Abstract
In animals, exposure to severe stress can damage the hippocampus. Recent human studies show smaller hippocampal volume in individuals with the stress-related psychiatric condition posttraumatic stress disorder (PTSD). Does this represent the neurotoxic effect of trauma, or is smaller hippocampal volume a pre-existing condition that renders the brain more vulnerable to the development of pathological stress responses? In monozygotic twins discordant for trauma exposure, we found evidence that smaller hippocampi indeed constitute a risk factor for the development of stress-related psychopathology. Disorder severity in PTSD patients who were exposed to trauma was negatively correlated with the hippocampal volume of both the patients and the patients’ trauma-unexposed identical co-twin. Furthermore, severe PTSD twin pairs—both the trauma-exposed and unexposed members—had significantly smaller hippocampi than non-PTSD pairs.
Animal research has provided compelling evidence that exposure to severe and chronic stress can damage the hippocampal formation1,2, a region best known for its role in declarative memory3,4. Such studies point to a neurotoxic role for corticosteroids, elevated levels of which cause atrophy and/or cell death in hippocampal neurons. This has led to the proposal that a similar process may occur in humans, and thereby mediate specific stress-related disease processes. Of particular relevance is the psychiatric condition of posttraumatic stress disorder (PTSD), a constellation of disabling behavioral and emotional symptoms that occur in some individuals who experience severe psychological trauma such as combat, sexual abuse or natural disaster. Indeed, several structural magnetic resonance imaging (MRI) studies report smaller hippocampal volume in patients diagnosed with chronic, unremitting forms of PTSD5–8. These results have generated intense interest regarding a potential pathogenesis for this disorder, and they raise the possibility that psychological trauma may in fact induce neurological damage in humans.
Controversy exists, however, over the nature and source of smaller hippocampal volume in PTSD9–12. The fundamental question at the heart of this controversy is whether volumetric differences represent the consequence of traumatic exposure or a pre-existing trait that predisposes people to pathological stress reactions to a traumatic event. This latter formulation is consistent with the fact that only some individuals exposed to trauma go on to develop PTSD13,14. The National Vietnam Veterans Readjustment Study13, for example, has estimated the prevalence of PTSD in Vietnam combat veterans to be 30.6%. Furthermore, animal research shows that inherited variations in hippocampal size can influence behavioral outcomes in stress-mediated conditioning procedures15–17 and can alter neuroendocrine responses to stress18. To date, there have been no human studies that directly address this important controversy.
In the present study, we used a ‘case-control’ design (Fig. 1) to examine samples of male monozygotic twin pairs in which one twin was a Vietnam combat veteran (exposed, Ex) and his identical co-twin had no combat exposure (unexposed, Ux). In some twin pairs, the combat-exposed brother developed chronic PTSD, whereas in other twin pairs the combat veteran never developed PTSD. Based on the diagnosis of the combat-exposed brother, we classified twin pairs into two groups: PTSD (P+) and non-(that is, never had) PTSD (P−). The P+ or P− designation always refers to the combat-related PTSD status of the exposed twin (no unexposed twin in this study had PTSD). Because monozygotic twins are genetically identical, any differences in hippocampal volume between brothers were interpreted as evidence for environmental effects, such as stress-induced neurotoxicity. Alternatively, any differences in hippocampal volume between the unexposed brothers of PTSD combat veterans (UxP+) versus the unexposed brothers of non-PTSD combat veterans (UxP−) were taken as evidence for a pre-existing trait. Amygdala and total brain volume served as controls. Our results indicate that smaller hippocampal volume constitutes a pre-existing vulnerability factor for pathological response to stress.
Fig. 1.
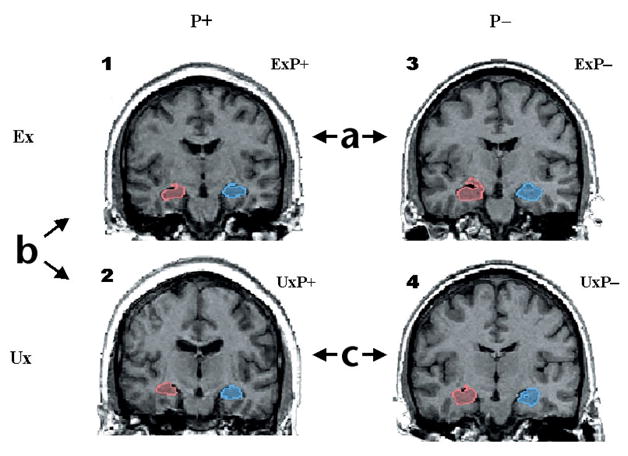
Discordant monozygotic twin paradigm for assessing MRI differences in PTSD. Sample coronal MRI images of right (red) and left (blue) hippocampi in a PTSD and a non-PTSD twin pair. Images represent four subject groups: (1) combat-exposed (Ex) subjects who developed chronic PTSD (ExP+); (2) their combat-unexposed (Ux) co-twins with no PTSD themselves (UxP+); (3) Ex subjects who never developed PTSD (ExP−) and (4) Ux co-twins also with no PTSD (UxP−). Contrast (a) provides a replication of previous work demonstrating smaller hippocampal volumes in combat veterans with versus without PTSD. Contrast (b) identifies the neurotoxicity effect—hippocampal reduction—as environmentally acquired, by contrasting hippocampal volumes in combat-exposed PTSD veterans with their unexposed co-twins. Contrast (c) examines pre-existing vulnerability by contrasting hippocampal volumes in the two groups of combat-unexposed co-twins whose combat-exposed brothers did versus did not develop PTSD. Model is tested by a diagnosis (P+ versus P−) × exposure (Ex versus Ux) ANOVA. Diagnosis refers to combat-exposed twin only. If hippocampal volume represents a vulnerability factor, the model predicts a significant main effect of diagnosis in the absence of a diagnosis × exposure interaction (that is, PTSD combat-exposed veterans and their unexposed co-twins show the same pattern). If hippocampal reduction results from neurotoxicity, the model predicts a significant main effect of exposure and/or a significant diagnosis × exposure interaction.
