Abstract
In a single case longitudinal study, a 70 year old female subject who has had a subcortical stroke 8 years prior, was tested three times in fMRI using an interactive MRI-compatible VR environment. The subject performed sequential finger movements with her right (unaffected) hand. Her hand motion (recorded with the data glove) animated either the ipsilateral (corresponding) or contralateral (mirrored) virtual hand model. In a visual feedback control condition, the virtual hand models were replaced with ellipsoids. In between the second and third session, the patient participated in an intensive, two-week long VR-based training of her affected upper extremity. When comparing activation in the mirrored versus the non-mirrored virtual visual feedback condition, no significant activation was noted in motor or premotor areas in the baseline 1 or baseline 2 sessions. However, increased activation in the ipsilesional motor cortex occurred as a result of training, despite the absence of active involvement of the ipsilesional motor cortex in this condition. The left motor cortex was also recruited in this condition (though weaker) despite the subtracted out ellipsoid condition (in which subjects also moved their hand). Thus, the contralateral (mirrored) visual feedback may have had a facilitory effect bilaterally. These findings might have some important implications for the development of novel therapies in the acute phase, when paresis and the potential for neural remapping are greatest.
I. Introduction
Impaired force generation in the fingers and hand (paresis) occurs in 70–85% of individuals who have had a stroke [1, 2]. Paresis is characterized by reduced strength and diminished or absent motor evoked potentials (MEP), and can occur even due to partial damage of the corticospinal tract (CST) system [3–6]. The prognosis to recover from paresis is higher for patients in whom MEPs can be elicited in the few weeks after stroke [5, 7] and for individuals in whom cortical activation (measured with functional MRI, fMRI) shifts from the contralesional hemisphere (that often over-compensates after a stroke) to the ipsilesional hemisphere [8, 9], [10–12]. Thus, empirical data suggest that recovery is dependent on entraining the intact ipsilesional CST system to assume the functions of the lesioned areas; a process of remapping functions onto new circuits.
This paper presents preliminary data that demonstrate that training a patient who is in the chronic phase after stroke on a set of virtual reality (VR)-based training exercises may bolster the remapping of ipsilesional motor cortex. For this, the patient trained for 2 weeks (10 sessions) on a battery of hand and arm tasks embedded in a VR environment. To asses cortical remapping, the patient participated in three fMRI sessions. The first two baseline sessions were separated by 6 months of no therapy and controlled for effects due to spontaneous recovery. The last session occurred after the training protocol. During the fMRI session, the subject performed sequential finger movements with the unaffected right hand which actuated either a left or a right virtual hand model. During these blocks, the affected hand remained motionless. Additional control blocks in which the virtual hand models were replaced by obliquely rotating ellipsoids controlled for non-specific activation. We hypothesized that time-locking movement of the unaffected hand (involving the contralesional hemisphere) with the opposite virtual hand would collide an interhemispherically transferred motor program with high-fidelity visual feedback in the ipsilesional motor cortex. Thus, if remapping in the ipsilesional motor cortex occurred as a result of training, it should manifest as increased activation despite the absence of active involvement of the ipsilesional motor cortex in this condition.
II. METHODS
A. Subject
A right-handed [13], 70 year old female subject participated after signing informed consent approved by the IRB Committees of NYU and NJIT. The subject sustained a right hemispheric subcortical stroke in 2000. Her medical presentation is significant for hypertension which is controlled medically and asthma. She presents with mild left side hemiparesis resulting in a Chedoke-McMaster Impairment Inventory stage of 7 for the arm, 4 for her hand, 6 for the leg and 4 for her foot. She presents with 1/4 spasticity of her finger flexors and plantar flexors. She ambulates in the community without an assistive device or orthosis. She is independent in all basic and instrumental activities of daily living without adaptive equipment, and is the primary home-maker for her family.
B. Task
The subject performed sequential finger movements with her right (unaffected) or left (affected) hand. Her hand motion (recorded with the data glove) animated either the ipsilateral (corresponding) or contralateral (mirrored) virtual hand model (Fig 1). Two visual feedback control conditions were also added. In these conditions, the virtual hand models were replaced with ellipsoids that rotated about an oblique axis at a rate of 1 Hz (a comparable rate to the subject’s finger movement). The non-anthropomorphic ellipsoids controlled for non-specific visual effects related to size, color, object movement, location of the object in the visual field, and eye movement. Four functional fMRI runs were performed with the subject using the left hand, and four with the right hand. The visual feedback conditions were interleaved within each run (10 trials per condition). The inter-trial interval was randomly varied between 3–7 seconds to introduce temporal jitter into the fMRI acquisition.
