Abstract
The mammalian muscle nicotinic acetylcholine receptor (AChR) is composed of five membrane-spanning subunits and its composition differs between embryonic and adult muscles. In embryonic muscles, it is composed of two α-, one β-, one δ-, and one γ-subunit; the γ-subunit is later replaced by the ε-subunit during postnatal development. This unique temporal expression pattern of the γ-subunit suggests it may play specific roles in embryonic muscles. To address this issue, we examined the formation and function of the neuromuscular junction in mouse embryos deficient in the γ-subunit. At embryonic day 15.5, AChR clusters were absent and the spontaneous miniature endplate potentials were undetectable in the mutant muscles. However, electrical stimulation of the nerves triggered muscle contraction and elicited postsynaptic endplate potential (EPP) in the mutant muscles, although the magnitude of the muscle contraction and the amplitudes of the EPPs were smaller in the mutant compared to the wild-type muscles. Reintroducing a wild-type γ-subunit into the mutant myotubes restored the formation of AChR clusters in vitro. Together, these results have demonstrated that functional AChRs were present in the mutant muscle membrane, but at reduced levels. Thus, in the absence of the γ-subunit, a combination of α, β, and δ subunits may assemble into functional receptors in vivo. These results also suggest that the γ-subunit maybe involved in interacting with rapsyn, a cytoplasmic protein required for AChR clustering.
Keywords: Neuromuscular junction, Mice, γ-Subunit, Muscle nicotinic acetylcholine receptor
Introduction
At the developing vertebrate neuromuscular junction (NMJ), nicotinic acetylcholine receptors (AChRs) are clustered in the central region of the muscle. The onset of the nascent AChRs clusters coincides with, but independent of, the arrival of the nerves (Lin et al., 2001; Yang et al., 2001; Yang et al., 2000). This has led to an emerging hypothesis that the postsynaptic specialization, such as the clustering of AChRs, is prepatterned in the developing muscles (Arber et al., 2002; Goda and Davis, 2003; Witzemann, 2006). This view challenges the long-held “neural centric” dogma, but also raises many new questions. For example, it is not clear how postsynaptic prepattern is generated, and to what extent the nascent AChRs contribute to synaptogenesis. One possibility is that fetal AChRs may serve as scaffolds for the incoming nerves (Fambrough, 1979; Albuquerque et al., 1974; Fertuck and Salpeter, 1974). Alternatively, the nascent AChRs may be essential for sensing the neurotransmitter acetylcholine released from the nerves and inducing local postsynaptic responses such as elevating postsynaptic Ca2+ to facilitate synaptogenesis (Wan and Poo 1999; Zhang and Poo 2001).
The mammalian muscle nicotinic AChRs are pentamers composed of five membrane-spanning subunits in a stoichiometry of α2βγδ (fetal AChRs, or γ-AChRs), or α2βεδ (adult AChRs, or ε-AChRs) (Changeux et al., 1992; Mishina et al., 1986). The γ-subunit is typically expressed during embryonic and neonatal stages, and is replaced by the ε-subunit during the first 2 weeks after birth (γ/ε switch). The physiological role of this switch is not well understood, but evidence suggests that γ-subunit and ε-subunit each play specific roles in embryonic and adult NMJs, respectively. Targeted deletion of the γ-subunit in mice leads to an absence of spontaneous muscle action potentials and premature death (Takahashi et al., 2002), aberrant patterning of the NMJs and increased motoneuron survival (Liu et al., 2008). Genetically replacing the γ-subunit with a chimeric γ/ε-subunit (Koenen et al., 2005) or with a γ/GFP which results in reduced γ-subunit expression also leads to abnormal nerve branching and increased motoneuron survival (Yampolsky et al., 2008). Blocking fetal AChRs with conotoxins in chick embryos rescues developing motoneurons from programmed cell death (Oppenheim et al., 2008). In the present study, we further examine the formation and function of the NMJs in mouse embryos deficient in the AChR γ-subunit.
Material and Methods
Morphological Analysis
Mice deficient in the γ-subunit (γ−/−) have been described previously (Takahashi et al., 2002). Wholemount immunocytochemistry, in situ hybridization and acetylcholinesterase (AChE) staining were carried out as previously described (Lin et al., 2001).
Electrophysiology
Phrenic nerve/diaphragm muscles were acutely dissected from mouse embryos (embryonic day 15.5 (E15.5) to E18.5) in normal mouse Ringer’s solution (Liley, 1956). Spontaneous miniature endplate potentials (mEPPs) and evoked endplate potentials (EPPs) were recorded using an intracellular amplifier (AxoClamp-2B). EPPs were induced by a supra threshold stimulation of the phrenic nerve via a suction electrode connected to an extracellular stimulator (SD9).
