Abstract
Objective
There is continuing controversy regarding the effect of glucocorticoids on a systemic inflammatory process. Based on a model of glucocorticoid action that includes both pro- and anti-inflammatory effects, we used the human experimental endotoxemia model to test the hypothesis that a transient elevation of plasma cortisol to stress-associated levels would enhance a subsequent (delayed) systemic inflammatory response to bacterial endotoxin.
Design
Prospective, randomized, double-blind, placebo-controlled clinical investigation.
Setting
Academic medical center.
Subjects
Thirty-six healthy human volunteers.
Interventions
Participants were randomized to receive a 6-hr intravenous infusion of saline (control), an intermediate dose of cortisol (Cort80; 6.3 mg/hr/70 kg), or a high dose of cortisol (Cort160; 12.6 mg/hr/70 kg) on day 1. On day 2, participants received an intravenous injection of 2 ng/kg Escherichia coli endotoxin followed by serial measurements of plasma cytokine concentrations.
Measurements and Main Results
Baseline participant characteristics and cortisol and cytokine concentrations were similar in all three groups. The plasma cortisol response to endotoxemia on day 2 was similar in all three groups. The interleukin-6 response to endotoxemia was significantly increased in the Cort80 Group compared with the control Group (p = .004), whereas the interleukin- 10 response was significantly suppressed (p = .034). Corresponding results for the Cort160 Group were not significantly different from control Group values.
Conclusions
In this study, transient elevation of in vivo cortisol concentrations to levels that are observed during major systemic stress enhanced a subsequent, delayed in vivo inflammatory response to endotoxin. This appeared to be a dose-dependent effect that was more prominent at intermediate concentrations of cortisol than at higher concentrations of cortisol.
Keywords: cytokine, glucocorticoid, experimental endotoxemia, interleukin-6, sepsis, human
Glucocorticoids (GCs) have been widely used to suppress inflammation since 1949 when Hench et al first described the anti-inflammatory effect of GCs in humans (1). Over the ensuing 50 years, most GC research focused on the anti-inflammatory and immune-suppressive properties of GCs, whereas comparatively little research examined the stimulatory properties of GCs that were, in fact, widely acknowledged before 1949 (2– 4). More recently, investigators have again examined the stimulatory effects of GCs on inflammatory processes (5). Recent work has shown that GCs induce increased expression of receptors for inflammatory cytokines, including interleukin (IL)-1 (6), IL-2 (7), IL-4 (8), IL-6 (9), IL-7 (10) and interferon-γ (11) as well as granulocyte– macrophage colony-stimulating factor (12). GCs also stimulate effector cell functions, including phagocytosis by monocytes (13) and neutrophils (14), proliferative responses of T-cells (7) and macrophages (15), and tissue inflammatory responses to injury (16).
To account for both suppressive and stimulatory effects of GCs on inflammatory processes, a model of GC action has been proposed that describes a concentration- and time-dependent biphasic (i.e., both stimulatory and suppressive) effect of GCs on integrated in vivo defense mechanisms (6, 17–20). According to this model, normal diurnal GC concentrations support the activity of defense mechanisms in a permissive and preparative (time-delayed) manner, whereas higher stress-induced concentrations act acutely to suppress inflammation and prevent tissue injury from excess inflammation. The hypothesized peak of cortisol stimulatory actions, based on the binding constant of cortisol to the glucocorticoid receptor (18), is approximately 80 nM free cortisol. Recent studies have, in fact, demonstrated biphasic effects of GCs on effector cell inflammatory events, including inflammatory cytokine mRNA concentrations and protein release from monocytes (21–23), Fc-mediated phagocytosis of macrophages (24), acute-phase protein gene expression in hepatocytes (25), delayed-type hypersensitivity reactions (26), and wound healing (27). In these studies, stimulatory GC effects were observed at lower concentrations, whereas higher GC concentrations produced no effect or suppressive effects.
To date, very little published data support either stimulatory or biphasic effects of GCs on in vivo human inflammatory processes. Barber et al reported that an experimental increase in plasma cortisol to concentrations of 75 to 85 µg/dL would, as expected, suppress the systemic inflammatory response (tumor necrosis factor-α [TNF-α] and IL-6) to experimental endotoxemia (EE) if the cortisol concentration was increased immediately before and during the endotoxin response. However, if participants in this study were exposed to the same plasma cortisol concentration for a 6-hr period ending 12 or 144 hrs before injection of endotoxin, they manifested an increased inflammatory response to EE (28). Therefore, time may be as important as concentration in determining GC effects on in vivo inflammation. Based on their report and on animal studies showing delayed inflammatory effects of GCs (29 –31), we hypothesized that in vivo exposure of humans to lower cortisol concentrations of approximately 35 to 45 µg/dL total plasma cortisol (approximately 80 nM free cortisol) would also enhance inflammatory responses to a subsequent, delayed inflammatory stimulus (EE). The results reported here suggest that a “preparative” or priming effect of GCs on the human innate immune inflammatory response can be observed after transient in vivo exposure to cortisol concentrations that are close to concentrations that are commonly observed during human systemic stress.
MATERIALS AND METHODS
This study was approved by the Dartmouth College Committee for the Protection of Human Subjects (Institutional Review Board) and written informed consent was obtained from all participants.
Participants
Participants (n = 36) were healthy male and female volunteers between the ages of 18 and 55 years.