Results
Brain volume correlations with post-trauma symptoms
Within-pair correlations for MRI brain volumes in the total sample were all highly significant (total brain volume: r = 0.90, P < 0.0001; total hippocampus: r = 0.73, P < 0.0001; total amygdala: r = 0.67, P < 0.0001). Within the ExP+ subjects, there was a significant negative relationship (r = −0.64, P = 0.006) between total hippocampal volume and PTSD symptom severity, as measured by the Clinician-Administered PTSD Scale (CAPS) score (Fig. 2a). Thus, the hippocampal volume of exposed individuals was smaller in those with more severe PTSD symptoms. Importantly, there was also a significant negative correlation between hippocampal volume in UxP+ subjects and PTSD severity in their ExP+ brothers (r = −0.70, P = 0.002; Fig. 2b), indicating that smaller hippocampal volume in identical co-twins who were not themselves exposed to combat was nonetheless related to more severe PTSD symptoms in their combat-exposed brothers. Adjusting for total brain volume, PTSD severity in the ExP+ twin remained significantly associated with both ExP+ (r = −0.54, P = 0.03) and UxP+ (r = −0.61, P = 0.01) hippocampal volumes. This indicates that the association between more PTSD symptoms in veterans and smaller hippocampal volumes in themselves and their co-twins were not explained by smaller overall brain volume. We did not find any significant correlations between PTSD severity and amygdala or total brain volume.
Fig. 2.
Hippocampal volume correlations with post-trauma symptoms. Scatter plots illustrate relationship of symptom severity in combat veterans with PTSD to: (a) their own hippocampal volumes and (b) the hippocampal volumes of their identical twin brothers who were not exposed to combat. Symptom severity represents the total score received on the Clinician-Administered PTSD Scale (CAPS).
Combat severity (measured by a standardized combat exposure scale; see Methods) was not significantly related to total hippocampal volume in any of the subject groups (ExP+, r = −0.32, P = 0.21; all Ex combined, r = −0.08, P = 0.64; UxP+, r = −0.11, P = 0.66; all Ux combined, r = 0.01, P = 0.97). Thus, the intensity level of stressful exposure in combat was not predictive of hippocampal volume in either exposed veterans or in their unexposed co-twins. A continuous measure of alcohol abuse history (Michigan Alcoholism Screening Test, MAST) was found to be related only to right hippocampal volume in ExP+ subjects (r = −0.51, P = 0.04). However, this relationship was not evident in UxP+ subjects (r = 0.09, P = 0.73). Furthermore, the relationship between total hippocampal volume in unexposed co-twins and PTSD symptom severity in their combat-exposed brothers remained significant after controlling for the effects of their own alcohol history (r = −0.70, P = 0.004). Thus, whereas alcohol history had some relationship to hippocampal volume in combat veterans with PTSD, it was not related to hippocampal volume in their combat-unexposed brothers.
Brain volume differences in twin pair groups
Comparison of severe PTSD cases (total CAPS > 65; see Methods) with non-PTSD cases (Fig. 3) yielded a highly significant main effect of diagnosis on total hippocampal volume (Table 1). This result was unchanged after controlling for age (analysis of covariance (ANCOVA): F′1,65 = 8.63, P = 0.005), combat severity in the Ex twin (F′1,65 = 6.72, P = 0.01) and number of non-combat traumatic life events (F′1,62 = 4.67, P = 0.03). Neither the main effect of exposure, nor the diagnosis × exposure interaction, was significant. Thus, hippocampal volumes were smaller in both the exposed and unexposed members of twin pairs in which the combat-exposed brother developed more severe PTSD, but there was no difference in hippocampal volume between brothers, regardless of combat or PTSD status. We did not find any significant effects for comparisons involving amygdala or total brain volumes.
Fig. 3.