Primary Myotube Culture
Primary myotube cultures were prepared from E18.5 embryos as previously described (Lin et al., 2005). Cells were plated onto 1% gelatin-coated plates at a density of 0.5 × 106 per 35 mm dish. Myotube cultures were transfected on day 5 (when cells reached 70–80% confluency) with FuGENE6 by drop-wisely adding serum-free DMEM (100 µl to each 35 mm dish) containing a premixed 3 µl FuGENE6 and 2 µg DNA (pRK5-γ/GFP; Gensler et al., 2001). Myotube fusion was induced by replacing the culture medium with DMEM plus 2% horse serum.
Results and Discussion
Establishment of Innervation Pattern at Developing NMJs
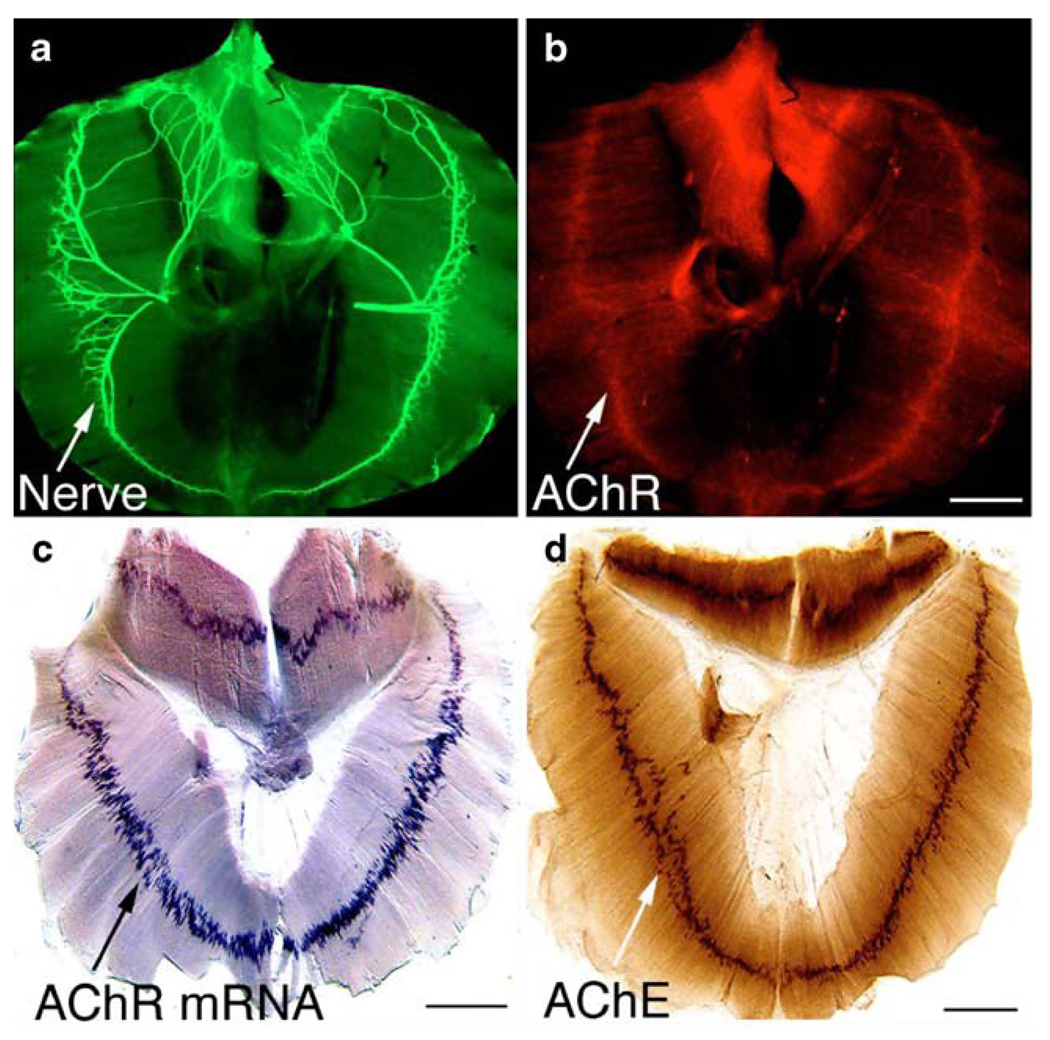
The rodent embryonic diaphragm muscle is a convenient preparation for analyzing neuromuscular development: the muscle is thin enough for wholemount staining to reveal the overall innervation and endplate pattern. In mice, the phrenic nerves originate from motor neuron pools at cervical levels (C3–C6), descend caudally, and innervate the diaphragm at thoracic levels (T8–T9; Allan and Greer, 1997a; b). The phrenic nerves reach the primordial diaphragm as early as embryonic day 12 (Noakes et al., 1983). During the subsequent stages of development, the nerves travel and extend intramuscularly along the central region of the muscle (often referred to as “the endplate band”), and by E15.5, the entire diaphragm is fully innervated (Fig. 1a). Within the endplate band, AChRs (Fig. 1b), AChR mRNA (Fig. 1c), and AChE (Fig. 1d) are all highly concentrated.
