Abstract
Ghrelin acts as an endocrine link connecting physiological processes regulating food intake, body composition, growth, and energy balance. Ghrelin is the only peptide known to undergo octanoylation. The enzyme mediating this process, ghrelin O-acyltransferase (GOAT), is expressed in the gastrointestinal tract (GI; primary source of circulating ghrelin) as well as other tissues. The present study demonstrates that stomach GOAT mRNA levels correlate with circulating acylated-ghrelin levels in fasted and diet-induced obese mice. In addition, GOAT was found to be expressed in both the pituitary and hypothalamus (two target tissues of ghrelin’s actions), and regulated in response to metabolic status. Using primary pituitary cell cultures as a model system to study the regulation of GOAT expression, we found that acylated-ghrelin, but not desacyl-ghrelin, increased GOAT expression. In addition, growth-hormone-releasing hormone (GHRH) and leptin increased, while somatostatin (SST) decreased GOAT expression. The physiologic relevance of these later results is supported by the observation that pituitary GOAT expression in mice lacking GHRH, SST and leptin showed opposite changes to those observed after in vitro treatment with the corresponding peptides. Therefore, it seems plausible that these hormones directly contribute to the regulation of pituitary GOAT. Interestingly, in all the models studied, pituitary GOAT expression paralleled changes in the expression of a dominant spliced-variant of ghrelin (In2-ghrelin) and therefore this transcript may be a primary substrate for pituitary GOAT. Collectively, these observations support the notion that the GI tract is not the only source of acylated-ghrelin, but in fact locally-produced des-acylated-ghrelin could be converted to acylated-ghrelin within target tissues by locally active GOAT, to mediate its tissue-specific effects.
Keywords: Ghrelin O-Acyl Transferase (GOAT); mouse models (fasting, obesity, knockouts); stomach; pituitary; hypothalamus
1. Introduction
The primary source of circulating ghrelin is the stomach, where pro-ghrelin can be modified by the addition of an O-linked octanoyl side group added to its serine-3 residue, via the actions of ghrelin O-acyltransferase (GOAT) to produce acylated-proghrelin (Kojima et al., 1999; Ariyasu et al., 2001; Garg, 2007; Gonzalez et al., 2008; Gutierrez et al., 2008; Yang et al., 2008; Gomez et al., 2009; Sakata et al., 2009). Then, prohormone convertase 1/3 (PC1/3) can further process pro-ghrelin (acylated or desacylated) to generate the mature 28-amino acid peptide ghrelin (Garg, 2007). Only a small proportion of circulating ghrelin is acylated, while the remaining is in the unmodified or des-acylated form, where both forms have biological effects (van der Lely et al., 2004; Kojima and Kangawa, 2005). Acylated-ghrelin is the primary endogenous ligand for the growth hormone secretagogue receptor (GHSR), and it is now recognized that the acyl-ghrelin/GHSR system exerts actions on multiple tissues, many of them related to regulation of metabolic functions (for review, (van der Lely et al., 2004)). In particular, acylated-ghrelin works at the level of the hypothalamus as a potent orexigenic signal (Kojima and Kangawa, 2005), and at the level of the pituitary to modulate hormone release including augmentation of growth hormone (GH), adrenocorticotropin (ACTH) and prolactin release (Kojima and Kangawa, 2005; Gahete et al., 2009). In addition, acylated ghrelin acts systemically to regulate glucose homeostasis and adiposity, where the deletion of the genes encoding ghrelin or its receptor prevents diet-induced obesity, improves insulin sensitivity and enhances glucose-stimulated insulin secretion (Yada et al., 2008).
Although the GI track is considered the major source of acylated ghrelin, other tissues express ghrelin, PC1/3 and GOAT (Gonzalez et al., 2008; Gutierrez et al., 2008; Yang et al., 2008) (for review, (van der Lely et al., 2004)). Therefore, it is possible that locally produced non-acylated proghrelin might be converted to acylated-proghrelin within target tissues by locally-active GOAT, to mediate tissue specific effects. Local production of acyl-ghrelin is supported by a recent report demonstrating that acylated ghrelin can be produced by a thyroid and a pituitary cell line that express ghrelin, proconvertase 1/3 and GOAT if n-octanoic acid is available as a substrate (Takahashi et al., 2009). Given the fact that hypothalamus and pituitary are targets for acylated-ghrelin, coupled with the fact that these tissues also express ghrelin (for review, (van der Lely et al., 2004)), GOAT (Gonzalez et al., 2008; Gutierrez et al., 2008; Yang et al., 2008) and PC1/3 (Dong and Day, 2002; Nillni, 2007), it is possible that local production of acylated-ghrelin, as well as that produced by the GI tract, may play an important role in regulating this neuroendocrine axis in response to metabolic signals. Therefore, the goals of the current study were 1) to determine whether GOAT expression in the stomach correlates with circulating acylated-ghrelin levels in mice under conditions of metabolic stress (i.e., fasting, and obese mouse models), 2) to confirm if GOAT is expressed within the hypothalamus and pituitary and if expression levels are mediated by metabolic stress and 3) to determine whether primary regulatory factors for the pituitary-metabolic interface can directly regulate GOAT expression, by using primary mouse pituitary cell cultures as a model system.
2. Material and Methods
2.1. Animals and cell culture
All experimental procedures were approved by the Animal Care and Use Committees of the University of Cordoba, University of Illinois at Chicago and the Jesse Brown VA Medical Center. Mice were housed under standard conditions of light (12-h light, 12-h dark cycle; lights on at 0700 h) and temperature (22–24 C), with free access to tap water and food (standard rodent chow; LabDiet, St. Louis, MO; USA; Catalog no. 5008; fat, 17 kcal%, carbohydrate, 56 kcal%; protein, 27 kcal%). Leptin deficient ob/ob mice and their corresponding controls were purchased from Jackson laboratories, while growth hormone releasing hormone (GHRH), somatostatin (SST) and neuropeptide Y (NPY) knockout mice were bred in house. Original breeders were obtained from Dr. Ute Hochgeschwender (SST-KO), Dr. Richard D. Palmiter (NPY-KO) and Dr. Roberto Salvatori (GHRH-KO) as previously reported (Alba and Salvatori, 2004; Park et al., 2005; Luque et al., 2006; Luque and Kineman, 2006; Luque and Kineman, 2007; Luque et al., 2007b; Luque et al., 2007c). Mice were handled daily at least 1 week prior to euthanasia to acclimate them to personnel and handling procedures and were sacrificed by decapitation, without anesthesia, under fed conditions unless otherwise specified. Trunk blood was immediately mixed with MiniProtease inhibitor (Roche, Nutley, NJ) and placed on ice, centrifuged and plasma was stored at −80C until analysis of total- and acylated- ghrelin by ELISA (Linco, St. Charles, MO) following the manufacturer’s instructions, including the addition of hydrochloric acid at a final concentration of 0.05N in order to prevent rapid des-acylation of ghrelin after the plasma collection. Tissues (stomachs, pituitaries and hypothalami) were immediately frozen in liquid nitrogen and stored at −80°C until further analysis of mRNA levels by quantitative real-time RT-PCR (qrtRT-PCR; see below).
