparation period, at the end of the TSST or control task, 20-minutes post-task, and after the post-task skin test assessment (approximately 30-minutes post-task). Plasma samples were frozen at -70° C and assayed by HPLC with ElectroChemical Detection using Standards and Chemistry (alumina extraction) from Thermo-Alko (Beverly, MA). The intra-assay variation for norepinephrine is 3%, the inter-assay variation is 6%, and sensitivity is 15 pg/ml; the respective figures for epinephrine are 6%, 13%, and 6 pg/ml.
Immunological Assays
Changes in IL-6 production across three assays provided data on the TH1 to TH2 shift. IL-6 changes following the TSST have been documented with each of the three assays below, and thus it was our cytokine of choice for this study. Blood for IL-6 was sampled following the relaxation period, immediately following the TSST or control task, and after the post-task skin test assessment (approximately 30-minutes post-task).
Stimulated IL-6 Production
4×106 PBLs were isolated from whole blood preparations and incubated at 37°C for 72 hours in 2 mls of RPMI-1640 media (Gibco) supplemented with 10% human male serum (Sigma) and 5ug/mL of Con A (Sigma) or 5ug/mL of der P1 (Greer Laboratories, Lenoir, NC). Control cells were also prepared in the same media but without the Con A or der P1. The supernatants were harvested and frozen at -86°C until assayed for IL-6.
Dexamethasone Inhibition of LPS-stimulated IL-6 production
Ten mL heparinized whole blood was transferred to a 15mL polypropylene tube and placed on a rotator for 10 minutes. After mixing, 1.8mL of blood was transferred to each of five polypropylene tubes. Dexamethasone was added to 3 of the 5 tubes at a concentration of 0.1 uM, 0.5uM and 1uM. Calcium, magnesium free phosphate buffered saline (CMF/PBS) was added to the positive and negative control tubes. After incubation at room temperature for 15 minutes, LPS (Sigma) at a final concentration of 3ng/mL was added to each tube except the negative control tube; CMF/PBS was added to the negative control tube. Two mL blood from each tube was put into wells of 24-well plates and incubated at 37° C, 5% CO2, 92% humidity for 16-18 hours. After incubating, the plates were centrifuged for 10 minutes at 2000 rpm. The supernatants were removed and stored at -80°C until assayed for IL-6 production.
Plasma IL-6
IL-6 levels in plasma were determined using a Sector Imager (Meso Scale Discovery) by electro-chemiluminescense. Standard curves using a range of .3 pg to 2500 pg were employed to determine the level of IL-6 in each plasma sample.
Statistical Methods
Unless stated otherwise, analyses consisted of mixed effect linear models fit to each dependent variable. These models accounted for correlation in measurements from the same subject across time and visits. Independent variables assessed in each model were visit (stress versus control), mean baseline anxiety (henceforth “anxiety”), and time (where applicable), as well as their interactions; gender and the baseline (pre-task) level of the dependent variable were included as a covariates. Log-transformations were used where appropriate to better achieve normality (cortisol, epinephrine, norepinephrine). Post hoc tests were performed to investigate significant interactions and pairwise differences, using Holm's or Tukey's procedure as appropriate to adjust for multiple comparisons. A two-sided significance level of alpha = 0.05 was used. All analyses were performed in SAS® version 9.1.
Results
Self-Report Data
State anxiety
Subjects' mean score on the state anxiety scale of the STAI (Spielberger et al., 1970) was 32.50 (SD=7.81). STAI normative data for working adults (mean=35.72, SD=10.40), college students (mean=35.20, SD=10.61), and patients with an anxiety disorder (mean=54.43, SD=13.02) provide numbers for comparison purposes (Spielberger et al., 1970). In addition, data from a large study of allergic respiratory diseases in a French population (mean=39.71) (Annesi-Maesano et al., 2006), and a sample of Italian AR patients (mean=55.1) (Addolorato et al., 1999) are in accord with the broader evidence of more clinical anxiety disorders and higher levels of anxiety symptoms in allergic samples compared to nonallergic controls (Cuffel et al., 1999; Katon et al., 2004; Stauder and Kovacs, 2003).
Stress appraisals
The ratio of perceived threat (primary appraisal) to coping (secondary appraisal) (Heffner et al., 2002; Tomaka et al., 1993) was higher at the stress visit than at the non-stress visit, and higher anxiety was associated with a higher ratio at the stress visit, producing a significant anxiety by visit interaction, F(1,26)=4.66, p = .04.