Total hippocampal volumes for four subject groups. Scatter plot illustrates absolute hippocampal volumes (ml) for combat-exposed individuals with and without PTSD, as well as for their respective unexposed co-twins. Data are only presented for PTSD twin pairs in which the combat-exposed twin had a CAPS score >65.
Table 1.
MRI brain volumes (ml) for severe PTSD subjects (CAPS > 65).
| PTSD (n = 24) |
Non-PTSD (n = 46) |
Two-factor ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exposed (n = 12) |
Unexposed (n = 12) |
Exposed (n = 23) |
Unexposed (n = 23) |
Diagnosis | Exposure | Interaction | ||||
| F1,66 | P | F1,66 | P | F1,66 | P | |||||
| Total brain volume | 1221 (108) | 1239 (125) | 1258 (106) | 1246 (112) | 0.61 | 0.44 | 0.01 | 0.92 | 0.28 | 0.60 |
| Total hippocampus | 6.66 (0.83) | 6.75 (0.90) | 7.41 (0.93) | 7.25 (0.69) | 8.73 | 0.004 | 0.03 | 0.87 | 0.34 | 0.56 |
| Right hippocampus | 3.32 (0.59) | 3.26 (0.39) | 3.76 (0.54) | 3.61 (0.48) | 9.63 | 0.003 | 0.62 | 0.43 | 0.13 | 0.72 |
| Left hippocampus | 3.34 (0.46) | 3.49 (0.57) | 3.65 (0.50) | 3.63 (0.45) | 3.35 | 0.07 | 0.29 | 0.59 | 0.40 | 0.53 |
| Total amygdala | 4.65 (0.87) | 4.53 (1.24) | 4.45 (0.67) | 4.61 (0.86) | 0.07 | 0.80 | 0.01 | 0.92 | 0.40 | 0.53 |
| Right amygdala | 2.37 (0.52) | 2.58 (0.64) | 2.34 (0.39) | 2.46 (0.48) | 0.37 | 0.54 | 1.76 | 0.19 | 0.12 | 0.73 |
| Left amygdala | 2.27 (0.49) | 1.95 (0.68) | 2.11 (0.47) | 2.15 (0.58) | 0.02 | 0.90 | 1.06 | 0.31 | 1.76 | 0.19 |
Data given as mean (s.d.).
Follow-up t-tests:
Total hippocampus: ExP+ versus ExP−, t33 = 2.32, P = 0.03; UxP+ versus UxP−, t33 = 1.83, P = 0.08; ExP+ versus UxP+, t11 = 0.38, P = 0.71; ExP− versus UxP−, t22 = 1.11, P = 0.28. Right hippocampus: ExP+ versus ExP−, t33 = 2.23, P = 0.03; UxP+ versus UxP−, t33 = 2.17, P = 0.04; ExP+ versus UxP+, t11 = 0.30, P = 0.77; ExP− versus UxP−, t22 = 1.42, P = 0.17. Left hippocampus: ExP+ versus ExP−, t33 = 1.74, P = 0.09; UxP+ versus UxP−, t33 = 0.85, P = 0.40; ExP+ versus UxP+, t11 = 1.31, P = 0.22; ExP− versus UxP−, t22 = 0.09, P = 0.93.
The main effect of diagnosis on hippocampal volume remained significant after removal of all subjects who reported childhood sexual or physical abuse (F1,54 = 4.77, P = 0.03), and hippocampal volumes did not differ between abused and non-abused subjects in P+ twin pairs (t32 = 0.40, P = 0.69) or in the sample as a whole (t78 = 0.95, P = 0.34). Therefore, a previous history of childhood abuse was not relevant to the overall results.
The same pattern of statistical significance persisted with the addition of the excluded PTSD outlier (Methods) and when regional volumes were tested as a percentage of total brain volume. In fact, diagnosis remained a highly significant factor when controlling for overall brain volume (ANCOVA, F′1,65 = 8.32, P = 0.005) and amygdala volume (F′1,65 = 9.95, P = 0.002). Thus, the observed hippocampal volume differences were specific relative to other brain regions examined. No significant main effects or interactions were observed in the two-factor ANOVA for hippocampal volumes in the full sample, which included PTSD subjects with total CAPS scores less than 65. Therefore, group differences emerged only when examining PTSD subjects with more severe symptoms and their co-twins.
