Abstract
Introduction
Traumatic injury may result in an exaggerated response to subsequent immune stimuli such as nosocomial infection. This “second hit” phenomenon, and molecular mechanism(s) of immune priming by traumatic lung injury, specifically pulmonary contusion, remains unknown. We used an animal model of pulmonary contusion to determine if the injury resulted in priming of the innate immune response and to test the hypothesis that resuscitation fluids could attenuate the primed response to a second hit.
Methods
Male, 8-9 wk, C57/BL6 mice with a pulmonary contusion were challenged by a second hit of intratracheal administration of the Toll like receptor (TLR) 4 agonist, lipopolysaccharide (LPS, 50mcg) 24hrs after injury (injury+LPS). Other experimental groups were injury+vehicle or LPS alone. A separate group were injured and resuscitated by 4cc/kg of hypertonic saline (HTS) or Lactated Ringer's (LR) resuscitation prior to LPS challenge. Mice were euthanized 4hrs after LPS challenge and blood, bronchoalveolar lavage (BAL), and tissue were isolated and analyzed. Data were analyzed using one way ANOVA with Bonferroni multiple comparison post-test for significant differences (*, p≤0.05).
Results
Injury+LPS showed immune priming observed by lung injury histology and increased BAL neutrophilia, lung myeloperoxidase, and serum IL-6, CXCL1 and MIP-2 levels when compared to injury+vehicle or LPS alone. After injury, resuscitation with HTS, but not LR was more effective in attenuating the primed response to a second hit.
Conclusion
Pulmonary contusion primes innate immunity for an exaggerated response to a second hit with the TLR4 agonist, LPS. We observed synergistic increases in inflammatory mediator expression in the blood and a more severe lung injury in injured animals challenged with LPS. This priming effect was reduced when HTS was used to resuscitate the animal after lung contusion.
Keywords: trauma, pulmonary contusion, second hit, immune priming, resuscitation, chemokines, inflammation, rodent model, innate immunity
Introduction
It has long been recognized in the clinical arena that significant traumatic injury seemingly primes the cells of the immune system for an exaggerated response to subsequent infectious challenge. This has been termed the “second hit” phenomenon, and is of clinical significance in that it is associated with the onset of multiple organ dysfunction and death1. Prior studies have linked the second hit response to enhanced Toll-like receptor (TLR) activity in cells of the innate immune system2;3. TLRs are a phylogenetically conserved family of receptors responsible for the initiation of inflammatory responses to putative pathogen-associated molecular patterns, such as bacterial lipopolysaccharide (LPS) and peptidoglycan (PGN), as well as several endogenous ligands (danger-associated molecular patterns)4;5. Of the 13 known TLRs, TLR4 is essential for cellular responsiveness to LPS, a component of the outer membrane of gram negative organisms. Once triggered, TLR4 signaling activates innate immune mechanisms resulting in NFκB activation and expression of a number of pro-inflammatory mediators including IL-1β, IL-6, and the chemokine, IL-86. Enhanced TLR4-mediated signaling has been shown to be involved in the second hit response in animal models of burn injury and ischemia/reperfusion2;7;8.
The use of fluids in the resuscitation of critically injured patients has been investigated in clinical trials as well as in several animal models9-15. Resuscitation with hypertonic fluids results in a transient increase in serum osmolarity. The subsequent redistribution of fluid from the extravascular to the intravascular space reduces fluid volume for effective resuscitation. The advantages of such a resuscitation strategy are a reduction in consequences of third space fluid sequestration (cerebral edema and pulmonary edema) and improved cardiac contractility. There is mounting evidence that suggests that resuscitation with 7.5% saline has immunomodulatory properties as well. In vitro and in vivo studies have shown that neutrophil activation, chemotaxis, and degranulation are reduced in the face of hypertonicity7;16-18. Likewise, hyperosmolar conditions have been shown to prevent macrophage activation in a dose-dependent manner19. Moreover, in a second hit model of ischemia/reperfusion followed by LPS challenge, hypertonic resuscitation resulted in abridged neutrophil activation and oxidative burst and a reduction in alveolar macrophage NFκB translocation20. Other studies have suggested that hyperosmolar conditions may cause phenotypic changes in circulating monocytes and reduce expression of proinflammatory mediators18. Thus, hypertonic saline is a beneficial resuscitation fluid, not only for its immediate effects on hemodynamics and fluid balance, but also due to immunomodulatory properties and potential to inhibit the second hit response after injury.
Blunt chest trauma resulting in pulmonary contusion is a common injury, affecting 10-17% of all trauma admissions with estimates of mortality at 10-25%21;22. Sequelae of pulmonary contusion vary widely and include infection (pneumonia), local organ failure (Acute Respiratory Distress Syndrome, ARDS), and remote organ failure (Multiple Organ Dysfunction/Failure, MODS/MOF)23. We have previously demonstrated in an animal model that lung contusion results in activation of innate immunity with the localized production of a variety of inflammatory cytokines and chemokines, followed by neutrophil infiltration into the areas of injury5;24;25. Additionally, we have shown that the initial innate response to lung contusion is at least in part mediated through TLR dependent signaling mechanisms5;25. However, it remains unknown whether isolated blunt chest trauma primes the innate immune system for a second hit response and enhanced TLR4 reactivity. We hypothesized that blunt chest trauma would prime effector cells of innate immunity for a second hit and observed the exaggerated response to the TLR4 ligand, LPS, when administered after injury. We used this second hit model to evaluate the effect of fluid resuscitation and to test the hypothesis that resuscitation would attenuate the second hit response.