Fig. 1.
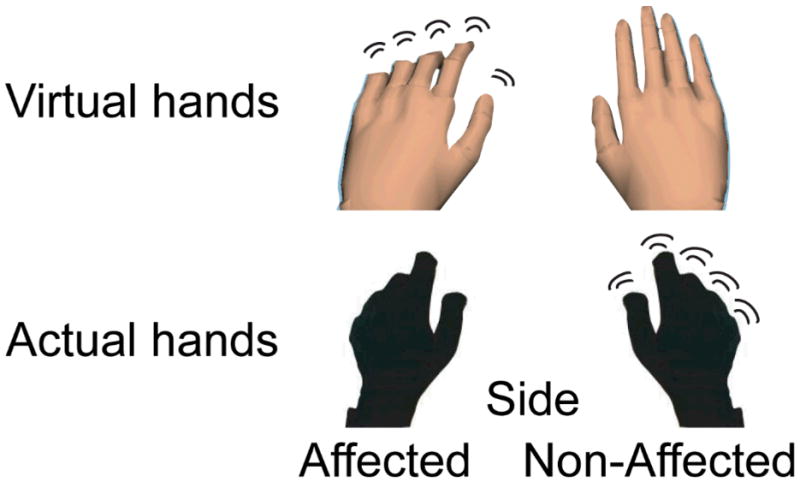
Top: The virtual environment used in the current paradigm. We extracted the essential component common to all of our virtual environments, the virtual hands, over a plane background. Bottom: picture of subject’s hands wearing 5DT data gloves that actuated motion of the virtual hand models.
C. The Virtual Reality System
The setup has been described elsewhere [14]. Briefly, hand kinematics were collected with an MRI-compatible left- and MRI-compatible right hand 5DT Data Glove 16 MRI (Fifth Dimension Technologies, 5DT Data Glove 16 MRI, http://www.5dt.com) that was interfaced with the virtual environment (developed with Virtools and VRPack plugin that communicated with the open source VRPN (Virtual Reality Peripheral Network, [15]). The data gloves use fiber optics to measure each metacarpophalangeal [16] joint, proximal interphalangeal (PIP) joint, and finger abduction angles. In the technician’s room, the fiber optic signals are digitized and connected to the serial port of a PC running the simulation. The VR simulation was projected through a rear-view display and subjects viewed it through a rear-facing mirror. Prior to the experiment, the data gloves were calibrated to each hand and the subject verified that the quality of her movement corresponded to that of the virtual hand models. The onset of the virtual simulation and of the data glove acquisition was triggered by back-tic TTL transmitted from the scanner.
D. FMRI Data Acquisition and Preprocessing
Magnetic resonance imaging was performed using a 3-T Siemens Allegra head-only scanner with a Siemens standard head coil. T1-weighted structural (TR=2500ms, TE=3.93ms, FOV=256mm, Flip angle=8°, thickness=1mm, voxel size=1×1×1mm, resolution=256) and functional images (TR=2500ms, TE=30ms, FOV=192mm, flip angle=85°, voxel size=3×3×3mm, resolution=64, bandwidth=4112 Hz/px, echo-spacing=0.31ms, 46 slices, thickness=3mm, number of volumes=120) Two dummy images were acquired (but not saved) at the start of each run to account for field inhomogeneity. FMRI data were preprocessed and analyzed with SPM5. Images were realigned, co-registered, and spatially normalized to the Montreal Neurological Institute template, and smoothed (8mm kernel).
