Title VIII of the American Recovery and Reinvestment Act of 2009 authorizes the expenditure of $1.1 billion to conduct research comparing “clinical outcomes, effectiveness, and appropriateness of items, services, and procedures that are used to prevent, diagnose, or treat diseases, disorders, and other health conditions.” Federal support of “comparative effectiveness” research has been viewed as a cornerstone in controlling runaway health care costs.
Although cost is not mentioned explicitly in the comparative effectiveness legislation, the American College of Physicians and others have called for cost-effectiveness analysis — assessment of the added improvement in health outcomes relative to cost — to be on the agenda for comparative effectiveness research.1,2 This approach has come under harsh criticism from some who view it as the first step in health care rationing by the government — that cost cutting will mean the withdrawal of expensive treatments with small (but still positive) benefits. Some politicians have therefore tried to restrict any efforts to use comparative effectiveness to guide U.S. health care policy.3
COMPARATIVE EFFECTIVENESS VERSUS COST-EFFECTIVENESS
Many supporters of comparative effectiveness research contend that there is little need to confront cost-effectiveness in order to contain costs. Some clinical practices, once subjected to rigorous evaluation, have been found to be of no benefit, if not harmful. Moreover, there is considerable variation in health care expenditures and a weak or even negative association between spending and outcomes, such as mortality at the regional level4 and quality measures at the state level.5 This evidence has been interpreted to mean that cutting back on these putatively useless or harmful services would simultaneously reduce cost and improve health.4,6 In contrast, several cross-sectional studies that have shown positive associations between spending and outcomes have been interpreted to show that more spending leads to better outcomes.7
We question whether these associations — either negative or positive — are being interpreted correctly. An association between higher spending and poorer outcomes does not imply causality. Such negative associations may result if physicians and hospitals in lower-cost areas are more skilled — or if they use resources for more cost-effective services.
Whether additional spending yields improved outcomes depends critically on what the money is spent on. Clinical trials of treatments such as coronary reperfusion in patients with acute myocardial infarction, implantable cardioverter–defibrillator therapy, fusion surgery for spinal stenosis, and new drugs for patients with cancer or the AIDS have established their comparative benefits.8–13 Several of the cost-effectiveness ratios for these treatments are well under $100,000 per quality-adjusted life-year (QALY) gained, indicating good value for the money (Table 1).
Table 1.
Cost per Quality-Adjusted Life-Year (QALY) Gained from Selected Clinical Strategies.*
| Switch to an aromatase inhibitor for early-stage breast cancer vs. continued tamoxifen8 | $22,900 |
| Implant a cardioverter-defibrillator (primary prevention) vs. continued medical management9 | $37,400 to $77,200 |
| Perform fusion surgery for degenerative spondylolisthesis with spinal stenosis vs. conservative management10 | $120,000 |
| Prescribe trastuzumab for metastatic breast cancer vs. standard chemotherapy11 | $150,000 |
| Prescribe erlotinib for advanced pancreatic cancer vs. gemcitabine alone12 | $370,000 to $500,000 |
| Perform helical computed tomographic screening for lung cancer in 60-year-old former heavy smokers vs. no screening13 | $2,300,000 |
Values are given in 2008 U.S. dollars, with adjustment for inflation according to the Consumer Price Index. Numbers are the ratios of the added cost per person to the gain in QALYs per person.
But cost-effectiveness studies reveal a stunning range of incremental cost per QALY gained, ranging from a negative net cost to millions of dollars per QALY gained.14 Preventive services are no more and no less likely to save money than treatments.15 For example, annual screening for cervical cancer costs about $800,000 more for every life-year gained than does biennial screening.16 Small variations in the mix of utilization across the spectrum of therapeutic, diagnostic, and preventive technologies could produce large geographic variations in overall costs and health outcomes.
