Abstract
P-Rex1, a novel Rac activator, has been identified in the first biochemical purification of a guanine nucleotide exchange factor for GTPases of the Rho family. P-Rex1 is synergistically activated by PIP3 and Gβγ and may act as a coincidence detector for these signaling molecules.
Small GTPases of the Rho family regulate a vast spectrum of functions in eukaryotic cells, from cyto-skeletal rearrangements to vesicle transport to transcriptional regulation. Rho GTPases act as molecular switches, cycling between an ‘inactive’ GDP-bound form and an ‘active’ GTP-bound form. Activation is accomplished by guanine-nucleotide exchange factors (GEFs) which catalyze GDP dissociation, thereby facilitating GTP loading. Members of the Rac subfamily of Rho GTPases play an important role in transcriptional activation, the production of reactive oxygen species, and actin polymerization during lamellipodial formation [1].
A recent study [2] has identified a novel Rac activator, P-Rex1, which may act as a coincidence detector for signals transduced by phosphoinositide lipids and trimeric G proteins. The road to the discovery of P-Rex1 began with a biochemical assay for the production of reactive oxygen species in neutrophil lysates. Reactive oxygen species are produced downstream of G-protein-coupled receptors in neutrophils and other innate immune cells, and represents an essential component of the pathogen killing process [3]. Both phosphatidylinositol 3-kinase (PI 3-kinase) and Rac have been implicated in reactive oxygen species production, and activated Rac is sufficient to drive production of reactive oxygen species. Welch et al. [2] found that the PI 3-kinase lipid product PIP3 can also stimulate reactive oxygen species formation in neutrophil lysates, and this stimulation is blocked by proteins that sequester GEFs for Rac.
These data suggested that PIP3 activates exchange factors for Rac in neutrophil lysates. Using a direct assay for PIP3 activation of Rac, Welch et al. [2] purified a novel exchange factor for Rac, which they named P-Rex1, for PIP3-dependent Rac exchanger [2]. P-Rex1 was the major PIP3-dependent Rac GEF in neutrophil lysates, representing about 65% of the total Rac GEF activity. As Gβγ also suffices to stimulate reactive oxygen species production in neutrophil lysates, Welch et al. [2] tested whether Gβγ can activate P-Rex1. Surprisingly, they found that Gβγ directly activates P-Rex1, and synergizes with PIP3 for P-Rex1 activation in vitro and in vivo. Antisense-mediated knock-down of P-Rex1 levels in a neutrophil-like cell line decreased agonist-induced formation of reactive oxygen species by 40–45%, suggesting that P-Rex1 is necessary for full reactive oxygen species production in vivo [2].
The identification of P-Rex1 is particularly significant in several respects. This is the first biochemical purification of an exchange factor for a Rho GTPase based on its activity. Most other mammalian Rho GEFs have been identified either as oncogene products or by homology to known GEFs identified by genetic screens in a model species. The best known Rho GEF activators have been protein kinases. PI 3-kinase has been reported to regulate several of these GEFs, but it primarily appears to do so indirectly, by affecting GEF phosphorylation by protein kinases. Where PIP3 has been reported to directly regulate GEFs in vitro, the observed degree of GEF activation was significantly lower than for P-Rex1: for example, PIP3 activated Vav1 [4] and Pix [5] less than 2.5-fold, compared to 20-fold activation of P-Rex1 by PIP3 [2]. Finally, P-Rex1 is the first example of an exchange factor for Rho GTPases that is directly stimulated by Gβγ. Other Rho GTPase exchange factor are known to act downstream of Gβγ, but the yeast GEF Cdc24 requires adaptor proteins to bind to and be activated by Gβγ [6], and the mammalian GEF Ras-GRF is regulated indirectly by Gβγ through tyrosine phosphorylation [7].
How does P-Rex1 fit into the known picture of Rac activation in neutrophils? In neutrophils and other haematopoietic cells, Gβγ release and PIP3 production are linked by the lipid kinase PI 3-kinase-γ and its adapter protein p101 (Figure 1A) [8]. Therefore, both coactivators of P-Rex1 are naturally produced upon activation of a G-protein-coupled receptor. In contrast, activation of some tyrosine kinase receptors which fail to generate Gβγ can lead to significant production of PIP3 but no detectable Rac activation. In some [9,10] but not all [11] experiments, Rac activation in neutrophils is dependent on PI 3-kinase, possibly reflecting the relative importance of Gβγ versus PIP3 at different time points for Rac activation.