Helplessness/Control
Subjects' ratings of their feelings of helplessness and lack of control (Breier et al., 1987a) after the TSST or control task also produced a significant anxiety by visit interaction, F(1,26)=5.93, p=0.02. Subjects felt more helpless at the stress visit than at the non-stress visit, and higher anxiety was associated with higher scores at the stress visit.
IES
Participants had more perseverative thoughts following the stress visit (mean=5.82, SD=6.31) compared to the control visit (mean=3.48, SD=4.47), F(1,25)=6.68, p = .02, reflected in higher IES scores. Although in the expected direction, the anxiety by visit interaction did not reach significance, F(1,25)=3.03, p = .09.
PANAS
A repeated-measures cumulative logistic regression model was used to evaluate PANAS negative affect scores, which ranged from 10 to 19 and were not normally distributed. The time by visit interaction was significant for negative affect, X2(1)=4.32, p = .04. The decrease in negative affect scores from post-task (time 2) to pre-discharge (time 3) was greater at the stress visit than at the control visit (p = .03). The changes in negative affect from baseline to post-task and from pre-discharge to day 2 did not differ significantly between visits. There was also a significant anxiety effect, X2(1)=4.98, p = .03. For each unit increase in anxiety the odds of having higher negative affect increased 12%.
Higher anxiety was marginally associated with lower positive affect, F(1,130)=3.54, p = .06. This marginal anxiety effect did not differ by visit or time.
Health behaviors
More anxious individuals reported poorer sleep on the PSQI, F(1,27)=21.36, p <.001. Anxiety was not related to frequency or duration of exercise, body mass index, or caffeine or alcohol use.
Catecholamines and Cortisol
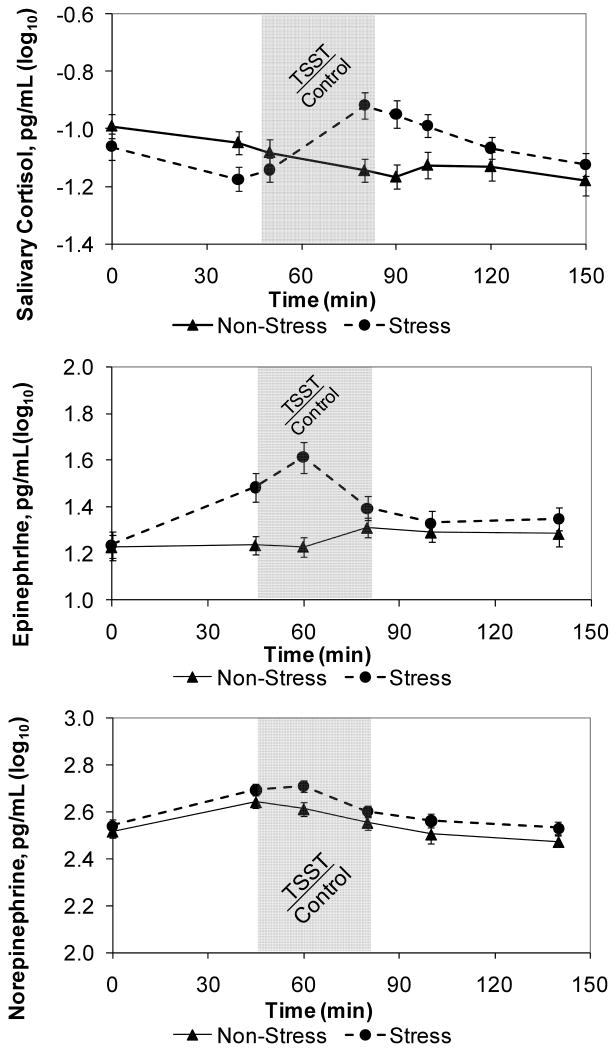
Consistent with past research, the TSST stimulated cortisol production, as seen in the visit by time interaction for log10 salivary cortisol, F(6,296)=11.29, p < .001 (Figure 1a). The increase in salivary cortisol from task preparation to post-task was greater at the stress visit than at the control visit (adj p = .01).
Figures 1a,b,c.
Unadjusted mean (± SEM) cortisol (1a), epinephrine (1b), and norepinephrine (1c) levels through the admission as a function of stressor (TSST) or control condition assignment for the day. Data are log10-transformed. The shaded portion indicates the interval for the stressor or control tasks. The TSST significantly stimulated cortisol and epinephrine production, as seen in the significant visit by time interactions for both, while the interaction was not significant for norepinephrine.
There was a significant visit by time interaction for log10 epinephrine, F(4,198)=10.15, p < .001 (Figure 1b). From after the task was explained (time 2) to just before the start of the task (time 3) there was a larger increase in epinephrine at the stress visit than at the control visit (adj p = .06). From just before the start of the task (time 3) to after the tape viewing (time 4), epinephrine decreased at the stress visit and increased at the control visit (adj p < .001). Although following much the same pattern as epinephrine, norepinephrine did not differ significantly by anxiety, visit, time, or gender (Figure 1c).