Figure 1.
The pattern of developing neuromuscular junction. Wholemount embryonic diaphragm muscles (wild type; a–b, E15.5) were immunostained with anti-neurofilament antibody (a) and Texas-red conjugated α-bungarotoxin (b). The nerve terminals and AChR clusters were confined to the central region of the muscle. c The pattern of AChR subunit (α-subunit) gene transcription revealed by wholemount in situ hybridization in the diaphragm muscle (E17.5). d The pattern of AChE distribution in wholemount diaphragm muscle (E16.5). Scale bars a, b 400 µm, c 500 µm, d 500 µm
How is such a unique pattern established during development? What are the underlying cellular and molecular mechanisms? We hypothesized that the fetal AChRs play critical roles in establishing the innervation pattern. We tested this hypothesis by analyzing the mutant mice deficient in the AChR γ-subunit, an essential component of the fetal AChRs. We have demonstrated that the γ-subunit is required for clustering of the nascent AChR. In these mutants, AChR clusters are completely absent during initial stages (E13–E15.5); innervation pattern is disrupted and the nerves branch extensively across the entire muscle surface (Liu et al., 2008). Interestingly, AChR clusters emerge at later stages (E16.5–E18.5) in the γ-subunit mutant muscles, likely due to the onset of the ε-subunit expression (Liu et al., 2008).
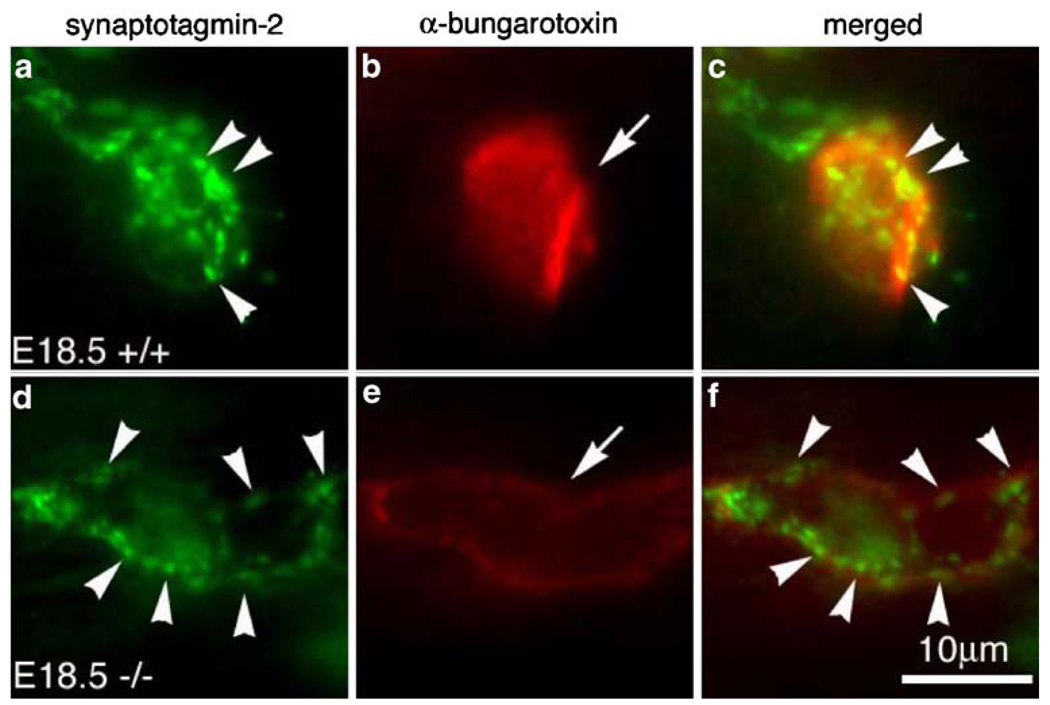
To further examine the spatial relationship between the differentiating nerve terminals and the endplates, we double-labeled diaphragm muscles (E18.5) with antibodies against the synaptic vesicle protein synaptotagmin-2 (Syt2) and α-bungarotoxin, and examined individual synapses under high magnification (× 100 water immersion objective, N.A. 1.0). Figure 2 shows typical individual endplates in the wild-type and mutant muscles. Syt2 were preferentially accumulated at the nerve terminals in both the wild type (arrowheads in Fig. 2a) and mutant (arrowheads in Fig. 2d). Consistent with the previous report (Marques et al., 2000), the nerve terminals in the wild type were localized within the endplate, but did not completely cover the entire endplate: some endplate regions were devoid of nerve terminal contacts (see merged images in Fig. 2c). Such spatial pattern was retained in the mutant (Fig. 2f). The size of the endplate in the mutant (Fig. 2e) was considerably larger than the wild type (Fig. 2b), but the fluorescence intensity labeled by α-bungarotoxin was considerably reduced (compare Fig. 2e and b), suggesting the density of the AChRs in the mutant was greatly decreased.
