Abstract
Phosphatidylinositol 3-kinase lipid products and the Rho GTPases play a central role in transmitting information from chemotactic receptors to the effectors of cell polarity, and recent advances in the field have allowed us to understand these roles more clearly. Emergent properties of positive and negative regulation of these molecules may account for the establishment of cell polarity during chemotaxis for a wide range of cells from Dictyostelium to fibroblasts to neutrophils.
Introduction
Many eukaryotic cells have the capacity to polarize and migrate in response to external gradients of chemoattractant. This crucial ability allows single-celled organisms to hunt and mate, axonal projections to find their way in the developing nervous system, and cells in the innate immune system — such as neutrophils — to find and kill invading pathogens. In contrast to our significant knowledge of the temporal mechanism by which bacteria interpret and respond to chemotactic gradients, we are just beginning to understand the inner workings of the ‘eukaryotic compass’ (see below). Several questions need to be answered; for example, which signaling molecules carry information from the outside world to internal cellular responses? And how is this information processed to produce appropriately oriented cell polarity? In this review, I will summarize recent advances in eukaryotic chemotaxis, particularly the central roles of phosphatidylinositol 3-kinase (PI3K) lipid products and the Rho GTPases in the establishment of cell polarity and transmitting information from chemotactic receptors to the cytoskeleton.
When presented with a gradient of chemoattractant, neutrophils, neurons, budding yeast and Dictyostelium respond with highly oriented polarity and motility towards the source of chemoattractant (Figure 1). This behavior is exhibited for very subtle gradients of chemoattractant and for doses of ligand spanning several orders of magnitude. Several basic ingredients are required for highly oriented polarity (Figure 2). First, the cells need receptors to transmit a signal from the extracellular ligand to the cell interior. For neutrophils, budding yeast and Dictyostelium, these are generally G-protein-coupled receptors (GPCRs). Second, the cell needs to interpret the gradient. In other words, the cell must identify the portion of its surface that receives the greatest external signal. We can think of this interpretation as pointing the cell’s compass. This interpretation requires a mechanism of comparing signaling levels throughout the cell surface and restricting activity to the most highly stimulated region. Here, I discuss the points in the signal transduction cascade where this interpretation is likely to occur; I then discuss some important components of this process, including the role of negative feedback, adaptation and the possible role of positive feedback. The final component of chemotaxis is the stimulation of the effectors of actin polymerization and other cytoskeletal rearrangements — the readout of the compass. I will review data implicating the products of PI3K and the Rho GTPases in this role.
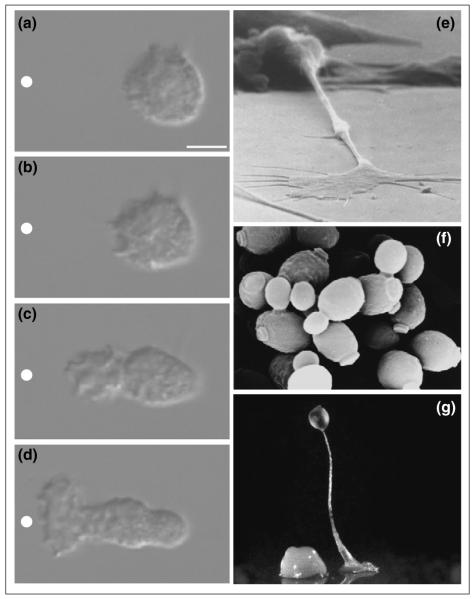
Figure 1.
Examples of directed cell polarity. (a–d) Polarization of neutrophil in response to gradient of chemoattractant. Nomarski images of unpolarized neutrophil responding to a micropipette containing the chemoattractant FMLP (white circle) at (a) 5 s, (b) 30 s, (c) 81 s and (d) 129 s. Bar = 5μm. Figure reprinted from [3], with permission. (e) Similar processes are required for axons to find their way in the developing nervous system (courtesy of Ken Balazovich and Kathryn Tosney), (f) for Saccharomyces cerevisiae to bud and mate (courtesy of Angela Dunn and Mick Tuite), and (g) for Dictyostelium to form multicellular aggregates (courtesy of Rob Kay).
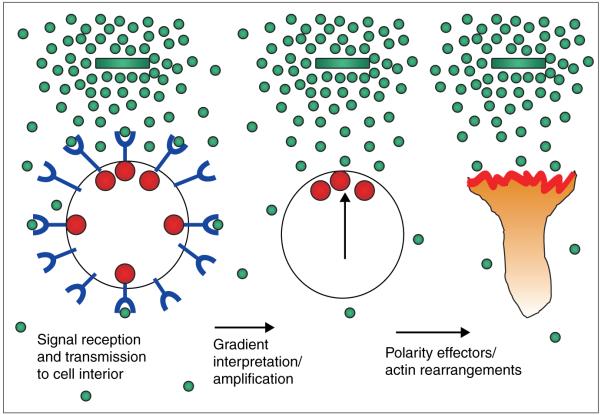
Figure 2.