2.2. Influence of metabolic stress on circulating total and acylated ghrelin and stomach, pituitary and hypothalamic GOAT mRNA levels
For these studies, serum and tissues previously processed and analyzed for other endpoints were used, where each study is listed below followed by a brief summary of the experimental design. Fasting–Ten week-old male C57Bl/6J mice were either fed ad libitum or fasted for 12h (food removed at 1900 h), 24h or 48h (food removed at 0700 h) (Luque et al., 2007c). An additional group of 8–9 week-old male C57Bl/6 x FVBN mice (n=5/group) were also fed ad libitum or fasted for 24h (Kineman et al., 2007). For these and subsequent studies all tissues and blood were collected between 0700h–0900h. Diet-induced obesity (DIO) - Male C57Bl/6J mice (n = 6–7) were fed a low-fat (LFD; 10% kcal from fat) or a high-fat (HFD; 60% kcal from fat) diet starting at 4 week of age and killed at 20 wk (Luque and Kineman, 2006). Leptin-deficient mice (ob/ob)–Blood and tissues were analyzed from 10 week old ob/ob mice and their littermates-controls (ob/?), as well as from ob/ob mice treated with vehicle (control 1), leptin (7d via osmotic mini-pumps released at a rate of 0.5 l/h, delivering a total of 15.6 g of leptin each day) or from a group of ob/ob mice pair-fed along with the leptin-treated animals that was used as an additional control (control 2) to match the food intake of leptin-treated mice (Luque and Kineman, 2006; Luque et al., 2007a) in order to differentiate between direct effects of leptin and those mediated indirectly by leptin-induced reduction in food intake and weight loss.
2.3. Direct regulation of GOAT mRNA levels by acylated-ghrelin, desacyl-ghrelin, GHRH, SST, leptin, NPY, insulin and IGF-I in mouse primary pituitary cell cultures
To examine whether primary regulatory hormones for the pituitary-metabolic interface can directly control pituitary GOAT expression, pituitaries of 8–12 wk old male C57Bl6 mice obtained from “Centro de Instrumentacion Cientifica” (University of Granada, Spain) or from Jackson Laboratories (Bar Harbor, ME, USA)) were enzymatically dispersed (n=3–5 pituitaries pooled/experiment, 6–7 separate experiments) into single cells and cultured (250,000 cells/well, 24-well plates) in serum containing α-MEM, as previously described (Luque et al., 2006; Luque and Kineman, 2006). After 24–48h of culture, media was removed and wells were washed in serum free media and subsequently treated with mouse acylated-ghrelin, desacyl-ghrelin, GHRH, NPY, insulin, IGF-I (10nM each), SST-14 (100nM) and mouse leptin (10ng/ml), for 24h (Sigma, Saint Louis, MO or Phoenix, Burlingame, CA; USA), and then total cellular RNA was extracted for determination of GOAT mRNA levels by qrtRT-PCR (see below). In order to determine if the direct actions of GHRH, SST, NPY or leptin could be important in maintaining in vivo expression of pituitary GOAT, total RNA obtained from pituitaries of 9–11 week old GHRH-knockout (KO) (B6129PF1/J), SST-KO mice (C57Bl/6J), NPY-KO mice (129/sv), leptin-KO (ob/ob; C57Bl/6J) and their respective littermate controls used in other studies (Alba and Salvatori, 2004; Park et al., 2005; Luque et al., 2006; Luque and Kineman, 2006; Luque and Kineman, 2007; Luque et al., 2007b; Luque et al., 2007c), were also evaluated from GOAT mRNA levels by qrtRTPCR.
2.4. RNA isolation and reverse transcription (RT)
Tissues and cell cultures were processed for recovery of total RNA using the Absolutely RNA RT-PCR Miniprep Kit with Deoxyribonuclease treatment (Stratagene, La Jolla, CA) as previously described (Luque et al., 2006; Luque and Kineman, 2006; Luque and Kineman, 2007; Luque et al., 2007c). The amount of RNA recovered was determined using the Ribogreen RNA quantification kit (Molecular Probes, Eugene, OR). Total RNA (1μg for whole tissue extract and 0.25μg for primary cell cultures) was reversed transcribed with enzyme and buffers supplied in the cDNA First Strand Synthesis kit (MRI Fermentas, Hanover, MD). cDNA was treated with ribonuclease H (1U; MRI Fermentas) and duplicate aliquots (1μl) were amplified by qrtRT-PCR, in which samples were run against synthetic standards to estimate mRNA copy number, as described below.