Figure 2.
Differentiation of the NMJ in the absence of the γ-subunit. High magnification views of a single endplate from wholemount diaphragm muscle at E18.5, double-labeled with synaptotagmin-2 (Syt-2) antibody (green) and Texas-red conjugated α-bungarotoxin (red). Individual endplate (AChR cluster) in the mutant (e, arrow) appeared less intensely labeled by α-bungarotoxin, but their sizes are bigger, compared to the wild type (b, arrow). In both WT and mutant muscles, Syt-2 antibody staining was highly concentrated at the nerve terminals (arrowheads in a, d). Scale bar 10 µm
Function of the Neuromuscular Synapse in γ−/− Muscles
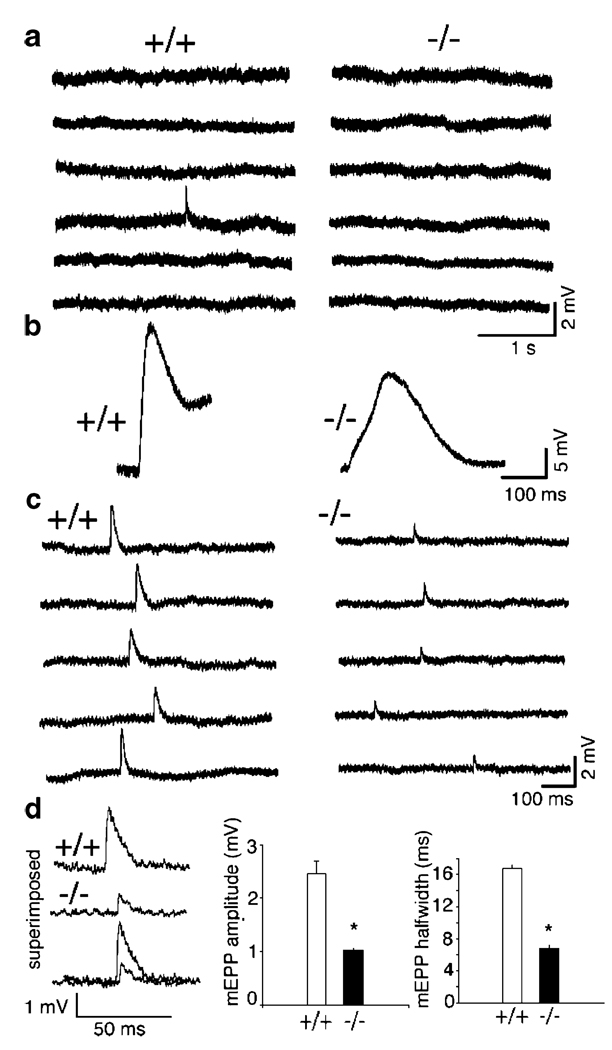
To examine the function of the neuromuscular synapses in γ−/− muscles, we carried out electrophysiological analyses in acutely isolated phrenic nerve/diaphragm muscles. The resting membrane potentials were around −50 mV for both wild-type and mutant muscles (wild type, −48±2.3 mV; mutant −49±6.4 mV), consistent with the previous reports for mammalian embryonic muscles (Dennis et al., 1981; Diamond and Miledi, 1962). At E15.5, mEPPs were detected in wild-type muscles (with a frequency less than 1 per minute), but not in the mutant muscles (Fig. 3a). However, E15.5 mutant muscles contracted in response to electrical stimulation of the phrenic nerves. The contraction in E15.5 mutant muscles appeared in a slow and wavy motion, whereas the contraction of E15.5 wild-type muscles was noticeably faster and stronger. Figure 3b shows typical examples of EPPs recorded from E15.5 muscle fibers in response to phrenic nerve stimulation—the EPP in the mutant muscle was slower and smaller in amplitude, compared to the wild-type muscle. These results suggest that functional AChRs were present at the mutant muscle membrane, but at reduced levels.