As long as there are opportunities to substitute more cost-effective clinical strategies for less cost-effective ones, costs can be lowered without adversely affecting health. But at some point, difficult choices must be made. Should the Medicare program continue to pay for cancer drugs that improve survival by a median of 10 days and have cost-effectiveness ratios of up to $500,000 per QALY added?12,17
RELATING HEALTH CARE EXPENDITURES TO OUTCOMES
Figure 1 shows different levels of efficiency (as measured in QALYs gained per expenditure) in two hypothetical health care delivery systems.18 The purple curve represents an efficient system; along this curve, higher expenditure leads to better outcomes.19 Each segment of the curve represents either the addition of an intervention for a particular clinical condition or the substitution of an intervention that is more effective but more expensive for an intervention that is less expensive but less effective. Each slope represents value for money — the incremental gain in health per dollar spent (steeper is better) — and the length of the segment reflects the degree to which the service is utilized. The incremental value of an addition or substitution to the mix of services often depends on what other services are already in the mix; for example, the added value of coronary catheterization may be lower when the hospital is already providing appropriate medical management for patients with acute myocardial infarction.20
Figure 1. Levels of Efficiency in Allocating Health Care Resources.
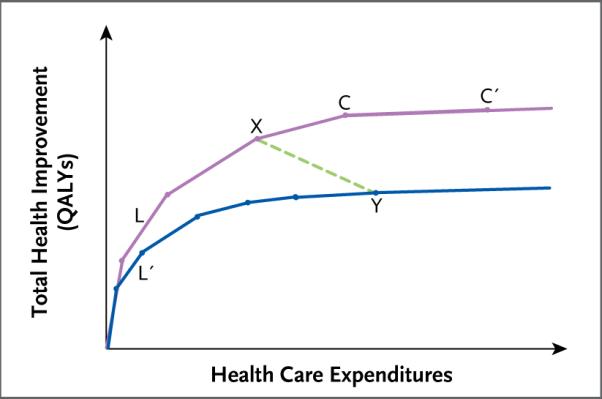
Shown is the relationship between expenditures and health gains (as measured in quality-adjusted life-years [QALYs] gained) in two hypothetical health care delivery systems. The purple upper curve represents an allocation of expenditures to health interventions that maximizes health improvements for any level of expenditure. The blue lower curve represents an allocation that is inefficient in the sense that it falls short of the maximum attainable health gain for any level of expenditure. The line segments that make up the curves represent incremental additions of health interventions or substitutions of more beneficial but more expensive interventions for less expensive but less beneficial alternatives. For example, line segments L and L′ both represent a hypothetical intervention that is very cost-effective relative to the other interventions to the right. However, in the inefficient allocation (lower curve), this highly cost-effective intervention is underutilized, as represented by the shorter length of L′ as compared with L. The line segments become progressively less steep from left to right, reflecting diminishing health value as expenditures increase. The points on the curves represent possible allocations of health expenditures. For example, point X on the upper curve represents an allocation that achieves more health gains at a lower cost than the allocation represented by point Y on the lower curve. The shallow slope of the line segment connecting points C and C′ reflects the fact that the intervention it represents is a relatively cost-ineffective one, although it is still beneficial.
The blue curve in Figure 1 represents an inefficient health delivery system, in which some cost-effective health services are underutilized. For example, the health service that is represented by segment L′ in the inefficient system is shorter than the segment L in the efficient system. As examples, antihypertensive treatment, screening for colorectal cancer, and counseling for smoking cessation are all underutilized in the United States.21,22 In both the more efficient system and the less efficient system, it is possible to increase expenditures to the point where they yield little or no health improvement, which is sometimes referred to as “flat-of-the-curve” medicine.23
In Figure 1, the efficient system spends less but obtains better health outcomes at point X than the inefficient system at point Y, even though the latter is doing nothing that is overtly harmful. The inefficient system does worse because it falls short in utilizing beneficial health practices — errors of omission, not commission. At point X on the curve, the efficient system forgoes some potentially valuable spending (between X and C) but still ends up at a higher level of health than at Y.
At a point in time, any statistical comparison between X and Y will show a negative association between spending and health outcomes, as shown by the dashed green line between the two curves. Indeed, one might conclude from cross-sectional data that spending more harms individual patients. In the case shown in Figure 1, however, cutting spending in health system Y back to the level of system X would result in worse outcomes. However, system Y could save money without sacrificing health by shifting up to the higher curve — substituting more cost-effective health services for less cost-effective health services.
