Abstract
Background
The Public Health Service (PHS) Guideline for Treating Tobacco Use and Dependence (1) recommends supporting autonomy and perceived competence to facilitate tobacco abstinence.
Purpose
To evaluate the effectiveness of an intensive tobacco-dependence intervention based on self-determination theory (SDT) and intended to support autonomy and perceived competence in facilitating long-term tobacco abstinence.
Methods
One thousand and six adult smokers were recruited into a randomized cessation-induction trial. Community care participants received cessation pamphlets and information on local treatment programs. Intervention participants received the same materials and were asked to meet four times with counselors over six months to discuss their health in a manner intended to support autonomy and perceived competence. The primary outcome was 24-month prolonged abstinence from tobacco. The secondary outcome was 7-day point prevalence tobacco abstinence at 24 months post-intervention.
Results
Smokers in the intervention were more likely to attain both tobacco abstinence outcomes and these effects were partially mediated by change in both autonomous self-regulation and perceived competence from baseline to six months. Structural equation modeling confirmed the SDT model of health-behavior change in facilitating long-term tobacco abstinence.
Conclusions
An intervention based on SDT and consistent with the PHS Guideline, which was intended to support autonomy and perceived competence, facilitated long-term tobacco abstinence.
Keywords: autonomy, perceived competence, self-determination theory, tobacco abstinence
Tobacco use remains the leading cause of death in the United States (2), although its use is voluntary. Smokers who quit live longer than those who continue to smoke (3) and those who receive intensive treatment receive a mortality benefit (4, 5). Thus, intensive tobacco-dependence interventions should become the standard form of clinical treatment for tobacco use. The Public Health Service (PHS) Guideline for Treating Tobacco Use and Dependence (1) suggests that intensive treatment include at least four contacts of more than 10 minutes each, total contact time more than 30 minutes, provision of medical advice about health risks and benefits of cessation and pharmacotherapy, and delivery of additional counseling.
Support for autonomy and perceived competence is a central aspect of medical practice (6, 7). The PHS Guideline (1) suggests that “motivation interventions are most likely to be successful when the clinician is empathic, promotes patient autonomy (choice among options), avoids arguments, and supports patient self-efficacy” (p. 31). However, no empirical evidence was provided for the effect of such interventions on smokers’ autonomy or their perceived competence, or on tobacco outcomes.
The Smoker’s Health Study (SHS) was funded by NIH to examine the efficacy of delivering the PHS Guideline for intensive treatment to smokers in a manner that supported their autonomy and perceived competence, relative to community care, in facilitating long-term tobacco abstinence. The theoretical framework for the SHS is self-determination theory (SDT) (8, 9), which posits that humans’ innate orientation toward physical and psychological health is supported by satisfaction of the basic psychological needs for autonomy, competence, and relatedness. As such, this study aimed to integrate the principles of SDT with the PHS Guideline.
The Self-Determination Theory Model of Health-Behavior Change
SDT proposes that health-behavior change involves a biopsychosocial process, which recognizes the importance of both psychological and social factors, in addition to biological predispositions, in facilitating and maintaining healthy behaviors (10). Two psychological factors that SDT has identified as paramount to the initiation and maintenance of health-behavior change are patients’ perceiving themselves to be autonomous and competent concerning health behavior.
People perceive themselves to be autonomous when they experience volition in the regulation of their behavior, whereas they perceive themselves to be controlled when they feel pressure or coercion from interpersonal or intrapsychic sources to think, feel, or behave in certain ways. People perceive themselves to be competent when they feel able to obtain important outcomes, whereas they perceive themselves to be incompetent when they feel unable to obtain those outcomes. Research suggests that both autonomous self-regulation and perceived competence are important for the initiation and maintenance of health-behavior change (11, 12). Importantly, studies indicate that when people feel autonomous in the regulation of their behavior, they feel more competent to initiate and maintain health-behavior change (12).
Internalization refers to the natural, active process through which people transform socially-sanctioned norms into personally-held values, a process that allows for the experiences of autonomy and perceived competence (8). The process of internalization is facilitated through the provision of autonomy support, which, in the health-care domain, is defined as the extent to which providers elicit and acknowledge patients’ perspectives, provide a clear rationale and effective options for change, support patients’ initiatives for change, and minimize pressure and control (10). Thus, to the extent that the health-care climate is perceived as autonomy supportive, internalization is expected to promote autonomous self-regulation and perceived competence. Importantly, the process of internalization is necessary for maintenance of health-behavior change because, even after the intervention has ended, autonomously self-regulated behaviors are expected to be enacted willingly. The efficacy of the SDT model of health-behavior change has been empirically demonstrated in a diverse set of health-related behaviors (13).
The Present Research
The aim of this study was to determine whether providing an intervention designed to support smokers’ autonomy and perceived competence would facilitate long-term tobacco abstinence. Previous findings have supported the efficacy of the intervention in promoting autonomous self-regulation, perceived competence, medication use, and tobacco abstinence at six (14) and at 18 (15) months following randomization. Herein, we focus on long-term tobacco outcomes. Participants were re-contacted, given informed consent, and paid an additional $5 honorarium. Thus, this is a separate study with additional funding to determine whether the intervention facilitated long-term (i.e., 24-month post-intervention) tobacco abstinence.