Methods
Animals
Male and age matched (8-9 weeks) C57/BL6 mice obtained from Jackson Laboratories (Bar Harbor, ME) were utilized in this study. All animals were bred and maintained under specific pathogen-free conditions at the animal facility at Wake Forest University School of Medicine. The protocol used in this study was approved by the Animal Care and Use Committee (#A07-246).
Animal injury model
Blunt chest injury resulting in pulmonary contusion was induced using the Cortical Contusion Impactor (CCI) as described previously5. Briefly, mice were anesthetized with 2% isoflurane at a flow rate of 1L/min. The mouse was positioned left lateral decubitus and during inspiration, the right chest was struck with the CCI along the posterior axillary line, 1cm above the costal margin.
Second hit model without and with resuscitation
At 24 hours following blunt chest injury, the animals were re-anesthetized with isoflurane. A midline neck incision exposed the trachea, and 50mcg of LPS in 50mcl of Phosphate Buffered Saline (PBS, Sigma, St. Louis, MO) was injected directly into the trachea of the mouse using a 26G needle and the incision was closed. Control animals were (1) injured and received intratracheal instillation of PBS alone or (2) received intratracheal LPS without prior injury. Resuscitated animals received 4cc/kg of HTS (7.5% saline) or Lactated Ringer's (LR) via tail vein injection 1hr prior to the second hit with LPS. At 4 hours serum and tissue samples were collected after death by isoflurane overdose and cervical dislocation.
Histopathology
Lung specimens were fixed in 10% formalin, sectioned, and stained with hematoxylin and eosin (H&E). Slides (100× magnification) were evaluated and graded for the presence of interstitial neutrophillic infiltrate, intra-alveolar hemorrhage, and pulmonary septal edema as described previously5;25.
Bronchoalveolar lavage (BAL)
After sacrifice, BAL was performed by cannulation of the trachea and lungs lavaged with 4ml of PBS (Sigma Biochemical, St. Louis, MO) at 4°C. BAL was centrifuged at 300 × g, 4°C for 10 minutes and supernatant collected and stored at -70°C until use. The cell pellet was counted and differentiated as previously described5;25.
Cytokine and chemokine expression
IL-6, CXCL1 and MIP-2 (CXCL2/3) were measured in the serum using commercially available ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. Samples were assayed in duplicate.
Myeloperoxidase activity
MPO activity was used as a quantitative measure of neutrophil accumulation in the lung. Lung tissue samples were collected in RPMI 1640 with 5% FCS at 4°C. The lung was cut into small pieces and digested for 60 minutes at 37°C in 10ml of PBS containing 300U/ml collagenase type I (Worthington Biochemical Corp., Lakewood, NJ), and 150μl of a DNase I (10mg/ml, Worthington Biochemical Corp., Lakewood, NJ). The lung digest was passed through a 40μm sieve with two washes of RPMI 1640 with 5% FCS, and cells collected by centrifugation at 300g and 4°C. After lysis of red blood cells with ACK lysis buffer (Lonza, Walkersville, MD), the remaining cells were washed twice with PBS and cell extracts prepared by three freeze thaw cycles in 1ml HTAB (Sigma-Aldrich, St. Louis, MO) buffer (0.5% HTAB in MPO buffer; MPO buffer contains 6.8g KH2PO4 + 8.7g K2HPO4 in 1L dH2O). The extracts were cleared by centrifugation at 10,000g for 10 minutes. Seven microliters of extract was assayed for MPO activity with 200ul development reagent (16.7mg O-dianisidine dihydrochloride in 100ml of a solution containing 90 ml dH2O, 10ml MPO buffer and 0.005% H2O2) by measuring the change in absorbance (450nm) over a period of 5 minutes and results reported as Vmax.
Neutrophil CD11b expression
Whole blood was collected in a heparinized syringe, transferred to a 1.5ml microcentrifuge tube and mixed thoroughly before placing at 4°C. Fc receptor binding was reduced by adding 0.5ug of Mouse BD Fc Block™ (BD Biosciences, San Jose, CA) to 100ul of whole blood for 5 minutes at 4°C. All subsequent steps were carried out in the dark at 4°C. Staining of CD11b was done by adding 0.5ug of FITC anti-mouse CD11b (eBioscience, Inc., San Diego, CA) to blocked samples for 30 minutes. A FITC labeled isotype control (eBioscience, Inc.) sample was stained in parallel to assess non-specific antibody binding. Next, red blood cells were lysed with an RBC lysis buffer (eBioscience, Inc.) for 5 minutes, cells collected by centrifugation at 300g, washed twice with PBS, and resuspended in fixation buffer (BD Biosciences). After 20 minutes, fixed cells were centrifuged, washed with PBS and resuspended in stain buffer (FBS) (BD Biosciences). Fixed and stained cells were analyzed by flow cytometry using a BD FACSCalibur™ system (BD Biosciences) and BD CellQuest™ Pro software (BD Biosciences).
Statistical analysis
Data are reported using GraphPad Prism (v 4.03, San Diego, CA) and expressed as the mean ± SEM of independent observations as indicated in the Figure legends. One-way analysis of variance (1way ANOVA) with multiple comparison post test (Bonferroni) was used to compare the means between experimental groups. A p-value ≤0.05 was considered to be significant. Samples were assayed in duplicate.