2.5. Quantification of GOAT and ghrelin mRNA levels by qrtRT-PCR
Details regarding the development, validation, and application of a qrtRT-PCR to measure expression levels of mouse transcripts, including ghrelin mRNA levels, have been reported previously (Luque et al., 2006; Luque and Kineman, 2006; Kineman et al., 2007; Luque et al., 2007a; Luque and Kineman, 2007; Luque et al., 2007c). Specific primer sequences, GenBank accession numbers and product sizes for mouse GOAT, ghrelin and cyclophilin used in this study were as follows: GOAT (Mboat4; forward 5′-ATTTGTGAAGGGAAGGTGGAG-3′ and reverse 5′-CAGGAGAGCAGGGAAAAAGAG-3′, NM_001126314, 120bp); Ghrelin (forward 5′-TCCAAGAAGCCACCAGCTAA-3′ and reverse 5′-AACATCGAAGGGAGCATTGA-3′, NM_021488, 126bp) and cyclophilin (forward 5′-TGGTCTTTGGGAAGGTGAAAG-3′ and reverse 5′-TGTCCACAGTCGGAAATGGT-3′, NM_008907, 109bp). Primers were selected using Primer 3 software (Rozen and Skaletsky, 2000) with selection parameters set to identify primer sets that: 1) span an intron (when possible), 2) differ by no more that 1°C in annealing temperature, 3) are at least 20bp in length, 4) have a GC content between 45–55%, but 5) exclude primers that may form primer-dimers. Sequences of selected primers were used in BLAST (NCBI) searches to check for potential homology to sequences other than the designated target. Initial screening of primer efficiency using real-time detection was performed by amplifying 2-fold dilutions of RT products, where optimal efficiency was demonstrated by a difference of one cycle threshold between dilutions and a clear melting peak followed by a graded temperature-dependent dissociation to verify that only one product was amplified. The thermocycling profile consisted of one cycle of 95 C for 10 min, 40 cycles of 95 C for 30 sec, 61 C for 1 min, and 72 C for 30 sec. PCR products were then column-purified (QIAGEN, Valencia, CA) and sequenced to confirm target specificity. After confirmation of primer efficiency and specificity, the concentration of purified products was determined using Molecular Probe’s Picogreen DNA quantification kit, and PCR products were serial diluted to obtain standards containing 1, 101,102, 103, 104, 105, and 106 copies of synthetic template. Standards were then amplified by real-time PCR, and standard curves were generated using Stratagene Mx3000p software. The slope of a standard curve for each template examined was approximately 1, indicating that the efficiency of amplification of our primers was 100%, meaning that all templates in each cycle were copied. To determine the starting copy number of cDNA, RT samples were PCR amplified and the signal was compared with that of a specific standard curve of each transcript run on the same plate. In addition, total RNA samples that were not reversed transcribed and a no DNA control were run on each plate to control for genomic DNA contamination and to monitor potential exogenous contamination, respectively. Also, to control for variations in the amount of RNA used in the RT reaction and the efficiency of the RT reaction, mRNA copy number of the transcript of interest was adjusted by the mRNA copy number of cyclophilin A (used as housekeeping gene), where cyclophilin A mRNA levels did not significantly vary between experimental groups, within tissue type or treatment group (data not shown).
2.6. Data presentation and statistical analysis
Samples from all groups within an experiment were processed at the same time. The effects of fasting and leptin replacement were assessed by one-way ANOVA followed by a Newman-Keuls test for multiple comparisons, while the effects of obesity, genotype and the in vitro effects of acylated-ghrelin, desacyl-ghrelin, GHRH, SST, NPY, leptin, insulin and IGF-I were assessed by Student’s t-test. P < 0.05 was considered significant. All data are expressed as means ± SEM. The in vivo effects of fasting/obesity/genotype were obtained from a minimum of 5 animals per group. Results from in vitro studies were obtained from 3–7 separate, independent experiments (3–5 wells/treatment) carried out in different days and with different cells preparation. Endpoints displaying heterogeneity of variance were log transformed prior to analysis. All statistical analyses were performed using the GB-STAT software package (Dynamic Microsystems, Inc. Silver Spring, MD, USA).
3. Results and Discussion
3.1. Quantification of GOAT mRNA levels in mouse stomachs, pituitaries and hypothalami
Using qrtRT-PCR, we found that GOAT mRNA levels were 5-fold greater in the stomach as compared to the pituitary and hypothalamus (Table 1). These results are consistent with other reports showing relative expression levels of GOAT in both human (Gutierrez et al., 2008) and rodent (Gonzalez et al., 2008; Yang et al., 2008; Sakata et al., 2009) tissues. Interestingly, the expression level of GOAT transcripts in the mouse tissues analyzed in the current study (table 1) paralleled the mRNA levels of ghrelin in the same tissues (Stomach ≫ pituitary = hypothalamus), as previously reported (Kineman et al., 2007).
Table 1.
Absolute cDNA copy number/0.05μg total RNA of GOAT gene transcripts in the stomach, pituitary and hypothalamus of male C57Bl/6 mice, as determined by quantitative real-time RT-PCR.
| GOAT mRNA copy# | |
|---|---|
| Whole tissue: | |
| -Stomach | 357 ± 46 |
| - Pituitary | 67 ± 8 |
| - Hypothalamus | 73 ± 5 |
| Primary pituitary cell culture: | 63 ± 2 |
Values represent means ± SEM (n=27–43 control tissues or control culture-wells/experimental or treatment group)
Since it has been previously reported that the enzyme prohormone convertase 1/3 which is required for the conversion of preprohormones, including preproghrelin to its 28 amino acid form, is also expressed in the pituitary and hypothalamus (Dong and Day, 2002; Nillni, 2007), as well as the GI tract (Macro et al., 1996), it is possible that the source of acylated-ghrelin within the pituitary and hypothalamus may include locally produced ghrelin, in addition to that found in the circulation. Therefore, the current study compared the impact of metabolic stress (fasting and obesity) on circulating ghrelin (acylated and total) levels, as well as GOAT and ghrelin mRNA levels in the stomach, pituitary and hypothalamus. Specifically, Figure 1 illustrates the impact of fasting, diet-induced obesity (DIO) and obesity caused by leptin deficiency (ob/ob mice) on circulating acylated- and total-ghrelin levels and stomach GOAT mRNA levels (Panels A, B and C, respectively), as well as pituitary and hypothalamic GOAT mRNA levels (Panels D, E and F, respectively). It should be noted that, in some cases we have previously published the regulation of stomach, pituitary and hypothalamic ghrelin mRNA levels in these model systems (Kineman et al., 2007; Luque et al., 2007a; Luque et al., 2007c). These results are summarized in Figure 1 as the relative changes in ghrelin mRNA levels (=, ↑, ↓), as compared to controls, as shown below each graph of tissue-specific GOAT expression.
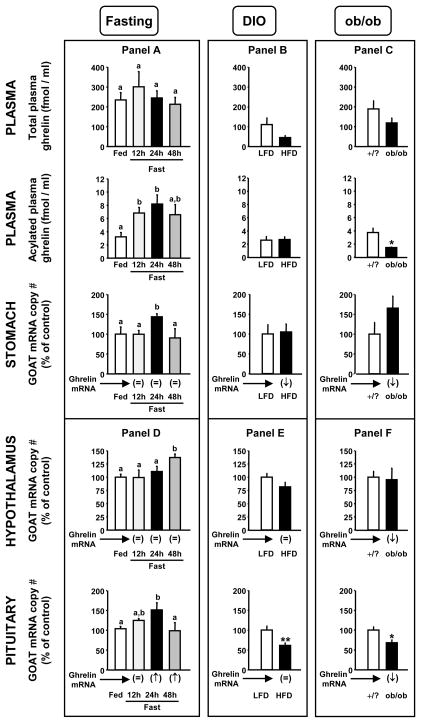
Figure 1.