Skin Prick Tests
Our average subject had 3.75 (SD=1.85) SPTs read as positive at baseline. Each subject's positive SPT responses were averaged, providing a summary response across positive allergens at each assessment point.
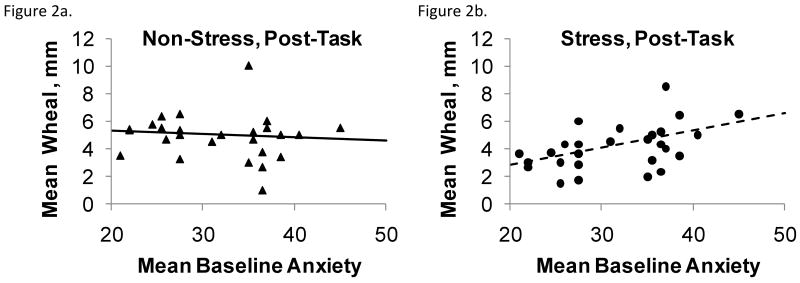
Changes in the mean saline-corrected skin test wheal produced a significant anxiety by visit interaction F(1,77)=6.74, p = .01 (Figures 2a-d). Evaluated at each measurement time point subsequent to the stressor/control task, the anxiety by visit interaction was significant at post-task (adjusted p = .02) but not at day 2 (p = .98). At the post-task measurement higher anxiety was associated with larger mean wheal size following the stressor and slightly smaller mean wheal size following the control task. At day 2, higher anxiety was associated with larger mean wheal size at both visits; this association did not differ by stress/control condition. Additionally, males had larger wheals than females F(1,77)=7.69, p = .01, consistent with epidemiological data (Turkeltaub, 2000). One subject with an outlying mean baseline anxiety of 56 was excluded from the mean wheal analysis due to highly influencing the model fit. The anxiety by visit interaction for skin prick test results did not differ based on randomized order of visit F(1,77)=0.02, p=.90.
Figures 2a-d.
Mean wheal diameter for all positive allergens as a function of anxiety, plotted separately by stress and control condition for skin tests immediately after (2a,b) and the morning following (2c,d) the GCRC session; all SPT data are expressed as the difference between the allergen response and the concurrent saline control. Plotted points are unadjusted wheal diameter observations and lines are baseline-adjusted regressions of mean wheal on anxiety. Anxiety heightened the magnitude of SPT wheals following the stressor, and higher anxiety was associated with larger wheals on the day after both the stressor and control tasks.
Late Phase Responses
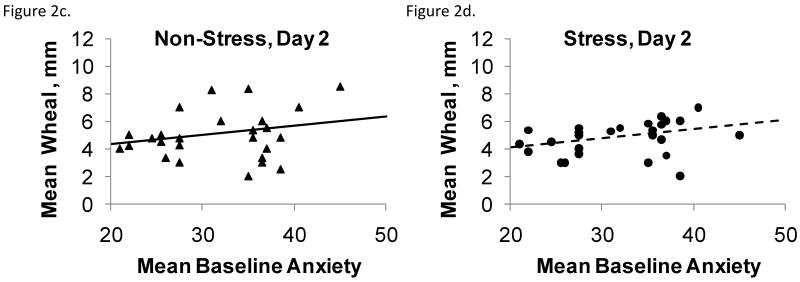
The mixed effect logistic regression model used for analysis of late phase responses demonstrated a significant interaction between anxiety and visit, F(1,127)=6.70, p = .01 (Figures 3a-c). For every 5 point increase in anxiety, the odds of a late phase response increased 1.2 times at the stress visit and decreased 2.3 times at the control visit.
Figures 3a-c.
The probability of late phase responses following allergen skin tests as a function of anxiety and stress or control condition. Skin tests were performed (3a) before the stressor or control tasks, (3b) immediately after the stressor or control tasks, and (3c) the morning following the GCRC stressor or control session as a function of stress or control condition and anxiety. These responses develop 6-8 hours following challenge, and thus skin tests performed before the stressor could still be influenced by it. Anxiety enhanced the effects of stress on late phase responses at each of the three time points.