Results
Pulmonary contusion primes innate immunity for an exaggerated response to a second hit with the TLR4 agonist, LPS
To determine if pulmonary contusion primes the innate immune response for a second hit, we compared the response of animals undergoing blunt chest trauma followed by intratracheal instillation of LPS (IT LPS) with animals administered vehicle alone (saline) after injury and animals given IT LPS only with no antecedent trauma. Upon histological examination, second hit animals had a dramatic pulmonary neutrophilic infiltrate and septal edema when compared to animals given IT LPS only and injury+vehicle animals (Figure 1). Hemorrhage and structural distortion were evident in animals receiving blunt chest trauma. To evaluate the involvement of remote organs in this process, we examined hepatic histology. No evidence of acute hepatic inflammation was observed, suggesting that the priming response was localized in the lung (data not shown). As the presence of a neutrophilic infiltrate strongly correlates with acute lung injury we examined bronchoalveolar lavage specimens and measured lung MPO activity to assess neutrophil infiltration in the primed response. As shown in Figure 2, second hit animals had significantly increased BAL neutrophilia (Figure 2A) and lung MPO activity (Figure 2B) when compared to animals given LPS alone or animals with injury+vehicle. A maximal priming effect was observed when the second hit was given at 24hrs after injury (data not shown).
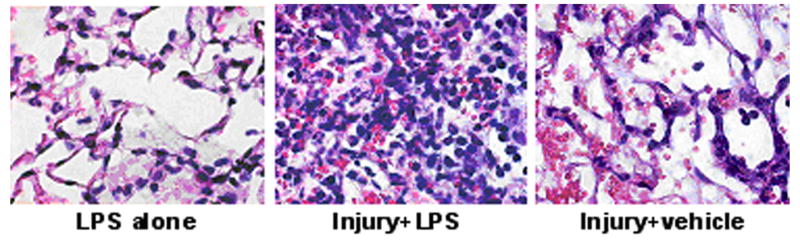
Figure 1. Lung histology shows that pulmonary contusion primes innate immunity for enhanced neutrophil response.
We compared lung histology of animals given LPS with no antecedent trauma (LPS alone), animals undergoing blunt chest trauma followed by intratracheal instillation of LPS (Injury+LPS) and injured animals administered intratracheal saline (Injury+vehicle). Second hit animals (Injury+LPS) show an increased pulmonary neutrophilic infiltrate and alveolar septal edema when compared to animals given LPS alone. Hemorrhage and structural distortion is evident in injured animals. Data shown (100× magnification) is representative of histology for at least 3 animals in each group.
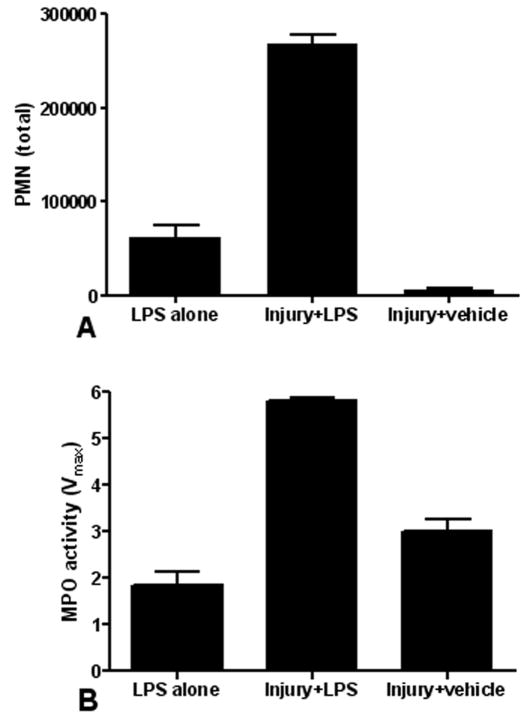
Figure 2. Pulmonary contusion primes innate immunity for enhanced neutrophil response.
We compared the neutrophil response of animals given LPS with no antecedent trauma (LPS alone) to animals given a blunt chest trauma followed by IT instillation of LPS (Injury+LPS) or injured animals administered IT saline (Injury+vehicle). Second hit animals (Injury+LPS) have increased BAL neutrophilia (A) and lung MPO activity (B) when compared to animals given LPS alone or injured animals administered IT saline (Injury+vehicle). Data shown is the mean ± SEM. Differences are significant (p≤0.01) between all groups (n=5 for each group).
We next evaluated the systemic innate response of second hit animals by measuring serum levels of IL-6 and the chemokines, CXCL1 and MIP-2. We previously demonstrated that both mediators play a central role in the pathogenesis of lung injury after pulmonary contusion. Relevant to our second hit model, these mediators are also produced in an NFkB-dependent fashion after LPS challenge6. We now show a synergistic increase in the expression of these mediators with injury+LPS when compared to animals given LPS alone or animals with injury+vehicle (Figure 3). These data support the observation that isolated blunt chest trauma primes for an enhanced innate immune response to TLR4 activation.
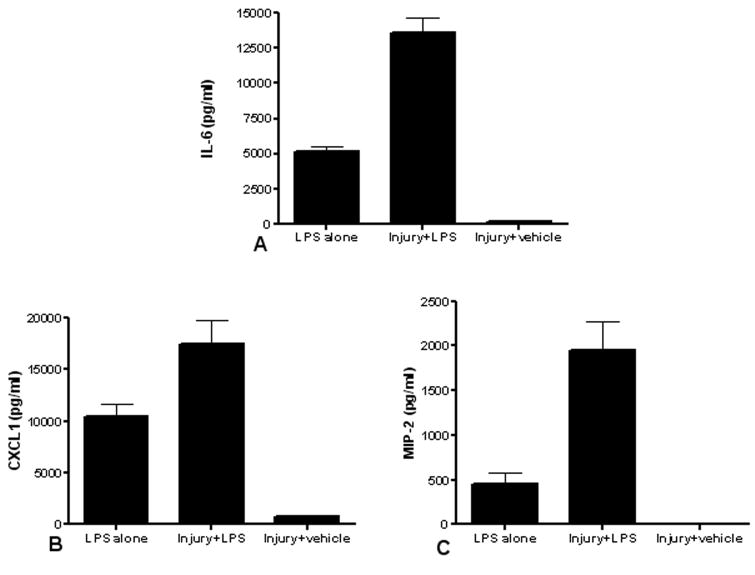
Figure 3. Pulmonary contusion primes the systemic innate immune response.