Figure 3.
Neuromuscular synaptic activity in γ−/− embryos. a mEPPs were detected in the wild type (+/+), but not in the mutant (−/−) at E15.5. b Electrical stimulation of the phrenic nerve elicited EPPs in both +/+ and −/− muscles (E15.5), but with smaller amplitude and slower response in the mutant. Visual inspection of the muscles during stimulation revealed a slow-wavy contraction in the mutant, vs. a fast contraction in the wild type. c MEPPs were recorded from the wild type and the mutant at E18.5. d Quantification of the mEPPs: the amplitudes and half-width of the mEPPs were significantly (*p<0.001) reduced in the mutant, compared to the wild type
At E18.5, mEPPs were readily detectable in the mutant muscles (Fig. 3c). The frequency of mEPPs in the mutants was comparable to that in the wild type, but their amplitude and half-width were markedly reduced. In the wild-type muscles, the average mEPP amplitudes were 2.46±0.25 mV, and the half-widths were 16.80±0.30 ms (n = 7 muscle cells), whereas in the mutant, the average mEPP amplitudes were 1.19±0.07 mV, half-widths were 7.98±0.58 ms (n=30 muscle cells; Fig. 2d). Decrease in mEPP amplitude in the mutants is consistent with reduced α-bungarotoxin labeling (Fig. 2e). The decreased half-width of mEPP in the mutants implies that the AChRs in the mutants contain adult-type (ε-AChR), since the ε-AChR channels have shorter mean open time (Mishina et al., 1986; Sakmann and Brenner, 1978; Schuetze and Role, 1987; Villarroel and Sakmann, 1996).
γ-Subunit is Sufficient for AChR Clustering in Myotubes In Vitro
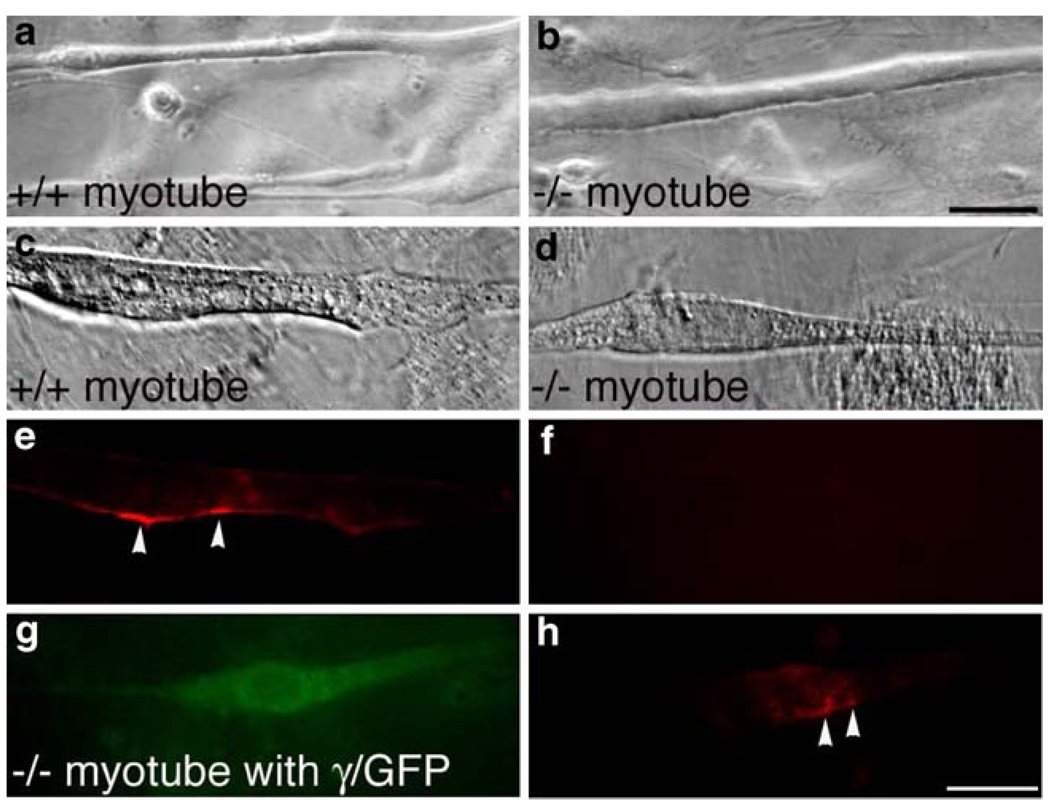
To test if γ-subunit is sufficient for AChR clustering, we turned to in vitro myotube cultures. We prepared primary myotube cultures from E18.5 embryos and observed myotube fusion 2 days following serum reduction. Myotubes isolated from the γ−/− muscles appeared healthy and indistinguishable from the wild type (Fig. 4a, b). To examine AChR clustering in myotube cultures, we applied agrin (10 ng/ml) to the myotube culture, since spontaneous clustering of AChRs in myotube cultures is rare in primary myotube cultures (Martinou and Merlie, 1991). As shown in Fig. 4e and f, AChR clusters were detected in the wild-type myotube (Fig. 4e), but not in γ−/− myotube (Fig. 4f). These results demonstrate that while AChR clusters were present in E18.5 γ−/− muscles in vivo, they were absent from myotube culture in vitro, likely due to the lack of expression of the ε-subunit in vitro.
Figure 4.