First, we hypothesized that the SDT-based intervention, relative to community care, would increase the likelihood of attaining 24-month prolonged abstinence from tobacco and concurrent 7-day point prevalence tobacco abstinence. Second, we hypothesized that the effects of treatment condition on long-term tobacco abstinence would be partially mediated by change in both autonomous self-regulation and perceived competence from baseline to six months. Third, we hypothesized that the components of the SDT model of health-behavior change (viz., treatment condition, change in both autonomous self-regulation and perceived competence, medication use, and long-term tobacco abstinence) would be significantly and positively correlated in the measurement phase of the structural equation modeling analysis. Fourth, we hypothesized that the following relations would be fully mediated (i.e., reduced to non-significance) in the structural phase of the structural equation modeling analysis: (1) treatment condition to change in perceived competence, (2) medication use to long-term tobacco abstinence, and (3) change in autonomous self-regulation to long-term tobacco abstinence. More specifically, we expected that (1) the relation of treatment condition to change in perceived competence would be mediated by change in autonomous self-regulation, (2) the relation of medication use to long-term tobacco abstinence would be mediated by change in perceived competence, and (3) the relation of change in autonomous self-regulation to long-term tobacco abstinence would be mediated by change in perceived competence. These specific predictions concerning mediation were based on the SDT model of health-behavior change.
Method
Participants, Study Design, and Conditions
Eligible participants had smoked more than 100 cigarettes in their lifetime and had smoked five or more cigarettes per day during the week prior to enrollment, were at least 18 years of age, read and spoke English, reported no prior history of psychotic illness (although anxiety and depression were allowed), and had a life expectancy of at least 18 months. Smokers were recruited using newspaper advertisements and signs in physicians’ offices. Interested participants were told that the study examined how people change health behaviors and how health-care providers can facilitate health-behavior improvement. Importantly, smokers were accepted into the study whether or not they intended to quit. Participants were asked to have two fasting lipid profiles seven days apart prior to their baseline appointment, which provided a risk-estimate of heart disease. All participants were paid $75.
Randomization was stratified by whether participants’ fasting lipid profiles indicated they met the National Cholesterol Education Program’s LDL-C goal (16). Participants who met this goal were randomized either to the tobacco intervention or to community care (CC). Those who did not were randomized to one of three conditions: tobacco and dietary intervention, tobacco intervention and dietary CC, or tobacco and dietary CC. The dietary intervention included two visits with a nutritionist focused exclusively on dietary change, and the contact time for the dietary component was considerably less than the contact time for smoking cessation. Thus, we collapsed across the dietary- and tobacco-intervention conditions and focused only on the tobacco intervention and outcomes. Seventy percent of the participants (n = 714) were randomized to the intervention, whereas 30% (n = 292) were randomized to CC. This ratio was used to minimize the number of people who would have less positive outcomes, as the intervention was expected to have a more pronounced effect on cessation (1), while still allowing for a test of the study hypotheses. Previous analyses confirmed this expectation (14), so all CC participants were offered intensive treatment at the end of the 24-month post-intervention follow-up. This study was approved by the University of Rochester Human Subjects Review Board.
Community care condition
Participants assigned to CC were given the following materials: the “You Can Quit Smoking” and “Clearing the Air: Quit Smoking Today” smoking-cessation pamphlets (e.g., 17), the results of their cholesterol tests, and a list of all local smoking-cessation resources, including the New York State Quit Line. Participants were encouraged to enlist in a smoking-cessation program and to consult with their physician about their smoking and cholesterol. Accordingly, this study examined whether the intervention facilitated improvement in tobacco outcomes relative to typical care available in the community.
Intervention condition
Participants assigned to the intervention received the same materials and advice as those in CC. Additionally, they were asked to meet four times with the SHS counselors during the following six months to discuss their health. The initial visit lasted 50 minutes, whereas follow-up visits lasted 20 minutes. The intervention was operationalized by taking a medical and smoking history, eliciting and acknowledging participants’ perspectives on their smoking and the health risks smoking poses, and discussing how stopping might improve health. The counselors presented to participants their 10-year absolute risk for developing coronary artery disease (18) and the expected reduction in that risk if they stopped smoking completely (19), and asked how the participants felt about that information. Participants discussed life aspirations and were asked how smoking helped and/or hindered their attaining those goals (20). Participants’ willingness and perceived ability to stop smoking were elicited.
Counselors were trained to consider smokers’ autonomy as the primary clinical goal, so, after the initial history and discussion of smoking risks and benefits of cessation, participants were asked whether they were ready to stop using tobacco. If yes, counselors provided competence support, including problem-solving and skills-building, intra-treatment support, and reviewing medications available for tobacco-dependence treatment (1). Participants who wanted to use medication were given the choice of obtaining it from their own physician or from a study prescriber. Available medications included all first-line medications approved for smoking cessation at the time of this trial (i.e., nicotine replacement therapies, Bupropion SR). If they were not ready to quit, participants were asked to return in about two months to discuss again. There was no limit placed on the number of contacts within the 6-month intervention period.