Circulating total- and acylated-ghrelin and stomach, hypothalamus and pituitary GOAT mRNA levels in: mice fed ad libitum or fasted for 12-, 24-, and 48-h (Panel A and D), mice fed a low-fat diet (LFD control group) or high-fat diet (HFD obese group) (Panel B and E) and lean controls (/?) and obese (ob/ob) mice (Panel C and F). Values are shown as mean ± SEM (n=5–8 mice/group) and expressed as percent of the respective control group (set at 100%). Group means that do not share a common letter (a,b) significantly differ (p<0.05; Panel A and D). Asterisks (*, p<0.05, **, p<0.01) indicate values that significantly differ from lean control LFD or/? values. The tissue-specific regulation of ghrelin mRNA levels in these mice models as previously reported (Kineman et al., 2007; Luque et al., 2007a; Luque et al., 2007c) are shown below each graph of tissue-specific GOAT expression and are provided as the relative changes (=, ↑, ↓) as compared to controls.
3.2. Effects of nutrient deprivation on GOAT/ghrelin axis
As shown in Figure 1, panel A, fasting did not alter circulating levels of total-ghrelin or stomach ghrelin expression, but did increase circulating acylated-ghrelin (12 and 24h) and stomach GOAT mRNA levels (24h). The impact of 24h fasting on stomach GOAT mRNA levels was confirmed in tissues taken from an independent set of male mice from another study (Kineman et al., 2007), using two different primer sets for amplification of the GOAT transcripts (supplemental Fig. 1). These results differ from those of a recent report by Kirchner et al., (Kirchner et al., 2009) showing that fasting (12h, 24h and 36h) significantly increased total-ghrelin levels and tended to increase acylated-ghrelin levels, but unexpectedly suppressed stomach GOAT mRNA in C57Bl/6 male mice. It is possible that such differences may be related to the time of food withdrawal, time of sample collection, type of standard rodent chow provided and/or the method of euthanasia which were not clearly defined in that study. In fact, Kirchner et al., (Kirchner et al., 2009) observed a diurnal pattern of circulating ghrelin and stomach ghrelin/GOAT mRNA levels, as well as an association between stomach GOAT expression and the type of dietary lipids supplied. It is also possible that the differences observed are related to the analytical techniques applied, where circulating ghrelin levels were assessed by commercial assays in the current study, but in the case of Kirchner et al., (Kirchner et al., 2009), were assessed by MALDI-TOF mass spectrometry of immunoprecipitated ghrelin. Finally, with respect to GOAT mRNA levels, although both the current study and that of Kirchner et al., (Kirchner et al., 2009) used real-time RT-PCR, the primer sets used and the characteristics and properties of the primers were not the same (length of primers, GC content, etc.). Nonetheless, our current observations are consistent with other reports showing a clear rise in acylated-ghrelin levels in fasted humans and other species (for review: (Casanueva and Dieguez, 2002; Gottero et al., 2004; Kojima and Kangawa, 2005; Williams and Cummings, 2005; Cummings, 2006)), including mice (Perreault et al., 2004; Luque et al., 2006; Luque et al., 2007c; Zizzari et al., 2007), as well as an increase in stomach GOAT mRNA levels in rats subjected to 21-days of caloric-restriction (Gonzalez et al., 2008). However, it should be noted that like Kirchner et al., Liu et al. failed to detect a rise in circulating acylated ghrelin in fasted humans (Liu et al., 2008). It should also be emphasized that in the current study the fasting-induced rise in acylated-ghrelin was observed at 12 and 24h, but began to fall after 48h, while the fasting-induced rise in GOAT mRNA was only observed at 24h. These results suggest that an increase in GOAT expression levels in the stomach may only in part contribute to the rise in circulating acyl-ghrelin, where enhanced GOAT activity and/or substrate availability may precede that process. However, after 48h of fasting circulating acylated-ghrelin levels and GOAT mRNA levels did not differ from controls (Fig. 1, panel A), where 48h of fasting in a mouse represent a severe catabolic state (Luque et al., 2007c). This observation is consistent with reports showing that the proportion of circulating acylated-ghrelin falls after long-term fasting in humans (61.5h-fasting) (Liu et al., 2008) and rats (48h-fasting) (Toshinai et al., 2001; Gonzalez et al., 2008) and with the recent observation indicating that 48h of fasting did not significantly alter stomach GOAT mRNA expression in rats (Gonzalez et al., 2008; Takahashi et al., 2009). Taken together our current results suggest that changes in stomach GOAT expression may contribute in part to fasting-induced changes in circulating acylated-ghrelin levels, a hypothesis previously put forth by other laboratories (Gonzalez et al., 2008; Gualillo et al., 2008). However, given the only other report examining this relationship in mice gave different results (Kirchner et al., 2009), and the fact that there are many potential levels of GOAT regulation (transcriptional, translational, enzyme activity level, substrate availability), it will be important for other laboratories to validate and confirm these findings.
3.3. Effects of obesity on GOAT/ghrelin axis
As shown in Figure 1, panel B, diet-induced obesity tended (50% reduction, p=0.13) to suppress total ghrelin levels in mice without altering acylated-ghrelin levels, consistent with a significant decline in stomach ghrelin expression, while GOAT mRNA levels remained unchanged. In contrast, obesity as a result of leptin-deficiency (ob/ob mice, Fig. 1 panel C) led to a significant decline in circulating acylated-ghrelin levels (and a downward trend in total-ghrelin levels), changes which were accompanied by a significant decrease in stomach ghrelin but not GOAT mRNA levels, similar to DIO mice. This is the first report to explore the impact of DIO on stomach GOAT and ghrelin expression, while a previous study showed similar results for stomach GOAT and ghrelin mRNA levels in ob/ob mice (Kirchner et al., 2009). In addition, others have reported a significant decline in total- or desacyl-ghrelin in DIO (Ueno et al., 2007) and obese Ay (Nonogaki et al., 2006) mice and a reduction in total- and acylated-ghrelin levels in obese humans (Casanueva and Dieguez, 2002; Gottero et al., 2004; Ghigo et al., 2005; Kojima and Kangawa, 2005), where DIO-induced alterations in acylated-ghrelin levels may be dependent on the time of day sampled (Perreault et al., 2004). Taken together these results indicate that the decline in circulating total-ghrelin levels in the obese state could be in part due to a decline in stomach ghrelin gene expression, however alterations in circulating acylated-ghrelin may be related to factors independent of GOAT expression, such as the rate of degradation of acylated-ghrelin, GOAT enzyme activity levels or substrate (ghrelin or fatty acid) availability.