We compared the serum levels of IL-6, CXCL1 and MIP-2 in animals given LPS with no antecedent trauma (LPS alone) to animals given a blunt chest trauma followed by IT instillation of LPS (Injury+LPS) or injured animals administered IT saline (Injury+vehicle). Second hit animals have increased IL-6 (A, n=6), CXCL1 (B, n=5) and MIP-2 (C, n=5) levels when compared to animals given IT LPS or injured animals administered IT saline. Data shown is the mean ± SEM. Differences are significant (p≤0.05) between all groups; the exception is MIP-2 levels in Injury+vehicle compared to LPS alone where no significant difference (p>0.05) is observed.
Small volume resuscitation with 7.5% hypertonic saline ameliorates enhanced TLR4 reactivity seen after chest injury
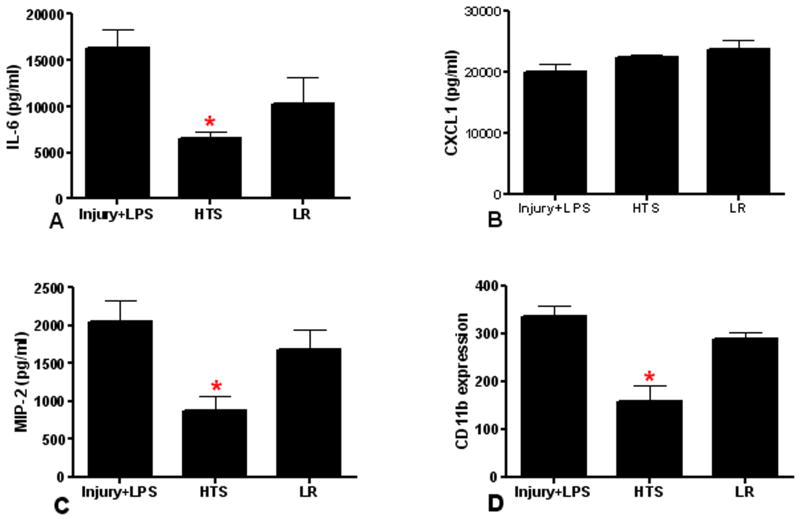
Given the priming effect observed in our model of blunt chest trauma, we next sought to test two post-injury resuscitation strategies for the ability to attenuate the second hit response. Small volume resuscitation with HTS was compared to an equivalent volume of resuscitation with Lactated Ringer's (LR). Resuscitation was performed after lung injury but prior to the second hit. As shown in Figure 4, HTS resuscitation prior to the second hit with LPS significantly reduced BAL neutrophilia and lung MPO activity. Resuscitation with LR showed no significant effect in reducing the neutrophil response to a second hit. Serum levels of IL-6 and MIP-2 were significantly reduced with HTS resuscitation in second hit animals (Figure 5). In contrast, serum CXCL1 levels were unaffected by either HTS or LR resuscitation. Finally, to evaluate neutrophil activation, we measured CD11b expression in second hit animals and in animals resuscitated with HTS or LR. As shown in Figure 5, we observed an anticipated rise in systemic neutrophil CD11b in second hit animals. We further found that CD11b expression was reduced in animals resuscitated with HTS, but not after resuscitation with LR. These data indicate that after isolated chest injury, innate immune responses to a second hit can be attenuated by resuscitation with HTS.
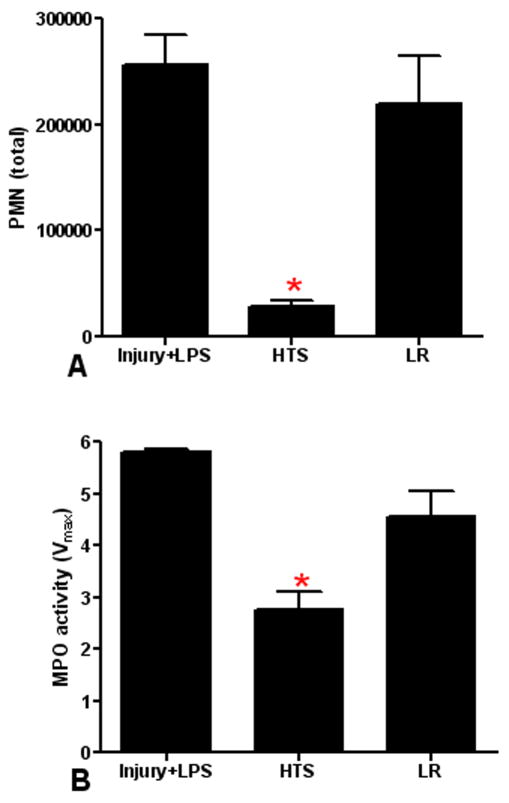
Figure 4. HTS resuscitation reduces the neutrophil response to a second hit.
We compared the neutrophil response of injured animals resuscitated with HTS or LR prior to a second hit with IT LPS. Second hit animals resuscitated with HTS show a significant decrease (*p≤0.001) in BAL neutrophilia (A) and lung MPO activity (B) when compared to second hit animals without resuscitation (Injury+LPS). There is no significant effect of LR resuscitation (p>0.05) on BAL neutrophilia or MPO activity. Data shown is the mean ± SEM (n=5).