Demographic and comorbidity features in twin pairs
ExP+ subjects had greater combat severity and PTSD symptom severity than ExP− subjects (Table 2). Age and education were similar between groups, although P+ pairs were slightly older. The highly significant interaction between diagnosis and exposure on the MAST indicates that combat veterans with PTSD had more severe alcohol abuse histories than the other three groups. No significant MAST score difference was found between UxP+ and UxP− subjects (CAPS > 65 subsample comparison, t30 = 1.0, P = 0.31), indicating that severity of alcohol abuse history did not explain the hippocampal differences in unexposed co-twins of PTSD versus non-PTSD combat veterans. For number of potentially traumatic lifetime events (non-combat related), PTSD combat veterans reported more of these than did non-PTSD combat veterans, and more events than their own unexposed co-twins. No significant difference was found in the reported number of traumatic lifetime events between UxP+ and UxP− subjects (CAPS > 65 subsample comparison, t31 = 1.01, P = 0.32), thus arguing against the relevance of lifetime non-combat trauma in the unexposed subjects as the explanation for the observed hippocampal volume differences in our sample. Within non-combat traumas, 29% of ExP+ subjects versus 13% of ExP− subjects (P = 0.25, Fisher’s exact test) and 24% of UxP+ versus 9% of UxP− subjects (P = 0.37) reported childhood sexual or physical abuse.
Table 2.
Demographic and clinical characteristics of PTSD and non-PTSD twin pairs.
| PTSD | Non-PTSD | Two-factor ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exposed (n = 17) |
Unexposed (n = 17) |
Exposed (n = 23) |
Unexposed (n = 23) |
Diagnosis | Exposure | Interaction | ||||
| F1,76 | P | F1,76 | P | F1,76 | P | |||||
| Age (years) | 53.1 (3.3) | 53.1 (3.3) | 51.8 (2.3) | 51.8 (2.3) | 3.8 | 0.05 | - | - | - | - |
| Education (years) | 13.5 (2.6) | 14.3 (2.8) | 14.7 (2.4) | 14.7 (2.6) | 1.8 | 0.18 | 0.45 | 0.51 | 0.55 | 0.46 |
| MAST* | 20.2 (17.6) | 6.8 (10.4) | 2.4 (4.5) | 2.5 (4.0) | 22.8 | <0.001 | 8.4 | 0.005 | 8.5 | 0.005 |
| Non-combat trauma† (number of events) | 7.9 (2.6) | 5.3 (3.8) | 5.1 (4.0) | 4.2 (3.0) | 6.1 | 0.02 | 4.9 | .03 | 1.3 | 0.26 |
| Combat severity | 7.9 (1.9) | - | 3.5 (2.6) | - | 67.4 | <0.001 | - | - | - | - |
| CAPS‡ | 72.2 (16.6) | - | 6.2 (7.3) | - | 577.7 | <0.001 | - | - | - | - |
Data given as mean (s.d.).
Michigan Alcoholism Screening Test. Data is missing for one PTSD pair and two non-PTSD pairs. Follow-up t-tests: ExP+ versus ExP−, t37 = 4.63, P < 0.0001; UxP+ versus UxP−, t35 = 1.70, P = 0.09; ExP+ versus UxP+, t15 = 2.92, P = 0.01; ExP− versus UxP−, t20 = 0.14, P = 0.89.
Lifetime number of non-combat potentially traumatic events. Data is missing for one PTSD pair and two non-PTSD pairs. Follow-up t-tests: ExP+ versus ExP−, t37 = 2.51, P = 0.02; UxP+ versus UxP−, t36 = 0.96, P = 0.34; ExP+ versus UxP+, t15 = 2.51, P = 0.02; ExP− versus UxP, t20 = 0.64, P = 0.53.
Clinician-Administered PTSD Scale (total symptom score)
Lifetime comorbid alcohol abuse and dependence diagnoses were more frequent in ExP+ (82%) versus ExP− (43%) veterans (P = 0.02), but there was no significant difference in rates between UxP+ (47%) and UxP− (30%) co-twins (P = 0.34). The same pattern was found for group rates of lifetime other substance abuse or dependence disorders (53% for ExP+ versus 9% for ExP−, P = 0.003; 6% for UxP+ versus 13% for UxP−, P = 0.62) and lifetime Major Depressive Disorder (59% for ExP+ versus 13% for ExP−, P = 0.005; 6% for UxP+ versus 4% for UxP−, P = 0.99). Therefore, with regard to history of alcohol/substance abuse and major depression, combat veterans with PTSD showed significantly elevated rates compared with combat veterans without PTSD, whereas the unexposed co-twins of the former did not show significantly elevated rates compared to the unexposed co-twins of the latter.
Discussion
Consistent with previous reports5–8, we found smaller hippocampal volume in trauma-exposed persons diagnosed with more severe, unremitting PTSD. The finding of a 10% difference in total hippocampal volume between individuals with versus without PTSD is in line with previously reported volumetric differences, as is the finding of predominantly right hippocampal differences5,19,20.
The key finding here concerns the identical twins of the higher-severity PTSD combat veterans who were not themselves exposed to combat; they showed hippocampal volumes that were comparable to their combat-exposed brothers but significantly smaller than those of combat veterans without PTSD and their non-combat–exposed twins. These data indicate that smaller hippocampi in PTSD represent a pre-existing, familial vulnerability factor rather than the neurotoxic product of trauma exposure per se. Further support for this conclusion comes from the highly significant correlation that we found between the hippocampal volume of combat-unexposed co-twins and the PTSD severity of their combat-exposed brothers. The high concordance of hippocampal volume within twin pairs, as well as the lack of a significant combat exposure effect or diagnosis × exposure interaction in our statistical model, provide clear evidence against the neurotoxicity hypothesis, as monozygotic co-twins provide the ideal biological control for detecting exposure-based differences. In light of the current findings, reference to hippocampal ‘atrophy’ in PTSD may be a misnomer.