3.4. Impact of metabolic stress (fasting and obesity) on hypothalamic and pituitary GOAT expression
As shown in Figure 1, panel D, fasting also enhanced expression of GOAT at both the hypothalamic [at 48h (p=0.0016)] and pituitary [24h (p=0.017) and 12h (p=0.051)] level. Interestingly, it should be noted that the timing of the GOAT mRNA rise was different from that observed in the stomach (Fig. 1, panel A). Conversely, DIO (Fig. 1, panel E) and obesity caused by leptin-deficiency (Fig. 1, panel F) did not alter hypothalamic GOAT expression, but did result in a significant decline in pituitary GOAT mRNA levels. As in the stomach, hypothalamic and pituitary GOAT expression did not always parallel local changes in ghrelin expression. Taken together, these results suggest local production (ghrelin gene expression) and/or modification (via GOAT gene expression) of ghrelin may contribute to regulation of hypothalamic and pituitary function independent of circulating ghrelin levels produced by the stomach which would be supported by a recent study indicating that various cell lines, including the mouse pituitary cell line AtT20, can produce acylated-ghrelin when both ghrelin and GOAT are present in the cells and when substrate (fatty acid) is available in the culture medium (Takahashi et al., 2009)
3.5. Direct regulation of GOAT mRNA levels by ghrelin, desacyl-ghrelin, GHRH, SST, leptin, NPY, insulin and IGF-I in mouse primary pituitary cell cultures
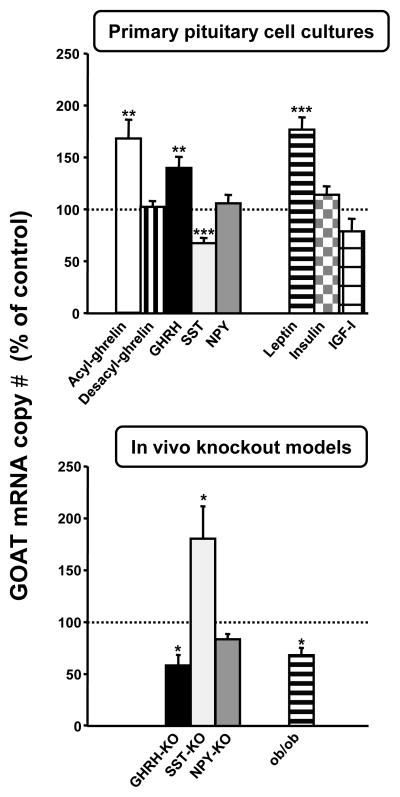
Given the dynamic changes in pituitary GOAT expression in both fasting (up-regulation; Fig. 1, panel D) and obesity (down-regulation; Fig. 1, panel E and F), we used primary mouse pituitary cell cultures as a model system to explore which central or systemic factors might mediate these changes. First, to confirm that mouse primary pituitary cells maintain differentiated function (ie. GOAT expression) after dispersion and culture, the absolute mRNA levels (copy numbers/0.05 μg total RNA) of GOAT were compared between whole tissue extracts and extracts prepared from pituitary cultures 24h after incubation in serum-free media, and the results are shown in Table 1. Transcript levels did not significantly vary between in vivo and in vitro samples, indicating that the cell preparation and culture conditions were appropriate to maintain GOAT expression. Based on reports from our laboratory and others showing that the levels and/or actions of ghrelin, GHRH and NPY are up-regulated in fasting (Henry et al., 2001; Park et al., 2005; Luque et al., 2006; Luque et al., 2007c; Kirchner et al., 2009) and down-regulated in obesity (Tannenbaum et al., 1990; Ahmad et al., 1993; Maccario et al., 1999; Lin et al., 2000; Perreault et al., 2004; Luque and Kineman, 2006; Nonogaki et al., 2006; Luque et al., 2007a) while those for SST, leptin, insulin and IGF-I are down-regulated in fasting (Henry et al., 2001; Frystyk, 2004; Park et al., 2005; Luque et al., 2006; Gonzalez et al., 2008) and up-regulated in obesity (Zhou et al., 1997; Frystyk, 2004; Luque and Kineman, 2006; Luque et al., 2007b; Luque et al., 2008), we incubated primary pituitary cell cultures with these hormones for 24h and measured the impact on GOAT mRNA levels. As shown in Figure 2, top panel, acylated-ghrelin clearly stimulated GOAT expression compared to vehicle-treated controls (set at 100%). In order to confirm that this effect on GOAT mRNA levels was directly exerted by acylated-ghrelin and not by desacyl-ghrelin (as result of a des-octanoylation of acylated-ghrelin in the pituitary cell cultures after 24h of incubation), pituitary cell cultures were treated with desacyl-ghrelin for 24h. The results clearly indicate that treatment with desacyl-ghrelin did not alter GOAT mRNA levels. In addition, as shown in figure 2 (top panel), GHRH and leptin stimulated and SST inhibited pituitary GOAT expression, while NPY, insulin and IGF-I had no effect, compared to vehicle-treated controls (set at 100%). In order to determine, if these direct actions might also be important in maintaining pituitary GOAT expression in vivo, we examined pituitary expression of GOAT in available mouse models that lack GHRH, SST, NPY and leptin. Accordingly, we found an opposite effect of GHRH-KO, SST-KO and leptin-KO on pituitary GOAT expression, as compared to that observed after in vitro treatment with the corresponding hormone (Fig. 2, bottom panel). It is also interesting to note that when the impact of leptin replacement was examined in the ob/ob model (Luque et al., 2007a), we observed that pituitary and stomach GOAT mRNA levels were greater than that observed in pair-fed controls (Supplemental Figure 2), which is consistent with the report of Gonzalez et al., (Gonzalez et al., 2008) showing that administration of exogenous leptin markedly increased expression levels of GOAT in the stomach of fasted rats. Taken together these results indicate that acylated-ghrelin may in fact regulate its own production at the level of the pituitary by increasing the expression of GOAT. In addition, these results clearly demonstrate that GHRH, SST and leptin are key regulators of pituitary GOAT expression, an observation which may in part explain changes in pituitary GOAT expression observed in response to metabolic extremes. Intriguingly, the substrate of GOAT activity in the mouse pituitary may not be limited to native ghrelin being locally produced within the pituitary, because our laboratory has identified a spliced mRNA ghrelin variant containing exon2, intron2 and exon3, but lacking exon1, 4 and 5 (In2-ghrelin variant; (Kineman et al., 2007)) which is expressed at higher levels than the native ghrelin transcript in the pituitary (139±24 copies for In2-ghrelin variant versus 18±3 copies for native-ghrelin/0.05μg RNA). If this variant is translated, it would encode a protein that would include the first 36 amino acid of the pre-proghrelin, where residues 24–28 represent the first five amino acids of mature ghrelin and thus contain the Ser-3 acylation site. It is interesting to note that when we compared the impact of metabolic stress (fasting and DIO) or GHRH, SST and leptin deficiency on pituitary expression of GOAT, native ghrelin and In2-ghrelin, the levels of GOAT expression paralleled those of In2-ghrelin variant but not native ghrelin, in all cases (summarized in Table 2, and details presented in Supplemental Figure 3). Based on these observations it seems reasonable to propose that the In2-ghrelin variant could be a primary substrate for GOAT in the anterior pituitary gland.