Immunological Data
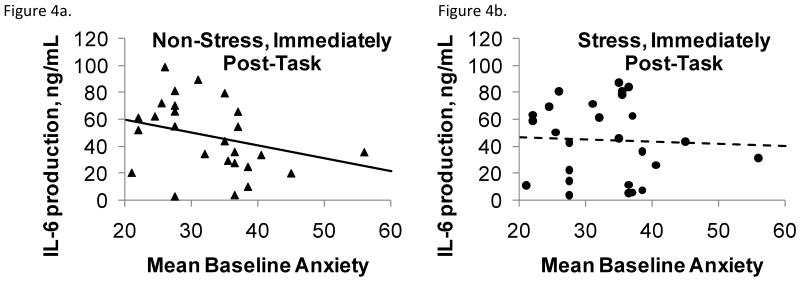
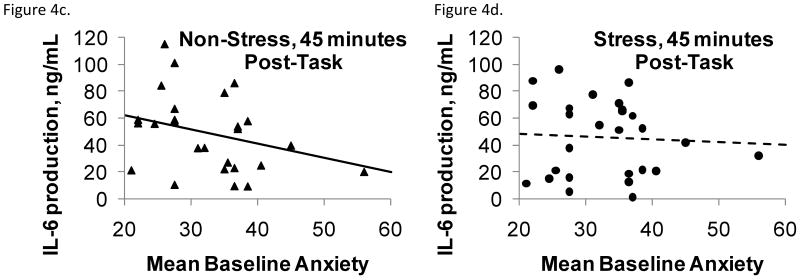
The significant anxiety by visit interaction for Con A stimulated IL-6 production by PBLs reflected the fact that higher anxiety was associated with lower IL-6 production at the control visit compared to the stress visit after controlling for baseline, F(1,72)=4.33, p = .04 (Figures 4a-d). In further analyses that used Con A stimulated IL-6 at each time point (including baseline) as the response variable and late phase response or wheal size as independent variables, having a late phase response was associated with higher IL-6 production, F(1,118)=8.55, p = .004. Also, larger wheal size was marginally associated with higher IL-6 production, F(1,121)=3.61, p = .0598, and IL-6 production at 45 min was larger than at baseline, F(2,121)=3.66, p = .03. Der P 1 stimulated IL-6 production, plasma IL-6 levels, and glucocorticoid sensitivity showed no significant effects or interactions for anxiety, visit, or time.
Figure 4a-d.
Con A stimulated IL-6 production as a function of baseline anxiety, plotted separately by stress or control condition immediately following (4a,b) and 45 minutes after (4c,d) the stressor or control task. Plotted points are unadjusted IL-6 observations and lines are baseline-adjusted regressions of IL-6 on anxiety. Higher anxiety was associated with lower IL-6 production at the control visit compared to the stress visit after controlling for baseline.
Discussion
Anxiety heightened the magnitude of SPT wheals following the stressor. As anxiety increased the SPT responses increased after the stressor, compared to a slight decrease following the control task. Anxiety also enhanced the effects of stress on late phase responses; indeed, even skin tests performed the day after the stressor reflected the continuing impact of the event among the more anxious participants. The inflammation that occurs during late phase allergic responses is thought to promote “priming” such that the dose of allergen required to elicit subsequent acute responses substantially decreases (Skoner, 2001). Moreover, priming can lead to hyperresponsiveness to other allergens as well as to nonspecific irritant triggers such as smoke, exercise, and noxious odors; these effects can be particularly troublesome, because the nonspecific triggers can then promote additional clinical symptoms even after allergen exposure has ended (Skoner, 2001). Our data suggest that both stress and anxiety function to enhance late phase SPT responses as well as some aspects of inflammation; accordingly, stress and anxiety may also promote priming and hyperresponsiveness to irritant triggers as well as to other allergens.
Late phase symptoms include postnasal drainage and nasal congestion, fatigue, drowsiness, impairments in concentration, irritability, and disrupted sleep, and these symptoms interfere with work and school performance as well as disrupting social interactions (Bender, 2005). The evidence that stress and anxiety amplify late phase SPT responses has potential clinical significance for AR patients. While the immediate phase symptoms can be readily treated or even prevented in most patients with the use of antihistamines, late phase responses are poorly responsive to antihistamine treatment (Marshall, 2004a). Our data suggest that the highly anxious patient, after exposure to an acute stressor, can have increased immediate as well as late phase responses that would be less responsive to first line therapies such as antihistamines. If similar immune changes occur in other inflammatory diseases with acute stress superimposed on chronic anxiety, this could account (at least in part) for the known association between stress and adverse clinical reactions such as asthma exacerbation, autoimmune disease exacerbation and even acute cardiovascular events.