We have also addressed the potential impact of confounding factors in the interpretation of hippocampal volume differences. In most non-twin studies, the presence of comorbid conditions precludes clear attribution of biological alterations to the psychiatric condition of PTSD alone. Most notably, major depression and alcohol abuse constitute comorbid conditions with high prevalence rates in PTSD21 that could ostensibly also influence hippocampal volume22–25. Although previous studies have attempted to statistically control for such confounds, the controls can still be unsound if the covariate differs between groups26. If alcohol abuse and depression are secondary consequences of more severe PTSD, the effect of ‘controlling for’ these variables may simply be to remove variance associated with more severe PTSD. Our study design uniquely circumvented these difficulties. Specifically, the PTSD combat veterans with smaller hippocampi predictably showed higher rates of major depression and more severe alcohol histories, but their combat-unexposed twin brothers, who showed comparably small hippocampi, did not. These results effectively exclude these comorbid conditions as a source of hippocampal volume differences in PTSD. Moreover, the association of hippocampal volume with PTSD remained significant after adjusting for combat severity, which in this study was not significantly associated with hippocampal volume. This argues against the possibility that subjects with smaller hippocampi were more likely to be selected for high combat roles. These data do not counter the idea that more severe combat exposure results in more severe PTSD; rather, they suggest hippocampal volume to be a predictor of PTSD severity independent of combat severity.
A limitation of the present study relates to the fact that hippocampal differences, as revealed by structural MRI, may not apply to all populations of individuals diagnosed with PTSD. More specifically, pre-existing decreased hippocampal volume may only be related to severe and unremitting forms of post-traumatic stress responses. All studies to date that have found smaller hippocampal volume in PTSD, including the findings reported here, have involved individuals with chronic, unremitting forms of the disorder; that is, intense symptoms which persist for years, and in many cases, decades. In fact, group differences in hippocampal volume only emerged in our sample when we examined PTSD individuals with a CAPS symptom severity score above 65. Failures to replicate findings of reduced hippocampal volume in PTSD have typically been reported in studies that involved subjects with PTSD of lower severity and/or shorter duration27–29. Over 40% of those diagnosed with PTSD show remission within the first year after traumatic exposure30,31, with a continued, more gradual remission rate for approximately six years14. Such individuals may clearly differ from those who develop long-standing, unremitting posttraumatic symptoms.
Hippocampal morphology and function have been implicated in conditioning and extinction of fear responses in animals, and may be involved in the contextual processing of fear32,33. Rodents with hippocampal lesions show stronger conditioned fear, as evidenced by more rapid acquisition of an avoidance response to an auditory cue paired with shock, as well as more fear behavior following acquisition, than do non-lesioned animals34,35. Similar alterations in fear-mediated performance have also been shown in mice with genetically smaller hippocampi15–17. Smaller hippocampal volume may also predispose an animal to diminished neuroendocrine regulation of the hypothalamic–pituitary–adrenal axis, as has been shown in monkeys who inherit smaller hippocampi and respond to stressful rearing conditions with larger cortisol elevations18. As a vulnerability factor for PTSD, smaller hippocampal volume might therefore predispose individuals to acquire stronger and/or more persistent conditioned emotional responses, or stronger hormonal stress responses, when exposed to a traumatic event36.
Heredity is the most likely explanation for the origin of the smaller hippocampi observed in PTSD combat veterans and their twins in this study18,37–40. In the absence of dizygotic twin subjects, however, the effects of heredity could not be separated from those of shared environment. We did find a non-significant trend for P+ pairs to share higher rates of childhood abuse, but this did not account for the observed hippocampal differences. Additionally, unexposed co-twins of PTSD veterans did not share with their brothers a general increase in lifetime number of reported non-combat trauma or stressor incidents, further diminishing the relevance of shared environment. Moreover, any stress-based interpretation of the smaller hippocampal volume observed in the combat-unexposed co-twins of the PTSD veterans would need to explain why the extra stress of military combat and consequent PTSD did not exert any further reduction in hippocampal volume in the PTSD veterans. Indeed, the finding that individuals who were exposed to combat, but did not develop PTSD, had larger hippocampi than individuals who were not exposed to combat but were merely the brothers of combat veterans with PTSD argues strongly against a stress–neurotoxic interpretation of the hippocampal diminution. Nevertheless, further research that includes dizygotic twin pairs is needed to tease apart the contributions of genetics and shared environment to smaller hippocampi in PTSD.