Figure 5. HTS resuscitation reduces the systemic innate immune response to a second hit.
We compared the serum levels of IL-6, CXCL1, MIP-2 and CD11b expression in injured animals resuscitated with HTS or LR prior to a second hit with IT LPS. HTS resuscitated animals have a significant decrease (*p≤0.05) in serum IL-6 (A, n=8), MIP-2 (C, n=5) and CD11b expression (D, n=5) compared to second hit animals. There is no significant change (p>0.05) in CXCL1 by either resuscitation strategy (B, n=5, 8, 8 for Injury+LPS, HTS, and LR, respectively). LR resuscitated animals are also not significantly changed (p>0.05). Data shown is the mean ± SEM.
Discussion
Without question, injury disrupts the normal host immune response and predisposes patients to the development of opportunistic infection. Clinicians have recognized that initial serious injury can result in a priming of the immune system for an exaggerated response to subsequent infectious challenge. Studies in both humans and animals show that innate immune cells such as neutrophils, macrophages/monocytes, and dendritic cells produce increased levels of inflammatory mediators in response to microbial stimuli after injury26-29. This “second hit” phenomenon is supported by epidemiologic studies linking the magnitude of the initial systemic inflammatory response to injury with the onset of late post-injury multiple organ failure and death due to invasive infection30.
We sought to determine whether pulmonary contusion would prime innate immune mechanisms in our murine model of isolated blunt chest trauma. As gram negative organisms often cause the nosocomial infections seen after injury, we used the TLR4 ligand, LPS (injury+LPS), to test for post-injury immuno-hyperreactivity in response to this second hit. Synergistic increases in pulmonary neutrophilia and systemic mediator expression (IL-6, CXCL1, and MIP-2) were seen in second hit animals when compared to injury+vehicle or LPS alone. These results indicate that isolated blunt chest trauma results in a primed effect on innate immune responses that enhance TLR4 reactivity and augmented lung injury. Our findings of enhanced post-injury TLR reactivity are in agreement with other animal models of injury and enhanced TLR responses to a second hit2;3;7. However, in a blast chest injury model and using cecal ligation and puncture (CLP) as the second hit, Perl et al. found that chest injury had a suppressive effect on macrophage and lymphocytic function and resulted in increased morality31. These results may, in part, be explained by the use of a blast injury model that imparts significantly more energy to a broad area of the chest than in our model of direct, concussive chest injury. In addition, CLP is a primary, polymicrobial peritoneal infection that is remote from the lung and will likely activate many TLR dependent responses compared to our specific intratraceal instillation of LPS. The diffuse chest injury and variable infectious stimuli of CLP (and potentially other non-TLR stimuli) are likely to activate immune responses that may be significantly different than in our second hit injury model that uses a focused and localized injury and a well characterized TLR4 ligand.
There are multiple cell types present in the lung both before and after injury that express TLR4 and are capable of producing IL-6 and CXC chemokines including alveolar macrophages, endothelial cells, and alveolar epithelial cells32;33. Paterson et al., in a burn injury model, demonstrated that macrophage and dendritic cells show enhanced IL-6 production after an LPS-mediated second hit2. Interestingly, they did not observe a change in TLR4 expression. They later found that injury enhanced TLR4 response was mediated, in part, by enhanced intracellular activity of the mitogen activated protein kinase, p38 and suggested a role for CD4+CD25+ T regulatory cells in controlling this innate response28;34. Moreover, in a model of ischemia/reperfusion, Fan et al. demonstrated enhanced CINC-1 expression primarily by alveolar macrophages in animals challenged with LPS intratracheally shortly after reperfusion35. They also showed that shock had little effect on TLR4 expression in the lung and that ischemia appeared to prevent LPS-induced reduction in TLR4 mRNA36. The mechanisms involved in TLR4 priming after blunt chest injury and which cells within the lung that mediate enhanced TLR4 response(s) are currently being investigated.
HTS resuscitation from hemorrhagic shock has been demonstrated to be an effective resuscitation strategy in the polytrauma patient. HTS has immunomodulatory properties that led us to hypothesize that HTS given after injury could potentially attenuate the clinically important second hit response in our mouse model of isolated chest injury. Distinct from the ischemia/reperfusion injury models, our model does not involve obvious blood loss. We found that HTS resuscitation after chest injury did reduce immune response to TLR4 as demonstrated by a marked reduction in lung PMN influx and reduced systemic levels of IL-6 and MIP2 (CXCL2/3) while resuscitation with LR did not have these effects.
Typically, neutrophil recruitment to the lung after injury involves localized CXC chemokine expression, neutrophil activation and adhesion receptor expression (CD11b/18), neutrophil rolling (selectins), and firm adhesion (β2 Integrins:ICAM-1)33. Our previous studies showed that CXCL1 is primarily involved in the innate response to traumatic lung injury5;25. CXCL1 and MIP-2 (CXCL2/3) are the murine equivalents of IL-8, the principle chemokine involved in neutrophil chemotaxis after traumatic lung injury in humans37. Alveolar macrophages and to a lesser extent, alveolar epithelial cells have been demonstrated to produce CXC chemokines after lung injury38. In our study, we found that both CXCL1 and MIP-2 were increased in second hit animals, and unexpectedly, resuscitation with HTS decreased MIP2, but not CXCL1, expression. Other investigators have observed a reduction in CXC chemokine expression when HTS was used as a resuscitation fluid. In a second hit model of ischemia/reperfusion followed by IT LPS in the rat, Fan et al. reported a reduction in alveolar macrophage expression of the CXC chemokine, CINC-1, but not MIP-2, when HTS was used35. They concluded that CINC-1 was primarily involved in the second hit response. Our findings demonstrate a similar differential effect on chemokine expression in second hit animals resuscitated with HTS and suggest that CXCL1 and MIP-2 have divergent roles in a second hit response. Differing pharmacokinetics for these chemokines may also participate in the second hit and may also explain our results. Evaluation of immune response kinetics will help to define the participation of these mediators.
