Abstract
Recent experiments in vivo and in vitro have advanced our understanding of the sites and mechanisms involved in mammalian respiratory rhythm generation. Here we evaluate and interpret the new evidence for two separate brainstem respiratory oscillators and for the essential role of emergent network properties in rhythm generation. Lesion studies suggest that respiratory cell death might explain morbidity and mortality associated with neurodegenerative disorders and ageing.
Breathing is a deceptively simple yet remarkable behaviour in vertebrates that regulates gas exchange in the lungs to support metabolism, regulate pH and, in non-primate mammals, regulate temperature (BOX 1). Breathing persists from birth until death. In a human lifespan of 80 years, respiratory movements repeat more than 5 × 108 times. Breathing at rest remains relatively unchanged throughout healthy adulthood, but breathing rates are exceptionally labile to acute challenges such as changes in posture, exercise and sleep. At rest, a 70 kg adult human male uses ∼250 ml O2 per minute. Because the body's reservoir of O2 is only ∼1 litre, and as low levels of blood O2 for more than a few minutes can cause irreversible brain damage, the need to breathe continuously is obvious. During sustained movements driven by large muscles, such as when chasing prey or fleeing predators, O2 consumption increases significantly. Even during a more modest movement such as walking, human O2 consumption triples to ∼800 ml per minute. Given the limited O2 reservoir, breathing must rapidly increase to satisfy the metabolic demands of sustained movement and to maintain consciousness. Breathing also adapts readily to accommodate slower changes associated with development, disease, pregnancy and ageing.
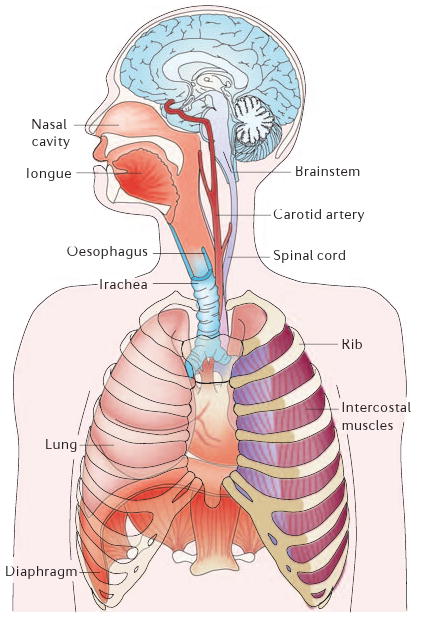
Box 1. Anatomy and physiology of the respiratory system.
When the thoracic cavity expands during inspiration by contraction of the diaphragm and external intercostal muscles, the lungs expand and air flows in at a rate dependent on airway resistance. Expiration is often passive, especially at rest, as the lungs and ribcage recoil to their equilibrium positions.
There are two classes of motor output, which involve pump muscles and resistance muscles. The lung expands during inspiration owing to contraction of the diaphragm (a pump muscle), a dome-like sheet of muscle that separates the thorax from the abdomen. The diaphragm is the principal inspiratory muscle and is unique to mammals. Skeletal muscles of the mouth, nose and throat, including the tongue and glottis, and smooth muscles of the bronchi modulate airway resistance.
In breathing, the brain links sensory information to motor output. Respiratory rhythm must be transformed into an energy efficient pattern of movement appropriate to the ongoing metabolic demand. The chemosensory organs that monitor blood O2 and CO2 levels are located primarily in the carotid bodies and in the brainstem. The carotid bodies are located at the bifurcation of the carotid arteries and produce signals that relate mostly to O2 levels in arterial blood. In the absence of functioning carotid bodies, ventilation becomes mostly sensitive to changes in CO2 and pH, which are generally accepted to be sensed in many regions of the brainstem, including the preBötzinger Complex and the ventral medullary surface23. Most recently, Guyenet and colleagues26,28 presented compelling evidence to suggest that the original view, that central chemoreception is the province of the ventral medullary surface, is correct, and that the retrotrapezoid nucleus (BOX 2) is a critical site.
Lung and cardiac pathologies are the main causes of breathing disorders, but dysfunctions relating to the neural control of breathing also have a significant effect on public health. Such dysfunctions include sleep apnoea, and possibly sudden infant death syndrome (SIDS)1. Several genetic disorders manifest in abnormal respiration, including Rett syndrome2 and congenital central hypoventilation syndrome (CCHS, also known as Ondine's curse)3,4. Death due to central respiratory arrest during sleep is probably common in neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS), Parkinson's disease and multiple systems atrophy (MSA), and perhaps in the elderly (see below).
What are the mechanisms that underlie the generation and modulation of breathing in humans? What perturbations to these mechanisms cause respiratory pathologies? With the possible and rare exception of single gene deletions that produce a respiratory phenotype in humans5 (such as the methyl CpG-binding protein 2 (MeCP2) mutation that causes Rett syndrome2), these questions cannot be addressed directly in humans for ethical and technical reasons — for example, significant limitations associated with brainstem anatomy and perfusion impede the use of functional imaging technologies6.
Our modern understanding of the neural mechanisms of breathing originates from experiments in anaesthetized or decerebrate dogs, cats and rabbits7. More recently, especially after the demonstration that the neonatal rat brainstem and spinal cord isolated in vitro, dubbed the en bloc preparation, could generate a rhythmic respiratory-related motor pattern8, rodents became the model of choice. Two subsequent experimental discoveries, addressing the site and mechanism of rhythm generation, fuelled most of the research we report here. First, respiratory rhythm persists in thin brainstem slices that encompass a small region of the ventrolateral medulla named the preBötzinger Complex9,10 (preBötC) (BOX 2c,d). These experiments first identified the preBötC and led to the hypothesis that the preBötC was the source of respiratory rhythm9–12. Second, rhythm persists en bloc13,14 and in slices13–17 after the attenuation of postsynaptic inhibition. This led to the hypothesis that intrinsically rhythmic pacemaker neurons drive the respiratory rhythm10–12,14,18.
Box 2. Respiratory regions in lateral rhombencephalon.
The preBötzinger Complex (preBötC) and retrotrapezoid nucleus/parafacial respiratory group (RTN/pFRG) are part of a continuous ventral respiratory column (VRC). Caudally within this column, excitatory inspiratory and expiratory premotor neurons are concentrated in regions termed the rostral (rVRG) and caudal (cVRG) ventral respiratory groups, respectively. The preBötC contains rhythm-generating interneurons. The BötC contains expiratory inhibitory interneurons. The RTN/pFRG contains interneurons that might generate expiratory rhythms. Panel a shows transverse sections (1–5) of adult rat brainstem with retrogradely labelled neurons (red) following FluoroGold injection in the rostral VRG (at 5). Arrows in panel b show the approximate levels of sections 1–5. Panel c shows a transverse slice encompassing the preBötC that is used for in vitro experiments. The brainstem location of the slice shown in panel c is indicated by the red bar in panel d (6). Panels b and d represent sagittal sections of rat brainstem depicting retrograde labelling (b) after tracer injection in the preBötC, and the functionally defined respiratory sites (d). Cutting planes X and Y in panel b indicate levels where more rostral brainstem removal does not interfere with expiratory motor output (X) or eliminates expiratory motor output (Y). Panel d shows how adult rhombencephalic regions map to their embryonic rhombomeres (black arrows). Rhombomeres 4–5 (r4–5) are shown to include the facial nucleus (7) because it originates in r4–5 but migrates developmentally to r6. 5n, trigeminal nerve; 7n, facial nerve; 12n, hypoglossal nerve; A5, A5 noradrenergic neurons; AmbC, nucleus ambiguus, compact part; Itr, intertrigeminal nucleus; KF, Kölliker-Fuse nucleus; LPBr, lateral parabrachial region; LRt, lateral reticular nucleus; Mo5, motor nucleus of the trigeminal nerve; Pn, basilar pontine nuclei; scp, superior cerebellar peduncle; SO, superior olive; VL pons, ventrolateral pons. Panels b and d modified, with permission, from REF. 108.

Here we evaluate the evidence for these hypotheses in relation to the sites and mechanism(s) of rhythmogenesis, and we propose modifications of both on the basis of recent developments. We posit that there are two distinct respiratory rhythm generators (RRGs) in the medulla19, which are normally coupled: the preBötC, discovered in 1990 (REFS 9,10), and the retrotrapezoid nucleus (RTN). The latter was discovered in 1989 (REF. 20) and postulated as a candidate for a RRG in 1990 (REF. 9), but was largely ignored as such until 2003–2005 when a related, apparently overlapping and perhaps identical, newly named region, the parafacial respiratory group (pFRG), was proposed as a RRG21. It is not yet known whether the RTN and pFRG are anatomically and functionally distinct, and here we use the designation RTN/pFRG to refer to this general region (BOX 2). We suggest that in adult mammals at rest the rhythm is dominated by the preBötC22. Furthermore, we propose that emergent properties of the preBötC engender a network oscillator in which bursting-pacemaker neurons can be embedded but are not obligatory for rhythm generation.
The restricted scope of this article precludes discussion of topics essential for a comprehensive understanding of the neurobiology of breathing, including chemoreception23–28, pontine sites and their contributions to respiratory behaviours29, expiratory pattern generation in vivo7,30,31, airway protective reflexes, development32–38, plasticity23,39, and the role of glutamate, GABA (γ-aminobutyric acid), glycine, noradrenaline, serotonin, acetylcholine, and other neurotransmitters and modulators in shaping respiratory pattern11, and in pathologies affecting respiratory rhythm (for an example, see REF. 40).