Interventions
On day 1 of the study, participants were randomized in a double-blind manner to receive a 6-hr intravenous infusion from 9:00 am to 3:00 pm of normal saline at 10 mL/hr (control), an intravenous infusion of an intermediate dose of hydrocortisone (Solu-Cortef; Pfizer, New York, NY) in normal saline at 1.5 µg/kg/min (6.3 mg/hr/70 kg) designed to produce the hypothesized peak cortisol stimulatory concentration of approximately 80 nM free salivary cortisol (Cort80), or a high dose of hydrocortisone at 3.0 µg/kg/min (12.6 mg/hr/70 kg; the same dose used by Barber et al [28]) designed to achieve twice the free cortisol concentration or approximately 160 nM free salivary cortisol (Cort160). The next day (day 2), at 7:00 am, participants were admitted to a hospital acute care facility where an intravenous catheter was inserted in a proximal arm vein. Continuous electrocardiographic and pulse oximetry measurements were initiated and blood pressure and core temperature were measured noninvasively every 15 mins for the next 5 hrs and every 30 mins for the subsequent 4 hrs. Participants received 10 mL/kg lactated Ringer’s solution intravenously over the first 2 hrs followed by 1.5 mL/kg/hr. At 8:00 am, participants were injected with 2 ng/kg Escherichia coli endotoxin (Clinical Center Reference Endotoxin; Lot 67801; Pharmacy Development Section, National Institutes of Health, Bethesda, MD) intravenously over 2 mins. Participants remained in the acute care facility for the remainder of the day with continuous nursing care and under the direct supervision of a physician. Heparinized peripheral blood samples were obtained from the intravenous catheter or by venipuncture at the times noted. Saliva samples were obtained for determination of salivary-free cortisol using a commercial collection device (Salivette; Sarstedt, Newton, NC).
Measurements—Cortisol
Total plasma and salivary-free cortisol measurements were made on day 1, whereas plasma cortisol only was measured on day 2. Saliva samples were stored at −80°C until analysis by Salimetrics Expanded Range High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit (State College, PA; analytical sensitivity = 3 ng/dL). Plasma was separated from heparinized whole blood and stored at −80°C until analysis for total plasma cortisol on an Immulite 1000 analyzer using a Siemens Cortisol kit (Los Angeles, CA; analytical sensitivity = 0.2 µg/dL; intra-assay variability = 4.7%; interassay variability = 6.3%).
Measurements—Adrenocorticotropic Hormone
Adrenocorticotropic hormone determinations of frozen plasma samples were performed on an Immulite 1000 analyzer using a Siemens adrenocorticotropic hormone kit (Los Angeles, CA; analytical sensitivity = 9 pg/mL; intra-assay variability = 5.6%; interassay variability = 7.8%).
Measurements—C-reactive Protein
C-reactive protein (CRP) measurements were performed immediately on unfrozen blood samples using a Roche P Module and Roche High Sensitivity CRP kit (Indianapolis, IN; analytical sensitivity = 0.1 mg/L; intra-assay variability = 1.3%; interassay variability = 3.9%).
Measurements—Complete Blood Count
Complete blood counts of EDTA-anticoagulated blood were performed immediately on an Advia 120 Hematology Analyzer using Siemens reagents (Ramsey, MN).
Measurements—Cytokine Analysis
Plasma samples were collected and immediately frozen at −80°C for batched measurement of TNF-α, IL-6, and IL-10 concentrations. TNF-α was measured using a TNF-α sandwich enzyme-linked immunosorbent assay (paired antibodies; BD Biosciences Pharmingen, San Diego, CA); IL-6 levels were determined using an IL-6 enzyme-linked immunosorbent assay kit (Peprotech, Rocky Hill, NJ); IL-10 was measured using an IL-10 enzyme-linked immunosorbent assay kit (Biosource, Camarillo, CA). Interassay variabilities for individual cytokines were in the range of 1.0% to 9.8% and intra-assay variabilities were in the range of 3.6% to 12.6%.
Analysis
The primary end point of this study was the plasma cytokine response to endotoxin assessed by enzyme-linked immunosorbent assay determination of three different cytokines: TNF-α, which is transiently released from 1 to 2 hrs after endotoxin administration; IL-6, which persists in plasma for several hours after endotoxin; and IL-10, which has a peak concentration at 3 hrs after endotoxin administration (28, 32). We hypothesized that pretreatment of participants with a 6-hr intravenous infusion of cortisol would induce an increased inflammatory cytokine (IL-6) response during EE. Power analysis was calculated using differences in plasma IL-6 concentrations between participants in the control Group and participants in the Stress Group at 2 hrs after endotoxin injection based on previously published data (28). Using these data, we calculated a type I error of 0.025 to account for two comparisons and power of 0.90 with 12 subjects per group. Nonparametric rank tests and Student’s t tests, with appropriate transformation to normality, were used to compare control and treatment groups at each time period. To account for the correlated nature of the data that resulted from multiple measures on each subject over time, we used generalized estimating equations as the primary analytic tool for intergroup comparisons that considers all time points and to characterize changes over time (33).
RESULTS
Clinical
All study participants completed the protocol as planned. Participants were evenly matched among the three groups with regard to sex, age, and weight (Table 1). Four females were taking birth control medication, one each in the control and Cort80 Groups and two in the Cort160 Group. Endotoxin injection induced the expected clinical response, which included a maximum increase in heart rate of approximately 30 beats/minute and increased body temperature to approximately 38.0°C to 38.5°C (Table 2). Fever was preceded in all cases by headache, myalgia, and chills. Several subjects reported transient nausea. Hypotension (systolic arterial blood pressure <90 mm Hg) was not observed in any study participant. There were no significant differences between groups in any of the clinical measurements.
Table 1.
Participants were randomized in a double-blind manner into one of three groups: control, Cort80, or Cort160a
| Control | Cort80 | Cort160 | |
|---|---|---|---|
| Sex, male/female | 5/6 | 5/7 | 6/7 |
| Age, years | 35.5 (4.3) | 33.0 (2.5) | 36.3 (3.5) |
| Weight, kg | 75.8 (3.6) | 78.7 (4.1) | 77.5 (3.7) |
| Plasma cortisol pre, µg/dL | 10.5 (3.3) | 10.3 (1.3) | 13.1 (1.5) |
| Plasma cortisol post, µg/dL | 8.1 (1.0) | 54.1 (6.2)b | 101 (19.6)b,c |
| Salivary cortisol pre, nM | 12.4 (3.2) | 7.2 (1.0) | 13.8 (3.7) |
| Salivary cortisol post, nM | 6.1 (1.7) | 77.8 (9.8)b | 169 (17.4)b,d |
Data presented as mean (SE).