Full-length γ-subunit restores AChR clustering in vitro. a, b Live myotube cultures visualized under Nomarski (7 days after plating). Spontaneous myotube fusion was observed in both wild type and γ−/− culture. c, d Fixed myotubes 5 days after serum deprivation. AChR clusters were present in wild-type myotube (e), but not in the γ−/− myotube (f). g, h Rescue of AChR clustering in γ−/− myotubes (h) after transfection with γ/GFP (g). Scales a, b 50 µm, c–h 20 µm
To test if the γ-subunit was sufficient for AChR clustering, we reintroduced a wild-type γ-subunit tagged with GFP (γ/GFP) into the γ−/− myotubes. We chose this γ/GFP because it has been shown to assemble into functional AChRs in wild-type muscles (Gensler et al., 2001). As shown in Fig. 4h, γ/GFP restored AChR clustering in the GFP-positive muscle cell (Fig. 4g). These results demonstrate that the γ-subunit is sufficient for AChR clustering.
Previous studies have shown that, in the absence of the γ-subunit, a combination of α, β, and δ subunits may assemble into functional receptors in vitro, such as α2βδ2 (Sine and Claudio, 1991). Our electrophysiological data at E15.5 is consistent with this idea. Our data also demonstrate that clustering of AChRs requires the inclusion of either the γ-subunit or the ε-subunit into the receptor. AChR clusters are completely absent during the early stages of neuromuscular synaptogenesis (E13–E15.5) in the γ-subunit mutant, but emerge at later stages (E16.5–E18.5) coinciding with the onset of the ε-subunit expression. Why do AChRs fail to cluster in the absence of the γ-subunit or ε-subunit? It is plausible that the γ-, or ε-subunit, is uniquely required for interacting with rapsyn, a 43-kDa cytoplasmic protein closely associates with the intracellular domain of AChRs (Froehner et al., 1981). Clustering of AChRs fails to occur in mutant mice deficient in rapsyn (Gautam et al., 1995), and rapsyn is absent from synaptic sites in AChR mutants in zebrafish (Ono et al., 2004; Ono et al., 2002). Thus, identifying specific domain(s) of the γ- or ε-subunit that interacts with rapsyn should provide further insights into how AChRs are clustered at the postsynaptic membrane.
Acknowledgments
We thank Dr. Thomas Südhof (UT Southwestern Medical Center) for synaptotagmin-2 antibody and Dr. Veit Witzemann (Max Planck Institute, Germany) for pRK5-γ/GFP plasmid. This work was supported by grants (to W. Lin) from NIH/NINDS (NS055028), the Edward Mallinckrodt, Jr. Scholar Program and the Cain Foundation in Medical Research.