Figure 2 displays the relationship between expenditures and outcomes among various providers (shown by the circles) in managing a hypothetical condition. This picture, which is consistent with empirical data from a variety of studies, shows little or no association between spending and health outcomes. One might find in such a seemingly random scatter plot either a positive or negative (or no) association, but the wide variations in both expenditures and outcomes suggest that factors other than spending itself must be affecting outcomes. In particular, this kind of pattern can be generated by wide variations in the use of interventions that are cost-effective (good value for the money) and those that are cost-ineffective (flat-of-the-curve interventions). Differences in the skill levels of providers or administrative efficiency might also contribute to the variation.
Figure 2. Expenditures and Health Outcomes According to Various Production Functions.
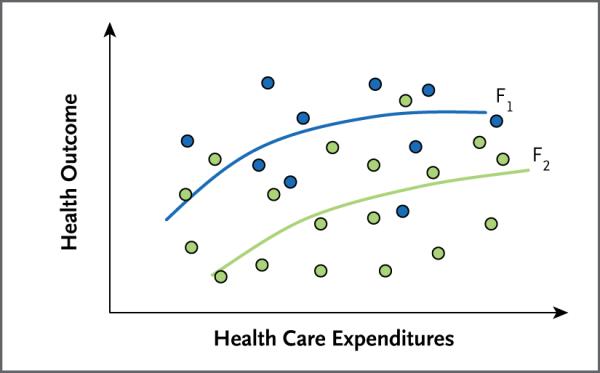
Shown is the relationship between expenditures and outcomes among various providers (circles) in managing a hypothetical condition. The blue circles represent providers who use a comparatively cost-effective mix of interventions for this condition, albeit at different levels of intensity (expenditure), and the green circles represent providers who use a less cost-effective mix of services. The curves F1 and F2 show the associations between expenditures and outcomes among providers who practice more (F1) or less (F2) cost-effective medicine.
To provide some structure to the scatter plot, we can distinguish between two types of providers: those who are close to the more cost-effective curve, F1 (blue circles), and others who are close to the less cost-effective curve, F2 (green circles). Some of the differences in outcomes, at any level of spending, arise because some providers utilize a more cost-effective mix of services than others.
Empirical support for Figure 2 comes from a recent study24 that used chart-review data from the 1994–1995 Cooperative Cardiovascular Project to categorize hospitals as either high-adopting facilities or low-adopting facilities, according to their rates of use of aspirin, beta-blockers, and coronary reperfusion in the treatment of acute myocardial infarction. The researchers found that the high-adopting hospitals had consistently better rates of risk-adjusted survival, at no additional cost to Medicare. But after stratification according to the hospitals' adoption rates, there was a positive but diminishing effect of spending on the health outcome (12-month survival), similar to curves F1 and F2 in Figure 2. The cost-effectiveness ratios at the margin were $95,000 per life-year or more but with slightly better returns for the hospitals that were slower to adopt cost-effective practices (curve F2).
Another study showed that regions that had high rates of revascularization for patients with acute myocardial infarction received good health value for the expenditure on the intervention.25 Despite this, there was essentially a zero association between spending and outcomes across regions. The explanation is that the high-revascularization areas were also less likely to use beta-blockers and aspirin for their patients. Thus, like the region represented by point Y in Figure 1 they started out with poorer outcomes because of their failure to adopt cost-effective interventions, but they caught up to the other regions by devoting more resources to invasive interventions that were more expensive yet reasonably cost-effective.
A third example comes from a study of colon-cancer treatment, in which once again there was no association between overall spending and outcomes.26 The authors found greater use of inappropriate chemotherapy in high-spending areas, where adverse effects shifted the providers down from outputs that were more efficient (F1) to those that were less efficient (F2). However, these providers were also more likely to use highly appropriate (yet expensive) adjuvant chemotherapy, which would move them along and up the F2 curve, resulting in no difference in overall outcomes from the lower-intensity areas, despite the higher cost.
As Figure 2 illustrates, the apparent lack of correlation at the aggregate level between health spending and outcomes does not disprove the existence of a positive association within a hospital or other health care delivery organization. Moreover, a negative association does not imply that more spending is harmful. That said, there are undoubtedly treatments — such as testing of prostate-specific antigen levels in older men with limited life expectancy,27 arthroscopic surgery for osteoarthritis of the knee,28 and ventricular reconstruction surgery29 — that offer no measurable value and could be scaled back without compromising outcomes. And there are many more therapies for which the comparative effectiveness is unknown.