Distinct respiratory oscillators
The nervous system could ensure that breathing remains robust and reliable by various means including multiple oscillator sites and multiple mechanisms for oscillation41, as well as through synaptic inhibition, which also serves in the shaping of the breathing pattern13,17,42–45. Here, we address the idea of multiple oscillators.
Inspiratory and expiratory rhythms
The idea that a dominant rhythm generator is localized in the preBötC is supported by several observations. First, medullary slices containing the preBötC generate a rhythm that is indistinguishable from the respiratory-related rhythm en bloc, although this pattern differs from that seen in vivo, which is probably due largely to the absence of peripheral and descending inputs, and low temperature (in vitro preparations are often studied at room temperature) and other environmental differences31,46,47. Second, almost complete bilateral lesion of a subclass of preBötC neurons in intact awake adult rats induces an irreversible pathological, ataxic breathing pattern that is quite different from normal breathing48. These data confirm the importance of the preBötC, but do not exclude the presence of other respiratory oscillators that might rely on the preBötC. Third, genetic deletion of the transcription factor MafB (v-maf musculoaponeurotic fibrosarcoma oncogene homologue B) results in markedly abnormal breathing patterns in neonatal mice49. The principal neuroanatomical disturbance in these mice is reported to be a marked reduction in the number of preBötC neurons. Fourth, an essentially normal inspiratory motor pattern persists after transection of the brainstem just rostral to the preBötC in rats, which removes all suprapontine and pontine respiratory-related circuits (and the RTN/pFRG)22. These data contradict long-standing claims that such transections irreversibly eliminate normal breathing and instead cause gasping50,51; these assertions have long influenced views of the brainstem organization of breathing circuits, and particularly the role of the pons52, which now requires re-evaluation.
Recent evidence suggests that a second site contributes to the rhythm of breathing. Neuronal population imaging of en bloc preparations reveals a cluster of rhythmically active neurons concentrated in the RTN/pFRG21 (FIG. 1). Most of these neurons are categorized as pre-inspiratory (pre-I) for their tendency to discharge before the inspiratory burst. (This nomenclature is potentially misleading because pre-I neurons are actively inhibited during inspiration and then typically rebound to discharge again during the post-inspiratory/early-expiratory phase.) Rhythmically active RTN/pFRG pre-I neurons are proposed to interact with preBötC neurons as a coupled oscillator system to regulate the rhythm of the intact mammal19,22,53 (BOX 2a,d; FIG. 1). Unlike the preBötC, the RTN/pFRG has not yet been captured in rhythmically active slice preparations in vitro, but this may just be a matter of time.
Figure 1. Ventral view of en bloc brainstem showing voltage-dependent respiratory neuronal activity.
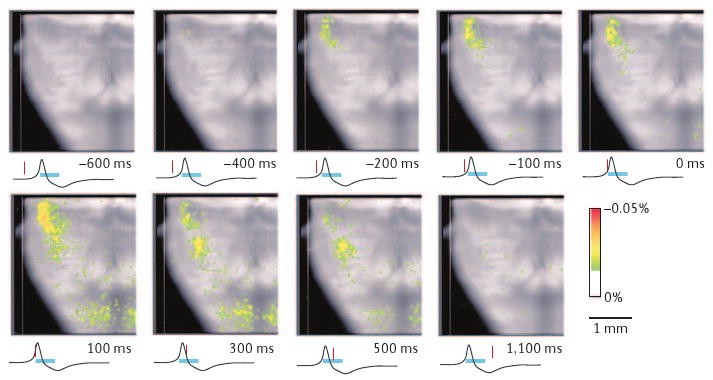
Frames of averaged (∼100 respiratory cycles) optical signals from bath-applied voltage-dependent dye (Di-2-ANEPEQ) reveal two discrete regions of respiratory-modulated neurons in the ventrolateral medulla. Averaging was synchronized to the onset of rhythmic inspiratory burst activity in the C4 ventral nerve root (black trace below each image; burst duration marked by blue bar). The red marker below each frame shows the time of each frame relative to C4 output. One cluster of activity appears at the top (rostral) of each frame ∼400 ms before inspiratory onset, whereas a second more caudal cluster appears ∼100 ms before. The rostral cluster corresponds to the retrotrapezoid nucleus/parafacial respiratory group and the caudal cluster corresponds to the preBötzinger Complex (BOX 2). Reproduced, with permission, from REF. 21.
Functional evidence for a second oscillator comes from experiments exploiting the differences in the pharmacological properties of most neurons in the preBötC compared with those in the RTN/pFRG. The μ-opiate agonist DAMGO (d-Ala(2),NMePhe(4),Gly-ol(5)enkephalin) hyperpolarizes a subset of preBötC inspiratory neurons16. At concentrations that eliminate motor output in en bloc preparations (>500 nM), DAMGO abolishes the synaptic drive to most inspiratory neurons of the ventral respiratory column, including many in the preBötC, but appears to have no direct postsynaptic effects on pre-I neurons, and does not affect their ability to oscillate in synchrony54. If the rhythm in pre-I neurons does not change in the presence of opiates, even though preBötC neurons are depressed, how do opiates slow breathing? Differences in the effects of opiates on respiratory motor output in slices versus en bloc preparations reveal a surprising explanation19.
Both slice and en bloc preparations preserve the preBötC, but only the en bloc preparation retains the RTN/pFRG (BOX 2d). In slices, low (200 nM) concentrations of DAMGO result in a continuous increase in inspiratory periods (FIG. 2a, top). In en bloc preparations, the same low concentrations of DAMGO also cause inspiratory periods to increase on average, but this slowing in breathing results from one or more skipped inspiratory bursts (FIG. 2a, bottom). In other words, in the en bloc preparation, DAMGO decreases the mean frequency of inspiratory motor nerve output, but the synchronized bursts in pre-I neurons continue at the control frequency and remain phase-locked to motor nerve output when it occurs (FIG. 2b, bottom)19. In addition, recordings from preBötC neurons during the intervals between inspiratory motor outputs show phasic synaptic potentials at the control frequency that presumably reflect inputs from the RTN/pFRG (FIG. 2b, top and centre)19.
Figure 2. Opiate agonists induce quantal slowing of inspirations without affecting frequency of active expirations.
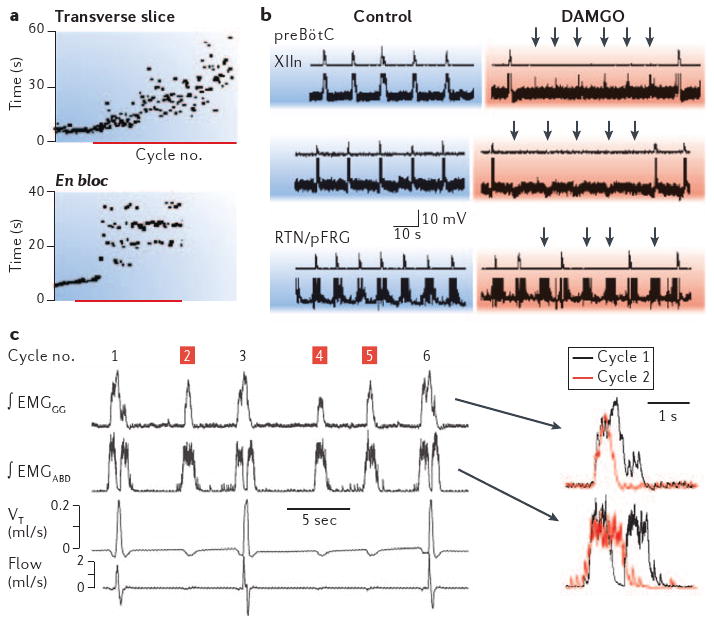
a | Sequential plots of inspiratory period in vitro before and after treatment with the μ-opiate agonist DAMGO (d-Ala(2),NMePhe(4),Gly-ol(5)enkephalin, red bar). The top trace shows continuous slowing of rhythm in the slice; the bottom trace shows quantal slowing of rhythm en bloc. b | Simultaneous recordings of inspiratory burst activity in control and DAMGO-treated XII nerve and preBötzinger Complex (preBötC) inspiratory neurons (top two pairs of traces) and pre-inspiratory (pre-I) neurons in the retrotrapezoid nucleus/parafacial respiratory group (bottom pair). Arrows in DAMGO traces indicate subthreshold events in preBötC neurons during skipped bursts, which occur at the approximate time expected for inspiratory bursts at control frequency, or unperturbed bursting in pre-I neurons. c | In vivo recordings in juvenile rats following fentanyl injection. Traces on the left show typical recording with normal cycles (1, 3, 6) interspersed with cycles without inspiratory activity (2,4,5). ∫EMGABD, integrated abdominal muscle electromyogram (EMG); ∫EMGGG, integrated genioglossus muscle EMG; flow, air flow (up or down for inspiratory or expiratory air flow, respectively); VT, tidal volume. Traces on the right show superimposition of ∫EMGGG and ∫EMGABD in a normal cycle (black) compared with an inspiratory skipped cycle (red). Note the marked differences, especially in the ∫EMGABD burst. Panels a and b reproduced, with permission, from REF. 19 © (2003) Cell Press. Panel c reproduced, with permission, from REF. 22.