Methods
Subjects
All subjects were recruited with the assistance of the Vietnam Era Twin (VET) Registry, which determined zygosity41 and combat status (via military records) of each twin pair. Combat severity was assessed using a standard 18-item combat exposure measure42. Complete descriptions of the development and characteristics of the VET Registry have appeared elsewhere43,44. All subjects had previously participated in a larger twin study of PTSD at our laboratory; a full description of the recruitment strategy appears elsewhere45. Twin pairs in which the combat-exposed brother never had PTSD were recruited directly from the Registry. Owing to competing demands on their time, twin pairs in which the combat-exposed brother had PTSD were unavailable from the Registry. Hence, these pairs were recruited by a mass mailing to Vietnam veterans who had a service-connected disability for PTSD; in fact, these pairs better approximated the PTSD veterans from our previous (non-twin) study of hippocampal volume6 with regard to clinical severity. Although concern might be raised regarding the different sources for the PTSD and non-PTSD twin pairs, the significant correlations observed between PTSD severity and hippocampal volume within the PTSD twin pairs alone mitigates the likelihood that recruitment differences can explain the observed group differences.
The protocol was approved by the Veterans Administration (Manchester, New Hampshire) Institutional Review Board and Human Subjects Subcommittee, and all subjects gave informed written consent prior to participation. PTSD diagnostic statuses of combat-exposed twins and their overall PTSD symptom severity were determined by an experienced doctoral-level psychologist using CAPS46. All subjects completed a stressful life events checklist (available upon request) that was designed to quantify the lifetime number of non-combat events that potentially met DSM-IV PTSD A (stressor) criteria. Subjects were also interviewed using the Structured Clinical Interview for DSM-IV (SCID)47 to determine the presence of other Axis I mental disorders. The Michigan Alcoholism Screening Test (MAST)48 was used as a measure of lifetime alcohol abuse. Subjects were excluded if they met DSM-IV criteria for a psychotic or bipolar disorder, or non-combat–related PTSD. Due to the high comorbidity of major depression and substance abuse disorders with PTSD, these disorders did not represent exclusion criteria. The final sample comprised 17 PTSD twin pairs and 23 non-PTSD twin pairs.
MRI image acquisition and volumetric analyses
MRI scanning was performed at the Brigham and Women’s Hospital in Boston with a 1.5-tesla General Electric Signa System (GE Medical Systems, Milwaukee, Wisconsin) using previously described techniques (see Supplementary Methods online)6,49. Whole-brain volume was calculated with automated multistep algorithms described in detail elsewhere49. Hippocampus and amygdala were outlined manually on a Sun Microsystems workstation by a rater who was blind to diagnostic information and twin status, using an established procedure for volumetric determination6,49. Regional volumes were also expressed as percentages of whole brain volume and reanalyzed to confirm the results of absolute volume analyses. A second blind rater performed volumetric analyses of the hippocampus and amygdala on five random twin pair cases. Reliability assessment of the two raters resulted in the following intraclass correlation coefficients: right hippocampus, 0.96; left hippocampus, 0.92; right amygdala, 0.98; left amygdala, 0.97.
Statistical analyses
All statistical analyses used two-tailed tests. Hippocampus and amygdala volumes were found to be normally distributed in the overall sample based upon a Shapiro-Wilk goodness-of-fit statistic with null hypothesis rejection set at P < 0.15. One case (ExP+) was removed from the analyses because of extreme outlier status on three characteristics of hippocampal morphology (right hippocampus, >2.5 s.d. above the full sample mean, right hippocampus 1.9 s.d larger than left hippocampus, and right hippocampus 1.7 s.d. larger than co-twin). No other subject showed similarly extreme values. This outlier and his twin were removed to avoid obscuring any potentially important correlations between variables. Nevertheless, critical group comparisons were performed both with and without this twin pair, to ensure that the observed differences were not due solely to their exclusion.
Pearson correlations between neuroanatomical volumes on the one hand, and clinical/psychometric characteristics on the other, were performed within PTSD twin pairs only, because of the low, restricted range of PTSD symptom severity in the non-PTSD twin pairs (75% of sample had total CAPS scores ≤10). Group differences were tested by two-factor ANOVA with one between-pair factor, diagnosis (P+ versus P− in Ex twin), in the exposed twin, and one within-pair factor, combat exposure (Ex versus Ux). As a CAPS score of 65 has been established as an optimal cutoff for creating an unambiguous PTSD group50, we conducted ANOVA tests both in the complete sample and in a redefined sub-sample that included only those ExP+ subjects who met this criterion for symptom severity and their twins.
Supplementary Material
Acknowledgments
This work was supported by Department of Veterans Affairs Merit Review Grants (to M.W.G. and S.P.O.), USPHS Grant R01-MH54636 (to R.K.P.) and USPHS Grant K02-MH01110 (to M.E.S.). The authors would like to thank M. Macklin, K. Sheldon, S. Williston, L. Paulus, H. Croteau and the VA Cooperative Studies VET Registry (M.E. Vitek, K. Bukowski, R. Havlicek, T. Colton, W.E. Nance, R.S. Paffenbarger, Jr., M.M. Weissman and R.R. Williams) for their assistance. We gratefully acknowledge the participation of the veterans of the VET Registry and the non-Registry twin participants.