Requirements for eukaryotic chemotaxis. In order to respond appropriately to chemotactic gradients, eukaryotic cells must contain receptors (blue) that transmit a signal to the cell interior (red spheres) upon binding chemoattractant (green spheres). Each cell must manipulate this information to determine which region of its surface is exposed to maximal chemoattractant (vertical arrow). Finally, the cell must transmit this information to the final effectors responsible for spatial regulation of actin rearrangements and cell motility. Adapted from [26] and reproduced with permission.
Where in the transduction cascade does gradient interpretation occur?
Where in the transduction cascade do cells convert relatively shallow gradients of chemoattractant to strongly polarized internal responses? The use of green fluorescent protein (GFP)-tagged signal transduction molecules has greatly increased our understanding of protein and lipid dynamics during chemotaxis in living cells. At the top of the signal transduction cascade, chemoattractant receptors are distributed uniformly, an important requirement for cells to interpret accurately changing external gradients [1,2]. At the bottom of the cascade, actin accumulation and effectors of actin polymerization are strongly polarized in response to the external gradient [3,4]. In the middle of the cascade, lipid products of PI3K exhibit strong asymmetries aligned with the chemotactic gradient. This asymmetric lipid distribution is observed during chemotaxis of neutrophils [5••], Dictyostelium [6••,7••], and fibroblasts [8••], suggesting significant conservation of the spatial dynamics of these lipids during chemotaxis (Figure 3).
Figure 3.
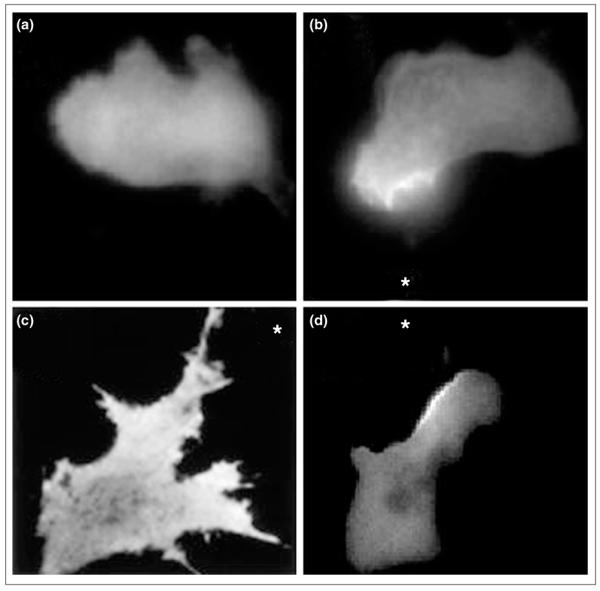
PI3K lipid products exhibit a polarized distribution during chemotaxis. The pleckstrin-homology domain of the protein kinase AKT/PKB fused to GFP was used as a probe for PI3K lipid products phosphatidylinositol 3,4-bisphosphate (PI[3,4]P2) and PIP3. (a) The probe is uniformly distributed throughout the cytosol of unstimulated neutrophil cells, but accumulates on the up-gradient face of cells exposed to chemoattractant. (b) Neutrophil-like HL60 cells exposed to a micropipette containing FMLP (asterisk). (c) 3T3 cell exposed to gradient of PDGF (asterisk). (d) Dictyostelium exposed to gradient of cAMP (asterisk). (a) and (b) are taken from [5••], (c) from [8••], and (d) was obtained courtesy of Satoru Funamoto and Rick Firtel.
Is this accumulation of PI3K lipid products important for chemotaxis? Several pieces of evidence suggest that it is. Inhibition of PI3K activation using specific toxins, dominant-negative mutations and genetic mutations interfere with chemotaxis and polarity in neutrophils, Dictyostelium and many other cell types [4,9, 10, 11•-13•]. Perhaps it is not surprising that these diverse systems use lipid metabolism as a regulatory mechanism for cell polarity. Large amounts of lipids can be generated and degraded rapidly, and large numbers of lipid effectors exist in cells, enabling lipid cues to direct a complex cellular programme [14].
The Rho GTPases Cdc42 and Rac are also necessary for chemotaxis in many systems. Dominant-negative mutants and genetic deletion of these proteins interfere with actin rearrangements and polarity in several organisms, from yeast [15] to macrophages [16].