Procedure and Time Line for Assessments
Baseline assessment
At baseline, participants completed a questionnaire packet assessing autonomous self-regulation and perceived competence and then were randomized to condition.
6-month assessment
Six months after baseline, participants were mailed a questionnaire packet assessing autonomous self-regulation, perceived competence, and medication use.
30-month outcomes
Thirty months after baseline (i.e., 24 months post-intervention), participants were mailed a questionnaire packet informing them of the additional study, providing an informed consent document, and assessing two indices of tobacco abstinence.
Measures
Autonomous self-regulation (ASR) for smoking cessation and medication use
The Treatment Self-Regulation Questionnaire (e.g., 12) presented participants with the following stems: “The reason I would stop smoking permanently or continue not smoking is…” and “The reason I would use medication as recommended (by a health-care practitioner or by medication package instructions) to stop smoking permanently is…” Participants rated pre-selected responses that assessed autonomous reasons for smoking cessation and medication use (six items for each; e.g., because I feel that I want to take responsibility for my own health). Responses were made on a 7-point Likert-type scale, ranging from 1 (not at all true) to 7 (very true). The reliabilities for these measures were as follows: ASR for smoking cessation α = .85 at baseline and α = .89 at six months, ASR for medication use α = .87 at baseline and α = .92 at six months. Change scores were computed as the standardized residuals of ASR for both smoking cessation and medication use. The two residual scores were averaged to form a composite ASR measure.
Perceived competence (PC)
The Perceived Competence Scale (e.g., 12) assessed participants’ experience of feeling able to stop smoking successfully (four items; e.g., I feel confident in my ability to stop smoking permanently). Responses were made on a 7-point Likert-type scale, ranging from 1 (strongly disagree) to 7 (strongly agree). The reliabilities for this measure were α = .90 at baseline and α = .94 at six months. A change score was computed as the standardized residual of PC.
Medication use
Participants were asked whether they had used any of the first-line medications approved for smoking cessation and available at the time of this trial (i.e., nicotine replacement therapies, Bupropion SR). Participants reported the number of days they had used each type of medication, which were summed to obtain the number of days using medication.
Smoking status
The primary outcome was 24-month prolonged abstinence (24mPA) from tobacco (21, 22), which was assigned if a participant had quit smoking completely at six months post-randomization (with a two week “grace period”) and had not used tobacco at all (including denying the use of a pipe, cigars, snuff, and chewing tobacco) between that time and 24 months post-intervention. The secondary outcome was 7-day point prevalence (7dPP) tobacco abstinence at 24 months post-intervention. Participants responded either “yes” or “no” to having smoked a cigarette, even a puff, in the past seven days and to having currently used a pipe, cigars, snuff, and chewing tobacco. To be classified as having attained 7dPP tobacco abstinence, participants must have responded “no” to having used each form of tobacco listed above.
Analytic Overview
Analyses were conducted using “intention-to-treat,” which included all participants. Missing data for ASR for smoking cessation and medication use, as well as PC, were replaced by participants’ last known reports; if those were not available, mean replacement was used. If smoking status was not available, participants were considered smoking. The amount of missing data was roughly equivalent across groups; thus, this approach provided a more conservative test of our hypotheses, compared to removing participants who did not provide data at 30 months.
Chi-square analyses were used to examine between-group differences in the tobacco outcomes (Hypothesis 1). Logistic and linear regression analyses were used to examine mediation of the effects of treatment condition on long-term tobacco abstinence by change in both ASR and PC (Hypothesis 2). In these analyses, mediation was tested using the Baron and Kenny (23) method, which specifies four necessary conditions for mediation: (1) the independent variable (IV; viz., treatment condition) must relate to the dependent variable (DV; viz., long-term tobacco abstinence); (2) the IV must relate to the mediating variable (MV; viz., ASR and PC); (3) while controlling for the relation of the IV to the DV, the MV must relate to the DV; and (4) the relation of the IV to the DV must be reduced significantly or to non-significance when the MV is added to the model. The procedure outlined by MacKinnon et al. (24) was used to test the significance of the indirect effect of the IV on the DV through the MV.
Structural Equation Modeling (SEM)1 with observed variables was used to examine the correlations among the components of the SDT model of health-behavior change in the measurement phase of model testing (Hypothesis 3).2 SEM with observed variables was used to examine mediation of the relations of (1) treatment condition to change in PC, (2) medication use to long-term tobacco abstinence, and (3) change in ASR to long-term tobacco abstinence (Hypothesis 4). In these analyses, mediation was tested using Holmbeck’s (25) method. Specifically, mediation is evident when (1) initially there is a significant relation of the IV to the DV, and (2) this relation is substantially reduced when controlling for the MV. Accordingly, two structural models were tested and compared. In the first (i.e., indirect effect, or fully mediated) model, those relations hypothesized to be mediated were omitted from the model. In the second (i.e., direct effect) model, those relations hypothesized to be mediated were added to the model.3 Mediation is evident when the addition of a direct path does not significantly improve model fit relative to the indirect effect model (tested using a χ2 difference test). The procedure outlined by MacKinnon et al. (24) was used to test the significance of the indirect effect of the IV on the DV through the MV.