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
Competing interests statement
The authors declare that they have no competing financial interests.
References
- 1.Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McEwen BS. In: The Cognitive Neurosciences. Gazzaniga MS, editor. MIT Press; Cambridge, Massachusetts: 1995. pp. 1117–1135. [Google Scholar]
- 3.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 4.Zola-Morgan S, Squire LR. Neuroanatomy of memory. Annu Rev Neurosci. 1993;16:547–563. doi: 10.1146/annurev.ne.16.030193.002555. [DOI] [PubMed] [Google Scholar]
- 5.Bremner JD, et al. MRI-based measurements of hippocampal volume in combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:973–978. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gurvits TV, et al. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry. 1996;40:1091–1099. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bremner JD, et al. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse: a preliminary report. Biol Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med. 1997;27:951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- 9.Pitman RK. Hippocampal diminution in PTSD: more (or less?) than meets the eye. Hippocampus. 2001;11:73–74. doi: 10.1002/hipo.1022. [DOI] [PubMed] [Google Scholar]
- 10.Bremner JD. Hypotheses and controversies related to effects of stress on the hippocampus: an argument for stress-induced damage to the hippocampus in patients with Posttraumatic Stress Disorder. Hippocampus. 2001;11:75–81. doi: 10.1002/hipo.1023. [DOI] [PubMed] [Google Scholar]
- 11.McEwen BS. Commentary on PTSD discussion. Hippocampus. 2001;11:82–84. [Google Scholar]
- 12.Yehuda R. Are glucocortoids responsible for putative hippocampal damage in PTSD? How and when to decide. Hippocampus. 2001;11:85–89. doi: 10.1002/hipo.1025. [DOI] [PubMed] [Google Scholar]
- 13.Kulka RA, et al. Trauma and the Vietnam War Generation: Report of Findings from the National Vietnam Veterans Readjustment Study. Brunner/Mazel; New York: 1990. [Google Scholar]
- 14.Kessler RC, Sonnega A, Bromet E, Huges M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 15.Crusio WE, Schwegler H, van Abeelen JHF. Behavioral responses to novelty and structural variation of the hippocampus in mice. II Multivariate genetic analysis. Behav Brain Res. 1989;32:81–88. doi: 10.1016/s0166-4328(89)80075-0. [DOI] [PubMed] [Google Scholar]
- 16.Wimer CC, Wimer RE, Roderick TH. Some behavioral differences associated with relative size of hippocampus in the mouse. J Comp Physiol Psychol. 1971;76:57–65. doi: 10.1037/h0031036. [DOI] [PubMed] [Google Scholar]
- 17.Schwegler H, Lipp HP. Hereditary covariations of neuronal circuitry and behavior: correlations between the proportions of hippocampal synaptic fields in the regio inferior and two-way avoidance in mice and rats. Behav Brain Res. 1983;7:1–38. doi: 10.1016/0166-4328(83)90002-5. [DOI] [PubMed] [Google Scholar]
- 18.Lyons DM, Yang C, Sawyer-Glover AM, Moseley ME, Schatzberg AF. Early life stress and inherited variation in monkey hippocampal volumes. Arch Gen Psychiatry. 2001;58:1145–1151. doi: 10.1001/archpsyc.58.12.1145. [DOI] [PubMed] [Google Scholar]
- 19.Freeman TW, Cardwell D, Karson CN, Komoroski RA. In vivo proton magnetic resonance spectroscopy of the medial temporal lobes of subjects with combat-related posttraumatic stress disorder. Magn Reson Med. 1998;40:66–71. doi: 10.1002/mrm.1910400110. [DOI] [PubMed] [Google Scholar]
- 20.Schuff N, et al. Reduced hippocampal volume and n-acetyl aspartate in posttraumatic stress disorder. Ann NY Acad Sci. 1997;821:516–520. doi: 10.1111/j.1749-6632.1997.tb48319.x. [DOI] [PubMed] [Google Scholar]
- 21.Keane TM, Kaloupek DG. Comorbid psychiatric disorders in PTSD: implications for research. Ann NY Acad Sci. 1997;821:24–34. doi: 10.1111/j.1749-6632.1997.tb48266.x. [DOI] [PubMed] [Google Scholar]
- 22.Bremner J, et al. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–127. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 23.Laakso MP, et al. A volumetric MRI study of the hippocampus in type 1 and 2 alcoholism. Behav Brain Res. 2000;109:177–186. doi: 10.1016/s0166-4328(99)00172-2. [DOI] [PubMed] [Google Scholar]