GETTING TO THE WIN-WIN BALANCE
Is it possible to contain health care costs without causing worse health outcomes? The answer, we suggest, is no and yes. If health delivery areas that are expensive and inefficient were to cut back without reordering their priorities from less to more cost-effective services, then they, too, could have worse outcomes. Thus, if Medicare spending per enrollee in Miami ($16,351 in 2006) were cut back to the national average ($8,304), the residents of Miami might suffer if health care providers cut back on cost-effective as well as ineffective treatments. If the expensive technologies that they use compensate, in part, for their failure to utilize cost-effective primary or secondary preventive services, as suggested by the previously mentioned study of survival after revascularization,25 then these areas may have even greater losses in outcome than the efficient areas would if they cut back on the same services. But the answer is also yes — we can save money without compromising outcomes — if we can induce providers to cut back on cost-ineffective services and replace them with more cost-effective but underutilized services.
There are many other approaches to improving quality of care that may or may not yield large cost savings but are still worth doing. The use of electronic medical records has the potential to reduce medical errors, thereby reducing costs and improving outcomes simultaneously. Improving coordination of care has the potential to reduce duplication of services, reduce administrative waste, and improve outcomes. Shared decision making can ensure that patients actually want invasive procedures, thereby reducing costs without compromising patients' well-being.30 Coupled with policies that shift resources away from cost-ineffective interventions, such innovations can result in a win-win situation: lower costs and better outcomes.
At some point, however, we will have to confront the problem of cost-effectiveness at the level of the patient. The limitless pipeline of effective clinical strategies — biologic drugs (e.g., etanercept for rheumatoid arthritis, imatinib for chronic myelogenous leukemia, and trastuzumab for breast cancer), enhanced imaging tests, and personalized medicine — offers improved outcomes, but the costs of development and production are often very high. Even if all hospitals, medical practices, and health plans became efficient in the sense that they adopted all interventions that are more cost-effective than anything they currently do, they would still have to draw the line on expenditures somewhere. There are trade-offs — reallocating resources from cost-ineffective treatments for late-stage pancreatic cancer to cost-effective treatments for diabetes may improve health outcomes in the aggregate but not for patients with late-stage pancreatic cancer.
Most industrialized countries have already confronted cost-effectiveness. For example, the National Institute for Health and Clinical Excellence (NICE) requires cost-effectiveness analyses of selected medical technologies as part of the basis for their coverage recommendations to the National Health Service in England and Wales, and it applies a criterion of £30,000 per QALY or less. Yet even in Britain, there are increasing complaints about denial of expensive but effective treatments for life-threatening conditions, such as metastatic cancers. As a result, NICE has implemented a compassionate care exception to the £30,000-per-QALY criterion for proven effective treatments of patients with a very poor prognosis.
Americans have less tolerance for command-and-control regulation than those in many other countries, so it is unlikely that a NICE-style structure of explicit rationing will be acceptable to Congress and the American people. On the other hand, Americans appear to be more accepting than Europeans of the role of price in allocating health care. Even conservative health economists who decry the possibility of rationing believe that reductions in health care utilization are acceptable if they occur through free choice in response to the price mechanism.31 Physicians and hospitals could be paid higher levels of compensation for more cost-effective services relative to their costs but lower levels of payment at or below cost for less cost-effective services. Consumers in many health plans are already used to higher copayments for prescriptions in higher tiers, so such stratification could be explicitly linked to cost-effectiveness.
Implementing value-based reimbursement or copayments is not without challenges. For example, many inexpensive drugs are cost-effective on average but ineffective for a minority of patients for whom only a more expensive drug is effective. To overcome the challenge of micro-managing prices according to characteristics of patients, price options could be offered to patients at the stage when they sign up for their insurance; a lower-cost insurance option might start patients on inexpensive drugs and switch them to more expensive drugs only if necessary, whereas a higher-cost option might provide immediate access to higher-cost drugs or to treatments without proven effectiveness. Any incentive schemes, whether linked to payments for individual services or to bundles of services through insurance packages, are subject to the caveat that patients may make unwise or irrational decisions.