This type of quantal slowing of inspiratory rhythm also occurs in vivo. Quantal slowing of inspiratory activity is seen in vagotomized, anaesthetized juvenile rats given mild doses (0.02 mg kg–1) of the μ-opioid agonist fentanyl53 (FIG. 2c). Interestingly, when expiratory motor nerve activity is also recorded under these conditions in vivo, its frequency is unaffected but its burst pattern is markedly affected19. Expiratory efforts and airflow occur at the same pace both before and after fentanyl treatment, whereas after fentanyl administration inspiratory muscle activity no longer occurs during every cycle (FIG. 2c). This is unlikely to be due to a simple block of inspiratory motor output, that is, of (pre)motor neurons, as the pattern of expiratory motor activity is quite different in cycles with and without inspiration (FIG. 2c, inset traces). During normal cycles, expiratory effort occurs just before, and again just after, inspiration. In cycles without inspiration, expiratory activity is a single burst that lacks the inspiratory-related pause, as if the mechanism for inspiration is inactive and expiratory activity evolves without interruption.
Lesions or transections can be used to separate the regions that generate inspiratory and expiratory activity. In en bloc preparations, bilateral lesion of the RTN/pFRG causes a marked reduction in respiratory frequency21, which suggests that the RTN/pFRG can modulate respiratory rhythm. In vivo, in vagotomized and anaesthetized juvenile rats, complete brainstem transections rostral to the VII nucleus (BOX 2b, line X) that leave the RTN/pFRG and preBötC intact do not markedly affect inspiratory or expiratory motor bursting activity, whereas transections at the caudal end of the VII nucleus (BOX 2b, line Y) that disconnect the rostral portion of the RTN/pFRG from the medulla abolish expiratory motor activity without affecting inspiratory rhythm. As mentioned above, these new data have resulted in the need for a re-evaluation of the dogma that such transections cause obligatory gasping (but see REF. 55 for an alternative view), and the idea that the pons is essential for normal rhythmogenesis52. These new observations, combined with the fact that transverse slices generate a respiratory-related rhythm, clearly establish a central role for the preBötC in rhythm generation, and show that in some conditions22 but not others48,56 in the absence of preBötC activity, the RTN/pFRG can also generate ventilation by active expiration and passive inspiration.
We propose that the preBötC generates inspiratory rhythm and the RTN/pFRG generates expiratory activity, and that these two oscillators are coupled22 (FIG. 3). This idea is further supported by the observation that partial ablation of the preBötC in awake adult goats57 or sudden noxious stimuli58 in young humans can result in quantal slowing of inspiration, that is, skipped inspiratory cycles, while rhythmic expiratory activity of the abdominal muscles persists. In addition, continuous lung inflation suppresses inspiratory activity and enhances expiratory drive, whereas lung deflations increase the rate of inspiration, while minimizing rhythmic expiratory efforts22.
Figure 3. Summary of our view of the gross organ ization of respiratory rhythmogenesis in the brainstem of mammals.
We propose that there are two oscillators that are differentially affected by various inputs, such as opiates and lung inflation and deflation. The more rostral oscillator is located in the region of the retrotrapezoid nucleus/parafacial respiratory group (RTN/pFRG) and appears to drive active expiratory activity. It might not be rhythmic in mammals at rest, when there is little or no active expiration. The more caudal oscillator is located in the preBötzinger Complex (preBötC) and appears to drive inspiratory activity. Substance P-saporin (SP-SAP) lesion of preBötC neurokinin 1 receptor (NK1R) neurons disrupts breathing. Transections between the two oscillators disrupt expiratory motor outflow, while inspiratory activity continues unabated. Lung inflation enhances the activity of the expiratory oscillator and depresses the inspiratory oscillator, which serves, ultimately, to reduce lung volume, whereas lung deflation has the opposite effect on the inspiratory and expiratory oscillators. We further speculate that the mechanism of rhythmogenesis in the preBötC involves a group pacemaker. However, this model does not resolve the question of why lesions of the preBötC NK1R neurons in adult rats should disrupt rhythm and render breathing ineffective.
If there are two distinct oscillators, three questions arise. First, how did a system with distinct oscillators evolve? Two key events characterize the evolution of breathing from fish to mammals (BOX 1). The first includes transform ations that support the transition from aquatic to terrestrial breathing in vertebrates. Breathing in fish is driven by cranial nerve innervation of the gills. As primitive fish (such as the ancestors of the lungfish) ventured onto land, rhythmic activity of muscles used for other purposes evolved to drive the emerging lung, and this activity might have had a neural origin that was independent of the rhythm driving the gill muscles. This speculative hypothesis is supported by observations in amphibians. Developing frogs have two distinct breathing patterns driven by different muscles and nerves: a buccal pattern that uses cranial nerve-innervated muscles to essentially swallow air, and a lung pattern that uses respiratory muscles to pump the lung. Either the buccal or the lung pattern can be suppressed without abolishing its counterpart, but when both are active they appear to be phase-locked59. Notably, the lung rhythm is opiate-sensitive (like the mammalian preBötC neurons and inspiration), whereas the buccal rhythm is not60 (like pre-I neurons and expiration). A prediction from this hypothesis is that the preBötC is absent in fish.
The second key event was the emergence of the diaphragm in mammals. Respiratory physiology in vertebrates has evolved to support higher resting and peak ventilation. For example, lizards have gular pumps that uncouple respiration from locomotor musculature, which enhances O2 consumption to support high-speed locomotion61. Birds use sacs to pump O2-rich air through non-compliant lungs62 to support flight. Uniquely, mammals have muscular diaphragms (BOX 1), a profoundly important ventilatory advance as it allows for continuous high basal rates of ventilation and for the high levels of ventilation necessary to support substantial increases in metabolism. The improvements in gas exchange associated with the development of the diaphragm were probably crucial for brain evolution, as it provided a platform for evolution of a brain that consumes extraordinary amounts of O2 and is generally intolerant of even transient anoxia. The neural circuits driving the primitive diaphragm could have evolved independently of pre-existing circuitry driving the rhythmic pumping machinery of the rib cage, abdominal or upper airway muscles; or it could have been based on the opiate-sensitive oscillator driving lung breathing in amphibians60. Nonetheless, a respiratory movement pattern consisting of alternating inspiratory activity to lower the diaphragm with expiratory motor activity has considerable mechanical advantages for efficient ventilation over a broad range of metabolic demand. Therefore, the neural oscillators for breathing, which may have arisen independently, might be expected to have evolved to be coupled.
Second, are these oscillators equal partners or does one or the other dominate rhythm and, if so, under what conditions? In vitro, RTN/pFRG neurons activate several hundred milliseconds before preBötC neurons (FIG. 1), which Onimaru and Homma interpret as evidence that the rostral RTN/pFRG is the trigger site for inspiratory rhythm21. However, in vivo, the functional role of RTN/pFRG seems to be more closely tied to expiration than inspiration22. Reptiles and amphibians with lower body temperatures, lower metabolic rates and no diaphragm have a breathing pattern of active expiration and passive inspiration. On the other hand, at rest, mammals, which have a diaphragm as well as higher body temperatures and metabolic rates, breathe with a pattern of active inspiration and passive expiration; the RTN/pFRG might not even be rhythmic until there is active expiration22, although its tonic activity would affect the excitability of the preBötC28. Breathing in behaving adult rats is pathologically disrupted by destruction of the preBötC19,48 (see below). Collectively, these results suggest that the balance in the dominance of these two oscillators shifts towards the preBötC in mammals under normal conditions. Under resting conditions, when there is typically no active expiration, it may only be the preBötC that is rhythmic.
There might be a special role for two oscillators at birth. In mammals there is a surge of endogenous opiates at birth63 (see discussion in REF. 22). This postnatal increase in opiate release would depress the excitability of the preBötC but not that of the RTN/pFRG, allowing the latter to act as an ‘anti-apnoea’ centre that would promote breathing immediately after birth64, either by rhythmically driving breathing or by providing the drive necessary to keep the preBötC rhythmic22. This view is supported by experiments in Krox20–/– (early growth response 2) mice, which have neuroanatomical defects that appear to result in deletion of the brainstem region containing the RTN/pFRG but do not seem to affect the preBötC32,64. These mutant mice die of fatal apnoeas within 18 h of birth, but brief postnatal administration of naloxone (which has no effect in control mice) rescues these pups by eliminating apnoea. So, without the RTN/pFRG to provide respiratory drive, the opiate surge at birth could fatally depress preBötC function. We suggest that in Krox20–/– mice, in the absence of the RTN/pFRG, the opiate-induced depression of the preBötC causes prolonged and ultimately fatal apnoeas; naloxone would reverse this depression, which is developmentally relieved within 2 days64, allowing the preBötC to function adequately for survival. This special role for the RTN/pFRG at birth is consistent with the two-oscillator model.
