Playing action-based video games has been shown to improve attentional processing. A study now finds that it also induces long-lasting improvements in contrast sensitivity, a basic visual function that commonly deteriorates with age. These improvements do not happen for an equivalent group who played a non-action video game.
How well we see primarily depends upon the physical condition of the structures of our eyes—components such as the cornea and lens that determine the amount and focus of light that impinges upon our retinas. During development, and over the course of a lifetime, changes in these structures or accidental damage to them can lead to visual impairments that have been alleviated for centuries through the use of corrective lenses (e.g. myopia) or more recently through surgical procedures (e.g. cataracts). However, even under ideal optical conditions, the quality of vision is limited by the neuronal architecture and function of the visual system instantiated in the brain1. Now a study in this issue of Nature Neuroscience2 demonstrates that normal-sighted people who play action-based video games can benefit from long lasting improvements in contrast sensitivity, one of the most basic visual functions that commonly deteriorates with aging among other causes. This improvement is likely to be due to changes in neural processing, rather than physical changes in eye structure.
Contrast perception is one of the most fundamental aspects of vision3. Contrast sensitivity refers to the ability to detect subtle changes in stimulus contrast. For example, contrast sensitivity permits us to see the faint glow of a pilot light, or the facial features of a person standing in a poorly lit room. The top of Figure 1 illustrates examples of high and low contrast stimuli. Unlike standard tests of visual acuity (Fig. 1 bottom left) that examine how well we can perceive objects of different sizes at high contrast, tests for contrast sensitivity (Fig. 1 bottom right) examine visual performance across a range of sizes and contrasts, thereby providing a broader assessment of basic visual functions. This distinction becomes important when considering certain visual impairments (such as cataracts) that can selectively impair contrast sensitivity, while leaving standard acuity intact.
Figure 1. Stimulus contrast and tests of visual function.
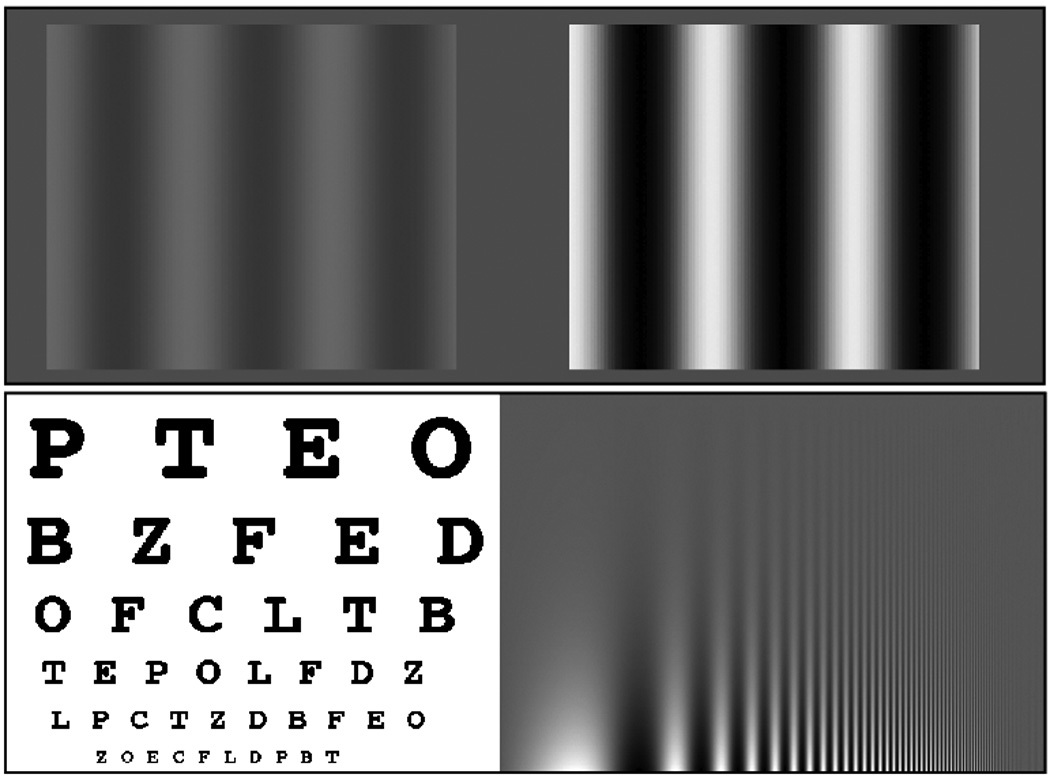
Top: Examples of low (left) and high (right) contrast sine wave gratings. Simple stimuli like these are often used in perceptual learning experiments.