Pointing the compass
Restricting activity using inhibitors
Negative regulation of phosphatidylinositol 3,4,5-trisphosphate accumulation
When presented with a gradient of chemoattractant, both PI3K lipid products (such as phosphatidylinositol 3,4,5-trisphosphate [PIP3]) and ruffling are restricted to the surface of the cell nearest the chemoattractant (Figure 3). In contrast, uniform stimulation elicits a short burst of PI3K activity, which then becomes undetectable in Dictyostelium [6••] or randomly polarized in neutrophils [5••]. Clearly, some mechanism of inhibition is necessary to restrict the spatial pattern of PI3K activity and ruffling during chemotaxis. What are good candidates for this inhibition? Because PI3K and Rho GTPases are strong candidates for the relevant products of receptor activation that carry spatial information to the cytoskeleton, negative regulators of PIP3 accumulation and negative regulators of Rho GTPase activation are potential targets of inhibition.
Negative regulators of PIP3 accumulation include the 3′ lipid phosphatase PTEN and the 5′ lipid phosphatase SHIP (Figure 4a). In mouse fibroblasts, a loss of PTEN activity results in spontaneous cell motility and activation of Cdc42 and Rac [17••], suggesting that PTEN is important in setting an appropriate level of PIP3 degradation to ensure that cells do not spontaneously activate in the absence of stimulation. However, levels of stimulated AKT activation (AKT is an effector of PIP3) are normal in PTEN-null cells, suggesting that stimulation of PTEN activity is not necessary for setting levels of PIP3 production following receptor activation [18]. SHIP or other 5′ phosphatases account for the bulk (>90%) of the phosphatase activity toward PIP3 in neutrophil lysates [19], suggesting that this may represent the major pathway for degrading PIP3 in neutrophils. SHIP-null cells exhibit a normal baseline-level PIP3 but potentiated PIP3 accumulation (both in extent and duration), suggesting that SHIP is an important stimulated degrader of PIP3 [20•]. However, SHIP-null cells exhibit no defect in chemotaxis [21], indicating either that there is redundancy in the system (there are multiple SHIP isoforms) or that more sensitive assays are necessary to uncover the chemotactic defect.
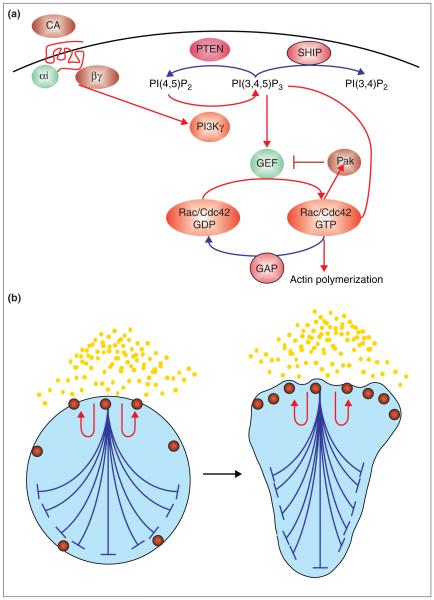
Figure 4.
Signal processing that may orchestrate cell polarity during chemotaxis. (a) Summary of signal transduction cascade during chemotaxis. Red arrows denote positive regulators of the signal to chemotactic effectors, whereas the blue arrow denotes negative regulators of the signal. Binding of chemoattractant (CA) to G-protein-coupled chemoattractant receptors (red) results in the dissociation of G protein heterotrimer Gαi and Gβγ. Dissociated G protein activates PI3K (for neutrophils, directly through activation of PI3Kγ). PI3Kγ phosphorylates phosphatidylinositol 4,5-bisphosphate [PI{4,5}P2] to generate PIP3), which activates GEFs for the Rho GTPases Rac and Cdc42. The latter two proteins induce localized actin polymerization through Arp2/3 complex activation and other mechanisms. Two negative regulators of PIP3 accumulation are the phosphatases PTEN and SHIP, which generate PI(4,5)P2 and PI(3,4)P2, respectively. A negative-feedback loop for Cdc42 activation (from S. cerevisiae) involves a Pak-like serine/threonine kinase, which phosphorylates and inhibits the Cdc42 GEF, thereby decreasing the amount of active GTP-bound Cdc42. Finally, GTPase-activating proteins (GAPs) catalyse the conversion of active (GTP-bound) Rac/Cdc42 to inactive (GDP-bound) Rac/Cdc42. (b) Model for cell polarity during chemotaxis. A product of receptor activation (red spheres) increases its abundance in a short-range positive-feedback manner (red arrows) and also acts in a long-range inhibitory fashion to inhibit activation elsewhere (blue). Note that for simplicity the positive- and negative-feedback effects are only shown for one of the sites of activity. In reality, this process is occurring at sites of activity throughout the cell. The net effect of this pattern-formation system is to develop an amplified internal gradient of activity at the surface of the cell nearest the chemoattractant, or, in extreme cases, in response to stochastic differences in a uniform chemoattractant. Good candidates for the short-range positive-feedback loop are PIP3 , Rac and Cdc42. Good candidates for the long-range negative-feedback loop are phosphatases for PIP3 and negative regulation of the GEFs for Rac and Cdc42 (see [a]). For several recent mathematical models for chemotaxis, see [42-44].