Third, are there possible benefits of two (or more) distinct rhythmogenic networks? The respiratory rhythm must be robust over about an order of magnitude in O2 consumption, persisting without substantive interruption from birth until death. At the same time, it must adjust rapidly to changes in metabolic demand, such as during exercise. In humans at rest, a 2% increase in arterial CO2 can produce a >30% increase in ventilation. However, high-gain systems are prone to instability, so there must be neural mechanisms to ensure stability while remaining labile to feedback65. Therefore, the RRG is embedded in a dense plexus of feedback sources, which includes lung, muscle and joint mechanoreceptors, lung and airway nociceptors, and chemoreceptors. A system of two RRGs that responds differently to various sensory or modulatory inputs might be helpful in this regard. If one RRG responds rapidly while the other is insensitive (FIG. 3), breathing could respond rapidly to various stimuli, yet remain stable. Excitatory and inhibitory coupling between inspiratory activity and RTN/pFRG neurons19, and different responses to opiates and to pulmonary afferents22 are consistent with this idea.
Effects of preBötC lesions
Breathing does not stop during wakefulness, except during transient behaviours such as speech, emesis or defecation. However, during sleep or unconsciousness breathing is less reliable, and sleep-disordered breathing, such as sleep apnoea, is quite common, even in otherwise healthy humans. Lesions of the preBötC can lead to significant disruption of, or even pathological, breathing56.
After local administration by micropipette of a potent and specific neurotoxin (substance P conjugated to saporin: SP-SAP) that slowly kills neurons that express neurokinin 1 receptors (NK1Rs) to the preBötC in adult rats, breathing deteriorates in a fixed sequence over a period of days48,56. Sustained and repeated apnoeas first appear during REM sleep, while breathing remains normal during wakefulness and non-REM sleep. These disturbances then spill over into non-REM sleep without any marked changes in breathing during wakefulness (FIG. 4). Finally, ataxic breathing develops that extends into wakefulness48. Humans rarely exhibit ataxic breathing during wakefulness, which suggests that such extensive loss of preBötC neurons is unlikely, perhaps because humans die long before they reach this stage. These data emphasize the essential importance of the preBötC for the maintenance of normal breathing, and suggest that deterioration of preBötC function probably requires significant neuronal loss48, which produces pathological breathing patterns that first manifest during sleep. These results are consistent with the two-oscillator model, yet clearly show that the RTN/pFRG is insufficient, on its own, to drive breathing after some types of lesion to the preBötC, at least in adult rats. The mechanisms that preclude independent oscillations in the RTN/pFRG of adults are unknown, but RTN/pFRG function might depend on some level of tonic preBötC activity that is lost after extensive lesions of the latter.
Figure 4. Lesions of the preBötzinger Complex significantly disturb breathing pattern in an unrestrained, unanaesthetized adult rat.

SP-SAP was injected bilaterally into the preBötzinger Complex (preBötC) and by (or shortly after) day 10 post-injection had killed >80% of preBötC neurokinin 1 receptor (NK1R) neurons. Under control conditions, breathing is continuous throughout the sleep–wake cycle, as determined from electroencephalogram (EEG) and neck electromyogram (EMG) signals (not shown). However, on day 8 after SP-SAP administration breathing pattern ceases to be normal during REM (rapid eye movement) sleep, when apnoea develops and ventilation is restored only on waking, a cycle that then repeats. DIAEMG, integrated diaphragmatic EMG; Iamp, inspiratory amplitude; NREM, non-REM sleep; W, wakefulness. Reproduced, with permission, from REF. 56.
Serious disruption of breathing during sleep is seen in at least three human neurodegenerative diseases: Parkinson's disease66,67, MSA68,69 and ALS70,71. During the late stages of Parkinson's disease and MSA there is substantial loss of NK1R neurons in a region of the human ventrolateral brainstem that includes the preBötC72. If this reflects loss of preBötC neurons, then significant disturbances of breathing during sleep, but not wakefulness, could result. In ALS, the neurodegenerative process might target neurons that lack Ca2+ buffers73. Many motor neuron pools, the degeneration of which underlies muscle atrophy in ALS, lack such buffers. However, other neurons also die. The Ca2+ buffer parvalbumin is widely distributed in neurons in the ventral respiratory column (BOX 2), with the notable exception of the preBötC74. Two other Ca2+ buffers, calretinin and calbindin, might also be lacking in preBötC neurons. Therefore, preBötC neurons might be vulnerable to neurodegeneration in ALS, and, if so, their loss could contribute to the respiratory failure and death during sleep that often accompanies late stage ALS.
Our data from rats indicate that although disrupted breathing during wakefulness is only apparent after substantial preBötC neuronal loss, disruptions in breathing during sleep are apparent earlier. In otherwise healthy humans, the incidence of central sleep apnoeas, as distinguished from obstructive sleep apnoeas, is relatively low until ∼65 years of age, and increases rapidly thereafter75. The likelihood of central sleep apnoeas increases with age in the elderly and may be part of the natural aging process75; we suggest that a cumulative lifetime loss of preBötC neurons contributes to this increase56. As mentioned above, preBötC neurons might be vulnerable to neurodegeneration. Recurring apnoeas (whether central or obstructive) could initiate a vicious cycle in which repeated episodes accelerate the loss of preBötC neurons, exacerbating apnoeas and ultimately increasing the threshold for arousal to wakefulness that is essential to restart breathing. As preBötC degeneration progresses, death by asphyxia resulting from a failure to arouse from apnoea or by an induced cerebro- or cardiovascular incident during sleep would become more likely56. These conditions that increase the susceptibility to death have been associated with sleep-disordered breathing, central apnoeas in particular, in elderly populations76.
Mechanisms of rhythm generation
The slice preparation isolates the preBötC and maintains inspiratory motor nerve activity. As an in vitro system, the slice lacks afferent inputs, and is studied below normal body temperature. Robust and long-lasting (hours) rhythm generation in slices requires an ionic or pharmacological boost, typically elevated extracellular K+, whereas en bloc preparations do not require such a boost. Rostral respiratory networks, including the RTN/pFRG, are not contained in slices (BOX 2c, FIG. 2). Therefore, the slice represents the minimal experimental model of inspiratory activity. Understanding the mechanisms that underlie inspiratory rhythm generation in the slice will serve as a foundation for unravelling the mechanisms in more intact preparations, including en bloc and in vivo.
The pacemaker hypothesis
The demonstration that in vitro rhythms continue unabated after postsynaptic inhibition is blocked14 led directly to the hypothesis that pacemaker neurons generate the respiratory rhythm10. Subsequently, preBötC neurons with bursting-pacemaker properties that depend on a persistent Na+ current (INaP) were identified10,77. Their voltage-dependent bursting mechanism is now well understood (BOX 3); it is unaffected by Cd2+ but is antagonized by riluzole, which blocks INaP78,79. The proportion of preBötC inspiratory neurons with voltage-dependent pacemaker properties is between 5 and 25%78,80–82. Another phenotype of pacemaker emerges developmentally, being <1% of the preBötC population before postnatal day (P)4, and becoming ∼8% of the preBötC population between P8 and P15 (there are no published data for >P15)82,83. In contrast to INaP pacemakers, these neurons are voltage-independent and their activity is reduced by Cd2+ or flufenamic acid (FFA), which suggests that bursting depends on Ca2+ and/or Ca2+-activated nonspecific and voltage-insensitive cation current (ICAN)81,82.
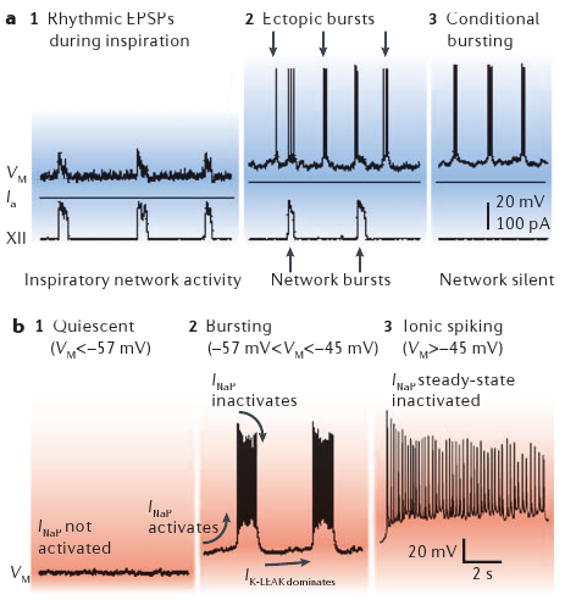
Box 3. Persistent Na+ current and the pacemaker hypothesis.
Bursting-pacemaker neurons are capable of oscillating between spiking and quiescent phases, when synaptically isolated. The duty cycle of bursting mimics respiratory rhythms in vitro.
A phenotype of voltage-dependent bursting neurons is found in the preBötzinger Complex (preBötC) of neonatal rodents (postnatal day (P)0–15). These neurons exhibit excitatory postsynaptic potentials (EPSPs) in phase with XII nerve motor output at baseline membrane potentials of −60 mV (or lower) but are otherwise silent (panels a1 and b1). Tonic depolarization above −57 mV causes ectopic bursting during their normally silent period, that is, bursts similar to those occurring during inspiration, which reflect an intrinsic burst-generating mechanism (panel a2). Pacemaker neurons maintain voltage-dependent bursting in the range of −57 to −45 mV after synaptic isolation (panels a3 and b2). When depolarization exceeds −45 mV, bursting gives way to tonic spiking (panel b3).