Results
Recruitment, Randomization, and Retention
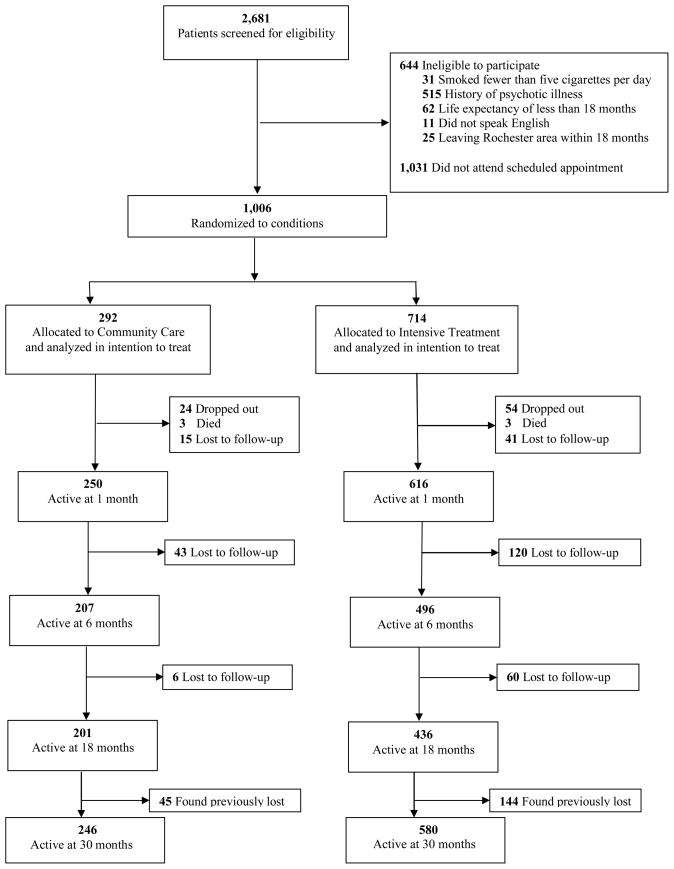
Between January 2000 and July 2002, 2,681 smokers were screened for eligibility, and 2,037 (76%) were eligible and provided phone consent for having two fasting lipid profiles. One thousand and six (49%) of those eligible came to an initial appointment, provided full informed consent, completed the baseline questionnaires, and were randomized to treatment condition. Randomization was effective, as the groups did not differ significantly on key demographic variables at baseline (14). Figure 1 depicts participant flow through the 30-month study period.
Figure 1.
CONSORT recruitment and retention of participants, reprinted from Niemiec, Ryan, Deci, and Williams (20).
Preliminary Analyses
Independent samples t-tests with Bonferroni protection revealed no significant differences on key demographic variables at baseline between participants who were retained and those who were lost to follow-up at the end of the 30-month study period. Independent samples t-tests with Bonferroni protection revealed no significant differences either on the motivation variables or on the tobacco outcomes between participants who received the dietary intervention (n = 174) and those who received dietary CC (n = 209), all t’s < 1, which supported our decision to collapse across the dietary- and tobacco-intervention conditions.
The Effect of Treatment Condition on Long-Term Tobacco Abstinence
Hypothesis 1 stated that the SDT-based intervention, relative to CC, would increase the likelihood of attaining 24mPA from tobacco and concurrent 7dPP tobacco abstinence. Chi-square analyses confirmed this hypothesis using the primary outcome, as the intervention had a significant effect on 24mPA from tobacco [4.1% vs. 1.4%; χ2 (1) = 4.73, p < .05; number needed to treat (NNT) = 37.0; odds ratio (OR) = 3.05; 95% confidence interval (CI): 1.06, 8.75]. Chi-square analyses confirmed this hypothesis using the secondary outcome, as the intervention had a significant effect on 7dPP tobacco abstinence (11.2% vs. 6.8%; χ2 (1) = 4.39, p < .05; NNT = 22.7; OR = 1.72; 95% CI = 1.03, 2.86). Thus, participants in the intervention, relative to those in CC, were more likely to report prolonged tobacco abstinence at 24 months post-intervention, which indicates that the intervention was effective in facilitating long-term tobacco abstinence.
Mediation of the Effect of Treatment Condition on Long-Term Tobacco Abstinence
Hypothesis 2 stated that the effect of treatment condition on long-term tobacco abstinence would be partially mediated by change in both ASR and PC. Results confirmed this hypothesis using both tobacco outcomes, as change in both ASR and PC partially mediated the effect of treatment condition on long-term tobacco abstinence (see Table 1).
Table 1.