A recent genetic clue to a negative regulator of PIP3 accumulation is the Dictyostelium SHK1 mutant [22•]. In the absence of SHK1 activity, Dictyostelium exhibit greatly potentiated activation of AKT. Both the duration and the intensity of PIP3 accumulation appear to be affected in this mutant; but even more informative are the spatial dynamics of PIP3. When presented with a gradient of chemoattractant, wild-type Dictyostelium respond with a sharp internal gradient of PIP3. In contrast, SHK1 mutants exhibit uniform activation of PIP3. Consistent with PIP3 as an instructive signal to the cytoskeleton, SHK1 mutants also exhibit a defect in polarized ruffling and migration in response to chemoattractant. SHK1 is an important candidate as a negative regulator of PIP3 accumulation during chemotaxis. Because this protein is not itself a lipid phosphatase, it will be important to determine specifically how this protein negatively regulates PIP3 accumulation through regulation of PIP3 metabolism or effects on GPCR activity.
Negative regulation of Rho GTPase activation
For an elegant example of a negative-feedback loop involving the Rho GTPase Cdc42, we look to budding yeast. Saccharomyces cerevisiae exhibit polarized growth during mating and at certain stages of the cell cycle. Both the spatial and temporal dynamics of polarized growth are thought to be directed through recruitment of the guanine nucleotide exchange factor (GEF) Cdc24, which locally activates Cdc42. Activated Cdc42 activates the serine/threonine kinase Cla4, which phosphorylates Cdc24. Phosphorylated Cdc24 dissociates from its adaptor protein and the plasma membrane, ending Cdc42 activation and polarized growth. Interruption of this negative-feedback loop during bud emergence leads to increased duration of Cdc24 association with bud tips, and hyperpolarized bud growth. In contrast, for cells arrested in G1 interrupting this negative-feedback loop inhibits Cdc42-mediated cytoskeletal polarization [23••]. These data suggest that this negative-feedback loop is important in setting both the temporal and spatial dynamics of Cdc42 activation during polarized growth.
A similar negative-feedback loop has also been observed in mammalian cells. Activation of the small GTPase Ras is catalysed by the GEF son of sevenless (SOS), which is recruited to the plasma membrane via the adaptor protein Grb2. Ras activation leads to mitogen-activated protein kinase (MAPK)/MAPK kinase (MEK)-dependent phosphorylation of SOS and dissociation of the Grb2–SOS complex, interrupting the ability of SOS to activate Ras [24]. Disruption of this negative-feedback loop through inhibition of MEK prolongs Ras activation. Whether a similar negative feedback loop is used to regulate the Rho GTPases during chemotaxis of mammalian cells is an open question.
Adaptation
Neutrophils and Dictyostelium are sensitive to chemoattractant over several orders of magnitude of ligand concentration. Exposure to chemoattractant elicits a number of transient responses, including actin polymerization, PIP3 production, Rho GTPase and adenyl cyclase activation, and myosin phosphorylation [25,26]. The cells become refractory to stimulation with a given concentration of chemoattractant but respond to stimuli of a greater intensity. This process is known as adaptation. Recent work has provided a great deal of understanding of which mechanisms are not necessary for adaptation during chemotaxis. A common mechanism for adaptation of G-protein-mediated signals is phosphorylation of the GPCR, leading to receptor internalization and/or inhibition of its coupling to G protein. Although phosphorylation of GPCRs is observed during chemotaxis, GPCR phosphorylation does not appear to be necessary for adaptation or chemotaxis. Non-phosphorylatable receptors in B cells [27] and Dictyostelium [28] fail to internalize, yet chemotaxis and adaptation of downstream pathways remain unaltered, suggesting that receptor phosphorylation is dispensable for these processes. More recently, an in vivo assay of G protein activation in Dictyostelium [29••] revealed that G proteins remain dissociated during continuous stimulation, suggesting that adaptation does not involve turn-off of the G protein, although the spatial dynamics of activation may be regulated under such conditions [30••]. The mechanism of adaptation during chemotaxis also remains very much an open question.