Voltage-dependent bursting depends on persistent Na+ current (INaP) and leakage K+ current (IK-LEAK). INaP causes burst depolarization and IK-LEAK regulates excitability and sets the baseline membrane potential. Bursts self-terminate owing to INaP inactivation.
Bursting is conditional because it depends on baseline membrane potential. Depolarizing the baseline membrane potential imposes steady-state inactivation that limits the amount of INaP available for subsequent bursts. By limiting INaP, baseline depolarization decreases burst duration and reduces the time needed for de-inactivation of INaP after a burst. Therefore, depolarization shortens the burst cycle and increases burst frequency. Tonic inputs can also hyperpolarize the baseline to −57 mV (or lower), which fails to activate INaP and the neuron remains quiescent (panel b1). Depolarization to −45 mV (or higher) steady state inactivates INap to such an extent that burst cycles are no longer possible and the neuron spikes tonically (panel b3).
INaP is ubiquitous in preBötC neurons and pacemaker properties occur in a subset with appropriate INaP/IK-LEAK ratios. Whether these neurons contribute to or are essential to rhythm generation is discussed in the text. VM, membrane potential of a rhythm-generating neuron. Ia, applied current.
The pacemaker hypothesis, which in its strongest form posits that pacemaker neurons are obligatory for rhythm generation, predicts that abolishing endogenous bursting activity should severely disrupt or abolish respiratory rhythm. Surprisingly, if bath application of riluzole is used to silence synaptically isolated INaP pacemaker neurons, the frequency of rhythmic motor output in slices is unaffected81. Is it possible that ICAN-mediated bursting neurons, which are riluzole-insensitive and sparse (constituting <1% of neurons during P0–P5), drive the rhythm in the absence of INaP? A means of testing this experimentally would be to abolish bursting behaviour in both populations of pacemaker neurons simultaneously by pharmacologically blocking INaP and ICAN. If rhythm generation depends on either or both types of pacemaker neuron, then this manipulation should abolish respiratory rhythm. Co-application of riluzole and FFA does silence the respiratory rhythm in mouse slices in vitro81,82. However, as almost all preBötC neurons express INaP78,84 and ICAN81, these drugs also lower overall neuronal excitability throughout the network: blocking INaP hyperpolarizes baseline membrane potentials and blocking both currents removes inward currents that ordinarily enhance inspiratory synaptic drive. Therefore, the loss of rhythm could simply be due to riluzole and FFA lowering the excitability of many, or even all, neurons, regardless of their effects on pacemaker properties. Consistent with this idea, AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) and substance P excite preBötC neurons without creating the requisite region of negative slope in the current-voltage relationship16,85 that is necessary for endogenous rhythmic bursting in a synaptically isolated neuron. Yet bath-applied substance P (0.5–2 μM) or AMPA (0.5 μM) can restart the respiratory rhythm in the presence of riluzole and FFA81. These results challenge the obligatory rhythmogenic role of pacemaker neurons. Moreover, we suggest that in the absence of substantial corroborating evidence, one cannot interpret any effect on respiratory rhythm of riluzole, FFA or tetrodotoxin, in vitro, in situ or in vivo, as evidence that pacemaker neurons have any, much less an obligatory, role in respiratory rhythm generation.
Aspects of our view of the role of pacemaker neurons are disputed86. What is not disputed is that intrinsic INaP and ICAN are ubiquitous in preBötC neurons and contribute to burst generation in the context of inspiratory-related synaptic input. These currents are a crucial part of the group-pacemaker hypothesis11,81,87, which we discuss below, in that excitatory synapses trigger the activation of intrinsic conductances that ultimately augment synaptic excitation to generate robust inspiratory bursts.
The group-pacemaker hypothesis
Emergent systems are widespread in biology88. Without exception, such systems comprise autonomous agents that interact according to simple rules and produce meaningful population-level behaviours. When these behaviours are rhythmic, the underlying network always incorporates two essential features: positive feedback, which serves to coordinate individual elements and promote the formation of a collective temporal pattern, and negative feedback, which can temporarily halt or reverse the assembly process fuelled by positive feedback. These processes, by their nature, alternate. The rhythms are called emergent because individual agents interact following simple rules but none possesses a blueprint for the collective behaviour that results.
Our favoured model for rhythmogenesis is the group-pacemaker hypothesis, in which the behaviour is emergent. We posit that periodic inspiratory bursts result from recurrent synaptic connections that combine with the intrinsic membrane properties of individual neurons11,81,87 (FIG. 5). In this framework, excitatory interconnections between preBötC neurons initiate positive feedback through recurrent excitation. INaP and ICAN serve to amplify the synaptic depolarization and then generate high-frequency spiking to form the full inspiratory drive potential characteristic of all preBötC neurons. The small subset of bursting-pacemaker neurons, which can be rhythmically active even when synaptically isolated, participates in network activity but is not essential because most preBötC neurons discharge inspiratory bursts with the aid of INaP and ICAN induced by excitatory synaptic input. The idea of a group pacemaker is consistent with the observation that in the presence of riluzole and FFA, when pacemaker properties are abolished, respiratory rhythm can continue as long as pharmacological excitation is provided to compensate for the significant reduction of INaP and ICAN, and the attendant lowering of excitability81. Substance P or AMPA accentuate excitability by depolarizing preBötC neurons and revive network rhythmicity as predicted82,89.
Figure 5. Group-pacemaker hypothesis of respiratory rhythm generation.
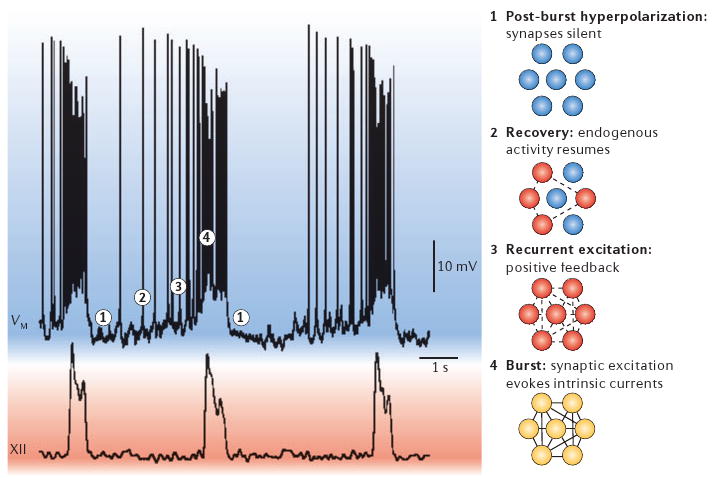
The membrane potential of a rhythm-generating neuron is shown (VM, top trace) with network activity, represented by XII motor output (XII, bottom). Images to the right of the traces depict neuronal activity at different stages of the cycle. 1 is the refractory state that follows inspiration, in which activity-dependent outward currents depress membrane potential, and excitatory synapses in the network are inactive. During epoch 2, the most excitable neurons recover from post-burst hyperpolarization and begin to spike at a low rate. By 3 these highly excitable cells begin to synaptically activate other neurons, leading to aggregation of network activity itself due to recurrent synaptic excitation — a positive-feedback process. The inspiratory burst (4) ensues once a critical number of cells in the network are activated by recurrent excitation. In this final step, synaptic inputs recruit burst-generating intrinsic currents such as Ca2+-activated nonspecific cationic current (ICAN) and persistent Na+ current (INaP), which give rise to large inspiratory burst potentials with high-frequency spike activity. Inspiratory bursts terminate owing to intrinsic properties of cells that can reverse the positive feedback process, including Ca2+-dependent K+ currents and electrogenic pumps, which are recruited by cationic influx during inspiration.
Recurrent glutamatergic excitation induces INaP and ICAN. Depolarization directly evokes INaP78,84,90 and voltage-gated Ca2+ channels91–95. Metabotropic glutamate receptors also activate periodically during inspiration96, which boosts ICAN through intracellular Ca2+ release and might also modulate channels.
Early models of rhythm generation proposed that high-threshold inhibitory neurons had an essential role7 in inspiratory phase termination and such neurons might be involved in adult inspiration in vivo97. Although glycinergic neurons are not essential for respiratory rhythms in neonates in vivo, both GABA-containing and glycinergic neurons contribute to controlling the membrane potential trajectory of preBötC neurons and participate in the formation of the normal respiratory pattern13,17,45,97,98. However, focusing on necessary mechanisms, GABA-containing and glycinergic neurons are not essential in vitro as the rhythm persists after the removal of post synaptic inhibition13,14,16,17. Activity-dependent outward currents such as Ca2+-dependent K+ currents and electrogenic pumps seem to underlie the negative feedback process that causes burst termination in vitro45,99,100 (FIG. 5, part 1).
PreBötC neurons slowly recover from post-inspiratory hyperpolarization in a process that is dominated by subthreshold K+ channels and tonic inputs. The most excitable neurons in the system recover midway through expiration and begin spiking before the next inspiratory cycle (FIG. 5, part 2). In simulations we find that early-onset spiking in a small fraction of neurons recruits other less excitable neurons to also start firing, and that this initiates the positive feedback that spreads rapidly in an aggregation process and ultimately consumes the whole network during inspiration101 (FIG. 5, parts 3 and 4).