Control, intravenous saline for 6 hours on day 1; Cort80, intravenous hydrocortisone at 1.5 g/kg/min for 6 hours on day 1; Cort160, intravenous hydrocortisone at 3 µg/kg/min for 6 hours on day 1; Pre, immediately before hydrocortisone infusion on day 1; Post, immediately after hydrocortisone infusion on day 1;
p < .001 vs. control;
p < .05 vs. Cort80;
p < .001 vs. Cort80.
Table 2.
Comparison of clinical and laboratory measurements before and after injection of Escherichia coli endotoxin in healthy human subjectsa
| Control | Cort80 | Cort160 | |
|---|---|---|---|
| Base heart rate, beats/min | 72 (1.8) | 73 (2.4) | 70 (3) |
| Peak heart rate, beats/min | 101 (2.9) | 111 (4.4) | 106 (4) |
| Base temperature, °C | 36.5 (0.17) | 36.6 (0.12) | 36.5 (0.1) |
| Peak temperature, °C | 38.4 (0.11) | 38.4 (0.14) | 38.1 (0.11) |
| Base white blood cell count ×103/mL |
6.3 (0.4) | 6.8 (0.5) | 7.0 (0.3) |
| 4-hour white blood cell count ×103/mL |
10.3 (0.9) | 9.1 (0.6) | 9.1 (1.0) |
| Base PMN per mL | 3770 (301) | 4312 (449) | 4842 (385) |
| 4-hour PMN per mL | 9705 (894) | 8353 (575) | 8390 (980) |
| Base Mono per mL | 300 (23) | 297 (39) | 264 (33) |
| 4-hour Mono per mL | 158 (29) | 111 (29)b | 85 (16)b |
| Base C-reactive protein, mg/L |
1.9 (0.7) | 2.6 (0.9) | 1.8 (1.1) |
| 24-hour C-reactive protein, mg/L |
40.4 (4.7) | 51.0 (4.6) | 44.0 (3.4) |
Data presented as mean (SE).
Base, immediately before injection of endotoxin; 4 hours, 4 hours after injection of endotoxin; PMN, peripheral blood polymorphonuclear cell count; Mono, peripheral blood monocyte count;
p < .05 vs. control.
Cortisol and Adrenocorticotropic Hormone
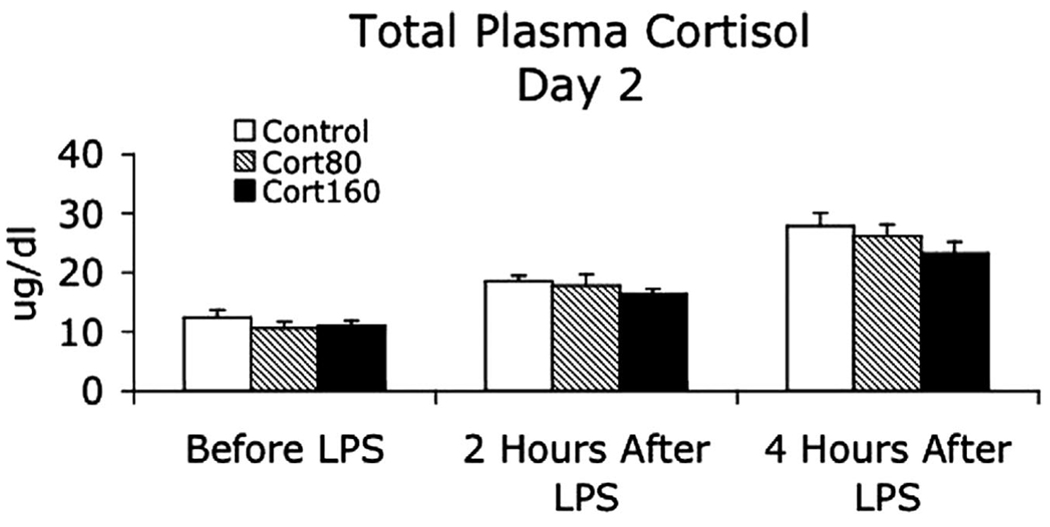
Total plasma and salivary-free cortisol concentrations on day 1 are shown in Table 1. Both measurements were significantly higher in both experimental groups at the end of the infusion compared with the control Group and in the Cort160 Group compared with the Cort80 Group. Plasma cortisol concentrations on the morning of day 2 were similar in all three groups and responded similarly during EE with the expected peak concentration occurring 4 hrs after endotoxin injection (34) (Fig. 1). Plasma adrenocorticotropic hormone concentrations (Fig. 2) were similar in all three groups on the morning of day 2, peaked at 3 to 4 hrs after endotoxin injection (35), and were greater in the Cort80 Group compared with the Cort160 Group at 3 and 4 hrs after endotoxin injection (p < .01) and compared with the control Group 4 hrs after endotoxin injection (p < .01).
Figure 1.
Total plasma cortisol immediately before (8:00 am) and 2 and 4 hrs after intravenous injection of Escherichia coli endotoxin (lipopolysaccharide), 2 ng/kg. Participants had been treated the day before with a 6-hr infusion of intravenous saline (control), 1.5 µg/kg/min hydrocortisone (Cort80), or 3.0 µg/kg/min hydrocortisone (Cort160). Data presented as mean (se). LPS, lipopolysaccharide.
Figure 2.