E. FMRI Analysis
Our first effect of interest was whether visual feedback of the virtual hand model corresponding to the affected side but actuated by the motion of the subject’s unaffected hand (contralateral visual feedback condition) would lead to increased activation in the motor cortex ipsilateral to the behaving hand (i.e. the non-acting ipsilesional hemisphere) (see Fig. 1). For this effect, we compared activation when the subject performed the task with her right (unaffected) hand but received real time feedback of her movement through the contralateral (left) virtual hand which corresponded to her affected side with the same condition but when the virtual hand models were replaced by the moving ellipsoids. Thus, everything was identical in both conditions except the viewed virtual object. Our second effect of interest was whether the above effect would be facilitated by having the subject train her affected hand and arm in a VR environment for 2 weeks. Thus, we repeated the fMRI experiment three times. The first and second sessions (baseline 1 and baseline 2) were separated by 6 months of no training to establish an absence of spontaneous neural changes. The second and third sessions (baseline 2 and post-test) were separated by 2 weeks of intense training on a different set of tasks in VR. Given the limited power in our preliminary data and to maximize the chances of observing even slight changes in activation, we used a liberal threshold of p<0.05 and a minimum extent of 10 voxels.
F. Description of VR-Based Training
The sensorimotor training of the hemiparetic upper extremity used a novel robotic system NJIT-RAVR,[17] with haptic effects and objects presented in three-dimensional VR environments. This system was used to train the hand and arm together as an integrated functional unit for 2–3 hour sessions for eight days. The training utilized four interactive VR environments that have been developed previously[18] Tracking of the arm endpoint in 3D space, as well as haptic assistance “as needed” was provided by the Haptic Master robotic arm (Moog-FCS, Netherlands) and finger tracking was done by an instrumented glove (CyberGlove, Immersion, USA). The subject improved her Jebsen Test of Hand Function [19] score from 121 sec to 84 sec, and her Wolf Motor Function Test score from 45 sec to 35 sec. The subject also improved in several kinematic measures of the arm and finger motion during the interactions with the VR simulations.
III. RESULTS
Fig. 2 shows the contrast for our effect of interest for each of the three testing sessions. Significant activation was noted in bilateral motor cortex during the post-test session (Fig. 2 and Table 1). No significant activation was noted in motor or premotor areas in the baseline 1 or baseline 2 sessions, even at a liberal threshold. Note that the right cortex activated in the post-test session represents the non-active hemisphere (i.e. corresponding to the hand that was resting in this condition). Interestingly, the left motor cortex was also recruited in this condition (though weaker) despite the subtracted out ellipsoid condition (in which subjects also moved their hand). Thus, the contralateral (mirrored) visual feedback may have had a facilitory effect bilaterally.
Fig. 2.
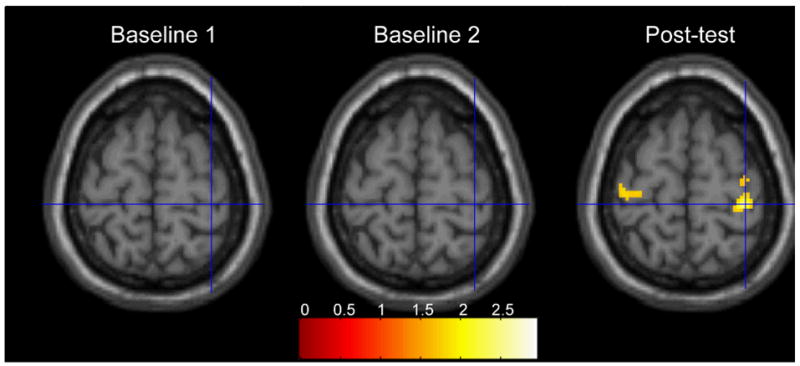
A chronic stroke patient performed a finger sequence with the less affected RIGHT hand. The panels show the activations that were significantly greater when viewing the corresponding finger motion of the LEFT virtual hand (i.e. activation related to ‘mirror’ viewing) than when viewing moving ellipsoids. The right panel shows that after two weeks of intensive training, viewing the LEFT virtual hand while moving the RIGHT hand led to significantly greater bilateral activation of the primary motor cortex, especially IPSILATERAL to the moving hand (i.e. contralateral to the observed virtual hand).
TABLE I.