The following observations suggest that ICAN is dendritically located11,87. Somatic depolarization fails to evoke inspiratory-like potentials with high-frequency spiking, seemingly because it is unable to evoke the intrinsic burst-generating conductances. In addition, the hyperpolarizing currents that activate at inspiratory burst termination cannot be reversed when the soma is held at membrane potentials of less than the reversal potential for K+, which suggests that the burst-generating conductances are electrotonically distant. At −70 mV, somatic Ca2+ fluxes (which readily occur at more depolarized membrane potentials) cease, yet inspiratory currents remain robust91, suggesting that burst generation relies on conductances and Ca2+ fluxes at dendritic locations.
Many details remain to be established with regard to the group-pacemaker hypothesis. Probably the most pressing issue about which we know least pertains to the connectivity among respiratory neurons and the role these connections have in governing network rhythms. Various connection schemes dramatically influence the temporal dynamics in a wide array of network systems in the physical and biological worlds, and influence the robustness of network function in the event of attack or insult, as well as the ability to respond to external inputs102–104.
Conclusions and future directions
Recent experiments in vivo and in vitro have provided crucial insights into the sites and mechanisms that underlie the rhythm of breathing in mammals7. With respect to site, we propose that there are two distinct, but normally coupled, respiratory oscillators in the brainstem, and that the rhythm in adult mammals is dominated by the preBötC22. We propose that the preBötC network functions as a self-organized group-pacemaker in slices, wherein individual pacemaker neurons can be embedded but are not essential for emergent network rhythms that depend on connectivity and synaptically activated burst-generating currents.
To make significant progress in terms of our current understanding of the neural mechanisms that underlie respiratory rhythm, we emphasize the need for development of more specific genetic manipulations that will allow selective modulation of intrinsic properties; an example would be the insertion of non-endogenous ion channels that could be used to reversibly silence105–107 putative rhythm-generating neurons. As we continue to gain information about the cellular and network mechanisms, we will need to develop realistic mathematical models that address the question of how interacting coupled respiratory oscillators stabilize breathing or enhance the ability to respond to physiological challenges. Models will also allow us to investigate emergent mechanisms of rhythmogenesis in networks. This multilevel approach should lead to an understanding of the central control of breathing under normal conditions and of the dysfunctions that underlie the morbidity and mortality of breathing disorders in human infants, the elderly and patients with neurodegenerative disorders.
DATABASES.
The following terms in this article are linked online to:
OMIM: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM
Amyotrophic lateral sclerosis | Congenital central hypoventilation syndrome | Parkinson's disease | Rett syndrome | Sudden infant death syndrome
Access to this interactive links box is free online.
Acknowledgments
The authors thank G. F. Alheid and D. R. McCrimmon of Northwestern University, Illinois, USA, for the figures and for editing the text in BOX 2. We thank our colleagues in the Systems Neurobiology Laboratory at the University of California, Los Angeles, USA, for their incisive comments on earlier versions of this manuscript. This work was supported by grants from the National Institutes of Health, USA, the Jeffress Memorial Trust, Richmond, Virginia, USA, and the Parker B. Francis Fellowship in Pulmonary Research, Parker B. Francis Foundation, Kansas City, Missouri, USA.
Glossary
- Rett syndrome
Mutation of the MeCP2 gene on the X-chromosome causes irregular breathing patterns during wakefulness and motor control deficits.
- Ondine's curse
Also known as congenital central hypoventilation syndrome). Characterized by episodes of sleep apnoea starting at birth that lead to pathophysiological respiration.
- Amyotrophic lateral sclerosis
ALS also known as Lou Gehrig's disease). A neurodegenerative disorder characterized by progressive motor neuronal cell death and severe muscular atrophy.
- Multiple systems atrophy
(MSA). A neurodegenerative disease of undetermined aetiology encompassing several clinical syndromes including (but not limited to) parkinsonism and autonomic dysfunction.
- Ataxia
Uncoordinated muscular movements symptomatic of some nervous disorders or pathological conditions.
Footnotes
Competing interests statement: The authors declare no competing financial interests.
Contributor Information
Jack L. Feldman, Department of Neurobiology, David Geffen School of Medicine at the University of California, Los Angeles, BOX 951763, Los Angeles, California 90095-1763, USA
Christopher A. Del Negro, Department of Applied Science, McGlothlin-Street Hall, The College of William and Mary, Williamsburg, Virginia 23187-8795, USA
References
- 1.Weese-Mayer DE, et al. Association of the serotonin transporter gene with sudden infant death syndrome: a haplotype analysis. Am J Med Genet A. 2003;122:238–245. doi: 10.1002/ajmg.a.20427. [DOI] [PubMed] [Google Scholar]
- 2.Amir RE, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nature Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 3.Chen ML, Keens TG. Congenital central hypoventilation syndrome: not just another rare disorder. Paediatr Respir Rev. 2004;5:182–189. doi: 10.1016/j.prrv.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Gozal D. New concepts in abnormalities of respiratory control in children. Curr Opin Pediatr. 2004;16:305–308. doi: 10.1097/01.mop.0000127159.74908.27. [DOI] [PubMed] [Google Scholar]
- 5.Blanchi B, Sieweke MH. Mutations of brainstem transcription factors and central respiratory disorders. Trends Mol Med. 2005;11:23–30. doi: 10.1016/j.molmed.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 6.McKay LC, Evans KC, Frackowiak RS, Corfield DR. Neural correlates of voluntary breathing in humans. J Appl Physiol. 2003;95:1170–1178. doi: 10.1152/japplphysiol.00641.2002. [DOI] [PubMed] [Google Scholar]
- 7.Feldman JL. In: Handbook of Physiology. Bloom FE, editor. IV. American Physiological Society; Bethesda, Maryland, USA: 1986. pp. 463–524. [Google Scholar]
- 8.Suzue T. Respiratory rhythm generation in the in vitro brain stem–spinal cord preparation of the neonatal rat. J Physiol (Lond) 1984;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldman JL, Connelly CA, Ellenberger HH, Smith JC. The cardiorespiratory system within the brainstem. Eur J Neurosci. 1990;3(Suppl):171. [Google Scholar]
- 10.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rekling JC, Feldman JL. PreBötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu Rev Physiol. 1998;60:385–405. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- 12.Smith JC, et al. Respiratory rhythm generation in neonatal and adult mammals: the hybrid pacemaker-network model. Respir Physiol. 2000;122:131–147. doi: 10.1016/s0034-5687(00)00155-9. [DOI] [PubMed] [Google Scholar]
- 13.Brockhaus J, Ballanyi K. Synaptic inhibition in the isolated respiratory network of neonatal rats. Eur J Neurosci. 1998;10:3823–3839. doi: 10.1046/j.1460-9568.1998.00396.x. [DOI] [PubMed] [Google Scholar]
- 14.Feldman JL, Smith JC. Cellular mechanisms underlying modulation of breathing pattern in mammals. Ann NY Acad Sci. 1989;563:114–130. doi: 10.1111/j.1749-6632.1989.tb42194.x. [DOI] [PubMed] [Google Scholar]
- 15.Onimaru H, Arata A, Homma I. Inhibitory synaptic inputs to the respiratory rhythm generator in the medulla isolated from newborn rats. Pflugers Arch. 1990;417:425–432. doi: 10.1007/BF00370663. [DOI] [PubMed] [Google Scholar]
- 16.Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shao XM, Feldman JL. Respiratory rhythm generation and synaptic inhibition of expiratory neurons in pre-Bötzinger complex: differential roles of glycinergic and GABAergic neural transmission. J Neurophysiol. 1997;77:1853–1860. doi: 10.1152/jn.1997.77.4.1853. [DOI] [PubMed] [Google Scholar]
- 18.Onimaru H, Arata A, Homma I. Firing properties of respiratory rhythm generating neurons in the absence of synaptic transmission in rat medulla in vitro. Exp Brain Res. 1989;76:530–536. doi: 10.1007/BF00248909. [DOI] [PubMed] [Google Scholar]
- 19.Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron. 2003;37:821–826. doi: 10.1016/s0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol. 1989;281:69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- 21.Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol (Lond) 2006;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005;436:108–111. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- 25.Williams SE, et al. Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science. 2004;306:2093–2097. doi: 10.1126/science.1105010. [DOI] [PubMed] [Google Scholar]