Testing Hypothesis 2: Mediation of the Effect of Treatment Condition on Long-term Tobacco Abstinence by Change in both Autonomous Self-Regulation (ASR) (Panel A) and Perceived Competence (PC) (Panel B)
| Panel A: | ||
|---|---|---|
| 24mPA | 7dPP | |
| Step 1: Treatment Condition Predicting Long-term Tobacco Abstinence | ||
| Intervention | b = 1.12* | b = .54* |
| Step 2: Treatment Condition Predicting Change in ASR | ||
| Intervention | β = .08* | β = .08* |
| Step 3: Change in ASR Predicting Long-term Tobacco Abstinence while Controlling for Treatment Condition | ||
| Chang in ASR | b = .46* | b = .43*** |
| Step 4: Treatment Condition Predicting Long-term Tobacco Abstinence while Controlling for Change in ASR | ||
| Intervention | b = 1.06* | b = .49+ |
| z′ statistic | 1.63** | 2.01** |
| Panel B: | ||
|---|---|---|
| 24mPA | 7dPP | |
| Step 1: Treatment Condition Predicting Long-term Tobacco Abstinence | ||
| Intervention | b = 1.12* | b = .54* |
| Step 2: Treatment Condition Predicting Change in PC | ||
| Intervention | β = .08* | β = .08* |
| Step 3: Change in PC Predicting Long-term Tobacco Abstinence while Controlling for Treatment Condition | ||
| Chang in PC | b = 1.51*** | b = .71*** |
| Step 4: Treatment Condition Predicting Long-term Tobacco Abstinence while Controlling for Change in PC | ||
| Intervention | b = .92 | b = .41 |
| z′ statistic | 2.47** | 2.43** |
Notes. ASR = Autonomous self-regulation, PC = Perceived competence, 24mPA = 24-month prolonged abstinence from tobacco, 7dPP = 7-day point prevalence tobacco abstinence. Step 1 refers to the relation of the independent variable (IV; viz., treatment condition) to the dependent variable (DV; viz., long-term tobacco abstinence). Step 2 refers to the relation of the IV to the mediating variable (MV; viz., ASR in Panel A, PC in Panel B). Step 3 refers to the relation of the MV to the DV while controlling for the IV. Step 4 refers to the relation of the IV to the DV while controlling for the MV. The z′ statistic refers to the significance of the indirect effect of the IV on the DV through the MV (24).
p < .10,
p < .05,
p < .01,
p < .001.
Subgroup Analyses
Subsequently, we examined the effect of treatment condition on long-term tobacco abstinence among the 528 participants who reported at baseline that they did not want to quit smoking within the first 30 days. Chi-square analyses suggested that the effect of treatment condition on 24mPA from tobacco was no longer significant, χ2 (1) = 2.52, p < .12. However, chi-square analyses confirmed that the intervention had a significant effect on 7dPP tobacco abstinence among those who did not want to quit smoking within the first 30 days (10.9% vs. 3.7%; χ2 (1) = 7.24, p < .01; NNT = 13.9; OR = 3.16; 95% CI = 1.31, 7.61).
Testing the Self-Determination Theory Model of Health-Behavior Change
24mPA from tobacco
Hypothesis 3 stated that the components of the SDT model of health-behavior change would relate positively to each other in the measurement phase of model testing. Results from the measurement model with 24mPA from tobacco as the outcome confirmed this hypothesis, as correlations among the observed variables indicated that treatment condition related positively to change in ASR (r = .08, p < .05), change in PC (r = .08, p < .01), medication use (r = .15, p < .001), and 24mPA from tobacco (r = .21, p < .001); change in ASR related positively to change in PC (r = .31, p < .001), medication use (r = .10, p < .01), and 24mPA from tobacco (r = .18, p < .001); change in PC related positively to medication use (r = .28, p < .001) and 24mPA from tobacco (r = .62, p < .001); and medication use related positively to 24mPA from tobacco (r = .23, p < .001). Thus, the associations among the components of the SDT model of health-behavior change were significant and in the anticipated direction.
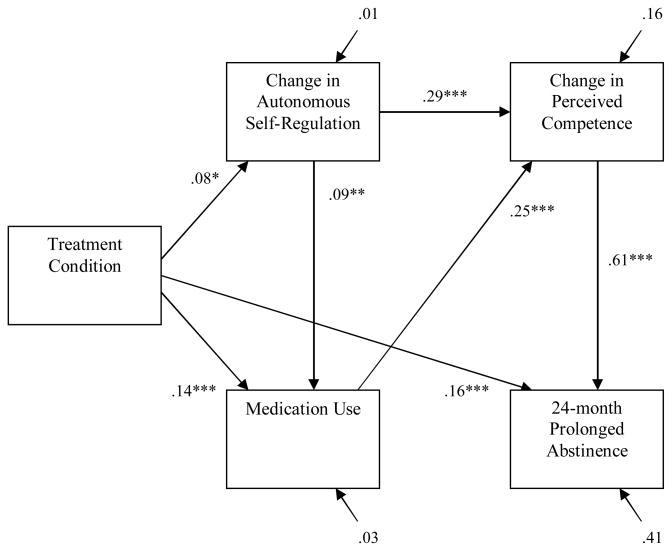
Hypothesis 4 stated that, in the structural phase of model testing, (1) the relation of treatment condition to change in PC would be mediated by change in ASR, (2) the relation of medication use to long-term tobacco abstinence would be mediated by change in PC, and (3) the relation of change in ASR to long-term tobacco abstinence would be mediated by change in PC. Results from the structural model with 24mPA from tobacco as the outcome confirmed this hypothesis. Specifically, the relation of treatment condition to change in PC was reduced from r = .08 (p < .01) to β = .02 (ns), and the indirect effect of treatment condition on change in PC through change in ASR was significant (z′ = 2.56, p < .01); the relation of medication use to 24mPA from tobacco was reduced from r = .23 (p < .001) to β = .04 (ns), and the indirect effect of medication use on 24mPA from tobacco through change in PC was significant (z′ = 8.13, p < .01); and the relation of change in ASR to 24mPA from tobacco was reduced from r = .18 (p < .001) to β = −.03 (ns), and the indirect effect of change in ASR on 24mPA from tobacco through change in PC was significant (z′ = 9.33, p < .01). The structural model that excluded these non-significant paths yielded excellent fit to the data, χ2 (3) = 4.74, ns; χ2/df = 1.58; CFI = 1.00; NNFI = .99; RMSEA = .02. Further, model fit was not improved by the addition of any of these non-significant paths (all Δχ2 (1) < 2.68, ns). The final structural model is presented in Figure 2.