Positive feedback
Most of the analyses of protein and lipid dynamics I have outlined thus far depended on analysis of cell signaling in response to stimulation of the cells at the level of the GPCR. More insight into information processing during chemotaxis can be obtained by analysing the effects of direct delivery of second messengers to cells. Using this approach, recent experiments implicate PIP3 metabolism in the patterning system for cell polarity. Direct delivery of membrane-permeable PIP3 to neutrophils ([31••]; O Weiner, unpublished data) or fibroblasts [32] induces cell polarity and motility. This powerful approach of directly introducing a signal transduction intermediate into living cells indicates that cell polarity can be established at the level of PIP3, or downstream. Therefore, PIP3 occupies the privileged position of being both the most upstream molecule known to exhibit asymmetry during chemotaxis and one of the most downstream molecules sufficient to induce cell polarity and motility. Surprisingly, PIP3-induced cell motility and MEK activation requires endogenous PI3K activity, suggesting that PIP3 may act in a positive-feedback loop [31••]. Paradoxical epistasis experiments with PI3K and Rho GTPases also suggest that these components may act in a positive-feedback loop. Inhibition of PI3K activity inhibits Rac and Cdc42 activation in neutrophils [33] and fibroblasts [9], suggesting that PI3K lies upstream of these proteins. However, inhibition of Rho GTPases blocks PIP3 accumulation in neutrophils [5••], inhibition of Rac blocks activation of AKT in T cells [34•] and mouse mast cells [35], and active Rac and Cdc42 can bind to — and in some cases potentiate — PI3K activity, suggesting that Rac and Cdc42 also lie upstream of PI3K activation [36-38]. A simple explanation for these conflicting results is that PI3K and Rho GTPases are both upstream and downstream of one another and act in a positive-feedback loop that regulates cell polarity (Figure 4).
Receptor transactivation and the role of platelet-derived growth factor and sphingosine phosphate in chemotaxis
Although most chemoattractants for neutrophils, Dictyostelium and S. cerevisiae use GPCRs for signal relay, several non-GPCR receptors are involved in chemotaxis of neutrophils, fibroblasts and neurons. Are similar mechanisms of gradient interpretation and cell polarization used for these distinct receptor inputs? Recent data regarding chemotaxis in response to platelet-derived growth factor (PDGF) suggest a central role for GPCRs during chemotaxis. PDGF stimulates signaling pathways through the PDGF receptor, a tyrosine kinase receptor. EDG-1 is a GPCR for sphingosine phosphate, a lipid produced intracellularly that is also capable of extracellular signaling. On the basis of the similarities between the phenotypes of mice disrupted for PDGF and for EDG-1, Hobson et al. [39••] examined the role of EDG-1 in PDGF signaling.
Amazingly, PDGF-induced chemotaxis is dependent on EDG-1 expression. Stimulation with PDGF results in activation of sphingosine kinase, production of sphingosine phosphate, and activation of the EDG-1 receptor. Interference with this receptor crosstalk, through inhibition of sphingosine kinase, inhibition of G-protein signaling or absence of EDG-1, inhibit motility and Rac activation in response to PDGF. These data suggest that chemotaxis in response to tyrosine kinase agonists also requires GPCR activity. Thus, dissection of GPCR-induced cell polarity may be important for understanding cell polarity in response to a wide variety of external signals beyond direct GPCR agonists.
Conclusions
How do the negative- and positive-feedback loops discussed in this review relate to the establishment of cell polarity during chemotaxis? One clue is a self-organizing pattern-formation system thought to be used in many developmental systems to amplify small gradients or even random differences in uniform signals into organizers [40]. The first ingredient for this system of pattern formation is a signal that amplifies itself in a short-range positive-feedback fashion. The second ingredient is that this signal also generates a more long-range inhibitor of signaling. Pattern-formation systems consistent with this model include retinotectal mapping, hydra regeneration, Dictyostelium morphogenesis and, more recently, eukaryotic chemotaxis [41]. (See Figure 4b for how this patterning system may relate to eukaryotic chemotaxis.)
In summary, PI3K lipid products and Rho GTPases play central roles in the control of eukaryotic chemotaxis. Emergent properties of positive and negative regulation of these molecules may account for the establishment of cell polarity during chemotaxis. On the basis of PDGF receptor crosstalk and possibly other inputs to GPCRs, this polarization programme may extend beyond direct GPCR agonists. Increased development of technologies for spatially and temporally assaying and interfering with signaling during chemotaxis will be essential to discern how these molecules interact to process spatial signals, and coordinate the many activities involved in cell polarity. This increased understanding is likely to yield valuable information for inflammation, cancer metastases, re-growth of axons in the central nervous system, and many other processes lying at the interface between cell polarity and the environment.
Acknowledgements
I thank the Dunn, Firtel, Kay, Meyer, Tosney, and Tuite labs for figure contributions, Henry Bourne, Lew Cantley, Marc Kirschner and John Sedat for support and guidance, and the Damon Runyon Cancer Research Fund for support.