References
- Albuquerque EX, Bernard EA, Porter CW, Warnick JE. The density of acetylcholine receptors and their sensitivity in the postsynaptic membrane of muscle endplates. Proceedings of the National Academy of Sciences of the United States of America. 1974;71(7):2818–2822. doi: 10.1073/pnas.71.7.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan DW, Greer JJ. Development of phrenic motoneuron morphology in the fetal rat. Journal Comparative Neurology. 1997a;382:469–479. doi: 10.1002/(sici)1096-9861(19970616)382:4<469::aid-cne4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Allan DW, Greer JJ. Embryogenesis of the phrenic nerve and diaphragm in the fetal rat. Journal Comparative Neurology. 1997b;382:459–468. doi: 10.1002/(sici)1096-9861(19970616)382:4<459::aid-cne3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Arber S, Burden SJ, Harris AJ. Patterning of skeletal muscle. Current Opinion in Neurobiology. 2002;12:100–103. doi: 10.1016/s0959-4388(02)00296-9. [DOI] [PubMed] [Google Scholar]
- Changeux JP, Devillers-Thiery A, Galzi JL, Revah F. The acetylcholine receptor: A model of an allosteric membrane protein mediating intercellular communication; Ciba Foundation Symposium; 1992. pp. 66–89. discussion 87–97. [DOI] [PubMed] [Google Scholar]
- Dennis MJ, Ziskind-Conhaim L, Harris AJ. Development of neuromuscular junctions in rat embryos. Developmental Biology. 1981;81:266–279. doi: 10.1016/0012-1606(81)90290-6. [DOI] [PubMed] [Google Scholar]
- Diamond J, Miledi R. A study of foetal and new-born rat muscle fibres. Journal of Physiology. 1962;162:393–408. doi: 10.1113/jphysiol.1962.sp006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fambrough DM. Control of acetylcholine receptors in skeletal muscle. Physiological Reviews. 1979;59:165–227. doi: 10.1152/physrev.1979.59.1.165. [DOI] [PubMed] [Google Scholar]
- Fertuck HC, Salpeter MM. Localization of acetylcholine receptor by 1251-labeled alpha-bungarotoxin binding at mouse motor endplates. Proceedings of the National Academy of Sciences of the United States of America. 1974;71(4):1376–1378. doi: 10.1073/pnas.71.4.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehner SC, Gulbrandsen V, Hyman C, Jeng AY, Neubig RR, Cohen JB. Immunofluorescence localization at the mammalian neuromuscular junction of the Mr 43, 000 protein of Torpedo postsynaptic membranes. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:5230–5234. doi: 10.1073/pnas.78.8.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam M, Noakes PG, Mudd J, Nichol M, Chu GC, Sanes JR, et al. Failure of postsynaptic specialization to develop at neuromuscular junctions of rapsyn-deficient mice. Nature. 1995;377:232–236. doi: 10.1038/377232a0. [DOI] [PubMed] [Google Scholar]
- Gensler S, Sander A, Korngreen A, Traina G, Giese G, Witzemann V. Assembly and clustering of acetylcholine receptors containing GFP-tagged epsilon or gamma subunits: Selective targeting to the neuromuscular junction in vivo. European Journal of Biochemistry. 2001;268:2209–2217. doi: 10.1046/j.1432-1327.2001.02093.x. [DOI] [PubMed] [Google Scholar]
- Goda Y, Davis GW. Mechanisms of synapse assembly and disassembly. Neuron. 2003;40:243–264. doi: 10.1016/s0896-6273(03)00608-1. [DOI] [PubMed] [Google Scholar]
- Koenen M, Peter C, Villarroel A, Witzemann V, Sakmann B. Acetylcholine receptor channel subtype directs the innervation pattern of skeletal muscle. EMBO Reports. 2005;6:570–576. doi: 10.1038/sj.embor.7400429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liley AW. An investigation of spontaneous activity at the neuromuscular junction of the rat. Journal of Physiology. 1956;132:650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Burgess RW, Dominguez B, Pfaff SL, Sanes JR, Lee KF. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature. 2001;410:1057–1064. doi: 10.1038/35074025. [DOI] [PubMed] [Google Scholar]