Systemic neutrophil activation is indicated by changes in surface expression of the adhesion receptor, CD11b39. CXCL1 and MIP-2 have both been demonstrated to increase CD11b receptor expression in neutrophils33. In our study, the decrease in systemic MIP-2 expression with HTS resuscitation correlated with a marked reduction in neutrophil CD11b expression. The reduction in pulmonary neutrophilia and neutrophil CD11b expression in HTS animals may be related to changes in systemic CXCL2/3 chemokine expression, or alternatively, a result of hypertonicity-induced changes in circulating neutrophils. Hypertonicity might impair neutrophil CD11b expression by impeding neutrophil swelling during transmigration and/or alterations in the actin cytoskeleton preventing translocation of the receptor in response to LPS40;41. We have not measured serum osmolarity; however this is a likely explanation for our findings as changes in serum osmolarity after equivalent does of intravenous HTS have been shown to last for up to 6 hours, with the highest levels being seen 1 hour after administration. HTS has also been shown reduce neutrophil L-selectin shedding and reduce pulmonary levels of ICAM-1, effects that could be mediated through hypertonicity or changes in CXC chemokine expression7. Thus, in our study, it is likely that decreased neutrophil recruitment and CD11b expression in HTS resuscitated animals involves multiple mechanisms.
In addition to alterations in chemokine expression, resuscitation with HTS showed a dramatic decrease in systemic IL-6 levels. Previous studies implicate the alveolar macrophage as the likely cellular source19;20;42. We evaluated hepatic histology and observed no apparent indication of involvement in our injury model; however, these findings are neither comprehensive nor conclusive and further study to identify extra-pulmonary organ involvement is indicated. HTS resuscitation has been shown to affect alveolar macrophage function. In vitro studies have shown that hypertonic pretreatment of macrophages reduces proinflammatory mediator production19. In animal studies, resuscitation with HTS reduced alveolar macrophage priming resulting in diminished NFkB nuclear translocation in response to LPS20. Others have suggested that resuscitation with HTS may also induce a phenotypic change in the monocyte population towards a less inflammatory profile18. The exact mechanisms surrounding these changes in macrophage function remain indistinct. Our data is consistent with the implication that HTS is having an effect on macrophage function in addition to its effects on PMN function.
From a clinical standpoint, the second hit response remains an attractive target for intervention. There have been multiple randomized studies evaluating the efficacy of HTS in the early management of injury patients9-11;14;15;18;43. All of these studies included patients who had prehospital hypotension and were treated with a single dose of HTS with or without dextran either in the field or early in the emergency department. No consistent survival advantage has been identified. The theoretic advantage of using HTS as a resuscitation fluid may lie in its ability to manipulate the subsequent inflammatory response to later infection. As suggested in our study, HTS significantly attenuated post-injury immune responses; decreased infectious morbidity and mortality may result. It remains unknown whether a single dose of HTS given in the prehospital setting is sufficient to modulate the ensuing inflammatory response or if repeated dosing is required. In this study, HTS was administered 1hr prior to the second hit, a difficult scenario to mimic clinically; however, it would suggest that repeated dosing may be beneficial. Lastly, clinical lung function was not measured in this study, although others have reported improvement with HTS resuscitation. Continued resuscitation studies and identification of the molecular and cellular mechanisms mediating the second hit are indicated.
In conclusion, our data demonstrate that blunt chest injury resulting in pulmonary contusion primes innate immunity for an exaggerated TLR4 response. Furthermore, resuscitation with HTS after blunt chest injury reduced the second hit response as indicated by decreased pulmonary neutrophilia and the decreased levels of systemic innate immune mediators. These data suggest that HTS may have a significant role in the treatment of patients with blunt chest trauma and pulmonary contusion.
Acknowledgments
This work was supported, in part, by the American College of Surgeons C. James Carrico Faculty Research Fellowship, ALA RG-52711-N, and GM083154 (JJH), RR023570 (CEM) and AI065791 (CEM, BKY).
Footnotes
Portions presented at the 67th Annual AAST meeting, Sept. 24-27, 2008, Maui, HI.