Plasma adrenocorticotrophic (ACTH) concentrations immediately before (8:00 am) and at 2, 3, and 4 hrs after intravenous injection of Escherichia coli endotoxin (lipopolysaccharide [LPS]), 2 ng/kg. Participants had been treated the day before with a 6-hr infusion of intravenous saline (control), 1.5 µg/kg/min hydrocortisone (Cort80), or 3.0 µg/ kg/min hydrocortisone (Cort160). **p <.01 Cort80 versus control; ## p < .01 Cort80 vs. Cort160. Data presented as mean (se).
Cytokine Responses
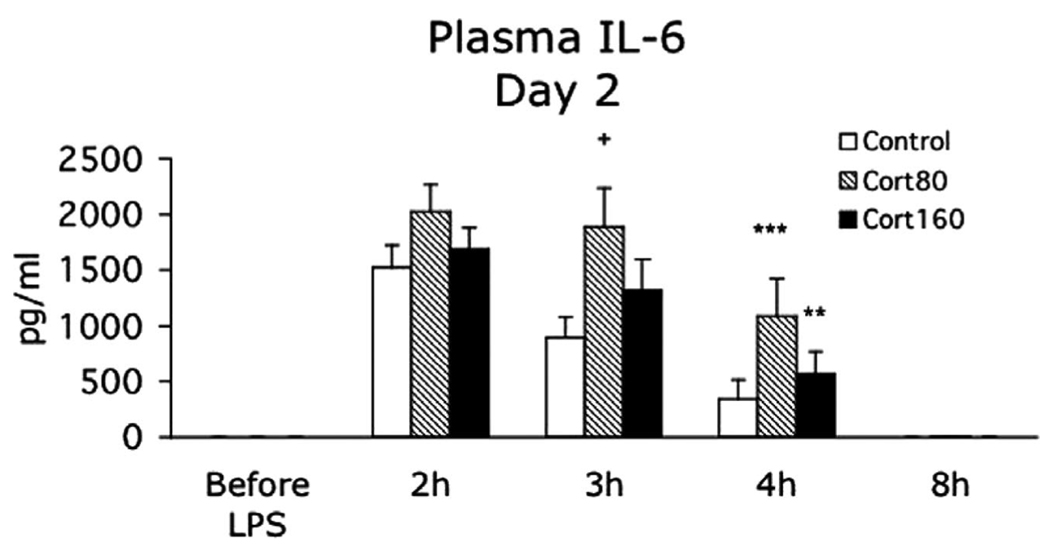
Plasma cytokine concentrations were measured immediately before endotoxin injection and at 2, 3, 4, and 8 hrs after endotoxin injection. Cytokine concentrations were also measured 24 hrs after endotoxin injection in the first 21 subjects and were found to be at baseline or below detection limits in all cases (data not shown). Plasma IL-6 reached a peak plasma concentration 2 hrs after endotoxin injection and remained elevated at 3 and 4 hrs after endotoxin. Analysis by generalized estimating equations to consider all measurement times showed that plasma IL-6 was significantly higher in the Cort80 Group compared with the control Group (p = .004) but not in the Cort160 Group (p = .14) (Fig. 3). At individual measurement times, the plasma IL-6 concentration was significantly greater in the Cort80 Group compared with the control Group at 3 hrs (p < .05) and 4 hrs (p < .001) and in the Cort160 Group compared with the control Group at 4 hrs (p < .01) after endotoxin injection. Plasma IL-10 peaked in all groups at 3 hrs after endotoxin injection. Analysis by generalized estimating equations to consider all measurement times showed that plasma IL-10 was significantly lower in the Cort80 Group compared with the control Group (p = .034) but not in the Cort160 Group (p = .4) (Fig. 4). At individual measurement times, the plasma IL-10 concentration was significantly lower in the Cort80 Group compared with the control Group at 2 and 3 hrs (p < .05) after endotoxin injection. Plasma TNF-α concentrations were highest 2 hrs after endotoxin injection, declined rapidly thereafter, and were not significantly different between groups (Fig. 5).
Figure 3.
Plasma interleukin (IL)-6 concentrations measured immediately before and at 2, 3, 4, and 8 hrs after intravenous injection of Escherichia coli endotoxin (lipopolysaccharide [LPS]), 2 ng/kg. Participants had been treated the day before with a 6-hr infusion of intravenous saline (control), 1.5 µg/kg/min hydrocortisone (Cort80), or 3.0 µg/kg/min hydrocortisone (Cort160). The plasma IL-6 response was significantly greater in the Cort80 group compared with the control group (p = .004). +p < .05 Cort80 vs. control; **p < .01 Cort160 vs. control; ***p < 0.001 Cort80 vs. control. Data presented as mean (se).
Figure 4.
Plasma interleukin (IL)-10 concentrations measured immediately before and at 2, 3, 4, and 8 hrs after intravenous injection of Escherichia coli endotoxin (lipopolysaccharide [LPS]), 2 ng/kg. Participants had been treated the day before with a 6-hr infusion of intravenous saline (control), 1.5 µg/kg/min hydrocortisone (Cort80), or 3.0 µg/kg/min hydrocortisone (Cort160). The plasma IL-10 response was significantly lower in the Cort80 group compared with the control group (p = .034). +p < .05 Cort80 vs. control. Data presented as mean (se).
Figure 5.
Plasma tumor necrosis factor (TNF)-α concentrations measured immediately before and at 2, 3, 4, and 8 hrs after intravenous injection of Escherichia coli endotoxin (lipopolysaccharide), 2 ng/kg. Participants had been treated the day before with a 6-hr infusion of intravenous saline (control), 1.5 µg/kg/min hydrocortisone (Cort80), or 3.0 µg/ kg/min hydrocortisone (Cort160). There were no significant differences in TNF-α response between groups.