- 26.Mulkey DK, et al. Respiratory control by ventral surface chemoreceptor neurons in rats. Nature Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- 27.Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nature Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- 28.Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci. 2005;25:8938–8947. doi: 10.1523/JNEUROSCI.2415-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alheid GF, Milsom WK, McCrimmon DR. Pontine influences on breathing: an overview. Respir Physiol Neurobiol. 2004;143:105–114. doi: 10.1016/j.resp.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 30.Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Richter DW, Spyer KM. Studying rhythmogenesis of breathing: comparison of in vivo and in vitro models. Trends Neurosci. 2001;24:464–472. doi: 10.1016/s0166-2236(00)01867-1. [DOI] [PubMed] [Google Scholar]
- 32.Borday C, et al. Developmental gene control of brainstem function: views from the embryo. Prog Biophys Mol Biol. 2004;84:89–106. doi: 10.1016/j.pbiomolbio.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Hilaire G, Duron B. Maturation of the mammalian respiratory system. Physiol Rev. 1999;79:325–360. doi: 10.1152/physrev.1999.79.2.325. [DOI] [PubMed] [Google Scholar]
- 34.Pagliardini S, Ren J, Greer JJ. Ontogeny of the pre-Bötzinger complex in perinatal rats. J Neurosci. 2003;23:9575–9584. doi: 10.1523/JNEUROSCI.23-29-09575.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viemari JC, Burnet H, Bevengut M, Hilaire G. Perinatal maturation of the mouse respiratory rhythm-generator: in vivo and in vitro studies. Eur J Neurosci. 2003;17:1233–1244. doi: 10.1046/j.1460-9568.2003.02561.x. [DOI] [PubMed] [Google Scholar]
- 36.Coutinho AP, et al. Induction of a parafacial rhythm generator by rhombomere 3 in the chick embryo. J Neurosci. 2004;24:9383–9390. doi: 10.1523/JNEUROSCI.2408-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fortin G, Borday C, Germon I, Champagnat J. Breathing at birth: influence of early developmental events. Adv Exp Med Biol. 2004;551:143–148. doi: 10.1007/0-387-27023-x_22. [DOI] [PubMed] [Google Scholar]
- 38.Thoby-Brisson M, Trinh JB, Champagnat J, Fortin G. Emergence of the pre-Bötzinger respiratory rhythm generator in the mouse embryo. J Neurosci. 2005;25:4307–4318. doi: 10.1523/JNEUROSCI.0551-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baker-Herman TL, et al. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nature Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- 40.Viemari JC, et al. Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. J Neurosci. 2005;25:11521–11530. doi: 10.1523/JNEUROSCI.4373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prinz AA, Bucher D, Marder E. Similar network activity from disparate circuit parameters. Nature Neurosci. 2004;7:1345–1352. doi: 10.1038/nn1352. [DOI] [PubMed] [Google Scholar]
- 42.Busselberg D, Bischoff AM, Paton JF, Richter DW. Reorganisation of respiratory network activity after loss of glycinergic inhibition. Pflugers Arch. 2001;441:444–449. doi: 10.1007/s004240000453. [DOI] [PubMed] [Google Scholar]
- 43.Dutschmann M, Paton JF. Glycinergic inhibition is essential for co-ordinating cranial and spinal respiratory motor outputs in the neonatal rat. J Physiol (Lond) 2002;543:643–653. doi: 10.1113/jphysiol.2001.013466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dutschmann M, Paton JF. Inhibitory synaptic mechanisms regulating upper airway patency. Respir Physiol Neurobiol. 2002;131:57–63. doi: 10.1016/s1569-9048(02)00037-x. [DOI] [PubMed] [Google Scholar]
- 45.Busselberg D, Bischoff AM, Richter DW. A combined blockade of glycine and calcium-dependent potassium channels abolishes the respiratory rhythm. Neuroscience. 2003;122:831–841. doi: 10.1016/j.neuroscience.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 46.Smith JC, Greer JJ, Liu GS, Feldman JL. Neural mechanisms generating respiratory pattern in mammalian brain stem–spinal cord in vitro. I. Spatiotemporal patterns of motor and medullary neuron activity. J Neurophysiol. 1990;64:1149–1169. doi: 10.1152/jn.1990.64.4.1149. [DOI] [PubMed] [Google Scholar]
- 47.Ramirez JM, et al. Respiratory rhythm generation: converging concepts from in vitro and in vivo approaches? Respir Physiol Neurobiol. 2002;131:43–56. doi: 10.1016/s1569-9048(02)00036-8. [DOI] [PubMed] [Google Scholar]
- 48.Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBötzinger complex neurokinin-1 receptor-expressing neurons. Nature Neurosci. 2001;4:927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blanchi B, et al. MafB deficiency causes defective respiratory rhythmogenesis and fatal central apnea at birth. Nature Neurosci. 2003;6:1091–1100. doi: 10.1038/nn1129. [DOI] [PubMed] [Google Scholar]
- 50.Lumsden T. Effects of bulbar anaemia on respiratory movements. J Physiol. 1924;59:Ivii–Ix. [Google Scholar]
- 51.St John WM. Differential alteration by hypercapnia and hypoxia of the apneustic respiratory pattern in decerebrate cats. J Physiol (Lond) 1979;287:467–491. doi: 10.1113/jphysiol.1979.sp012671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.St John WM, Paton JF. Role of pontile mechanisms in the neurogenesis of eupnea. Respir Physiol Neurobiol. 2004;143:321–332. doi: 10.1016/j.resp.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 53.Janczewski WA, Onimaru H, Homma I, Feldman JL. Opioid-resistant respiratory pathway from the preinspiratory neurones to abdominal muscles: in vivo and in vitro study in the newborn rat. J Physiol (Lond) 2002;545:1017–1026. doi: 10.1113/jphysiol.2002.023408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takeda S, et al. Opioid action on respiratory neuron activity of the isolated respiratory network in newborn rats. Anesthesiology. 2001;95:740–749. doi: 10.1097/00000542-200109000-00029. [DOI] [PubMed] [Google Scholar]
- 55.Batini C, Moruzzi G, Palestini M, Rossi GF, Zanchetti A. Persistent patterns of wakefulness in the pretrigeminal midpontine preparation. Science. 1958;128:30–32. doi: 10.1126/science.128.3314.30-a. [DOI] [PubMed] [Google Scholar]
- 56.McKay LC, Janczewski WA, Feldman JL. Sleep-disordered breathing following targeted ablation of preBötzinger complex. Nature Neurosci. 2005;8:1142–1144. doi: 10.1038/nn1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wenninger JM, et al. Large lesions in the pre-Bötzinger complex area eliminate eupneic respiratory rhythm in awake goats. J Appl Physiol. 2004;97:1629–1636. doi: 10.1152/japplphysiol.00953.2003. [DOI] [PubMed] [Google Scholar]
- 58.Southall DP, et al. Prolonged expiratory apnoea: a disorder resulting in episodes of severe arterial hypoxaemia in infants and young children. Lancet. 1985;2:571–577. doi: 10.1016/s0140-6736(85)90583-5. [DOI] [PubMed] [Google Scholar]
- 59.Wilson RJ, Vasilakos K, Harris MB, Straus C, Remmers JE. Evidence that ventilatory rhythmogenesis in the frog involves two distinct neuronal oscillators. J Physiol (Lond) 2002;540:557–570. doi: 10.1113/jphysiol.2001.013512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vasilakos K, Wilson RJ, Kimura N, Remmers JE. Ancient gill and lung oscillators may generate the respiratory rhythm of frogs and rats. J Neurobiol. 2004;62:369–385. doi: 10.1002/neu.20102. [DOI] [PubMed] [Google Scholar]
- 61.Owerkowicz T, Farmer CG, Hicks JW, Brainerd EL. Contribution of gular pumping to lung ventilation in monitor lizards. Science. 1999;284:1661–1663. doi: 10.1126/science.284.5420.1661. [DOI] [PubMed] [Google Scholar]
- 62.Podulka S, Rohrbaugh R, Bonney R. Cornell Lab of Ornithology Handbook of Bird Biology. Cornell Lab of Ornithology in association with Princeton Univ. Press; Ithaca, New York: 2005. [Google Scholar]
- 63.Jansen AH, Chernick V. Development of respiratory control. Physiol Rev. 1983;63:437–483. doi: 10.1152/physrev.1983.63.2.437. [DOI] [PubMed] [Google Scholar]
- 64.Jacquin TD, et al. Reorganization of pontine rhythmogenic neuronal networks in Krox-20 knockout mice. Neuron. 1996;17:747–758. doi: 10.1016/s0896-6273(00)80206-8. [DOI] [PubMed] [Google Scholar]
- 65.Csete ME, Doyle JC. Reverse engineering of biological complexity. Science. 2002;295:1664–1669. doi: 10.1126/science.1069981. [DOI] [PubMed] [Google Scholar]
- 66.Maria B, et al. Sleep breathing disorders in patients with idiopathic Parkinson's disease. Respir Med. 2003;97:1151–1157. doi: 10.1016/s0954-6111(03)00188-4. [DOI] [PubMed] [Google Scholar]
- 67.Stocchi F, Barbato L, Nordera G, Berardelli A, Ruggieri S. Sleep disorders in Parkinson's disease. J Neurol. 1998;245(Suppl 1):S15–S18. doi: 10.1007/pl00007731. [DOI] [PubMed] [Google Scholar]