REGIONS IN MNI SPACE SHOWING SIGNIFICANT ACTIVATION FOR THE REPORTED CONTRASTS.
| Region | X, Y, Z | K | T | P |
|---|---|---|---|---|
| Right motor cortex | 36, −30, 66 | 194 | 2.57 | 0.005 |
| Left motor cortex | −30, −22, 68 | 50 | 1.9 | 0.029 |
IV. DISCUSSION
Our preliminary data demonstrate that time-locking movement of the unaffected hand with high-fidelity movement of the opposite virtual hand that corresponds to the affected side is associated with activation in the ipsilesional, and to a lesser extent the contralesional, motor cortex. This effect was evident only after intense training of the hand and arm on a set of different tasks in a virtual reality environment. Our data suggest that training in virtual reality may bolster remapping in the ipsilesional motor cortex. Moreover, our data may provide a physiological explanation for the benefits reported in several small-scale studies that investigated the efficacy of mirror visual feedback therapy for recovery of hand and arm function in patients with stroke [20, 21].
The activation noted in the ipsilesional motor cortex is unlikely to result from uninstructed motion of the affected hand since inspection of hand movement (from the glove data) revealed that the subject complied with the task. Also, our findings are unlikely to result from familiarity with the virtual environment since no effects were noted at the second baseline session. Others have reported that intentional observation of actions can facilitate the magnitude of MEPs and influence corticocortical interactions in the motor and premotor areas [14, 22–25]. Further, it is known from retrograde tracer studies that rich intra-hemispheric cortico-cortical connections link the occipital, parietal, and frontal cortices [26–32] and from single unit studies that a substantial number of neurons in motor, premotor, and parietal areas are modulated by visual information [33–36]. Findings of our study are in line with this data and suggest that visual feedback may modulate the motor system without requiring overt movement and that this modulation may be bolstered by (a) training in virtual reality and (b) time-locking the visual feedback with interhemispheric transfer of motor commands. If these findings hold for other patients who are in the chronic phase of stroke, then it will be important to test this principle in the acute phase, when paresis and the potential for neural remapping are greatest.
Acknowledgments
This work was supported in part by the NIH grant HD 58301 and by the NIDRR Rehabilitation Engineering Research Center grant # H133E050011.
Contributor Information
Eugene Tunik, University of Medicine and Dentistry of New Jersey, Newark, NJ 07107 USA.
Sergei V. Adamovich, Email: sergei.adamovich@njit.edu, New Jersey Institute of Technology, Newark, NJ 07102 USA.
References
- 1.Dobkin BH. Impairments, disabilities, and bases for neurological rehabilitation after stroke. J Stroke Cerebrovasc Dis. 1997 Apr–May;6:221–6. doi: 10.1016/s1052-3057(97)80015-8. [DOI] [PubMed] [Google Scholar]
- 2.Dobkin BH. Functional MRI: a potential physiologic indicator for stroke rehabilitation interventions. Stroke. 2003 May;34:e23–8. doi: 10.1161/01.str.0000071140.00153.05. [DOI] [PubMed] [Google Scholar]
- 3.Heald A, Bates D, Cartlidge NE, French JM, Miller S. Longitudinal study of central motor conduction time following stroke. 1. Natural history of central motor conduction. Brain. 1993 Dec;116(Pt 6):1355–70. doi: 10.1093/brain/116.6.1355. [DOI] [PubMed] [Google Scholar]
- 4.Heald A, Bates D, Cartlidge NE, French JM, Miller S. Longitudinal study of central motor conduction time following stroke. 2. Central motor conduction measured within 72 h after stroke as a predictor of functional outcome at 12 months. Brain. 1993 Dec;116(Pt 6):1371–85. doi: 10.1093/brain/116.6.1371. [DOI] [PubMed] [Google Scholar]
- 5.Pennisi G, Rapisarda G, Bella R, Calabrese V, Maertens De Noordhout A, Delwaide PJ. Absence of response to early transcranial magnetic stimulation in ischemic stroke patients: prognostic value for hand motor recovery. Stroke. 1999 Dec;30:2666–70. doi: 10.1161/01.str.30.12.2666. [DOI] [PubMed] [Google Scholar]