Peripheral Blood Leukocyte Counts and C-reactive Protein
Peripheral blood leukocyte counts were measured immediately before injection of endotoxin (8:00 am, day 2) and 4 hrs after endotoxin administration (12:00 pm, day 2). Before endotoxin injection, there were no significant differences between groups in total white cell counts or in the total neutrophil or monocyte counts (Table 2). Total peripheral blood monocyte counts were significantly lower in the Cort80 and Cort160 Groups compared with control Group values (p < .05) 4 hrs after endotoxin administration (Table 2). By the next morning, all cell counts were again similar in all three groups (data not shown). C-reactive protein increased substantially in all treatment groups with no differences between groups (Table 2).
DISCUSSION
This study showed that endotoxininduced plasma concentrations of IL-6, which has predominately proinflammatory effects (36), were significantly increased when participants were exposed to an intermediate dose of cortisol 1 day before endotoxin injection. In addition, plasma concentrations of the prototypical anti-inflammatory cytokine IL-10 were decreased in the same intermediate-dose cortisol group. In sharp contrast to a dose-dependent increase in GC anti-inflammatory effects, this study is unique because it showed a dose-dependent GC inflammatory effect that was greater at intermediate cortisol concentrations with minimal effect at higher concentrations.
The concentration of cortisol that is needed to effectively suppress ongoing systemic inflammation in vivo remains a matter of controversy (37), but what is not controversial is that cortisol will acutely suppress coincident or ongoing systemic inflammation in a dose-dependent manner (32, 38). In this study, we used a different clinical paradigm that included a defined time interval between an increase in cortisol concentration and the onset of systemic inflammation. Barber et al previously showed that a minimal interval of 12 hrs is needed to observe delayed proinflammatory effects of GCs on the human inflammatory response to EE (28). Since then, there have been confirmatory reports of delayed in vivo inflammatory effects of GCs in animals. Intraperitoneal dexamethasone, for example, significantly increases serum TNF-α and nitric oxide levels in mice after endotoxin injection, but only with an intervening interval of 24 to 48 hrs (39). Similarly, acute stress augments a subsequent delayed-type hypersensitivity skin response in rats, an effect that is abrogated by adrenalectomy, which strongly suggests that it is mediated by GCs (40). We attempted to determine whether an inflammatory effect from GCs might be observed after in vivo increases in cortisol to concentrations that are more commonly observed in humans during acute systemic stress (34, 38). Although plasma cortisol concentrations are highly variable during ongoing systemic inflammation (41), they typically increase to the range of 30 to 45 µg/dL after an acute systemic inflammatory stimulus (28, 34, 38). The mean experimentally induced increase in plasma cortisol concentrations that we measured in the Cort80 Group was slightly higher than this level, whereas it was markedly higher in the Cort160 Group. More notably, an increased proinflammatory response to EE was observed after Cort80 treatment but not after Cort160 treatment. Therefore, transient exposure of healthy humans to the intermediate plasma cortisol concentrations observed after Cort80 treatment— but not to higher or lower (normal diurnal) cortisol concentrations—increased the inflammatory response to a succeeding systemic inflammatory stimulus.
This study did not examine potential mechanism(s) by which cortisol can augment the inflammatory response to endotoxin, although prior studies suggest several possibilities. The increased IL-6 concentrations that we observed in the Cort80 Group could have been a consequence of a diminished anti-inflammatory response during EE. However, cortisol pretreatment did not inhibit the acute plasma cortisol response to endotoxin in either experimental group. In addition, plasma adrenocorticotropic hormone values were the same in all groups on day 2 before endotoxin injection, further indicating that there was no residual adrenal cortical inhibitory effect in either treatment group. A diminished anti-inflammatory response could also have been the result of decreased GC activity as a consequence of decreased GC receptor (density in effector cells. Downregulation of the GC receptor α receptor is a well-described consequence of GC treatment (42, 43). However, any such GC effect on GC receptor density should have also been observed in the Cort160 Group, which did not show the same enhancement of IL-6 that we observed in the Cort80 Group. Finally, the plasma response of anti-inflammatory IL-10 was significantly lower in the Cort80 Group. This observation is an unlikely explanation for the augmented IL-6 response in the Cort80 Group because IL-10, when given after endotoxin injection, does not suppress inflammation and may, in fact, enhance the inflammatory response (44, 45).
A second explanation for cortisol enhancement of systemic inflammation during EE is a direct effect of GC pretreatment on synthesis and release of proinflammatory molecules from GC-sensitive effector cells. As noted, GCs increase the expression of inflammatory cytokine receptors on immune effector cells, including receptors for IL-1 and IL-6 (6, 46–48). Increased receptor density would be expected to enhance mediator activity with potentially both IL-1 and IL-6 acting to augment endogenous release of IL-6 by IL-6 receptor-bearing cells. Second, a previously reported GC-induced increase in the inflammatory response to endotoxin was associated with increased cytochrome p450 activity leading the authors to speculate that increased nitric oxide was responsible for enhanced TNF-α production (49). GCs, in turn, have been shown to induce cytochrome p450 (50). Third, recent studies have shown that enhanced toll-like receptor signaling can be a consequence of “sterile stress” in animals. A sterile burn injury, for example, leads to a delayed enhancement of ligand-stimulated production of IL-1β, IL-6, and TNF-α by spleen cells between 1 and 7 days after injury (51). Recent work has shown differential effects of intermediate and high GC concentrations on cytokine production that may be mediated through modulation of mitogen-activated protein kinase signal transduction (52). Signals transduced through these pathways are crucial for the generation of a rapid innate immune response (53). In addition to mediating effects on transcription, activation of mitogen-activated protein kinase pathways has been shown to stabilize mRNA expression of many proinflammatory cytokines, including IL-6 and TNF-α (54, 55). We have recently observed that intermediate doses of stress cortisol comparable to those administered in vivo to the Cort80 Group can enhance leukocyte IL-6 production in vitro and also suppresses expression of two negative regulators of mitogen-activated protein kinase signaling pathways, dual specificity phosphatases 2 and 10 (unpublished observations). Inhibition of dual specificity phosphatases is known to enhance both mitogen-activated protein kinase signal transduction and proinflammatory cytokine production (56). Finally, the observed GC enhancement of systemic inflammation in this study was probably the result of “overlapping” GC effects on different molecular events. Stimulation of mitogen-activated protein kinase signaling, for example, may have occurred in both the Cort80 and Cort160-treated groups but was not manifested in the Cort160 Group as a result of greater activation of other inflammation-inhibitory mechanisms.