If we can induce hospitals and health plans to improve efficiency and not just cut costs, then health costs in the United States will come down and outcomes will improve. Although it is necessary to confront the trade-off between cost and outcome at the margin, we can have our cake and eat it too if we induce inefficient providers to adopt cost-effective health practices instead of cost-ineffective ones.
Acknowledgments
Supported by a grant (PO1-AG19783, to Dr. Skinner) from the National Institute of Aging and by institutional sabbatical research funds from the Harvard School of Public Health and the Dartmouth Institute for Health Policy and Clinical Practice (to Dr. Weinstein).
We thank Douglas Staiger, Elliott Fisher, and James Weinstein for their advice and intellectual support.
Footnotes
Financial and other disclosures provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.American College of Physicians Information on cost-effectiveness: an essential product of a national comparative effectiveness program. Ann Intern Med. 2008;148:956–61. doi: 10.7326/0003-4819-148-12-200806170-00222. [DOI] [PubMed] [Google Scholar]
- 2.Garber AM. A menu without prices. Ann Intern Med. 2008;148:964–6. doi: 10.7326/0003-4819-148-12-200806170-00223. [DOI] [PubMed] [Google Scholar]
- 3.U.S. to compare medical treatments. New York Times. 2009 Feb 15;:A1. [Google Scholar]
- 4.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending. Part 2: Health outcomes and satisfaction with care. Ann Intern Med. 2003;138:288–98. doi: 10.7326/0003-4819-138-4-200302180-00007. [DOI] [PubMed] [Google Scholar]
- 5.Baicker K, Chandra A. Medicare spending, the physician workforce, and beneficiaries' quality of care. Health Aff (Millwood) 2004;(Suppl Web Exclusives):W184–W197. doi: 10.1377/hlthaff.w4.184. [DOI] [PubMed] [Google Scholar]
- 6.Skinner J, Fisher ES, Wennberg JE. The efficiency of Medicare. In: Wise D, editor. Analyses in the economics of aging. University of Chicago Press; Chicago: 2005. pp. 129–57. [Google Scholar]
- 7.Ong MK, Mangione CM, Romano PS, et al. Looking forward, looking back: assessing variations in hospital resource use and outcomes for elderly patients with heart failure. Circ Cardiovasc Qual Outcomes. 2009;2:548–57. doi: 10.1161/CIRCOUTCOMES.108.825612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson D, Taylor DC, Montoya EL, Winer EP, Jones SE, Weinstein MC. Cost-effectiveness of switching to exemestane following 2 to 3 years of therapy with tamoxifen in postmenopausal women with early-stage breast cancer. Value Health. 2007;10:367–76. doi: 10.1111/j.1524-4733.2007.00190.x. [DOI] [PubMed] [Google Scholar]
- 9.Sanders GD, Hlatky MA, Owens DK. Cost-effectiveness of implantable cardioverter–defibrillators. N Engl J Med. 2005;353:1471–80. doi: 10.1056/NEJMsa051989. [DOI] [PubMed] [Google Scholar]
- 10.Tosteson AN, Lurie JD, Tosteson TD, et al. Surgical treatment of spinal stenosis with and without degenerative spondylolisthesis: cost-effectiveness after 2 years. Ann Intern Med. 2008;149:845–53. doi: 10.7326/0003-4819-149-12-200812160-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elkin EB, Weinstein MC, Winer EP, Kuntz KM, Schnitt SJ, Weeks JC. HER-2 testing and trastuzumab therapy for metastatic breast cancer: a cost-effectiveness analysis. J Clin Oncol. 2004;22:854–63. doi: 10.1200/JCO.2004.04.158. [DOI] [PubMed] [Google Scholar]
- 12.Miksad RA, Schnipper L, Goldstein M. Does a statistically significant survival benefit of erlotinib plus gemcitabine for advanced pancreatic cancer translate into clinical significance and value? J Clin Oncol. 2007;25:4506–8. doi: 10.1200/JCO.2007.13.0401. [DOI] [PubMed] [Google Scholar]