- 68.Munschauer FE, Loh L, Bannister R, Newsom-Davis J. Abnormal respiration and sudden death during sleep in multiple system atrophy with autonomic failure. Neurology. 1990;40:677–679. doi: 10.1212/wnl.40.4.677. [DOI] [PubMed] [Google Scholar]
- 69.Vetrugno R, et al. Sleep disorders in multiple system atrophy: a correlative video-polysomnographic study. Sleep Med. 2004;5:21–30. doi: 10.1016/j.sleep.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 70.Arnulf I, et al. Sleep disorders and diaphragmatic function in patients with amyotrophic lateral sclerosis. Am J Respir Crit Care Med. 2000;161:849–856. doi: 10.1164/ajrccm.161.3.9805008. [DOI] [PubMed] [Google Scholar]
- 71.Barthlen GM, Lange DJ. Unexpectedly severe sleep and respiratory pathology in patients with amyotrophic lateral sclerosis. Eur J Neurol. 2000;7:299–302. doi: 10.1046/j.1468-1331.2000.00044.x. [DOI] [PubMed] [Google Scholar]
- 72.Benarroch EE, Schmeichel AM, Low PA, Parisi JE. Depletion of ventromedullary NK-1 receptor-immunoreactive neurons in multiple system atrophy. Brain. 2003;126:2183–2190. doi: 10.1093/brain/awg220. [DOI] [PubMed] [Google Scholar]
- 73.Alexianu ME, et al. The role of calcium-binding proteins in selective motoneuron vulnerability in amyotrophic lateral sclerosis. Ann Neurol. 1994;36:846–858. doi: 10.1002/ana.410360608. [DOI] [PubMed] [Google Scholar]
- 74.Alheid GF, Gray PA, Jiang MC, Feldman JL, McCrimmon DR. Parvalbumin in respiratory neurons of the ventrolateral medulla of the adult rat. J Neurocytol. 2002;31:693–717. doi: 10.1023/a:1025799830302. [DOI] [PubMed] [Google Scholar]
- 75.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–148. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 76.Ancoli-Israel S, et al. Morbidity, mortality and sleep-disordered breathing in community dwelling elderly. Sleep. 1996;19:277–282. doi: 10.1093/sleep/19.4.277. [DOI] [PubMed] [Google Scholar]
- 77.Johnson SM, Smith JC, Funk GD, Feldman JL. Pacemaker behavior of respiratory neurons in medullary slices from neonatal rat. J Neurophysiol. 1994;72:2598–2608. doi: 10.1152/jn.1994.72.6.2598. [DOI] [PubMed] [Google Scholar]
- 78.Del Negro CA, Koshiya N, Butera RJ, Jr, Smith JC. Persistent sodium current, membrane properties and bursting behavior of pre-Bötzinger complex inspiratory neurons in vitro. J Neurophysiol. 2002;88:2242–2250. doi: 10.1152/jn.00081.2002. [DOI] [PubMed] [Google Scholar]
- 79.Ptak K, et al. Sodium currents in medullary neurons isolated from the pre-Bötzinger complex region. J Neurosci. 2005;25:5159–5170. doi: 10.1523/JNEUROSCI.4238-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pagliardini S, Adachi T, Ren J, Funk GD, Greer JJ. Fluorescent tagging of rhythmically active respiratory neurons within the pre-Bötzinger complex of rat medullary slice preparations. J Neurosci. 2005;25:2591–2596. doi: 10.1523/JNEUROSCI.4930-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Del Negro CA, et al. Sodium and calcium dependent pacemaker neurons and respiratory rhythm generation. J Neurosci. 2005;25:446–453. doi: 10.1523/JNEUROSCI.2237-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pena F, Parkis MA, Tryba AK, Ramirez JM. Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron. 2004;43:105–117. doi: 10.1016/j.neuron.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 83.Del Negro CA, Morgado-Valle C, Feldman JL. Respiratory rhythm: an emergent network property? Neuron. 2002;34:821–830. doi: 10.1016/s0896-6273(02)00712-2. [DOI] [PubMed] [Google Scholar]
- 84.Rybak IA, Ptak K, Shevtsova NA, McCrimmon DR. Sodium currents in neurons from the rostroventrolateral medulla of the rat. J Neurophysiol. 2003;90:1635–1642. doi: 10.1152/jn.00150.2003. [DOI] [PubMed] [Google Scholar]
- 85.Pena F, Ramirez JM. Substance P-mediated modulation of pacemaker properties in the mammalian respiratory network. J Neurosci. 2004;24:7549–7556. doi: 10.1523/JNEUROSCI.1871-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ramirez JM, Tryba AK, Pena F. Pacemaker neurons and neuronal networks: an integrative view. Curr Opin Neurobiol. 2004;14:665–674. doi: 10.1016/j.conb.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 87.Rekling JC, Champagnat J, Denavit-Saubie M. Electroresponsive properties and membrane potential trajectories of three types of inspiratory neurons in the newborn mouse brain stem in vitro. J Neurophysiol. 1996;75:795–810. doi: 10.1152/jn.1996.75.2.795. [DOI] [PubMed] [Google Scholar]
- 88.Camazine S, et al. Self-organization in Biological Systems. Princeton Univ. Press; Princeton, New Jersey: 2001. p. 538. [Google Scholar]
- 89.Morgado-Valle C, Feldman JL. Depletion of substance P and glutamate by capsaicin blocks respiratory rhythm in neonatal rat in vitro. J Physiol (Lond) 2004;555:783–792. doi: 10.1113/jphysiol.2003.060350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ptak K, et al. The murine neurokinin NK1 receptor gene contributes to the adult hypoxic facilitation of ventilation. Eur J Neurosci. 2002;16:2245–2252. doi: 10.1046/j.1460-9568.2002.02305.x. [DOI] [PubMed] [Google Scholar]
- 91.Frermann D, Keller BU, Richter DW. Calcium oscillations in rhythmically active respiratory neurones in the brainstem of the mouse. J Physiol (Lond) 1999;515:119–131. doi: 10.1111/j.1469-7793.1999.119ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Koshiya N, Smith JC. Neuronal pacemaker for breathing visualized in vitro. Nature. 1999;400:360–363. doi: 10.1038/22540. [DOI] [PubMed] [Google Scholar]
- 93.Onimaru H, Ballanyi K, Richter DW. Calcium-dependent responses in neurons of the isolated respiratory network of newborn rats. J Physiol (Lond) 1996;491:677–695. doi: 10.1113/jphysiol.1996.sp021249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pierrefiche O, Champagnat J, Richter DW. Calcium-dependent conductances control neurones involved in termination of inspiration in cats. Neurosci Lett. 1995;184:101–104. doi: 10.1016/0304-3940(94)11179-m. [DOI] [PubMed] [Google Scholar]
- 95.Pierrefiche O, Haji A, Bischoff A, Richter DW. Calcium currents in respiratory neurons of the cat in vivo. Pflugers Arch. 1999;438:817–826. doi: 10.1007/s004249900090. [DOI] [PubMed] [Google Scholar]
- 96.Mironov SL, Richter DW. Hypoxic modulation of L-type Ca2+ channels in inspiratory brainstem neurones: intracellular signalling pathways and metabotropic glutamate receptors. Brain Res. 2000;869:166–177. doi: 10.1016/s0006-8993(00)02396-9. [DOI] [PubMed] [Google Scholar]
- 97.Busselberg D, Bischoff AM, Becker K, Becker CM, Richter DW. The respiratory rhythm in mutant oscillator mice. Neurosci Lett. 2001;316:99–102. doi: 10.1016/s0304-3940(01)02382-5. [DOI] [PubMed] [Google Scholar]
- 98.Liu YY, et al. GABAergic and glycinergic synapses onto neurokinin-1 receptor-immunoreactive neurons in the pre-Bötzinger complex of rats: light and electron microscopic studies. Eur J Neurosci. 2002;16:1058–1066. doi: 10.1046/j.1460-9568.2002.02163.x. [DOI] [PubMed] [Google Scholar]
- 99.Ballerini L, Bracci E, Nistri A. Pharmacological block of the electrogenic sodium pump disrupts rhythmic bursting induced by strychnine and bicuculline in the neonatal rat spinal cord. J Neurophysiol. 1997;77:17–23. doi: 10.1152/jn.1997.77.1.17. [DOI] [PubMed] [Google Scholar]
- 100.Darbon P, Tscherter A, Yvon C, Streit J. The role of the electrogenic Na/K pump in disinhibition-induced bursting in cultured spinal networks. J Neurophysiol. 2003;90:3119–3129. doi: 10.1152/jn.00579.2003. [DOI] [PubMed] [Google Scholar]
- 101.Stauffer D, Aharony A. Introduction to Percolation Theory. Taylor & Francis; London: 1992. p. 181. [Google Scholar]
- 102.Barabasi AL. Linked: The New Science of Networks. Perseus; Cambridge, Massachusetts: 2002. p. 280. [Google Scholar]
- 103.Newman ME. The structure and function of complex networks. SIAM Review. 2003;45:167–256. [Google Scholar]
- 104.Watts DJ. Six Degrees: The Science of a Connected Age. Norton; New York: 2003. p. 368. [Google Scholar]
- 105.Callaway EM. A molecular and genetic arsenal for systems neuroscience. Trends Neurosci. 2005;28:196–201. doi: 10.1016/j.tins.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 106.Slimko EM, Lester HA. Codon optimization of Caenorhabditis elegans GluCl ion channel genes for mammalian cells dramatically improves expression levels. J Neurosci Methods. 2003;124:75–81. doi: 10.1016/s0165-0270(02)00362-x. [DOI] [PubMed] [Google Scholar]
- 107.Slimko EM, McKinney S, Anderson DJ, Davidson N, Lester HA. Selective electrical silencing of mammalian neurons in vitro by the use of invertebrate ligand-gated chloride channels. J Neurosci. 2002;22:7373–7379. doi: 10.1523/JNEUROSCI.22-17-07373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McCrimmon DR, Alheid GF, Jiang M, Calandriello T, Topgi A. Converging functional and anatomical evidence for novel brainstem respiratory compartments in the rat. Adv Exp Med Biol. 2004;551:101–105. doi: 10.1007/0-387-27023-x_16. [DOI] [PubMed] [Google Scholar]