Abbreviations
- GEF
guanine nucleotide exchange factor
- GPCR
G-protein-coupled receptor
- PDGF
platelet-derived growth factor
- PI3K
phosphatidylinositol 3-kinase
- PIP3
phosphatidylinositol 3,4,5-trisphosphate
- SOS
son of sevenless
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
••of outstanding interest
- 1.Xiao Z, Zhang N, Murphy DB, Devreotes PN. Dynamic distribution of chemoattractant receptors in living cells during chemotaxis and persistent stimulation. J Cell Biol. 1997;139:365–374. doi: 10.1083/jcb.139.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Servant G, Weiner OD, Neptune ER, Sedat JW, Bourne HR. Dynamics of a chemoattractant receptor in living neutrophils during chemotaxis. Mol Biol Cell. 1999;10:1163–1178. doi: 10.1091/mbc.10.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiner OD, Servant G, Welch MD, Mitchison TJ, Sedat JW, Bourne HR. Spatial control of actin polymerization during neutrophil chemotaxis. Nat Cell Biol. 1999;1:75–81. doi: 10.1038/10042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Funamoto S, Milan K, Meili R, Firtel RA. Role of phosphatidylinositol 3′ kinase and a downstream pleckstrin homology domain-containing protein in controlling chemotaxis in Dictyostelium. J Cell Biol. 2001;153:795–810. doi: 10.1083/jcb.153.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5••.Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, Bourne HR. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science. 2000;287:1037–1040. doi: 10.1126/science.287.5455.1037.See annotation Haugh et al. (2000) [8••].
- 6••.Meili R, Ellsworth C, Lee S, Reddy TB, Ma H, Firtel RA. Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J. 1999;18:2092–2105. doi: 10.1093/emboj/18.8.2092.See annotation Haugh et al. (2000) [8••].
- 7••.Jin T, Zhang N, Long Y, Parent CA, Devreotes PN. Localization of the G protein betagamma complex in living cells during chemotaxis. Science. 2000;287:1034–1036. doi: 10.1126/science.287.5455.1034.See annotation Haugh et al. (2000) [8••].
- 8••.Haugh JM, Codazzi F, Teruel M, Meyer T. Spatial sensing in fibroblasts mediated by 3′ phosphoinositides. J Cell Biol. 2000;151:1269–1280. doi: 10.1083/jcb.151.6.1269.These four papers [5••-8••] analyse the spatial dynamics of phosphatidylinositol 3,4,5-trisphosphate (PIP3) during chemotaxis. Remarkable conservation of PIP3 asymmetry is observed in these migrating cells.
- 9.Hawkins PT, Eguinoa A, Qiu RG, Stokoe D, Cooke FT, Walters R, Wennstrom S, Claesson-Welsh L, Evans T, Symons M, et al. PDGF stimulates an increase in GTP-Rac via activation of phosphoinositide 3-kinase. Curr Biol. 1995;5:393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]
- 10.Niggli V, Keller H. The phosphatidylinositol 3-kinase inhibitor wortmannin markedly reduces chemotactic peptide-induced locomotion and increases in cytoskeletal actin in human neutrophils. Eur J Pharmacol. 1997;335:43–52. doi: 10.1016/s0014-2999(97)01169-2. [DOI] [PubMed] [Google Scholar]
- 11•.Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049.See annotation Sasaki et al. (2000) [13•].
- 12•.Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D. Roles of PLC-beta2 and -beta3 and PI3Kgamma in chemoattractant-mediated signal transduction. Science. 2000;287:1046–1049. doi: 10.1126/science.287.5455.1046.See annotation Sasaki et al. (2000) [13•].
- 13•.Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040.These three papers [11•-13•] establish the crucial role for the Gβγ-regulated phosphatidylinositol 3-kinase (PI3K) isoform PI3K-gamma for phosphatidylinositol 3,4,5-trisphosphate generation and cell migration during neutrophil chemotaxis. The specific nature of the migratory defect, as well as the possible contribution of other PI3K isoforms, are important areas for future research.
- 14.Hurley JH, Meyer T. Subcellular targeting by membrane lipids. Curr Opin Cell Biol. 2001;13:146–152. doi: 10.1016/s0955-0674(00)00191-5. [DOI] [PubMed] [Google Scholar]
- 15.Gulli MP, Peter M. Temporal and spatial regulation of Rho-type guanine-nucleotide exchange factors: the yeast perspective. Genes Dev. 2001;15:365–379. doi: 10.1101/gad.876901. [DOI] [PubMed] [Google Scholar]
- 16.Allen WE, Zicha D, Ridley AJ, Jones GE. A role for Cdc42 in macrophage chemotaxis. J Cell Biol. 1998;141:1147–1157. doi: 10.1083/jcb.141.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17••.Liliental J, Moon SY, Lesche R, Mamillapalli R, Li D, Zheng Y, Sun H, Wu H. Genetic deletion of the PTEN tumor suppressor gene promotes cell motility by activation of rac1 and cdc42 GTPases. Curr Biol. 2000;10:401–404. doi: 10.1016/s0960-9822(00)00417-6.Loss of PTEN results in constitutive motility and activation of Rac and Cdc42. This suggests that PTEN is important in setting the basal levels of phosphatidylinositol 3,4,5-trisphosphate.