AR symptoms can substantially disrupt sleep, enhancing AR-associated fatigue. Importantly, sleep deprivation can exacerbate allergic responses in turn; one study showed that AR patients had substantially greater wheal responses and IgE production following a night of sleep deprivation than after a rested baseline (Kimata, 2002). Furthermore, in our study more anxious participants reported poorer sleep than those who were less anxious, an expected finding because poorer sleep is a common anxiety symptom. Thus, sleep disruption provides another important avenue through which stress and anxiety can intensify AR symptoms.
Compared to those who were less anxious, more anxious participants felt more threatened by the speech stressor; afterwards they felt less in control and more helpless, and their PBLs had greater Con A-stimulated IL-6 production compared to the control condition. Moreover, having a late phase response was associated with higher IL-6 production. Relatedly, in another study asthmatic children who reported lower levels of perceived control had higher levels of asthma-relevant Th2 stimulated cytokine production, including IL-4, IL-5, and IL-13 (Griffin and Chen, 2006). Stressors that engender feelings of helplessness and lack of control augment and prolong psychological and physiological stress responses (Breier et al., 1987b; Brosschot et al., 2005); anxious individuals who worry more about negative outcomes are particularly susceptible because they have stronger anticipatory responses as well as slower recovery than those who are less anxious (Brosschot et al., 2005).
The probability of experiencing a late phase skin increased with the level of anxiety; indeed, in contrast to the immediate wheal diameter which was only different immediately post task, the late phase response was related to anxiety levels over the entire period of the experiment. This is not surprising given the increased inflammatory milieu associated with a late phase allergic response that is predominantly TH2 in nature, and the fact that TH2 predominance is enhanced by stress (Agarwal and Marshall, 1998; Marshall et al., 1998).
The fact that neither glucocorticoid resistance nor plasma IL-6 levels were related to either anxiety or stress is likely a function of the timing of the samples; the blood samples for these assays were drawn 45 minutes after the stressor. Stress- and distress-related differences in these assays are amplified 1.5-2 hours after stressors like the TSST (Pace et al., 2006; Steptoe et al., 2007).
One limitation of our study is the relatively low level of anxiety symptoms in our sample, which were well below those of clinical anxiety disorders. Indeed, in samples of AR patients seeking allergy treatment, the mean STAI anxiety scores were higher than those of our participants (Addolorato et al., 1999; Annesi-Maesano et al., 2006), consistent with the over-representation of clinical anxiety disorders in AR populations (Cuffel et al., 1999). Accordingly, our data are likely to underestimate the actual contribution of anxiety to AR symptoms, particularly in patients with clinically active disease.
In fact, even in our relatively small sample in which anxiety was in the normative range, we found that participants who were more anxious showed larger increases in negative affect than less anxious participants; this finding may be particularly relevant given the well-documented association between depression and allergic disorders (Goodwin et al., 2006; Kovacs et al., 2003; Meggs et al., 2001; Wamboldt et al., 2000). We did not conduct formal mental health assessments of subjects, and we limited exclusionary criteria in this regard to the absence of current psychotropic medications, so we do not know if any of our subjects had experienced syndromal mood disorders.
This study did not specifically assess the effects of an acute stressor on clinical symptoms. This was by design because clinical symptomatology can vary between individuals as well as within an individual from day to day based upon many factors in addition to their level of stress. By doing SPT measurements in participants who were currently asymptomatic, we avoided the complication of interpreting subjective clinical symptoms such as stuffiness or congestion in the context of anxiety and stress responses. SPTs can be a reliable surrogate; both total and specific IgE and allergic symptomatology correlate with reactivity to skin tests (Brown et al., 1979); indeed, correspondence between skin tests and inhalation challenge varies from 60-90% (Bernstein and Storms, 1995). Moreover, our data complement and extend the finding that the stress of academic examinations can augment allergic responses to inhaled allergens in asthmatics (Liu et al., 2002).
The allergic patient provides one of the most relevant and translatable models for studying the effects of various forms of stress, both acute and chronic, on complex immunoregulatory mechanisms (Marshall and Roy, 2007). The mast cell is a fundamental component of innate host defense, being able to respond to challenge in a matter of minutes. Its activity is made much more efficient by arming with allergen specific IgE via high affinity receptors (FcεR1). Both IgE and its receptor are produced under the control of TH2 cytokines such as IL-4. Stress has been associated with elevated IgE levels (Buske-Kirschbaum et al., 2004; Wright et al., 2004), and our SPT responses provided evidence of the ability of stress and anxiety to modulate allergen-specific IgE response. Of note, elevated IgE levels have been shown to be independent risk factors for allergic conditions such as asthma and atopic dermatitis (Wright, 2005), both of which are more prevalent in patients with underlying AR. However, it is the late phase response that is associated with higher morbidity in virtually all allergic syndromes including those of the nose (AR), lungs (asthma) and skin (atopic dermatitis).