Figure 1.
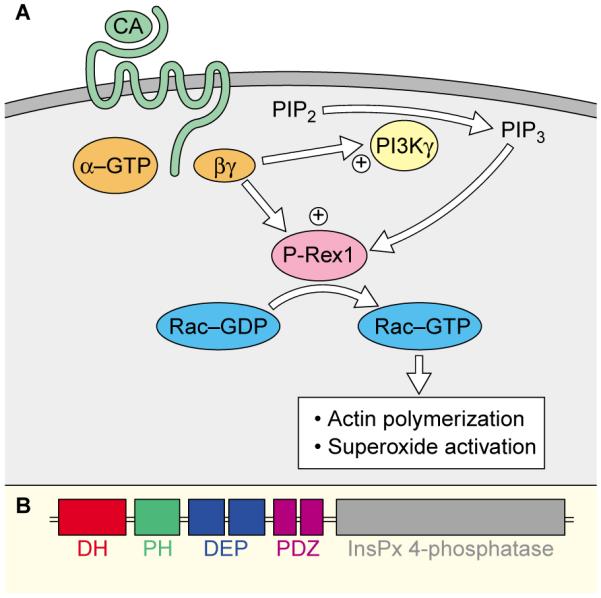
(A). Signal transduction cascade for P-Rex1 activation in neutrophils. Activation of a G-coupled receptor (green) by chemoattractant (CA) stimulates the activation of heterotrimeric G-proteins (orange) and dissociation of Gα from Gβγ. Gβγ directly stimulates PI 3-kinase-γ (PI3Kγ), leading to the production of PIP3. Both Gβγ and PIP3 synergize in activating P-Rex1 which catalyzes nucleotide exchange on Rac. Activated Rac then stimulates superoxide activation necessary for pathogen killing and actin polymerization necessary for lamellipodial formation and chemotaxis. (B) Domain structure of P-Rex1. Almost all exchange factors contain a Dbl-homology (DH) domain followed by a pleckstrin homology (PH) domain. P-Rex1 also contains tandem DEP homology domains, tandem PDZ domains, and an apparently enzymatically inactive inositol polyphosphate 4-phosphatase domain.
The fact that Gβγ and PIP3 synergize in P-Rex1 activation suggests that they bind separate domains of P-Rex1, though neither domain has yet been identified. The pleckstrin homology (PH) domain would seem the most obvious candidate for interaction with PIP3, although P-Rex1’s PH domain is not very similar to those of known PIP3-binding proteins, such as Akt, Grp1 and Btk. For interaction with Gβγ, the DEP domain is a good possibility as it is found in ‘regulator of G protein signalling’ (RGS) proteins, which interact with the Gα subunit of heterotrimeric G proteins and other signaling proteins that act at the plasma membrane (Figure 1B). Identifying the protein domains used by P-Rex1 to interact with Gβγ and PIP3 will be instrumental for identifying additional GEFs and other proteins that relay signals downstream of these important signaling molecules.
Numerous effectors for PIP3 have been characterized, and specific protein domains involved in PIP3 binding have been identified [12]. Several of these proteins, such as the serine-threonine kinase AKT, appear to translocate from the cytosol to sites of PIP3 production. In contrast, P-Rex1 exhibits significant basal association with the plasma membrane and is not significantly recruited upon PIP3 production [2]. Similarly, significant PI 3-kinase-γ is associated with the membrane even in the absence of stimulation [13]. This basal association of PI 3-kinase-γ and P-Rex1 with the plasma membrane may be necessary for the extremely rapid activation of Rac, which can peak in 10 seconds in neutrophils. Because these molecules may be allosterically activated, and not regulated solely by localization, this will present a challenge for assaying the spatial distribution of activated P-Rex1 and p101/110γ.