A direct comparison of our results with those of Barber et al is difficult as a result of methodologic difference in cytokine and hormone measurements and differences in study design because we studied larger group sizes in a blinded, randomized manner. The major difference, however, is that we varied the cortisol pretreatment dose followed by a fixed time interval, whereas Barber et al varied the time interval. The time interval that we used—17 hrs—is well within the 12- to 144-hr interval that was associated with cortisol enhancement of the inflammatory response. The results reported here do confirm and extend their observations by showing that a transient increase in plasma cortisol to intermediate stress-associated levels enhances subsequent endotoxin-induced IL-6 production while decreasing IL-10 production. Increases in plasma cortisol to higher levels had a substantially lesser effect on the plasma IL-6 response and no significant effect on the plasma IL-10 response.
In conclusion, several features of this study warrant emphasis. First, time was an important variable. Based on prior reports (28, 31, 39), our experimental protocol included a time interval between the experimentally induced increase in plasma cortisol and the subsequent initiation of systemic inflammation. Second, we did not administer cortisol during EE because prior reports clearly demonstrate anti-inflammatory effects from similar GC doses and plasma concentrations when the GCs are given during a systemic inflammatory response (28, 32, 38). Third, we looked for a dose-dependent effect of cortisol and found that cortisol affected the human systemic inflammatory response to EE in a biphasic manner with an intermediate cortisol concentration leading to a distinctly different—and greater—inflammatory response to EE compared with higher or lower cortisol concentrations. Finally, although the EE model was developed to study the human systemic inflammatory response syndrome as a component of human sepsis, as a result of the transient and isolated nature of the EE stimulus, results of this and similar studies clearly cannot be directly extrapolated to patients with sepsis (57, 58). EE is, however, an excellent model for studying preidentified components of the human systemic inflammatory response as reported here.
CONCLUSIONS
We have shown that a transient in vivo increase in cortisol concentration can significantly enhance a subsequent delayed human response to a systemic inflammatory stimulus and simultaneously suppress the anti-inflammatory response. This effect was observed by increasing plasma cortisol to concentrations that are typically measured in humans during and after an acute systemic stress. Our results suggest that the human response to repeated systemic stresses may be altered and even augmented by the cortisol response to the first stimulus, an observation that Selye made repeatedly in animals before the anti-inflammatory properties of GCs were discovered (2, 3).
Acknowledgments
Supported, in part, by a grant from the National Institutes of Health, National Institute of Allergy and Infectious Diseases AI051547 (PMG) and by Centers of Biomedical Research Excellence P20RR016437.
Footnotes
All work completed at the Dartmouth–Hitchcock Medical Center, Lebanon, NH.
ClinicalTrials.gov identifier: NCT00396344.
The authors have not disclosed any potential conflicts of interest.
REFERENCES
- 1.Hench P, Kendall E, Slocumb C, et al. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone: Compound E) and of pituitary adrenocorticotropic hormone on rheumatoid arthritis; preliminary report. Proc Staff Meet Mayo Clin. 1949;24:181–197. [PubMed] [Google Scholar]
- 2.Selye H. Role of the hypophysis in the pathogenesis of the diseases of adaption. Can Med Assoc J. 1944;50:426–433. [PMC free article] [PubMed] [Google Scholar]
- 3.Selye H, Dosne C, Bassett L, et al. On the therapeutic value of adrenal cortical hormones in traumatic shock and allied conditions. Can Med Assoc J. 1940;43:1–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Ingle D. The role of the adrenal cortex in homeostasis. J Endocrinol. 1952;8:23–37. [PubMed] [Google Scholar]
- 5.Yeager MP, Guyre PM, Munck AU. Glucocorticoid regulation of the inflammatory response to injury. Acta Anaesthesiol Scand. 2004;48:799–813. doi: 10.1111/j.1399-6576.2004.00434.x. [DOI] [PubMed] [Google Scholar]
- 6.Shieh J-H, Peterson RH, Moore MA. Cytokines and dexamethasone modulation of IL-1 receptors on human neutrophils in vitro. J Immunol. 1993;150:3515–3524. [PubMed] [Google Scholar]
- 7.Wiegers GJ, Labeur M, Stec I, et al. Glucocorticoids accelerate anti-T cell receptorinduced T cell growth. J Immunol. 1995;155:1893–1902. [Google Scholar]
- 8.Paterson RK, Or R, Domenico J, et al. Regulation of CD23 expression by IL-4 and corti-costeroid in human B lymphocytes. J Immunol. 1994;152:2139–2147. [PubMed] [Google Scholar]
- 9.Pietzko D, Zohlnhofer D, Graeve L, et al. The hepatic interleukin-6 receptor. J Biol Chem. 1993;268:4250–4258. [PubMed] [Google Scholar]