- Lin W, Dominguez B, Yang J, Aryal P, Brandon EP, Gage FH, et al. Neurotransmitter acetylcholine negatively regulates neuromuscular synapse formation by a Cdk5-dependent mechanism. Neuron. 2005;46:569–579. doi: 10.1016/j.neuron.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Liu Y, Padgett D, Takahashi M, Li H, Sayeed A, Teichert RW, et al. Essential roles of the acetylcholine receptor {gamma}-subunit in neuromuscular synaptic patterning. Development. 2008;135:1957–1967. doi: 10.1242/dev.018119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques MJ, Conchello JA, Lichtman JW. From plaque to pretzel: Fold formation and acetylcholine receptor loss at the developing neuromuscular junction. Journal of Neuroscience. 2000;20:3663–3675. doi: 10.1523/JNEUROSCI.20-10-03663.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinou JC, Merlie JP. Nerve-dependent modulation of acetylcholine receptor epsilon-subunit gene expression. Journal of Neuroscience. 1991;11:1291–1299. doi: 10.1523/JNEUROSCI.11-05-01291.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina M, Takai T, Imoto K, Noda M, Takahashi T, Numa S, et al. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature. 1986;321:406–411. doi: 10.1038/321406a0. [DOI] [PubMed] [Google Scholar]
- Noakes PG, Bennett MR, Davey DF. Growth of segmental nerves to the developing rat diaphragm: Absence of pioneer axons. Journal of Comparative Neurology. 1983;218:365–377. doi: 10.1002/cne.902180402. [DOI] [PubMed] [Google Scholar]
- Ono F, Mandel G, Brehm P. Acetylcholine receptors direct rapsyn clusters to the neuromuscular synapse in zebrafish. Journal of Neuroscience. 2004;24:5475–5481. doi: 10.1523/JNEUROSCI.0851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono F, Shcherbatko A, Higashijima S, Mandel G, Brehm P. The Zebrafish motility mutant twitch once reveals new roles for rapsyn in synaptic function. Journal of Neuroscience. 2002;22:6491–6498. doi: 10.1523/JNEUROSCI.22-15-06491.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim RW, Caldero J, Cuitat D, Esquerda J, McArdle JJ, Olivera BM, et al. The rescue of developing avian motoneurons from programmed cell death by a selective inhibitor of the fetal muscle-specific nicotinic acetylcholine receptor. Development in Neurobiology. 2008;68:972–980. doi: 10.1002/dneu.20636. [DOI] [PubMed] [Google Scholar]
- Sakmann B, Brenner HR. Change in synaptic channel gating during neuromuscular development. Nature. 1978;276:401–402. doi: 10.1038/276401a0. [DOI] [PubMed] [Google Scholar]
- Schuetze SM, Role LW. Developmental regulation of nicotinic acetylcholine receptors. Annual Review of Neuroscience. 1987;10:403–457. doi: 10.1146/annurev.ne.10.030187.002155. [DOI] [PubMed] [Google Scholar]
- Sine SM, Claudio T. Gamma- and delta-subunits regulate the affinity and the cooperativity of ligand binding to the acetylcholine receptor. Journal of Biological Chemistry. 1991;266:19369–19377. [PubMed] [Google Scholar]
- Takahashi M, Kubo T, Mizoguchi A, Carlson CG, Endo K, Ohnishi K. Spontaneous muscle action potentials fail to develop without fetal-type acetylcholine receptors. EMBO Reports. 2002;3:674–681. doi: 10.1093/embo-reports/kvf128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroel A, Sakmann B. Calcium permeability increase of endplate channels in rat muscle during postnatal development. Journal of Physiology. 1996;496(Pt 2):331–338. doi: 10.1113/jphysiol.1996.sp021688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Poo M. Activity-induced potentiation of developing neuromuscular synapses. Science. 1999;285:1725–1728. doi: 10.1126/science.285.5434.1725. [DOI] [PubMed] [Google Scholar]
- Witzemann V. Development of the neuromuscular junction. Cell Tissue Research. 2006;326(2):263–271. doi: 10.1007/s00441-006-0237-x. [DOI] [PubMed] [Google Scholar]
- Yampolsky P, Gensler S, McArdle J, Witzemann V. AChR channel conversion and AChR-adjusted neuronal survival during embryonic development. Molecular and Cellular Neurosciences. 2008;37:634–645. doi: 10.1016/j.mcn.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Yang X, Arber S, William C, Li L, Tanabe Y, Jessell TM, et al. Patterning of muscle acetylcholine receptor gene expression in the absence of motor innervation. Neuron. 2001;30:399–410. doi: 10.1016/s0896-6273(01)00287-2. [DOI] [PubMed] [Google Scholar]
- Yang X, Li W, Prescott ED, Burden SJ, Wang JC. DNA topoisomerase IIbeta and neural development. Science. 2000;287:131–134. doi: 10.1126/science.287.5450.131. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Poo MM. Electrical activity and development of neural circuits. Nature Neuroscience. 2001;4 Suppl:1207–1214. doi: 10.1038/nn753. [DOI] [PubMed] [Google Scholar]