Although the initial characterization of P-Rex1 focused on its role in reactive oxygen species formation, P-Rex1 is very likely to coordinate other Rac activities downstream of Gβγ and PIP3 in neutrophils, such as actin polymerization. When presented with a gradient of chemoattractant, neutrophils respond with highly oriented polarity and motility toward the source of chemoattractant. Actin polymerization and PI 3-kinase lipid products exhibit strong asymmetries aligned with the chemotactic gradient [14], and both PI 3-kinase and Rac are necessary for proper neutrophil chemotaxis [15]. P-Rex1 is an excellent candidate for linking gradients of PIP3 production during chemotaxis with Rac-mediated cytoskeletal rearrangements. The synergistic activation of P-Rex1 by Gβγ and PIP3 might also help sharpen the asymmetry of Rac activation during chemotaxis.
Do P-Rex1-like exchange factors function solely in the immune system and brain of mammals, or might they act more generally? Although P-Rex1 is primarily expressed in the immune system and the brain, there is a close homolog to P-Rex1 on chromosome 8 that may be more broadly expressed. There are good homologues of P-Rex1 in pig and mouse, but no exchange factors with obviously similar domain structure in Caenorhabditis elegans or Drosophila, suggesting either that P-Rex1-like molecules are not necessarily universal links between Gβγ, PIP3 and Rac activation or that the relevant architecture of P-Rex1 may lie beyond its currently identified domains. It is also important to note that at least one additional peak of PIP3-stimulated exchange factor activity for Rac was observed in neutrophil lysates [2], so it is likely that more PIP3-regulated GEFs for Rac will be discovered before long.
What about other signaling cascades where PIP3 has been implicated in Rac activation? A great deal of evidence suggests that PI 3-kinase activation is required to turn on Rac in response, not only to G-protein-coupled receptors, but also to tyrosine kinase receptors such as the platelet-derived fibroblast growth factor (PDGF) receptor in fibroblasts and other cells [16]. Remarkably, recent evidence suggests that, to activate Rac, the PDGF receptor requires transactivation of a G-protein-coupled receptor. In the absence of EDG-1 — a G-protein-coupled receptor for sphingosine phosphate — stimulation of the PDGF receptor fails to activate Rac. Stimulation with PDGF results in activation of sphingosine kinase, production of sphingosine phosphate and activation of EDG-1. Interference with this receptor cross-talk — by inhibition of sphingosine kinase or G-protein signaling, or the absence of EDG-1 — prevents the usual increase in motility and Rac activation in response to PDGF [17].
Why is there a requirement for the transactivation of G-protein-coupled receptors if the PDGF receptor can generate PIP3 and recruit GEFs for Rac on its own? One intriguing possibility is that the G-protein-coupled receptor provides Gβγ which synergizes with PIP3 generated by the PDGF receptor for full synergistic activation of P-Rex1 or a similar exchange factor. Indeed, a variety of signaling events for cell polarity appear to rely on heterotrimeric G-protein activation, including cell polarization in response to external cues in mammalian cells, budding yeast and Dictyostelium [14], and asymmetric cell division in Drosophila [18] and C. elegans [19], suggesting that some property of heterotrimeric G-protein signaling may naturally lend itself to polarity establishment.
Why construct an exchange factor to be sensitive to coincident generation of Gβγ and PIP3? Perhaps this permits Rac activation to be variably coupled to these signaling cascades. This may allow independent regulation of G-protein-coupled receptor pathways such as sensory transduction, or PIP3-regulated events such as proliferation and survival, to occur in instances where Rac activation and cell motility are not desired. In contrast, Rac activation and cell migration can be stimulated in cells such as neutrophils, where Gβγ release is directly coupled to PI 3-kinase activation, or fibroblasts, where cross-talk between the PDGF receptor and G-protein-coupled receptors provides the proper signals for P-Rex1 activation.