References
- 1.Rotstein OD. Modeling the two-hit hypothesis for evaluating strategies to prevent organ injury after shock/resuscitation. J Trauma. 2003;54:S203–S206. doi: 10.1097/01.TA.0000064512.62949.92. [DOI] [PubMed] [Google Scholar]
- 2.Paterson HM, Murphy TJ, Purcell EJ, et al. Injury primes the innate immune system for enhanced Toll-like receptor reactivity. J Immunol. 2003;171:1473–1483. doi: 10.4049/jimmunol.171.3.1473. [DOI] [PubMed] [Google Scholar]
- 3.Murphy TJ, Paterson HM, Kriynovich S, et al. Linking the “two-hit” response following injury to enhanced TLR4 reactivity. J Leukoc Biol. 2005;77:16–23. doi: 10.1189/jlb.0704382. [DOI] [PubMed] [Google Scholar]
- 4.Mollen KP, Anand RJ, Tsung A, Prince JM, Levy RM, Billiar TR. Emerging paradigm: toll-like receptor 4-sentinel for the detection of tissue damage. Shock. 2006;26:430–437. doi: 10.1097/01.shk.0000228797.41044.08. [DOI] [PubMed] [Google Scholar]
- 5.Hoth JJ, Hudson WP, Brownlee NA, et al. Toll-like receptor 2 participates in the response to lung injury in a murine model of pulmonary contusion. Shock. 2007;28:447–452. doi: 10.1097/shk.0b013e318048801a. [DOI] [PubMed] [Google Scholar]
- 6.Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol. 2006;117:979–987. doi: 10.1016/j.jaci.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 7.Rizoli SB, Kapus A, Fan J, Li YH, Marshall JC, Rotstein OD. Immunomodulatory effects of hypertonic resuscitation on the development of lung inflammation following hemorrhagic shock. J Immunol. 1998;161:6288–6296. [PubMed] [Google Scholar]
- 8.Zhang H, Wang HY, Bassel-Duby R, et al. Role of interleukin-6 in cardiac inflammation and dysfunction after burn complicated by sepsis. Am J Physiol Heart Circ Physiol. 2007;292:H2408–H2416. doi: 10.1152/ajpheart.01150.2006. [DOI] [PubMed] [Google Scholar]
- 9.Mattox KL, Maningas PA, Moore EE, et al. Prehospital hypertonic saline/dextran infusion for post-traumatic hypotension. The U.S.A. Multicenter Trial. Ann Surg. 1991;213:482–491. doi: 10.1097/00000658-199105000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vassar MJ, Perry CA, Holcroft JW. Prehospital resuscitation of hypotensive trauma patients with 7.5% NaCl versus 7.5% NaCl with added dextran: a controlled trial. J Trauma. 1993;34:622–632. [PubMed] [Google Scholar]
- 11.Shackford SR, Bourguignon PR, Wald SL, Rogers FB, Osler TM, Clark DE. Hypertonic saline resuscitation of patients with head injury: a prospective, randomized clinical trial. J Trauma. 1998;44:50–58. doi: 10.1097/00005373-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Horton JW, Maass DL, White J, Sanders B. Hypertonic saline-dextran suppresses burn-related cytokine secretion by cardiomyocytes. Am J Physiol Heart Circ Physiol. 2001;280:H1591–H1601. doi: 10.1152/ajpheart.2001.280.4.H1591. [DOI] [PubMed] [Google Scholar]
- 13.Deitch EA, Shi HP, Feketeova E, Hauser CJ, Xu DZ. Hypertonic saline resuscitation limits neutrophil activation after trauma-hemorrhagic shock. Shock. 2003;19:328–333. doi: 10.1097/00024382-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Rizoli SB, Rhind SG, Shek PN, et al. The immunomodulatory effects of hypertonic saline resuscitation in patients sustaining traumatic hemorrhagic shock: a randomized, controlled, double-blinded trial. Ann Surg. 2006;243:47–57. doi: 10.1097/01.sla.0000193608.93127.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bulger EM, Jurkovich GJ, Nathens AB, et al. Hypertonic resuscitation of hypovolemic shock after blunt trauma: a randomized controlled trial. Arch Surg. 2008;143:139–148. doi: 10.1001/archsurg.2007.41. [DOI] [PubMed] [Google Scholar]
- 16.Hampton MB, Chambers ST, Vissers MC, Winterbourn CC. Bacterial killing by neutrophils in hypertonic environments. J Infect Dis. 1994;169:839–846. doi: 10.1093/infdis/169.4.839. [DOI] [PubMed] [Google Scholar]
- 17.Rizoli SB, Rotstein OD, Kapus A. Cell volume-dependent regulation of L-selectin shedding in neutrophils. A role for p38 mitogen-activated protein kinase. J Biol Chem. 1999;274:22072–22080. doi: 10.1074/jbc.274.31.22072. [DOI] [PubMed] [Google Scholar]
- 18.Bulger EM, Cuschieri J, Warner K, Maier RV. Hypertonic resuscitation modulates the inflammatory response in patients with traumatic hemorrhagic shock. Ann Surg. 2007;245:635–641. doi: 10.1097/01.sla.0000251367.44890.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuschieri J, Gourlay D, Garcia I, Jelacic S, Maier RV. Hypertonic preconditioning inhibits macrophage responsiveness to endotoxin. J Immunol. 2002;168:1389–1396. doi: 10.4049/jimmunol.168.3.1389. [DOI] [PubMed] [Google Scholar]
- 20.Powers KA, Zurawska J, Szaszi K, Khadaroo RG, Kapus A, Rotstein OD. Hypertonic resuscitation of hemorrhagic shock prevents alveolar macrophage activation by preventing systemic oxidative stress due to gut ischemia/reperfusion. Surgery. 2005;137:66–74. doi: 10.1016/j.surg.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 21.Cohn SM. Pulmonary contusion: review of the clinical entity. J Trauma. 1997;42:973–979. doi: 10.1097/00005373-199705000-00033. [DOI] [PubMed] [Google Scholar]
- 22.Ciesla DJ, Moore EE, Johnson JL, Burch JM, Cothren CC, Sauaia A. A 12-year prospective study of postinjury multiple organ failure: has anything changed? Arch Surg. 2005;140:432–438. doi: 10.1001/archsurg.140.5.432. [DOI] [PubMed] [Google Scholar]