Figure 2.
Top panel: Effect of 24-h treatment with acylated-ghrelin, growth hormone releasing hormone (GHRH), somatostatin (SST), neuropeptide Y (NPY), leptin, insulin and IGF-I on GOAT mRNA levels in mouse primary pituitary cell cultures. Data represent the mean ± SEM (n=3–7 experiment performed in different cell preparations; 3–4 wells/treatment). Bottom panel: GOAT mRNA expression levels in pituitaries of growth hormone releasing hormone knockout (GHRH-KO), somatostatin knockout (SST-KO), neuropeptide Y knockout (NPY-KO) and ob/ob (leptin deficient) mice. Values are shown as mean ± SEM and expressed as percent of intact control mice (set at 100%; n=5–9 mice/group). Asterisks (*, p<0.05, **, p<0.01, ***, p<0.001) indicate values that significantly differ from respective controls (shown by the dotted line set at 100%).
Table 2.
Relative effect of metabolic condition (fasting, obesity) or inactivating mutations in growth hormone releasing hormone (GHRH), somatostatin (SST) or leptin genes on pituitary GOAT, ghrelin and In2-ghrelin variant mRNA levels, as determined by quantitative real-time RT-PCR. This summary represents data presented in the current report, combined with previously published reports (Kineman et al., 2007; Luque et al., 2007a; Luque et al., 2007c)..
| GOAT mRNA | Native ghrelin | In2-ghrelin | |
|---|---|---|---|
| Fasting 24h | ↑ | ↑ | ↑ |
| Diet induced obese | ↓ | No change | ↓ |
| GHRH-KO | ↓ | No change | ↓ |
| SST-KO | ↑ | ↑ | ↑* |
| Leptin-KO (ob/ob) | ↓ | ↓ | ↓ |
Differences observed only in females.
Although more experiments are required to fully elucidate the physiologic significance of our findings in the pituitary, our data give credence to the possibility that local production and/or modification of ghrelin (desacylated to acylated via GOAT gene expression) may contribute to the fine regulation of pituitary function in response to metabolic stress. For example, circulating GH levels are known to rise in response to fasting in the mouse, and this is associated with an increase in pituitary expression of GH, GHRH-R and GHS-R and a decline in SST receptor expression (rat and mouse; (Luque et al., 2007a; Luque et al., 2008)). The acute rise in systemic acylated-ghrelin, in combination with an increased sensitivity of the pituitary to ghrelin and GHRH actions, might initiate local production of acylated-ghrelin by augmentation of ghrelin and GOAT expression, thereby acting as a positive ultrashort feedback loop to enhance or facilitate GH release. In contrast, GH output is suppressed in obesity, and effect which is believed to be due in part to enhanced SST tone [for review see (Luque et al., 2008)] and a decline in pituitary of GHRH-R, GHS-R and GH (Luque and Kineman, 2006; Luque et al., 2008). In this context, our data would support the idea that part of the negative effects of SST on somatotrope function could include direct suppression of GOAT expression and a decline of locally produced acylated-ghrelin, further contributing to the decline in GH release observed with weight gain.
In summary, we report herein a series of novel analyses on the regulation of the mouse GOAT/ghrelin system at the circulating-stomach-pituitary-hypothalamic levels by energy status and relevant metabolic cues in the mouse. By applying a combination of studies involving in vivo models (fasting, obesity and knockout mice) and primary pituitary cell cultures, our results further characterize the impact of changes in body energy stores on the expression of GOAT in different tissues and define the role of key regulators, such as acylated-ghrelin itself, GHRH, SST and leptin on the control of GOAT expression in the mouse. The fact that GOAT mRNA levels are oppositely regulated in extreme metabolic states (fasting, DIO) and its regulation is tissue-dependent suggests that this enzyme may be of biological relevance in coordinating the neuroendocrine response to metabolic stress. Although much work remains to be done to fully understand how GOAT fits into the control of energy homeostasis, our results reinforce the contention that GOAT/acylated-ghrelin system could operate as a novel molecular conduit for the integration of energy balance, metabolism and pituitary function.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Ute Hochgeschwender (Oklahoma Medical Research Foundation, Oklahoma City, OK) for the SST knockout mice, Dr. Richard D. Palmiter (Howard Hughes Medical Institute, University of Washington, Seattle Seattle, WA) for the NPY knockout
This work is supported in part by grants from Programa Nacional de becas de FPU (FPU-AP20052473, to MDG) and Programa Ramon y Cajal del Ministerio de Educación y Ciencia, Spain (RYC-2007-00186, to RML); Ayudas predoctorales de formacion en investigacion en salud del Fondo de Investigación sanitaria del Instituto de Salud Carlos III (FI06/00804; to JCC) and by grants from Junta de Andalucía BIO-0139 and CTS-01705 and Ministerio de Ciencia e Innovacion/FEDER BFU2007-60180/BFI, BFU2008-01136/BFISpain, and NIDDK 30677 and the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development Merit Award (to RDK). CIBER is an initiative of Instituto de Salud Carlos III (Ministerio de Sanidad, Ministerio de Ciencia e Innovación, Spain).