AR has a substantial public health impact; it is the fifth most common chronic disease, affecting 10-30% of adults and up to 40% of children in the United States, and the prevalence of AR appears to be increasing worldwide (Skoner, 2001). Estimates of the medical costs associated with AR are $3.4 billion ($2.3 billion in medications and $1.1 billion in physician billings), not including the 3.5 million workdays and 154 million in wages lost because of seasonal nasal allergies (Storms et al., 1997).
However, the public health burden is actually much greater than suggested by these numbers, because AR is associated with a number of other allergic diseases, including asthma rhinosinusitis; survey data suggest that 38% of AR patients have coexisting asthma, and 78% of asthma patients have AR (Nathan, 2007). Improvement in AR can promote positive changes in asthma; conversely, deterioration in AR can worsen asthma (Nathan, 2007). Thus, by enhancing and prolonging allergic responses, stress and anxiety substantially impact public health.
The data also have implications for clinical practice. Indeed, these results should alert practitioners and patients alike to the adverse effects of stress and anxiety on allergic reactions in the nose, chest, skin and other organs that may seemingly resolve within a few minutes to hours after starting, but may reappear the next day when least expected. The evidence that anxiety fuels stress-related changes suggests that more systematic assessment of allergic patients for comorbid anxiety disorders might be in order. Indeed, management-based interventions (psychological, pharmacological) that target various forms of stress could be rapidly (minutes to hours) evaluated for their clinical potential by testing the impact of a given intervention on the immediate and late phase skin test responses. This would be a relevant surrogate system for testing the mitigating effects of such interventions on the clinical course of allergic disease activity. Further studies with this model in patients with other allergic diseases would allow investigations into the effects of anxiety and stress on various inflammatory cascades known to exist in the allergic response, as well as specific effects on individual cells and molecules.
References
- Addolorato G, Ancona C, Capristo E, Grazioseto R, Di Rienzo L, Maurizi M, Gasbarrini G. State and trait anxiety in women affected by allergic and vasomotor rhinitis. J Psychosom Res. 1999;46:283–289. doi: 10.1016/s0022-3999(98)00109-3. [DOI] [PubMed] [Google Scholar]
- Agarwal SK, Marshall GD. Glucocorticoid induced type-1/type-2 cytokine alterations in humans-a model for stress-related immune dysfunction. Interferon and Cytokine Research. 1998;18:1059–1068. doi: 10.1089/jir.1998.18.1059. [DOI] [PubMed] [Google Scholar]
- Annesi-Maesano I, Beyer A, Marmouz F, Mathelier-Fusade P, Vervloet D, Bauchau V. Do patients with skin allergies have higher levels of anxiety than patients with allergic respiratory diseases? Results of a large-scale cross-sectional study in a French population. Br J Dermatol. 2006;154:1128–1136. doi: 10.1111/j.1365-2133.2006.07186.x. [DOI] [PubMed] [Google Scholar]
- Bender BG. Cognitive effects of allergic rhinitis and its treatment. Immunology and Allergy Clinics of North America. 2005;25:301–312. doi: 10.1016/j.iac.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Bernstein IL, Storms WW. Practice parameters for allergy diagnostic testing. Ann Allergy Asthma Immunol. 1995;75:553–623. [PubMed] [Google Scholar]
- Breier A, Arora PK, Wolkowitzm OM, Pickar D, Paul SM. Metabolic stress produces rapid immunosuppression in humans. Arch Gen Psychiatry. 1987a;44:1108–1109. doi: 10.1001/archpsyc.1987.01800240084014. [DOI] [PubMed] [Google Scholar]