- 18.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 19.Stephens LR, Hughes KT, Irvine RF. Pathway of phosphatidylinositol(3,4,5)-trisphosphate synthesis in activated neutrophils. Nature. 1991;351:33–39. doi: 10.1038/351033a0. [DOI] [PubMed] [Google Scholar]
- 20•.Brauweiler A, Tamir I, Dal Porto J, Benschop RJ, Helgason CD, Humphries RK, Freed JH, Cambier JC. Differential regulation of B cell development, activation, and death by the Src homology 2 domain-containing 5′ inositol phosphatase (SHIP) J Exp Med. 2000;191:1545–1554. doi: 10.1084/jem.191.9.1545.Stimulated but not basal accumulation of phosphatidylinositol 3,4,5-trisphosphate (PIP3) is potentiated in B cells lacking the 5′-phosphatase SHIP, suggesting that SHIP is necessary for setting the stimulated level of PIP3 production.
- 21.Kim CH, Hangoc G, Cooper S, Helgason CD, Yew S, Humphries RK, Krystal G, Broxmeyer HE. Altered responsiveness to chemokines due to targeted disruption of SHIP. J Clin Invest. 1999;104:1751–1759. doi: 10.1172/JCI7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Moniakis J, Funamoto S, Fukuzawa M, Meisenhelder J, Araki T, Abe T, Meili R, Hunter T, Williams J, Firtel RA. An SH2-domain-containing kinase negatively regulates the phosphatidylinositol-3 kinase pathway. Genes Dev. 2001;15:687–698. doi: 10.1101/gad.871001.A negative regulator of phosphatidylinositol 3,4,5-trisphosphate (PIP3) accumulation is important for chemotaxis as well as for the spatial and temporal dynamics of PIP3.
- 23••.Gulli M, Jaquenoud M, Shimada Y, Niederhauser G, Wiget P, Peter M. Phosphorylation of the cdc42 exchange factor cdc24 by the PAK-like kinase Cla4 may regulate polarized growth in yeast. Mol Cell. 2000;6:1155–1167. doi: 10.1016/s1097-2765(00)00113-1.Activated Cdc42 acts through a Pak-like kinase to phosphorylate and inactivate the Cdc42 guanine nucleotide exchange factor. This negative-feedback loop is important in setting the spatial and temporal dynamics of Cdc42 activation during polarized growth in budding yeast.
- 24.Waters SB, Holt KH, Ross SE, Syu LJ, Guan KL, Saltiel AR, Koretzky GA, Pessin JE. Desensitization of Ras activation by a feedback disassociation of the SOS–Grb2 complex. J Biol Chem. 1995;270:20883–20886. doi: 10.1074/jbc.270.36.20883. [DOI] [PubMed] [Google Scholar]
- 25.Devreotes PN, Zigmond SH. Chemotaxis in eukaryotic cells: a focus on leukocytes and Dictyostelium. Annu Rev Cell Biol. 1988;4:649–686. doi: 10.1146/annurev.cb.04.110188.003245. [DOI] [PubMed] [Google Scholar]
- 26.Weiner OD, Servant G, Parent CA, Devreotes PN, Bourne HR. Cell polarity in response to chemoattractants. In: Drubin DG, editor. Cell Polarity: Frontiers in Molecular Biology. Oxford University Press; Oxford: 2000. [Google Scholar]
- 27.Arai H, Monteclaro FS, Tsou CL, Franci C, Charo IF. Dissociation of chemotaxis from agonist-induced receptor internalization in a lymphocyte cell line transfected with CCR2B. Evidence that directed migration does not require rapid modulation of signaling at the receptor level. J Biol Chem. 1997;272:25037–25042. doi: 10.1074/jbc.272.40.25037. [DOI] [PubMed] [Google Scholar]
- 28.Kim JY, Soede RDM, Schaap P, Valkema R, Borleis JA, Van Haastert PJM, Devreotes PN, Hereld D. Phosphorylation of chemoattractant receptors is not essential for chemotaxis or termination of G-protein-mediated responses. J Biol Chem. 1997;272:27313–27318. doi: 10.1074/jbc.272.43.27313. [DOI] [PubMed] [Google Scholar]
- 29••.Janetopoulos C, Jin T, Devreotes P. Receptor-mediated activation of heterotrimeric G proteins in living cells. Science. 2001;291:2408–2411. doi: 10.1126/science.1055835.First analysis of G-protein activation in living cells demonstrates that adaptation does not occur at the level of G-protein inactivation.