Figure 2.
The structural equation model, with parameter estimates, examining the structural relations among treatment condition, change in ASR and PC, medication use, and 24mPA from tobacco.
7dPP tobacco abstinence
Results from the measurement model with 7dPP tobacco abstinence as the outcome confirmed Hypothesis 3, as correlations among the observed variables indicated that treatment condition related positively to change in ASR (r = .08, p < .05), change in PC (r = .08, p < .01), medication use (r = .15, p < .001), and 7dPP tobacco abstinence (r = .12, p < .001); change in ASR related positively to change in PC (r = .31, p < .001), medication use (r = .10, p < .01), and 7dPP tobacco abstinence (r = .20, p < .001); change in PC related positively to medication use (r = .28, p < .001) and 7dPP tobacco abstinence (r = .35, p < .001); and medication use related positively to 7dPP tobacco abstinence (r = .14, p < .001). Thus, the associations among the components of the SDT model of health-behavior change were significant and in the anticipated direction.
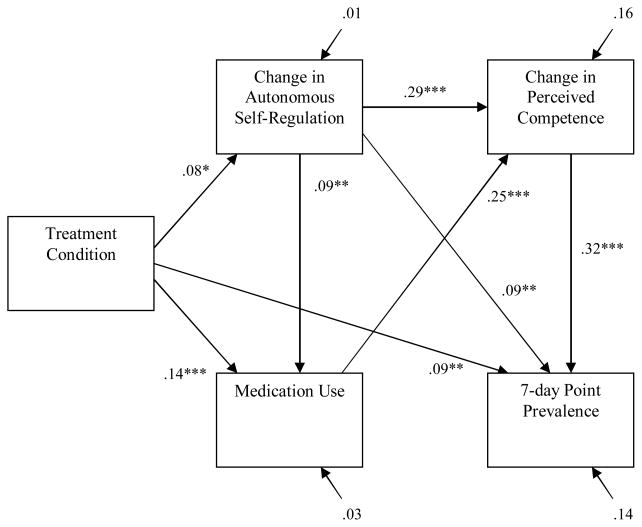
Results from the structural model with 7dPP tobacco abstinence as the outcome partially confirmed Hypothesis 4. Specifically, the relation of treatment condition to change in PC was reduced from r = .08 (p < .01) to β = .02 (ns), and the indirect effect of treatment condition on change in PC through change in ASR was significant (z′ = 2.56, p < .01); and the relation of medication use to 7dPP tobacco abstinence was reduced from r = .14 (p < .001) to β = .03 (ns), and the indirect effect of medication use on 7dPP through change in PC was significant (z′ = 6.65, p < .01). However, the relation of change in ASR to 7dPP tobacco abstinence remained significant β = .09 (p < .01), although the indirect effect of change in ASR on 7dPP tobacco abstinence through change in PC was significant (z′ = 7.21, p < .01). The structural model that excluded the three paths hypothesized to be mediated yielded adequate fit to the data, χ2 (3) = 9.76, ns; χ2/df = 3.25; CFI = .98; NNFI = .94; RMSEA = .05. However, the addition of a direct path from change in ASR to 7dPP tobacco abstinence significantly improved model fit (Δχ2 (1) = 8.18, p < .01). The final structural model is presented in Figure 3.
Figure 3.
The structural equation model, with parameter estimates, examining the structural relations among treatment condition, change in ASR and PC, medication use, and 7dPP tobacco abstinence.
Discussion
The results of this study demonstrated that an intensive tobacco-dependence intervention that focused on supporting smokers’ autonomy as the primary clinical goal facilitated two indices of long-term tobacco abstinence (viz., 24mPA from tobacco and 7dPP tobacco abstinence), relative to community care. The relative effect sizes for this intervention were moderate and likely to be both cost-effective (1, 26) and of clinical importance because of the remarkable health benefits of tobacco abstinence (3–5, 26). The intervention also facilitated 7dPP tobacco abstinence among participants who initially did not want to quit. The absolute rates of abstinence were small, although consistent with other published prolonged abstinence outcomes (22). It is important to emphasize, however, that smokers were invited to participate in the study regardless of whether they wanted to quit, and over half the participants were not interested in quitting smoking at the time they began the study. Thus, although the intervention had small absolute rates of abstinence, our results have potentially important public health and clinical implications for at least two reasons. First, the intervention reached a broader population of smokers compared to studies that have only included smokers who currently want to quit. Second, tobacco-dependence interventions are highly cost-effective (27).