- 10.Franchimont D, Galon J, Vacchio MS, et al. Positive effects of glucocorticoids on T cell function by up-regulation of IL-7 receptor α. J Immunol. 2002;168:2212–2218. doi: 10.4049/jimmunol.168.5.2212. [DOI] [PubMed] [Google Scholar]
- 11.Strickland RW, Wahl LM, Finblood DS. Corticoid steroids enhance the binding of recombinant interferon-V to cultured human monocytes. J Immunol. 1986;137:1577–1580. [PubMed] [Google Scholar]
- 12.Hawrylowicz C, Guida L, Paleolog E. Dexamethasone up-regulates granulocyte-macrophage colony- stimulating factor receptor expression on human monocytes. Immunology. 1994;83:274–280. [PMC free article] [PubMed] [Google Scholar]
- 13.van der Goes A, Hoekstra K, van den Berg TK, et al. Dexamethasone promotes phagocytosis and bacterial killing by human monocytes/ macrophages in vitro. J Leukoc Biol. 2000;67:801–807. doi: 10.1002/jlb.67.6.801. [DOI] [PubMed] [Google Scholar]
- 14.Freischlag J, Colburn M, Quinones-Baldrich M, et al. Alteration of neutrophil (PMN) function by heparin, dexamethasone, and enalapril. J Surg Res. 1992;52:523–529. doi: 10.1016/0022-4804(92)90322-q. [DOI] [PubMed] [Google Scholar]
- 15.Lloberas J, Soler C, Celada A. Dexamethasone enhances macrophage colony stimulating factor- and granulocyte macrophage colony stimulating factor-stimulated proliferation of bone marrow-derived macrophages. Int Immunol. 1998;10:593–599. doi: 10.1093/intimm/10.5.593. [DOI] [PubMed] [Google Scholar]
- 16.Dinkel K, MacPherson A, Sapolsky RM. Novel glucocorticoid effects on acute inflammation in the CNS. J Neurochem. 2003;84:705–716. doi: 10.1046/j.1471-4159.2003.01604.x. [DOI] [PubMed] [Google Scholar]
- 17.Munck A, Guyre P. Glucocorticoid physiology, pharmacology and stress. Adv Exp Med Biol. 1986;196:81–96. doi: 10.1007/978-1-4684-5101-6_6. [DOI] [PubMed] [Google Scholar]
- 18.Munck A, Guyre P, Holbrook N. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- 19.Sapolsky RM, Romero M, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 20.Guyre P, Yeager M, Munck A. Glucocorticoid effects on immune responses. Neuroimmune Biology. 2008;7:147–168. [Google Scholar]
- 21.Lim H-Y, Müller N, Herold M, et al. Glucocorticoids exert opposing effects on macrophage function dependent on their concentration. Immunology. 2007;122:47–53. doi: 10.1111/j.1365-2567.2007.02611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calandra T, Bucala R. Macrophage migration inhibitory factor (MIF): A glucocorticoid counter-regulator within the immune system. Crit Rev Immunol. 1997;17:77–88. doi: 10.1615/critrevimmunol.v17.i1.30. [DOI] [PubMed] [Google Scholar]
- 23.Yeager M, Pioli P, Wardwell K, et al. In vivo exposure to high or low cortisol has bi-phasic effects on inflammatory response pathways of human monocytes. Anesth Analg. 2008;107:1726–1734. doi: 10.1213/ane.0b013e3181875fb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warren M, Vogel S. Opposing effects of glucocorticoids on interferon-γ-induced murine macrophage Fc receptor and Ia antigen expression. J Immunol. 1985;134:2462–2469. [PubMed] [Google Scholar]
- 25.Eastman H, Fawcett T, Udelsman R, et al. Effects of perturbations of the hypothalamic-pituitary-adrenal axis on the acute phase response: Altered C/EBP and acute phase response gene expression in lipopolysaccharide-treated rats. Shock. 1996;6:286–292. doi: 10.1097/00024382-199610000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Natl Acad Sci USA. 1999;96:1059–1064. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsusue S, Walser M. Healing of intestinal anastomoses in adrenalectomized rats given corticosterone. Am J Physiol. 1992;263:R164–R168. doi: 10.1152/ajpregu.1992.263.1.R164. [DOI] [PubMed] [Google Scholar]
- 28.Barber AE, Coyle SM, Marano MA, et al. Glucocorticoid therapy alters hormonal and cytokine responses to endotoxin in man. J Immunol. 1993;150:1999–2006. [PubMed] [Google Scholar]
- 29.Smyth GP, Stapleton PP, Freeman TA, et al. Glucocorticoid pretreatment induces cytokine overexpression and nuclear factor-kappaB activation in macrophages. J Surg Res. 2004;116:253–261. doi: 10.1016/S0022-4804(03)00300-7. [DOI] [PubMed] [Google Scholar]
- 30.Maung A, Fujimi S, MacConmara M, et al. Injury enhances resistance to Escherichia coli infection by boosting innate immune system function. J Immunol. 2008;180:2450–2458. doi: 10.4049/jimmunol.180.4.2450. [DOI] [PubMed] [Google Scholar]
- 31.Kallapur S, Kramer B, Moss TJM. Maternal glucocorticoids increase endotoxin-induced lung inflammation in preterm lambs. Am J Physiol Lung Cell Mol Physiol. 2003;284:L633–L642. doi: 10.1152/ajplung.00344.2002. [DOI] [PubMed] [Google Scholar]
- 32.de Kruif MD, Lemaire LC, Giebelen IA, et al. Prednisolone dose-dependently influences inflammation and coagulation during human endotoxemia. J Immunol. 2007;178:1845–1851. doi: 10.4049/jimmunol.178.3.1845. [DOI] [PubMed] [Google Scholar]
- 33.Hardin J, Hilbe J. Generalized Estimating Equations. New York: Chapman & Hall; 2003. [Google Scholar]
- 34.Rassias AJ, Holzberger PT, Givan AL, et al. Decreased physiologic variability as a generalized response to human endotoxemia. Crit Care Med. 2005;33:512–519. doi: 10.1097/01.ccm.0000155908.46346.ed. [DOI] [PubMed] [Google Scholar]