- Breier A, Margot A, Pickar D, Zahn T, Owen M, Paul S. Controllable and uncontrollable stress in humans: Alterations in mood and neuroendocrine and psychophysiological function. Am J Psychiatry. 1987b;144:1419–1425. doi: 10.1176/ajp.144.11.1419. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. J Psychosom Res. 2006;60:113–124. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Pieper S, Thayer JF. Expanding stress theory: Prolonged activation and perseverative cognition. Psychoneuroendocrinology. 2005;30:1043–1049. doi: 10.1016/j.psyneuen.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Brown WG, Halonen MJ, Kaltenborn WT, Barbee RA. The relationship of respiratory allergy, skin test reactivity, and serum IgE in a community population sample. J Allergy Clin Immunol. 1979;63:328–335. doi: 10.1016/0091-6749(79)90127-1. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Fischbach S, Rauh W, Hanker J, Hellhammer D. Increased responsiveness of the hypothalamus-pituitary-adrenal (HPA) axis to stress in newborns with atopic disposition. Psychoneuroendocrinology. 2004;29:705–711. doi: 10.1016/S0306-4530(03)00100-8. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Geiben A, Hellhammer D. Psychobiological aspects of atopic dermatitis: An overview. Psychother Psychosom. 2001;70:6–16. doi: 10.1159/000056219. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Chen E, Miller G. Stress and inflammation in exacerbations of asthma. Brain Behav Immun. 2007;21:993–999. doi: 10.1016/j.bbi.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder WT, Wilson NW. Comparison of three methods of using the DermaPIK with the standard prick method for epicutaneous skin testing. Ann Allergy Asthma Immunol. 1995;75:434–438. [PubMed] [Google Scholar]
- Cuffel B, Wamboldt M, Borish L, Kennedy S, Crystal-Peters J. Economic consequences of comorbid depression, anxiety, and allergic rhinitis. Psychosomatics. 1999;40:491–496. doi: 10.1016/S0033-3182(99)71187-4. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: Implications for health. Nature Reviews Immunology. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Goodwin RD. Self-reported hay fever and panic attacks in the community. Ann Allergy Asthma Immunol. 2002;88:556–559. doi: 10.1016/S1081-1206(10)61885-6. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Castro M, Kovacs M. Major depression and allergy: Does neuroticism explain the relationship? Psychosom Med. 2006;68:94–98. doi: 10.1097/01.psy.0000195797.78162.f4. [DOI] [PubMed] [Google Scholar]
- Griffin MJ, Chen E. Perceived control and immune and pulmonary outcomes in children with asthma. Psychosom Med. 2006;68:493–499. doi: 10.1097/01.psy.0000221367.96439.da. [DOI] [PubMed] [Google Scholar]
- Hart EL, Lahey BB, Hynd GW, Loeber R, McBurnett K. Association of chronic overanxious disorder with atopic rhinitis in boys: A four-year longitudinal study. J Clin Child Psychol. 1995;24:332–337. [Google Scholar]
- Heffner KL, Ginsburg GP, Hartley TR. Appraisals and impression management opportunities: Person and situation influences on cardiovascular reactivity. Int J Psychophysiol. 2002;44:165–175. doi: 10.1016/s0167-8760(01)00200-8. [DOI] [PubMed] [Google Scholar]
- Horowitz M, Wilner N, Alvarez WA. Impact of Events Scale: A measure of subjective stress. Psychosom Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- Katon WJ, Richardson L, Lozano P, McCauley E. The relationship of asthma and anxiety disorders. Psychosom Med. 2004;66:349–355. doi: 10.1097/01.psy.0000126202.89941.ea. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U S A. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata H. Enhancement of allergic skin responses by total sleep deprivation in patients with allergic rhinitis. Int Arch Allergy Immunol. 2002;128:351–352. doi: 10.1159/000063854. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’- A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kodama A, Horikawa T, Suzuki T, Ajiki W, Takashima T, Harada S, Ichihashi M. Effect of stress on atopic dermatitis: Investigation in patients after the great Hanshin earthquake. J Allergy Clin Immunol. 1999;104:173–176. doi: 10.1016/s0091-6749(99)70130-2. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Stauder A, Szedmak S. Severity of allergic complaints: The importance of depressed mood. J Psychosom Res. 2003;54:549–557. doi: 10.1016/s0022-3999(02)00477-4. [DOI] [PubMed] [Google Scholar]
- Liu LY, Coe CL, Swenson CA, Kelly EA, Kita H, Busse WW. School examinations enhance airway inflammation to antigen challenge. Am J Respir Crit Care Med. 2002;165:1062–1067. doi: 10.1164/ajrccm.165.8.2109065. [DOI] [PubMed] [Google Scholar]
- Marshall GD. Internal and external environmental influences in allergic diseases. The Journal of the American Osteopathic Association. 2004a;104:S1–6. [PubMed] [Google Scholar]
- Marshall GD. Neuroendocrine mechanisms of immune dysregulation: Applications to allergy and asthma. Ann Allergy Asthma Immunol. 2004b;93:S11–S17. doi: 10.1016/s1081-1206(10)61482-2. [DOI] [PubMed] [Google Scholar]