- 13.Mahadevia PJ, Fleisher LA, Frick KD, Eng J, Goodman SN, Powe NR. Lung cancer screening with helical computed tomography in older adult smokers: a decision and cost-effectiveness analysis. JAMA. 2003;289:313–22. doi: 10.1001/jama.289.3.313. [DOI] [PubMed] [Google Scholar]
- 14.Cost-Effectiveness Analysis (CEA) Registry. The Center for the Evaluation of Value and Risk in Health; (Accessed January 4, 2010, at http://www.tufts-nemc.org/cearegistry.) [Google Scholar]
- 15.Cohen JT, Neumann PJ, Weinstein MC. Does preventive care save money? Health economics and the presidential candidates. N Engl J Med. 2008;358:661–3. doi: 10.1056/NEJMp0708558. [DOI] [PubMed] [Google Scholar]
- 16.Kim JJ, Wright TC, Goldie SJ. Cost-effectiveness of alternative triage strategies for atypical squamous cells of undetermined significance. JAMA. 2002;287:2382–90. doi: 10.1001/jama.287.18.2382. [DOI] [PubMed] [Google Scholar]
- 17.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 18.Weinstein MC. Spending health care dollars wisely: can cost-effectiveness analysis help?. Policy brief no. 30; 16th Annual Herbert Lourie Memorial Lecture on Health Policy; Syracuse, NY: Maxwell School of Citizenship and Public Affairs, Syracuse University; 2005. [Google Scholar]
- 19.Russell LB, Gold MR, Siegel JE, Daniels N, Weinstein MC. The role of cost-effectiveness analysis in health and medicine. JAMA. 1996;276:1172–77. [PubMed] [Google Scholar]
- 20.Stukel TA, Lucas FL, Wennberg DE. Long-term outcomes of regional variations in intensity of invasive vs medical management of Medicare patients with acute myocardial infarction. JAMA. 2005;293:1329–37. doi: 10.1001/jama.293.11.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348:2635–45. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 22.National Healthcare Quality Report 2007. Agency for Healthcare Research and Quality; Washington, DC: 2008. AHRQ publication number 08–0040. [Google Scholar]
- 23.Fuchs VR. Health, economics, and social choice. Basic Books; New York: 1974. Who shall live? [Google Scholar]
- 24.Skinner J, Staiger D. Working paper no. 14865. National Bureau of Economic Research; Cambridge, MA: Apr, 2009. Technology diffusion and productivity growth in health care. Accessed December 30, 2009, at http://www.dartmouth.edu/~jskinner/documents/SkinnerStaigerw14865.pdf. [Google Scholar]
- 25.Chandra A, Staiger D. Productivity spillovers in healthcare: evidence from the treatment of heart attacks. J Polit Econ. 2007;115:103–40. doi: 10.1086/512249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landrum MB, Meara ER, Chandra A, Guadagnoli E, Keating NL. Is spending more always wasteful? The appropriateness of care and outcomes among colorectal cancer patients. Health Aff (Millwood) 2008;27:159–68. doi: 10.1377/hlthaff.27.1.159. [DOI] [PubMed] [Google Scholar]
- 27.Walter LC, Bertenthal D, Lindquist K, Konety BR. PSA screening among elderly men with limited life expectancies. JAMA. 2006;296:2336–42. doi: 10.1001/jama.296.19.2336. [DOI] [PubMed] [Google Scholar]
- 28.Kirkley A, Birmingham TB, Litchfield RB, et al. A randomized trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2008;359:1097–107. doi: 10.1056/NEJMoa0708333. [DOI] [PubMed] [Google Scholar]
- 29.Jones RH, Velazquez EJ, Michler RE, et al. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med. 2009;360:1705–17. doi: 10.1056/NEJMoa0900559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Connor AM, Llewellyn-Thomas HA, Flood AB. Modifying unwarranted variations in health care: shared decision making using patient decision aids. Health Aff (Millwood) 2004;(Suppl Web Exclusives):VAR63–VAR72. doi: 10.1377/hlthaff.var.63. [DOI] [PubMed] [Google Scholar]
- 31.Feldstein M. Obama's plan isn't the answer. Washington Post: Jul 27, 2009. [Google Scholar]