Footnotes
Disclosures: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad I, Finkelstein JA, Downs TR, Frohman LA. Obesity-associated decrease in growth hormone-releasing hormone gene expression: a mechanism for reduced growth hormone mRNA levels in genetically obese Zucker rats. Neuroendocrinology. 1993;58(3):332–7. doi: 10.1159/000126558. [DOI] [PubMed] [Google Scholar]
- Alba M, Salvatori R. A mouse with targeted ablation of the growth hormone-releasing hormone gene: a new model of isolated growth hormone deficiency. Endocrinology. 2004;145(9):4134–43. doi: 10.1210/en.2004-0119. [DOI] [PubMed] [Google Scholar]
- Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86(10):4753–8. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- Casanueva FF, Dieguez C. Ghrelin: the link connecting growth with metabolism and energy homeostasis. Rev Endocr Metab Disord. 2002;3(4):325–38. doi: 10.1023/a:1020901624103. [DOI] [PubMed] [Google Scholar]
- Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav. 2006;89(1):71–84. doi: 10.1016/j.physbeh.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Dong W, Day R. Gene expression of proprotein convertases in individual rat anterior pituitary cells and their regulation in corticotrophs mediated by glucocorticoids. Endocrinology. 2002;143(1):254–62. doi: 10.1210/endo.143.1.8570. [DOI] [PubMed] [Google Scholar]
- Frystyk J. Free insulin-like growth factors -- measurements and relationships to growth hormone secretion and glucose homeostasis. Growth Horm IGF Res. 2004;14(5):337–75. doi: 10.1016/j.ghir.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Gahete MD, Duran-Prado M, Luque RM, Martinez-Fuentes AJ, Quintero A, Gutierrez-Pascual E, Cordoba-Chacon J, Malagon MM, Gracia-Navarro F, Castano JP. Understanding the multifactorial control of growth hormone release by somatotropes: lessons from comparative endocrinology. Ann N Y Acad Sci. 2009;1163:137–53. doi: 10.1111/j.1749-6632.2008.03660.x. [DOI] [PubMed] [Google Scholar]
- Garg A. The ongoing saga of obestatin: is it a hormone? J Clin Endocrinol Metab. 2007;92(9):3396–8. doi: 10.1210/jc.2007-0999. [DOI] [PubMed] [Google Scholar]
- Ghigo E, Broglio F, Arvat E, Maccario M, Papotti M, Muccioli G. Ghrelin: more than a natural GH secretagogue and/or an orexigenic factor. Clin Endocrinol (Oxf) 2005;62(1):1–17. doi: 10.1111/j.1365-2265.2004.02160.x. [DOI] [PubMed] [Google Scholar]
- Gomez R, Lago F, Gomez-Reino JJ, Dieguez C, Gualillo O. Expression and modulation of ghrelin O-acyltransferase in cultured chondrocytes. Arthritis Rheum. 2009;60(6):1704–9. doi: 10.1002/art.24522. [DOI] [PubMed] [Google Scholar]
- Gonzalez CR, Vazquez MJ, Lopez M, Dieguez C. Influence of chronic undernutrition and leptin on GOAT mRNA levels in rat stomach mucosa. J Mol Endocrinol. 2008;41(6):415–21. doi: 10.1677/JME-08-0102. [DOI] [PubMed] [Google Scholar]
- Gottero C, Broglio F, Prodam F, Destefanis S, Bellone S, Benso A, Gauna C, Arvat E, van der Lely AJ, Ghigo E. Ghrelin: a link between eating disorders, obesity and reproduction. Nutr Neurosci. 2004;7(5–6):255–70. doi: 10.1080/10284150400017363. [DOI] [PubMed] [Google Scholar]
- Gualillo O, Lago F, Dieguez C. Introducing GOAT: a target for obesity and anti-diabetic drugs? Trends Pharmacol Sci. 2008;29(8):398–401. doi: 10.1016/j.tips.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, Witcher DR, Luo S, Onyia JE, Hale JE. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci U S A. 2008;105(17):6320–5. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BA, Rao A, Tilbrook AJ, Clarke IJ. Chronic food-restriction alters the expression of somatostatin and growth hormone-releasing hormone in the ovariectomised ewe. J Endocrinol. 2001;170(1):R1–5. doi: 10.1677/joe.0.170r001. [DOI] [PubMed] [Google Scholar]
- Kineman RD, Gahete MD, Luque RM. Identification of a mouse ghrelin gene transcript that contains intron 2 and is regulated in the pituitary and hypothalamus in response to metabolic stress. J Mol Endocrinol. 2007;38(5):511–21. doi: 10.1677/JME-06-0026. [DOI] [PubMed] [Google Scholar]
- Kirchner H, Gutierrez JA, Solenberg PJ, Pfluger PT, Czyzyk TA, Willency JA, Schurmann A, Joost HG, Jandacek RJ, Hale JE, Heiman ML, Tschop MH. GOAT links dietary lipids with the endocrine control of energy balance. Nat Med. 2009;15(7):741–5. doi: 10.1038/nm.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85(2):495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- Lin S, Storlien LH, Huang XF. Leptin receptor, NPY, POMC mRNA expression in the diet-induced obese mouse brain. Brain Res. 2000;875(1–2):89–95. doi: 10.1016/s0006-8993(00)02580-4. [DOI] [PubMed] [Google Scholar]
- Liu J, Prudom CE, Nass R, Pezzoli SS, Oliveri MC, Johnson ML, Veldhuis P, Gordon DA, Howard AD, Witcher DR, Geysen HM, Gaylinn BD, Thorner MO. Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J Clin Endocrinol Metab. 2008;93(5):1980–7. doi: 10.1210/jc.2007-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque RM, Gahete MD, Hochgeschwender U, Kineman RD. Evidence that endogenous SST inhibits ACTH and ghrelin expression by independent pathways. Am J Physiol Endocrinol Metab. 2006;291(2):E395–403. doi: 10.1152/ajpendo.00038.2006. [DOI] [PubMed] [Google Scholar]
- Luque RM, Huang ZH, Shah B, Mazzone T, Kineman RD. Effects of leptin replacement on hypothalamic-pituitary growth hormone axis function and circulating ghrelin levels in ob/ob mice. Am J Physiol Endocrinol Metab. 2007a;292(3):E891–9. doi: 10.1152/ajpendo.00258.2006. [DOI] [PubMed] [Google Scholar]