- 35.Schreiber W, Pollmacher T, Fassbender K, et al. Endotoxin- and corticotropin-releasing hormone-induced release of ACTH and cortisol. Neuroendocrinology. 1993;58:123–128. doi: 10.1159/000126526. [DOI] [PubMed] [Google Scholar]
- 36.Gabay C. Proceedings: Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8 suppl 2:1–6. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med. 2003;348:727–734. doi: 10.1056/NEJMra020529. [DOI] [PubMed] [Google Scholar]
- 38.Yeager MP, Rassias AJ, Fillinger MP, et al. Cortisol antiinflammatory effects are maximal at postoperative plasma concentrations. Crit Care Med. 2005;33:1507–1512. doi: 10.1097/01.ccm.0000164565.65986.98. [DOI] [PubMed] [Google Scholar]
- 39.Fantuzzi G, Galli G, Zinetti M, et al. The upregulating effect of dexamethasone on tumor necrosis factor production is mediated by a nitric oxide-producing cytochrome P450. Cell Immunol. 1995;160:305–308. doi: 10.1016/0008-8749(95)80042-h. [DOI] [PubMed] [Google Scholar]
- 40.Dhabhar FS. Stress-induced augmentation of immune function—The role of stress hormones, leukocyte trafficking, and cytokines. Brain Behav Immun. 2002;16:785–798. doi: 10.1016/s0889-1591(02)00036-3. [DOI] [PubMed] [Google Scholar]
- 41.Bendel S, Karlsson S, Pettila V. Free cortisol in sepsis and septic shock. Anesth Analg. 2008;106:1813–1819. doi: 10.1213/ane.0b013e318172fdba. [DOI] [PubMed] [Google Scholar]
- 42.Shipman GF, Bloomfield CD, Gajl-Peczalska K, et al. Glucocorticoids and lymphocytes. III. Effects of glucocorticoid administration on lymphocyte glucocorticoid receptors. Blood. 1983;61:1086–1090. [PubMed] [Google Scholar]
- 43.Burnstein K, Bellingham D, Jewell C, et al. Autoregulation of glucocorticoid receptor gene expression. Steroids. 1991;56:52–58. doi: 10.1016/0039-128x(91)90124-e. [DOI] [PubMed] [Google Scholar]
- 44.Lauw F, Pajkrt D, Hack C, et al. Proinflammatory effects of IL-10 during human endotoxemia. J Immunol. 2000;165:2783–2789. doi: 10.4049/jimmunol.165.5.2783. [DOI] [PubMed] [Google Scholar]
- 45.Pajkrt D, Camoglio L, Tiel-van Buul MC, et al. Attenuation of proinflammatory response by recombinant human IL-10 in human endotoxemia: Effect of timing of recombinant human IL-10 administration. J Immunol. 1997;158:3971–3977. [PubMed] [Google Scholar]
- 46.Wiegers GJ, Reul JMHM. Induction of cytokine receptors by glucocorticoids: Functional and pathological significance. TiPS. 1998;19:317–321. doi: 10.1016/s0165-6147(98)01229-2. [DOI] [PubMed] [Google Scholar]
- 47.Spriggs MK, Lioubin PJ, Slack J, et al. Induction of an interleukin-1 receptor (IL-1R) on monocytic cells. J Biol Chem. 1990;265:22499–22505. [PubMed] [Google Scholar]
- 48.Snyers L, DeWitt L, Content J. Glucocorticoid up-regulation of high-affinity interleukin 6 receptors on human epithelial cells. Proc Natl Acad Sci USA. 1990;87:2838–2842. doi: 10.1073/pnas.87.7.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fantuzzi G, Demitri MT, Ghezzi P. Differential effect of glucocorticoids on tumour necrosis factor production in mice: Up-regulation by early pretreatment with dexamethasone. Clin Exp Immunol. 1994;96:166–169. doi: 10.1111/j.1365-2249.1994.tb06247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Honkakoski P, Negishi M. Regulation of cytochrome p450 (CYP) genes by nuclear receptors. Biochem J. 2000;347:321–337. doi: 10.1042/0264-6021:3470321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paterson H, Murphy T, Purcell E, et al. Injury primes the innate immune system for enhanced toll-like receptor reactivity. J Immunol. 2003;171:1473–1483. doi: 10.4049/jimmunol.171.3.1473. [DOI] [PubMed] [Google Scholar]
- 52.Zhang TY, Daynes RA. Glucocorticoid conditioning of myeloid progenitors enhances TLR4 signaling via negative regulation of the phosphatidylinositol 3-kinase-Akt pathway. J Immunol. 2007;178:2517–2526. doi: 10.4049/jimmunol.178.4.2517. [DOI] [PubMed] [Google Scholar]
- 53.Lang R, Hammer M, Mages J. DUSP meet immunology: Dual specificity MAPK phosphatases in control of the inflammatory response. J Immunol. 2006;177:7497–7504. doi: 10.4049/jimmunol.177.11.7497. [DOI] [PubMed] [Google Scholar]
- 54.Horwood NJ, Page TH, McDaid JP, et al. Bruton’s tyrosine kinase is required for TLR2 and TLR4-induced TNF, but not IL-6, production. J Immunol. 2006;176:3635–3641. doi: 10.4049/jimmunol.176.6.3635. [DOI] [PubMed] [Google Scholar]
- 55.Zhao W, Liu M, Kirkwood KL. p38alpha stabilizes interleukin-6 mRNA via multiple AUrich elements. J Biol Chem. 2008;283:1778–1785. doi: 10.1074/jbc.M707573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jeffrey KL, Camps M, Rommel C, et al. Targeting dual-specificity phosphatases: Manipulating MAP kinase signalling and immune responses. Nat Rev Drug Discov. 2007;6:391–403. doi: 10.1038/nrd2289. [DOI] [PubMed] [Google Scholar]
- 57.Marshall J. Sepsis: Rethinking the approach to clinical research. J Leukocyte Biol. 2008;83:471–482. doi: 10.1189/jlb.0607380. [DOI] [PubMed] [Google Scholar]
- 58.Lowry SF. Human endotoxemia: A model for mechanistic insight and therapeutic targeting. Shock. 2005;24 suppl 1:94–100. doi: 10.1097/01.shk.0000191340.23907.a1. [DOI] [PubMed] [Google Scholar]