- 24.Sheline Y, Sanghavi M, Mintin M, Gado M. Depression duration but not age predicts hippocampal volume loss in medical healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5041. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Bellis MD, et al. Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- 26.Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- 27.De Bellis MD, et al. Developmental traumatology part II: brain development. Biol Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 28.Schuff N, et al. Decreased hippocampal n-acetylaspartate in the absence of atrophy in Posttraumatic Stress Disorder. Biol Psychiatry. 2001;50:952–959. doi: 10.1016/s0006-3223(01)01245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonne O, et al. Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am J Psychiatry. 2001;158:1248–1251. doi: 10.1176/appi.ajp.158.8.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breslau N, Davis GC. Posttraumatic Stress Disorder in an urban population of young adults: risk factors for chronicity. Am J Psychiatry. 1992;149:671–675. doi: 10.1176/ajp.149.5.671. [DOI] [PubMed] [Google Scholar]
- 31.Mellman TA, Randolph CA, Brawman-Mintzer O, Flores LP, Milanes FJ. Phenomenology and course of psychiatric disorders associated with combat-related posttraumatic stress disorder. Am J Psychiatry. 1992;149:1568–1574. doi: 10.1176/ajp.149.11.1568. [DOI] [PubMed] [Google Scholar]
- 32.Selden NRW, Everitt BJ, Jarrard LE, Robbins TW. Complementary roles for amygdala and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience. 1991;42:335–350. doi: 10.1016/0306-4522(91)90379-3. [DOI] [PubMed] [Google Scholar]
- 33.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 34.Falls WA, Davis M. In: Neurobiological and Clinical Consequences of Stress: from Normal Adaptation to Post-Traumatic Stress Disorder. Friedman DJ, Charney DS, Deutch AY, editors. Lippincott-Raven; Philadelphia: 1995. pp. 177–202. [Google Scholar]
- 35.Antelman SM, Brown TS. Hippocampal lesions and shuttlebox avoidance behavior: a fear hypothesis. Physiol Behav. 1972;9:15–20. doi: 10.1016/0031-9384(72)90257-0. [DOI] [PubMed] [Google Scholar]
- 36.Orr SP, et al. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol. 2000;109:290–298. [PubMed] [Google Scholar]
- 37.Wimer RE, Wimer CC. A biometrical-genetic analysis of granule cell number in the area dentata of house mice. Brain Res. 1981;254:129–140. doi: 10.1016/0165-3806(81)90064-x. [DOI] [PubMed] [Google Scholar]
- 38.Beck KD, Powell-Braxton L, Widmer HR, Vlaverde J, Hefti F. Igfl gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron. 1995;14:717–730. doi: 10.1016/0896-6273(95)90216-3. [DOI] [PubMed] [Google Scholar]
- 39.Pennington BF, et al. A twin MRI study of size variations in the human brain. J Cogn Neurosci. 2000;12:223–232. doi: 10.1162/089892900561850. [DOI] [PubMed] [Google Scholar]
- 40.Thompson PM, et al. Genetic influences on brain structure. Nat Neurosci. 2001;4:1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- 41.Eisen S, Neuman R, Goldberg J, Rice J, True W. Determining zygosity in the Vietnam Era Twin Registry: an approach using questionnaires. Clin Genet. 1989;35:423–432. doi: 10.1111/j.1399-0004.1989.tb02967.x. [DOI] [PubMed] [Google Scholar]
- 42.Janes GR, Goldberg J, Eisen SA, True WR. Reliability and validity of a combat exposure index for Vietnam era veterans. J Clin Psychol. 1991;47:80–86. doi: 10.1002/1097-4679(199101)47:1<80::aid-jclp2270470112>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 43.Eisen SA, True WR, Goldberg J, Henderson W, Robinette CD. The Vietnam Era Twin (VET) Registry: method of construction. Acta Geneticae Medicae et Gemellologiae (Roma) 1987;36:61–66. doi: 10.1017/s0001566000004591. [DOI] [PubMed] [Google Scholar]
- 44.Henderson WG, et al. The Vietnam Era Twin Registry: a resource for medical research. Public Health Rep. 1990;105:368–373. [PMC free article] [PubMed] [Google Scholar]
- 45.Orr SP, et al. Physiologic responses to sudden, loud tones in monozygotic twins discordant for combat exposure: association with PTSD. Arch Gen Psychiatry. doi: 10.1001/archpsyc.60.3.283. (in press) [DOI] [PubMed] [Google Scholar]
- 46.Blake DD, et al. The development of a clinician-administered PTSD scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 47.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders, Version 2.0. Biometrics Research Department; New York: 1994. [Google Scholar]
- 48.Selzer ML. The Michigan Alcoholism Screening Test: the quest for a new diagnostic instrument. Am J Psychiatry. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- 49.Shenton MA, et al. Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imagery study. N Engl J Med. 1992;327:604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- 50.Weathers FW, Ruscio AM, Keane TM. Psychometric properties of nine scoring rules for the Clinican-Administered Posttraumatic Stress Disorder Scale. Psychol Assess. 1999;11:124–133. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