References
- 1.Tapon N, Hall A. Rho, Rac and Cdc42 GTPases regulate the cytoskeleton. Curr. Opin. Cell Biol. 1997;9:86–92. doi: 10.1016/s0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- 2.Welch HCE. [Google Scholar]
- 3.Reeves EP, Lu H, Jacobs HL, Messina CG, Bolsover S, Gabella G, Potma EO, Warley A, Roes J, Segal AW. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–297. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- 4.Han J, Luby-Phelps K, Das B, Shu X, Xia Y, Mosteller RD, Krishna UM, Falck JR, White MA, Broek D. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- 5.Yoshii S, Tanaka M, Otsuki Y, Wang DY, Guo RJ, Zhu Y, Takeda R, Hanai H, Kaneko E, Sugimura H. alphaPIX nucleotide exchange factor is activated phosphatidylinositol 3-kinase. Oncogene. 1999;18:5680–5690. doi: 10.1038/sj.onc.1202936. [DOI] [PubMed] [Google Scholar]
- 6.Gulli MP, Peter M. Temporal and spatial regulation of Rho-type guanine-nucleotide exchange factors: the yeast perspective. Genes Dev. 2001;15:365–379. doi: 10.1101/gad.876901. [DOI] [PubMed] [Google Scholar]
- 7.Kiyono M, Satoh T, Kaziro Y. G protein beta gamma subunit-dependent Rac- activity of Ras-GRF1/CDC25(Mm) Proc. Natl. Acad. Sci. U.S.A. 1999;96:4826–4831. doi: 10.1073/pnas.96.9.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stephens LR, Eguinoa A, Erdjument-Bromage H, Lui M, Cooke F, Coadwell J, Smrcka AS, Thelen M, Cadwallader K, Tempst P, Hawkins PT. The G beta gamma sensitivity of a PI3K is associated adaptor, p101. Cell. 1997;89:105–114. doi: 10.1016/s0092-8674(00)80187-7. [DOI] [PubMed] [Google Scholar]
- 9.Akasaki T, Koga H, Sumimoto H. Phosphoinositide 3-kinase-dependent and independent regulation of the small GTPase Rac2 in human neutrophils. J. Biol. Chem. 1999;274:18055–18059. doi: 10.1074/jbc.274.25.18055. [DOI] [PubMed] [Google Scholar]
- 10.Benard V, Bohl BP, Bokoch GM. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J. Biol. Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- 11.Geijsen N, van Delft S, Raaijmakers JA, Lammers JW, Collard JG, Koenderman L, Coffer PJ. Regulation of p21rac activation in human. Blood. 1999;94:1121–1130. [PubMed] [Google Scholar]
- 12.Hurley JH, Meyer T. Subcellular targeting by membrane lipids. Curr. Opin. Cell Biol. 2001;13:146–152. doi: 10.1016/s0955-0674(00)00191-5. [DOI] [PubMed] [Google Scholar]
- 13.Krugmann S, Cooper MA, Williams DH, Hawkins PT, Stephens LR. Mechanism of the regulation of type IB phosphoinositide 3OH-kinase by G-protein betagamma subunits. Biochem. J. 2002;362:725–731. doi: 10.1042/0264-6021:3620725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiner OD. Regulation of cell polarity during eukaryotic chemotaxis: the chemotactic compass. Curr. Opin. Cell Biol. 2002;14:196–202. doi: 10.1016/s0955-0674(02)00310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts AW, Kim C, Zhen L, Lowe JB, Kapur R, Petryniak B, Spaetti A, Pollock JD, Borneo JB, Bradford GB, et al. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity. 1999;10:183–196. doi: 10.1016/s1074-7613(00)80019-9. [DOI] [PubMed] [Google Scholar]
- 16.Hawkins PT, Eguinoa A, Qiu RG, Stokoe D, Cooke FT, Walters R, Wennstrom S, Claesson-Welsh L, Evans T, Symons M. PDGF stimulates an increase in GTP-Rac via activation of phosphoinositide 3-kinase. Curr. Biol. 1995;5:393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]
- 17.Hobson JP, Rosenfeldt HM, Barak LS, Olivera A, Poulton S, Caron MG, Milstien S, Spiegel S. Role of the sphingosine-1-phosphate receptor EDG-1 in PDGF-induced cell motility. Science. 2001;291:1800–1803. doi: 10.1126/science.1057559. [DOI] [PubMed] [Google Scholar]
- 18.Schaefer M, Petronczki M, Dorner D, Forte M, Knoblich JA. Heterotrimeric G proteins direct two modes of asymmetric cell division in the Drosophila nervous system. Cell. 2001;107:183–194. doi: 10.1016/s0092-8674(01)00521-9. [DOI] [PubMed] [Google Scholar]
- 19.Zwaal RR, Ahringer J, van Luenen HG, Rushforth A, Anderson P, Plasterk RH. G proteins are required for spatial orientation of early cell cleavages in C. elegans embryos. Cell. 1996;86:619–629. doi: 10.1016/s0092-8674(00)80135-x. [DOI] [PubMed] [Google Scholar]