Bottom Left: An example of a Snellen Eye chart used to measure visual acuity.
Bottom Right: An example of a Campbell-Robson contrast sensitivity function (CSF) chart. See your own CSF: towards the top of the chart the white-black modulations should ‘blend in’ with the grey background. The inverted-U shaped curve you see indicates that contrast sensitivity is better for mid-range spatial frequencies than low or high ones. Playing action-based video games improves contrast sensitivity, effectively ‘pushing’ this curve upwards.
Contrast sensitivity is a building block for a wide range of visual functions including object recognition4 and attention5. In addition, contrast sensitivity is particularly important for people such as radiologists and pilots, whose professions often depend on being able to perceive and recognize objects at low contrast. For example, a radiologist typically examines images in which subtle changes in contrast may reflect abnormal tissue densities arising from tumors, hemorrhages or other causes. For the rest of us, contrast sensitivity is important for common experiences like driving at night or in the fog, when the contrast of the environment is low. Therefore, the finding that contrast sensitivity can be improved simply by playing a video game represents a significant advance that may ultimately lead to improved vision above and beyond that which can be provided by corrective lenses. Such an improvement would not only apply to those with perceptual deficits or professional needs, but potentially to anybody willing to pick up a video game controller.
In their study, Li et al. carefully measured contrast sensitivity at several spatial frequencies in participants with either extensive practice playing action-based video games, or no video game experience. They found that the video game players (VGP) had increased contrast sensitivity at all but the lowest spatial frequencies when compared to age-matched non-video game players (NVGP). To establish a causal relationship between video game playing and improved vision, an additional group of NVGP underwent video game ‘training’, in which they played a video game for 50 hours over a nine-week period. Half of these participants played an action-based video game, while the other half played a non-action simulation-based video game. Contrast sensitivity was measured for these two groups, both before and after the training, and significant improvements were observed only for the action-based video game group. This result suggests that video game playing has a causal role in improving contrast sensitivity and, more specifically, identifies the visual processing associated with the action-based video game as the causal mechanisms for the improvement.
The present study adds to a rich history of experimental investigation into ways in which sensory training and experience can change and improve perceptual experience. In multiple sensory modalities including vision, it has been shown that performance on simple and basic perceptual tasks can improve with extensive training and practice, an ability referred to as perceptual learning 6. For example, observers can be trained to discriminate subtle differences in the orientation of line segments7or spatial frequency content8of grating stimuli similar to those shown in the top of Figure 1. Perceptual learning has also been used to improve visual functions in patients suffering from amblyopia9, a developmental disorder that leads to poor or reduced vision in an eye that has otherwise normal optical properties. Such learning paradigms typically involve extensive training over dozens of sessions and thousands of trials in which observers repeatedly perform near threshold perceptual tasks, such as discriminating or detecting different grating stimuli. Paradigms such as these have been used to induce long-lasting improvements to the acuity9and contrast sensitivity10–12 of amblyopic patients.
In assessing the importance of the current findings, it is important to consider by how much the video game playing actually improved the contrast sensitivity of the VGP. In quantifying the degree to which contrast sensitivity was improved Li et al. found the effects to be quite substantial, representing a ~50% enhancement in contrast sensitivity over NVGP. The size of this effect is comparable if somewhat smaller to that found using classical learning paradigms in normal sighted12 and amblyopic patients10–12. However, in the current study, these improvements were achieved by playing video games, not by performing thousands of tedious contrast detection tasks in a vision scientist’s laboratory. Importantly, and again similar to classic perceptual learning paradigms, the effects of action-based video game playing on contrast sensitivity remained for months and even years after training.
The results of this study raise several important questions for the field of perceptual learning. First and foremost, what aspects of playing action-based video games lead to the improved perceptual performance? Classic perceptual learning experiments use simple stimuli, such as sine wave gratings (top of figure 1), to improve the perceptual experience of those same classes of stimuli. In general, such learning effects are often specific and strictly limited to the stimuli used in the training. For example, learning to discriminate vertically oriented gratings will not generalize to gratings presented at other orientations8. In comparison, the perceptual learning that occurs through video game playing appears to be quite general, improving contrast sensitivity across a range of spatial frequencies without explicitly training the observers on detecting low contrast stimuli. However, the complex nature of the action-based video games makes it difficult to isolate the key components underlying the learning effect. One clue may be found in the fact that non-action based video games, although rich in the way they stimulate the visual system, operate at a much slower pace and do not require fast and precise visually-guided actions such as aiming weapons and navigating through complex virtual landscapes.