- 23.Miller PR, Croce MA, Bee TK, et al. ARDS after pulmonary contusion: accurate measurement of contusion volume identifies high-risk patients. J Trauma. 2001;51:223–228. doi: 10.1097/00005373-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Hoth JJ, Stitzel JD, Gayzik FS, et al. The pathogenesis of pulmonary contusion: an open chest model in the rat. J Trauma. 2006;61:32–44. doi: 10.1097/01.ta.0000224141.69216.aa. [DOI] [PubMed] [Google Scholar]
- 25.Hoth JJ, Wells JD, Brownlee NA, et al. Toll-like receptor 4 dependent responses to lung injury in a murine model of pulmonary contusion. Shock. 2008 doi: 10.1097/SHK.0b013e3181862279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly JL, O'Sullivan C, O'Riordain M, et al. Is circulating endotoxin the trigger for the systemic inflammatory response syndrome seen after injury? Ann Surg. 1997;225:530–541. doi: 10.1097/00000658-199705000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shahbazian LM, Jeevanandam M, Petersen SR. Release of proinflammatory cytokines by mitogen-stimulated peripheral blood mononuclear cells from critically ill multiple-trauma victims. Metabolism. 1999;48:1397–1401. doi: 10.1016/s0026-0495(99)90149-x. [DOI] [PubMed] [Google Scholar]
- 28.Murphy TJ, Ni CN, Zang Y, Mannick JA, Lederer JA. CD4+CD25+ regulatory T cells control innate immune reactivity after injury. J Immunol. 2005;174:2957–2963. doi: 10.4049/jimmunol.174.5.2957. [DOI] [PubMed] [Google Scholar]
- 29.Vuichard D, Ganter MT, Schimmer RC, et al. Hypoxia aggravates lipopolysaccharide-induced lung injury. Clin Exp Immunol. 2005;141:248–260. doi: 10.1111/j.1365-2249.2005.02835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore FA, Sauaia A, Moore EE, Haenel JB, Burch JM, Lezotte DC. Postinjury multiple organ failure: a bimodal phenomenon. J Trauma. 1996;40:501–510. doi: 10.1097/00005373-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Perl M, Gebhard F, Bruckner UB, et al. Pulmonary contusion causes impairment of macrophage and lymphocyte immune functions and increases mortality associated with a subsequent septic challenge. Crit Care Med. 2005;33:1351–1358. doi: 10.1097/01.ccm.0000166352.28018.a9. [DOI] [PubMed] [Google Scholar]
- 32.Schilling D, Thomas K, Nixdorff K, Vogel SN, Fenton MJ. Toll-like receptor 4 and Toll-IL-1 receptor domain-containing adapter protein (TIRAP)/myeloid differentiation protein 88 adapter-like (Mal) contribute to maximal IL-6 expression in macrophages. J Immunol. 2002;169:5874–5880. doi: 10.4049/jimmunol.169.10.5874. [DOI] [PubMed] [Google Scholar]
- 33.Gao H, Neff T, Ward PA. Regulation of lung inflammation in the model of IgG immune-complex injury. Annu Rev Pathol. 2006;1:215–242. doi: 10.1146/annurev.pathol.1.110304.100155. [DOI] [PubMed] [Google Scholar]
- 34.Maung AA, Fujimi S, Miller ML, MacConmara MP, Mannick JA, Lederer JA. Enhanced TLR4 reactivity following injury is mediated by increased p38 activation. J Leukoc Biol. 2005;78:565–573. doi: 10.1189/jlb.1204698. [DOI] [PubMed] [Google Scholar]
- 35.Fan J, Marshall JC, Jimenez M, Shek PN, Zagorski J, Rotstein OD. Hemorrhagic shock primes for increased expression of cytokine-induced neutrophil chemoattractant in the lung: role in pulmonary inflammation following lipopolysaccharide. J Immunol. 1998;161:440–447. [PubMed] [Google Scholar]
- 36.Fan J, Kapus A, Marsden PA, et al. Regulation of Toll-like receptor 4 expression in the lung following hemorrhagic shock and lipopolysaccharide. J Immunol. 2002;168:5252–5259. doi: 10.4049/jimmunol.168.10.5252. [DOI] [PubMed] [Google Scholar]
- 37.Bhatia RK, Pallister I, Dent C, Jones SA, Topley N. Enhanced neutrophil migratory activity following major blunt trauma. Injury. 2005;36:956–962. doi: 10.1016/j.injury.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Lentsch AB, Ward PA. Regulation of inflammatory vascular damage. J Pathol. 2000;190:343–348. doi: 10.1002/(SICI)1096-9896(200002)190:3<343::AID-PATH522>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 39.Das UN. Critical advances in septicemia and septic shock. Crit Care. 2000;4:290–296. doi: 10.1186/cc711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosengren S, Henson PM, Worthen GS. Migration-associated volume changes in neutrophils facilitate the migratory process in vitro. Am J Physiol. 1994;267:C1623–C1632. doi: 10.1152/ajpcell.1994.267.6.C1623. [DOI] [PubMed] [Google Scholar]
- 41.Hallows KR, Law FY, Packman CH, Knauf PA. Changes in cytoskeletal actin content, F-actin distribution, and surface morphology during HL-60 cell volume regulation. J Cell Physiol. 1996;167:60–71. doi: 10.1002/(SICI)1097-4652(199604)167:1<60::AID-JCP7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 42.Staudenmayer KL, Maier RV, Jelacic S, Bulger EM. Hypertonic saline modulates innate immunity in a model of systemic inflammation. Shock. 2005;23:459–463. doi: 10.1097/01.shk.0000160523.37106.33. [DOI] [PubMed] [Google Scholar]
- 43.Cooper DJ, Myles PS, McDermott FT, et al. Prehospital hypertonic saline resuscitation of patients with hypotension and severe traumatic brain injury: a randomized controlled trial. JAMA. 2004;291:1350–1357. doi: 10.1001/jama.291.11.1350. [DOI] [PubMed] [Google Scholar]