The results of this study further supported several important components of the SDT model of health-behavior change. First, as hypothesized, change in both ASR and PC partially mediated the effect of treatment condition on long-term tobacco abstinence. Second, SEM analyses confirmed the SDT model of health-behavior change in facilitating long-term tobacco abstinence. Specifically, in the measurement phase of model testing, the associations among the components of the SDT model of health-behavior change were significant and in the anticipated direction. Further, in the structural phase of model testing, the relations of treatment condition to change in PC and medication use to long-term tobacco abstinence were fully mediated by change in ASR and change in PC, respectively. Also, the relation of change in ASR to 24mPA from tobacco was fully mediated by change in PC; however, the relation of change in ASR to 7dPP tobacco abstinence remained significant. Although we hypothesized mediation, it is possible that change in ASR continued to motivate new, successful quit attempts that were not captured by the 24mPA from tobacco variable, but were captured by the less stringent criteria for attaining 7dPP tobacco abstinence. Future research may explore the differential relations of the proposed SDT mediators (viz., ASR and PC) to different indices of long-term tobacco abstinence.
One possible, alternative explanation for the finding that the SDT-based intervention, relative to community care, facilitated long-term tobacco abstinence is that participants in the intervention had easier access to medication to aid in smoking cessation, and that medication use contributed to the higher likelihood of attaining long-term tobacco abstinence among smokers in the intervention. Certainly, participants in the intervention had easier access to treatment for smoking cessation, part of which included consultation with a study prescriber. However, the results of this study do not support the hypothesis that easier access to medication accounts for the between-group differences in abstinence rates. Specifically, the SEM analyses suggested that the relation of medication use to long-term tobacco abstinence was fully mediated by change in PC. Thus, our results support the SDT model of health-behavior change in demonstrating the importance of ASR and PC in facilitating long-term tobacco abstinence.
Several limitations deserve mention. First, the community care group did not receive a uniform treatment. Second, the difference in contact time between groups favored the intervention and facilitated internalization of ASR and PC (15), which is consistent with the finding that contact time has a strong dose-response relation to abstinence (1). However, the specific, effective elements of the intervention cannot be identified. Third, biochemical validation was not used to verify smoking cessation at 30 months, although serum cotinine was used at six (14) and at 18 (15) months. The zero-order correlation between the validated and non-validated reports of 7dPP tobacco abstinence was .88 and .92 (both ps < .001) at six and at 18 months, respectively. Thus, we feel confident that the self-report results are consistent with biochemical validation.
In sum, the importance of supporting autonomy and perceived competence in facilitating long-term health-behavior change was supported by finding that the SDT-based intervention, relative to community care, facilitated increases in autonomous self-regulation and perceived competence, as well as higher rates of long-term tobacco abstinence. In addition, the PHS Guideline for intensive tobacco-dependence treatment was further validated.
Acknowledgments
This research was supported by grants R01-CA106668 from the National Cancer Institute and by R01-MH59594 that was co-funded by the National Institute of Mental Health and the National Cancer Institute. Requests for reprints may be made to Geoffrey C. Williams, M.D., Ph.D., University of Rochester, R.C. Box 270266, Rochester, NY 14627-0266.
Footnotes
In all SEM analyses, a tetrachoric correlation matrix was used as input for the estimation of the models because of the inclusion of the dichotomous long-term tobacco abstinence variable.
The measurement model was saturated, or just-identified, and thus had zero degrees of freedom. Therefore, all incremental fit indices [e.g., Comparative Fit Index (CFI)] and χ2 yielded perfect fit (i.e., CFI = 1.00, χ2 = 0), and other indices (e.g., NNFI, RMSEA) could not be computed, so fit indices will not be reported.
The direct effect model was also saturated, or just-identified.