These results prompt the question of mechanism: how does video game induced perceptual learning occur? Although the answer to this question remains unknown, the same group has previously demonstrated that action-based video game playing leads to an enhancement in attentional processes13. A variety of studies suggest a close link between attention and contrast sensitivity. Psychophysical methods have shown that the allocation of attention can increase contrast sensitivity5, but see 14. Furthermore, electrophysiological methods have shown that attention can modify the contrast response properties of neurons within visual cortex15. This leads to the hypothesis that the improved contrast sensitivity reflects changes within attentional rather than contrast sensitivity mechanisms. If so, it is possible that the potential therapeutic benefits of video game playing may be different, if not complimentary, to those observed using classic perceptual learning paradigms that likely operate directly on the mechanisms underlying contrast detection11.
The implications of using video games therapeutically to improve contrast sensitivity are far reaching. Notably, the improvement was demonstrated to occur for normal-sighted observers under ideal optical and foveal viewing conditions. This means that presumably, anybody who plays action-based video games can potentially benefit. Importantly, individuals such as radiologists or pilots who rely upon their vision for making critical decisions may directly benefit from such a training routine. Furthermore, decreased contrast sensitivity is associated with a wide range of visual impairments related to both the optical quality of the eye (e.g. cataracts) and neuronal deficits (e.g. amblyopia). The current finding adds to a growing literature indicating that perceptual learning may be able to compliment clinical procedures aimed at improving eyesight. In order to reach full potential, additional research will be required to isolate and identify the critical components of action-based video games that lead to the improvements, and to identify ways in which they may compliment other perceptual learning approaches. Such research may ultimately lead to interfaces that specifically optimize the perceptual benefits.
Finally, the current finding adds fuel to the fiery 21st century debate raging between parent and child over whether or not, how much or what kind of video game playing is appropriate.
References
- 1.Elliott D, Whitaker D, MacVeigh D. Vision Res. 1990;30:541–547. doi: 10.1016/0042-6989(90)90066-t. [DOI] [PubMed] [Google Scholar]
- 2.Li R, Polat U, Makous W, Bavelier D. Nat. Neurosci. doi: 10.1038/nn.2296. xxxx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell FW. Behav. Brain Res. 1983;10:87–97. doi: 10.1016/0166-4328(83)90154-7. [DOI] [PubMed] [Google Scholar]
- 4.Avidan G, Harel M, Hendler T, Ben-Bashat D, Zohary E, Malach R. J. Neurophysiol. 2002;87(6):3102–3116. doi: 10.1152/jn.2002.87.6.3102. [DOI] [PubMed] [Google Scholar]
- 5.Carrasco M. Prog Brain Res. 2006;154:33–70. doi: 10.1016/S0079-6123(06)54003-8. [DOI] [PubMed] [Google Scholar]
- 6.Fahle M, Poggio T. Perceptual Learning. Cambridge: MIT Press; 2002. [Google Scholar]
- 7.Fahle M. Vision Res. 1997;37(14):1885–1895. doi: 10.1016/s0042-6989(96)00308-2. [DOI] [PubMed] [Google Scholar]
- 8.Fiorentini A, Berardi N. Nature. 1980;287(5777):43–44. doi: 10.1038/287043a0. [DOI] [PubMed] [Google Scholar]
- 9.Levi DM, Li RW. Philos Trans R Soc Lond B Biol Sci. 2009;364(1515):399–407. doi: 10.1098/rstb.2008.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polat U, Ma-Naim T, Belkin M, Sagi D. Proc Natl Acad Sci U S A. 2004;27;101(17):6692–6697. doi: 10.1073/pnas.0401200101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Huang C, Xu P, Tao L, Qiu Z, Li X, Lu ZL. Vision Res. 2006;46(5):739–750. doi: 10.1016/j.visres.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 12.Huang CB, Zhou Y, Lu ZL. Proc Natl Acad Sci U S A. 2008;105(10):4068–4073. doi: 10.1073/pnas.0800824105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green CS, Bavelier D. Nature. 2003;29;423(6939):534–537. doi: 10.1038/nature01647. [DOI] [PubMed] [Google Scholar]
- 14.Prinzmetal W, Long V, Leonhardt J. Percept Psychophys. 2008;70(7):1139–1150. doi: 10.3758/PP.70.7.1139. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds JH, Pasternak T, Desimone R. Neuron. 2000;26(3):703–714. doi: 10.1016/s0896-6273(00)81206-4. [DOI] [PubMed] [Google Scholar]