- Luque RM, Kineman RD. Impact of obesity on the growth hormone axis: evidence for a direct inhibitory effect of hyperinsulinemia on pituitary function. Endocrinology. 2006;147(6):2754–63. doi: 10.1210/en.2005-1549. [DOI] [PubMed] [Google Scholar]
- Luque RM, Kineman RD. Gender-dependent role of endogenous somatostatin in regulating growth hormone-axis function in mice. Endocrinology. 2007;148(12):5998–6006. doi: 10.1210/en.2007-0946. [DOI] [PubMed] [Google Scholar]
- Luque RM, Kineman RD, Tena-Sempere M. Regulation of hypothalamic expression of KiSS-1 and GPR54 genes by metabolic factors: analyses using mouse models and a cell line. Endocrinology. 2007b;148(10):4601–11. doi: 10.1210/en.2007-0500. [DOI] [PubMed] [Google Scholar]
- Luque RM, Park S, Kineman RD. Severity of the catabolic condition differentially modulates hypothalamic expression of growth hormone-releasing hormone in the fasted mouse: potential role of neuropeptide Y and corticotropin-releasing hormone. Endocrinology. 2007c;148(1):300–9. doi: 10.1210/en.2006-0592. [DOI] [PubMed] [Google Scholar]
- Luque RM, Park S, Kineman RD. Role of endogenous somatostatin in regulating GH output under basal conditions and in response to metabolic extremes. Mol Cell Endocrinol. 2008;286(1–2):155–68. doi: 10.1016/j.mce.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Maccario M, Gauna C, Procopio M, Di Vito L, Rossetto R, Oleanders SE, Grottoli S, Ganzaroli C, Aimaretti G, Ghigo E. Assessment of GH/IGF-I axis in obesity by evaluation of IGF-I levels and the GH response to GHRH+arginine test. J Endocrinol Invest. 1999;22(6):424–9. doi: 10.1007/BF03343585. [DOI] [PubMed] [Google Scholar]
- Macro JA, Dimaline R, Dockray GJ. Identification and expression of prohormone-converting enzymes in the rat stomach. Am J Physiol. 1996;270(1 Pt 1):G87–93. doi: 10.1152/ajpgi.1996.270.1.G87. [DOI] [PubMed] [Google Scholar]
- Nillni EA. Regulation of prohormone convertases in hypothalamic neurons: implications for prothyrotropin-releasing hormone and proopiomelanocortin. Endocrinology. 2007;148(9):4191–200. doi: 10.1210/en.2007-0173. [DOI] [PubMed] [Google Scholar]
- Nonogaki K, Nozue K, Oka Y. Hyperphagia alters expression of hypothalamic 5-HT2C and 5-HT1B receptor genes and plasma des-acyl ghrelin levels in Ay mice. Endocrinology. 2006;147(12):5893–900. doi: 10.1210/en.2006-0418. [DOI] [PubMed] [Google Scholar]
- Park S, Peng XD, Frohman LA, Kineman RD. Expression analysis of hypothalamic and pituitary components of the growth hormone axis in fasted and streptozotocin-treated neuropeptide Y (NPY)-intact (NPY+/+) and NPY-knockout (NPY−/−) mice. Neuroendocrinology. 2005;81(6):360–71. doi: 10.1159/000089101. [DOI] [PubMed] [Google Scholar]
- Perreault M, Istrate N, Wang L, Nichols AJ, Tozzo E, Stricker-Krongrad A. Resistance to the orexigenic effect of ghrelin in dietary-induced obesity in mice: reversal upon weight loss. Int J Obes Relat Metab Disord. 2004;28(7):879–85. doi: 10.1038/sj.ijo.0802640. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–86. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Sakata I, Yang J, Lee C, Osborne-Lawrence S, Rovinsky S, Elmquist JK, Zigman J. Co-Localization of Ghrelin O-Acyltransferase (GOAT) and Ghrelin in Gastric Mucosal Cells. Am J Physiol Endocrinol Metab. 2009;297(1):E134–41. doi: 10.1152/ajpendo.90859.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Ida T, Sato T, Nakashima Y, Nakamura Y, Tsuji A, Kojima M. Production of n-octanoyl-modified ghrelin in cultured cells requires prohormone processing protease and ghrelin O-acyltransferase, as well as n-octanoic acid. J Biochem. 2009 doi: 10.1093/jb/mvp112. [DOI] [PubMed] [Google Scholar]
- Tannenbaum GS, Lapointe M, Gurd W, Finkelstein JA. Mechanisms of impaired growth hormone secretion in genetically obese Zucker rats: roles of growth hormone-releasing factor and somatostatin. Endocrinology. 1990;127(6):3087–95. doi: 10.1210/endo-127-6-3087. [DOI] [PubMed] [Google Scholar]
- Toshinai K, Mondal MS, Nakazato M, Date Y, Murakami N, Kojima M, Kangawa K, Matsukura S. Upregulation of Ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem Biophys Res Commun. 2001;281(5):1220–5. doi: 10.1006/bbrc.2001.4518. [DOI] [PubMed] [Google Scholar]
- Ueno N, Asakawa A, Inui A. Blunted metabolic response to fasting in obese mice. Endocrine. 2007;32(2):192–6. doi: 10.1007/s12020-007-9016-z. [DOI] [PubMed] [Google Scholar]
- van der Lely AJ, Tschop M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev. 2004;25(3):426–57. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- Williams DL, Cummings DE. Regulation of ghrelin in physiologic and pathophysiologic states. J Nutr. 2005;135(5):1320–5. doi: 10.1093/jn/135.5.1320. [DOI] [PubMed] [Google Scholar]
- Yada T, Dezaki K, Sone H, Koizumi M, Damdindorj B, Nakata M, Kakei M. Ghrelin regulates insulin release and glycemia: physiological role and therapeutic potential. Curr Diabetes Rev. 2008;4(1):18–23. doi: 10.2174/157339908783502352. [DOI] [PubMed] [Google Scholar]
- Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132(3):387–96. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Zhou X, De Schepper J, Vergeylen A, Luis O, Delhase M, Hooghe-Peters EL. Cafeteria diet-induced obese rats have an increased somatostatin protein content and gene expression in the periventricular nucleus. J Endocrinol Invest. 1997;20(5):264–9. doi: 10.1007/BF03350298. [DOI] [PubMed] [Google Scholar]
- Zizzari P, Longchamps R, Epelbaum J, Bluet-Pajot MT. Obestatin partially affects ghrelin stimulation of food intake and growth hormone secretion in rodents. Endocrinology. 2007;148(4):1648–53. doi: 10.1210/en.2006-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.