- 30••.Ueda M, Sako Y, Tanaka T, Devreotes P, Yanagida T. Single-molecule analysis of chemotactic signaling in Dictyostelium cells. Science. 2001;294:864–867. doi: 10.1126/science.1063951.Single-molecule imaging of cAMP and its receptor demonstrates an asymmetry in the rate of ligand association and dissociation in the front and back of polarized cells.
- 31••.Niggli V. A membrane-permeant ester of phosphatidylinositol 3,4,5-trisphosphate (PIP3) is an activator of human neutrophil migration. FEBS Lett. 2000;473:217–221. doi: 10.1016/s0014-5793(00)01534-9.A membrane-permeable PIP3 induces cell migration in an endogenous phosphatidylinositol 3-kinase-dependent fashion, suggesting a PIP3 positive-feedback loop during neutrophil polarization.
- 32.Derman MP, Toker A, Hartwig JH, Spokes K, Falck JR, Chen CS, Cantley LC, Cantley LG. The lipid products of phosphoinositide 3-kinase increase cell motility through protein kinase C. J Biol Chem. 1997;272:6465–6470. doi: 10.1074/jbc.272.10.6465. [DOI] [PubMed] [Google Scholar]
- 33.Benard V, Bohl BP, Bokoch GM. Characterization of Rac and Cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- 34•.Genot EM, Arrieumerlou C, Ku G, Burgering BM, Weiss A, Kramer IM. The T-cell receptor regulates Akt (protein kinase B) via a pathway involving Rac1 and phosphatidylinositide 3-kinase. Mol Cell Biol. 2000;20:5469–5478. doi: 10.1128/mcb.20.15.5469-5478.2000.Good evidence that Rac acts upstream and downstream of phosphatidylinositide 3-kinase (PI3K) in T cells, consistent with a positive-feedback loop involving Rac and PI3K.
- 35.Yang FC, Kapur R, King AJ, Tao W, Kim C, Borneo JB, Breese R, Marshall M, Dinauer MC, Williams DA. Rac2 stimulates Akt activation affecting BAD/Bcl-XL expression while mediating survival and actin function in primary mast cells. Immunity. 2000;12:557–568. doi: 10.1016/s1074-7613(00)80207-1. [DOI] [PubMed] [Google Scholar]
- 36.Zheng Y, Bagrodia S, Cerione RA. Activation of phosphoinositide 3-kinase activity by Cdc42Hs binding to p85. J Biol Chem. 1994;269:18727–18730. [PubMed] [Google Scholar]
- 37.Tolias KF, Cantley LC, Carpenter CL. Rho family GTPases bind to phosphoinositide kinases. J Biol Chem. 1995;270:17656–17659. doi: 10.1074/jbc.270.30.17656. [DOI] [PubMed] [Google Scholar]
- 38.Bokoch GM, Vlahos CJ, Wang Y, Knaus UG, Traynor-Kaplan AE. Rac GTPase interacts specifically with phosphatidylinositol 3-kinase. Biochem J. 1996;315:775–779. doi: 10.1042/bj3150775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39••.Hobson JP, Rosenfeldt HM, Barak LS, Olivera A, Poulton S, Caron MG, Milstien S, Spiegel S. Role of the sphingosine-1-phosphate receptor EDG-1 in PDGF-induced cell motility. Science. 2001;291:1800–1803. doi: 10.1126/science.1057559.Platelet-derived growth factor (PDGF) requires the EDG-1 sphingosine phosphate receptor for cell motility and Rac activation, strongly suggesting crosstalk between PDGF tyrosine kinase receptors and G-protein-coupled receptors during chemotaxis.
- 40.Meinhardt H, Gierer A. Applications of a theory of biological pattern formation based on lateral inhibition. J Cell Sci. 1974;15:321–346. doi: 10.1242/jcs.15.2.321. [DOI] [PubMed] [Google Scholar]
- 41.Meinhardt H, Gierer A. Pattern formation by local self-activation and lateral inhibition. BioEssays. 2000;22:753–760. doi: 10.1002/1521-1878(200008)22:8<753::AID-BIES9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 42.Meinhardt H. Orientation of chemotactic cells and growth cones: models and mechanisms. J Cell Sci. 1999;112:2867–2874. doi: 10.1242/jcs.112.17.2867. [DOI] [PubMed] [Google Scholar]
- 43.Narang A, Subramanian KK, Lauffenburger DA. A mathematical model for chemoattractant gradient sensing based on receptor-regulated membrane phospholipid signaling dynamics. Ann Biomed Eng. 2001;29:677–691. doi: 10.1114/1.1385805. [DOI] [PubMed] [Google Scholar]
- 44.Postma M, Van Haastert PJ. A diffusion-translocation model for gradient sensing by chemotactic cells. Biophys J. 2001;81:1314–1323. doi: 10.1016/S0006-3495(01)75788-8. [DOI] [PMC free article] [PubMed] [Google Scholar]