Contributor Information
Geoffrey C. Williams, University of Rochester
Christopher P. Niemiec, University of Rochester
Heather Patrick, University of Rochester
Richard M. Ryan, University of Rochester
Edward L. Deci, University of Rochester
References
- 1.Fiore M, Bailey W, Cohen S, et al. Treating Tobacco Use and Dependence. Rockville, MD: U.S. Department of Health and Human Services (DHHS); 2000. [Google Scholar]
- 2.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. J Amer Med Assoc. 2004;291:1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 3.Doll R, Peto R, Boreham J, Sutherland I. Mortality from cancer in relation to smoking: 50 years observations on British doctors. Brit J Cancer. 2005;92:426–9. doi: 10.1038/sj.bjc.6602359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anthonisen NR, Skeans MA, Wise RA, et al. The effects of a smoking cessation intervention on 14.5-year mortality: A randomized clinical trial. Ann Intern Med. 2005;142:233–9. doi: 10.7326/0003-4819-142-4-200502150-00005. [DOI] [PubMed] [Google Scholar]
- 5.Mohiuddin SM, Mooss AN, Hunter CB, Grollmes TL, Cloutier DA, Hilleman DE. Intensive smoking cessation intervention reduces mortality in high-risk smokers with cardiovascular disease. Chest. 2007;131:446–52. doi: 10.1378/chest.06-1587. [DOI] [PubMed] [Google Scholar]
- 6.Beauchamp TL, Childress JF. Principles of Biomedical Ethics. 5. New York: Oxford University Press; 2001. [Google Scholar]
- 7.Woolf SH, Chan EC, Harris R, et al. Promoting informed choice: Transforming health care to dispense knowledge for decision making. Ann Intern Med. 2005;143:293–300. doi: 10.7326/0003-4819-143-4-200508160-00010. [DOI] [PubMed] [Google Scholar]
- 8.Deci EL, Ryan RM. The “what” and “why” of goal pursuits: Human needs and the self-determination of behavior. Psychol Inq. 2000;11:227–68. [Google Scholar]
- 9.Niemiec CP, Ryan RM, Deci EL. Self-determination theory and the relation of autonomy to self-regulatory processes and personality development. In: Hoyle RH, editor. Handbook of Personality and Self-regulation. Malden, MA: Blackwell Publishing; in press. [Google Scholar]
- 10.Williams GC, Deci EL. Internalization of biopsychosocial values by medical students: A test of self-determination theory. J Pers Soc Psychol. 1996;70:767–79. doi: 10.1037//0022-3514.70.4.767. [DOI] [PubMed] [Google Scholar]
- 11.Williams GC, Gagne M, Ryan RM, Deci EL. Facilitating autonomous motivation for smoking cessation. Health Psychol. 2002;21:40–50. [PubMed] [Google Scholar]
- 12.Williams GC, McGregor HA, Zeldman A, Freedman ZR, Deci EL. Testing a self-determination theory process model for promoting glycemic control through diabetes self-management. Health Psychol. 2004;23:58–66. doi: 10.1037/0278-6133.23.1.58. [DOI] [PubMed] [Google Scholar]
- 13.Ryan RM, Patrick H, Deci EL, Williams GC. Facilitating health behaviour change and its maintenance: Interventions based on self-determination theory. The European Health Psychologist. 2008;10:2–5. [Google Scholar]
- 14.Williams GC, McGregor HA, Sharp D, et al. Testing a self-determination theory intervention for motivating tobacco cessation: Supporting autonomy and competence in a clinical trial. Health Psychol. 2006;25:91–101. doi: 10.1037/0278-6133.25.1.91. [DOI] [PubMed] [Google Scholar]
- 15.Williams GC, McGregor HA, Sharp D, et al. A self-determination multiple risk intervention trial to improve smokers’ health. J Gen Intern Med. 2006;21:1288–1294. doi: 10.1111/j.1525-1497.2006.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Cholesterol Education Program (NCEP) Cholesterol Lowering in the Patient with Coronary Heart Disease: Physician Monograph. Bethesda, MD: Institutes of Health, National Heart, Lung, and Blood Institute; 1997. NIH Publication No. 97–3794. [Google Scholar]
- 17.National Cancer Institute, National Institutes of Health. Clearing the Air: Quit Smoking Today. 2003 April; NIH Publication No. 03-1647. Accessed online: http://www.smokefree.gov/pubs/clearing_the_air.pdf.
- 18.Grundy SM, Pasternak R, Greenland P, Smith S, Jr, Fuster V. Assessment of cardiovascular risk by use of multiple-risk-factor assessment equations: A statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation. 1999;100:1481–92. doi: 10.1161/01.cir.100.13.1481. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Department of Health and Human Services. The benefits of smoking cessation: A report from the Surgeon General. Atlanta, GA: U.S. Department of Human Services, Public Health Service, Centers for Disease Control, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; DHHS; 1990. Publication No. (CDC) 90–8416. [Google Scholar]
- 20.Niemiec CP, Ryan RM, Deci EL, Williams GC. Aspiring to physical health: The role of aspirations for physical health in facilitating long-term tobacco abstinence. Patient Educ Couns. 2009;74:250–257. doi: 10.1016/j.pec.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: Issues and recommendations. Nico Tob Res. 2003;5:13–25. [PubMed] [Google Scholar]
- 22.Pierce JP, Gilpin EA. A minimum 6-month prolonged abstinence should be required for evaluating smoking cessation trials. Nico Tob Res. 2003;5:151–3. doi: 10.1080/0955300031000083427. [DOI] [PubMed] [Google Scholar]
- 23.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 24.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmbeck GN. Toward terminological, conceptual, and statistical clarity in the study of mediators and moderators: Examples from the child-clinical and pediatric psychology literatures. J Consult Clin Psych. 1997;65:599–610. doi: 10.1037//0022-006x.65.4.599. [DOI] [PubMed] [Google Scholar]
- 26.Maciosek MV, Edwards NM, Coffield AB, et al. Priorities among effective clinical preventive services: Methods. Am J Prev Med. 2006;31:90–6. doi: 10.1016/j.amepre.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 27.Woolf SH. Potential health and economic consequences of misplaced priorities. J Amer Med Assoc. 2007;297:523–526. doi: 10.1001/jama.297.5.523. [DOI] [PubMed] [Google Scholar]