- Marshall GD, Jr, Agarwal SK, Lloyd C, Cohen L, Henninger EM, Morris GJ. Cytokine dysregulation associated with exam stress in healthy medical students. Brain Behav Immun. 1998;12:297–307. doi: 10.1006/brbi.1998.0537. [DOI] [PubMed] [Google Scholar]
- Marshall GD, Roy S. Stress and allergic diseases. In: Ader R, editor. Psychoneuroimmunology. 4th. Elsevier Academic Press; Burlington, MA: 2007. pp. 799–824. [Google Scholar]
- Marshall PS, Colon EA. Effects of allergy season on mood and cognitive function. Ann Allergy. 1993;71:251–258. [PubMed] [Google Scholar]
- Meggs WJ, Bloch RM, Finestone DH. Depression and anxiety associated with allergic and irritant sensitivity. J Allergy Clin Immunol. 2001;107:S26–S27. [Google Scholar]
- Nathan RA. The burden of allergic rhinitis. Allergy Asthma Proc. 2007;28:3–9. doi: 10.2500/aap.2007.28.2934. [DOI] [PubMed] [Google Scholar]
- Niemeijer NR, Fluks AF, de Monchy JGR. Optimization of skin testing II: Evaluation of concentration and cutoff values, as compared with RAST and clinical history, in a multicenter study. Allergy. 1993;48:498–503. doi: 10.1111/j.1398-9995.1993.tb01105.x. [DOI] [PubMed] [Google Scholar]
- Pace TWW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1632. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Richardson LP, Lozano P, Russo J, McCauley E, Bush T, Katon W. Asthma symptom burden: Relationship to asthma severity and anxiety and depression symptoms. Pediatrics. 2006;118:1042–1051. doi: 10.1542/peds.2006-0249. [DOI] [PubMed] [Google Scholar]
- Ritz T, Steptoe A. Emotion and pulmonary function in asthma: reactivity in the field and relationship with laboratory induction of emotion. Psychosom Med. 2000;62:808–815. doi: 10.1097/00006842-200011000-00011. [DOI] [PubMed] [Google Scholar]
- Sandberg S, Paton JY, Ahola S, McCann DC, McGuinness D, Hillary CR, Oja H. The role of acute and chronic stress in asthma attacks in children. Lancet. 2000;356:982–987. doi: 10.1016/S0140-6736(00)02715-X. [DOI] [PubMed] [Google Scholar]
- Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108:S2–8. doi: 10.1067/mai.2001.115569. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1970. [Google Scholar]
- Stauder A, Kovacs M. Anxiety symptoms in allergic patients: identification and risk factors. Psychosom Med. 2003;65:816–823. doi: 10.1097/01.psy.0000088620.66211.b1. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain Behavior and Immunity. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Storms W, Meltzer EO, Nathan RA, Selner JC. The economic impact of allergic rhinitis. J Allergy Clin Immunol. 1997;99:S820–S824. [PubMed] [Google Scholar]
- Straub RH, Cutolo M, Zietz B, Scholmerich J. The process of aging changes the interplay of the immune, endocrine and nervous systems. Mech Ageing Dev. 2001;122:1591–1611. doi: 10.1016/s0047-6374(01)00289-5. [DOI] [PubMed] [Google Scholar]
- Tomaka J, Blascovich J, Kelsey RM, L LC. Subjective, physiological, and behavioral effects of threat and challenge appraisal. J Pers Soc Psychol. 1993;65:248–260. [Google Scholar]
- Turkeltaub PC. Percutaneous and intracutaneous diagnostic tests of IgE-mediated diseases (immediate hypersensitivity) Clinical Allergy and Immunology. 2000;15:53–87. [PubMed] [Google Scholar]
- Wamboldt MZ, Hewitt JK, Schmitz S, Wamboldt FS, Rasanen M, Koskenvuo M, Romanov K, Varjonen J, Kaprio J. Familial association between allergic disorders and depression in adult Finnish twins. American Journal of Medical Genetics (Neuropsychiatric Genetics) 2000;96:146–153. doi: 10.1002/(sici)1096-8628(20000403)96:2<146::aid-ajmg4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wright RJ. Stress and atopic disorders. J Allergy Clin Immunol. 2005;116:1301–1306. doi: 10.1016/j.jaci.2005.09.050. [DOI] [PubMed] [Google Scholar]
- Wright RJ, Finn P, Contreras JP, Cohen S, Wright RO, Staudenmayer J, Wand M, Perkins D, Weiss ST, Gold DR. Chronic caregiver stress and IgE expression, allergen-induced proliferation, and cytokine profiles in a birth cohort predisposed to atopy. J Allergy Clin Immunol. 2004;113:1051–1057. doi: 10.1016/j.jaci.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Wu CY, Sarfati M, Heusser C, Fournier S, Rubio-Trujillo M, Peleman R, Delespesse G. Glucocorticoids increase the synthesis of immunoglobulin E by interleukin 4-stimulated human lymphocytes. J Clin Invest. 1991;87:870–877. doi: 10.1172/JCI115092. [DOI] [PMC free article] [PubMed] [Google Scholar]