- 6.Dachy B, Biltiau E, Bouillot E, Dan B, Deltenre P. Facilitation of motor evoked potentials in ischemic stroke patients: prognostic value and neurophysiologic correlations. Clin Neurophysiol. 2003 Dec;114:2370–5. doi: 10.1016/s1388-2457(03)00252-9. [DOI] [PubMed] [Google Scholar]
- 7.Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007 Jan;130:170–80. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- 8.Jang SH, You SH, Hallett M, Cho YW, Park CM, Cho SH, Lee HY, Kim TH. Cortical reorganization and associated functional motor recovery after virtual reality in patients with chronic stroke: an experimenter-blind preliminary study. Arch Phys Med Rehabil. 2005 Nov;86:2218–23. doi: 10.1016/j.apmr.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 9.You SH, Jang SH, Kim YH, Hallett M, Ahn SH, Kwon YH, Kim JH, Lee MY. Virtual reality-induced cortical reorganization and associated locomotor recovery in chronic stroke: an experimenter-blind randomized study. Stroke. 2005 Jun;36:1166–71. doi: 10.1161/01.STR.0000162715.43417.91. [DOI] [PubMed] [Google Scholar]
- 10.Carey LM, Abbott DF, Puce A, Jackson GD, Syngeniotis A, Donnan GA. Reemergence of activation with poststroke somatosensory recovery: a serial fMRI case study. Neurology. 2002 Sep 10;59:749–52. doi: 10.1212/wnl.59.5.749. [DOI] [PubMed] [Google Scholar]
- 11.Carey LM, Abbott DF, Egan GF, O’Keefe GJ, Jackson GD, Bernhardt J, Donnan GA. Evolution of brain activation with good and poor motor recovery after stroke. Neurorehabil Neural Repair. 2006 Mar;20:24–41. doi: 10.1177/1545968305283053. [DOI] [PubMed] [Google Scholar]
- 12.Small SL, Hlustik P, Noll DC, Genovese C, Solodkin A. Cerebellar hemispheric activation ipsilateral to the paretic hand correlates with functional recovery after stroke. Brain. 2002 Jul;125:1544–57. doi: 10.1093/brain/awf148. [DOI] [PubMed] [Google Scholar]
- 13.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971 Mar;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 14.Tunik E, August KG, Merians A, Adamovich SV. A Virtual Reality-Based System Integrated with fMRI to Study Neural Mechanisms of Action Observation-Execution: A Proof of Concept Study. Restorative Neurology and Neuroscience. 2009 doi: 10.3233/RNN-2009-0471. vol. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor RM. The Virtual Reality Peripheral Network (VRPN) 2006. [Google Scholar]
- 16.Quaney B, Meyer K, Cornwall MW, McPoil TG. A comparison of the dynamic pedobarograph and EMED systems for measuring dynamic foot pressures. Foot Ankle Int. 1995 Sep;16:562–6. doi: 10.1177/107110079501600909. [DOI] [PubMed] [Google Scholar]
- 17.Adamovich S, Fluet G, Qiu Q, Mathai A, Merians A. Incorporating haptic effects into three-dimensional virtual environments to train the hemiparetic upper extremity. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2009 doi: 10.1109/TNSRE.2009.2028830. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merians AS, Tunik E, Fluet GG, Qiu Q, Adamovich SV. Innovative approaches to the rehabilitation of upper extremity hemiparesis using virtual environments. Eur J Phys Rehabil Med. 2008 Dec 21; [PMC free article] [PubMed] [Google Scholar]
- 19.Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Arch Phys Med Rehabil. 1969 Jun;50:311–9. [PubMed] [Google Scholar]
- 20.Ramachandran VS, Rogers-Ramachandran D. Synaesthesia in phantom limbs induced with mirrors. Proc Biol Sci. 1996 Apr 22;263:377–86. doi: 10.1098/rspb.1996.0058. [DOI] [PubMed] [Google Scholar]
- 21.Altschuler EL, Wisdom SB, Stone L, Foster C, Galasko D, Llewellyn DM, Ramachandran VS. Rehabilitation of hemiparesis after stroke with a mirror. Lancet. 1999 Jun 12;353:2035–6. doi: 10.1016/s0140-6736(99)00920-4. [DOI] [PubMed] [Google Scholar]
- 22.Stefan K, Cohen LG, Duque J, Mazzocchio R, Celnik P, Sawaki L, Ungerleider L, Classen J. Formation of a motor memory by action observation. J Neurosci. 2005 Oct 12;25:9339–46. doi: 10.1523/JNEUROSCI.2282-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strafella AP, Paus T. Modulation of cortical excitability during action observation: a transcranial magnetic stimulation study. Neuroreport. 2000 Jul 14;11:2289–92. doi: 10.1097/00001756-200007140-00044. [DOI] [PubMed] [Google Scholar]
- 24.Patuzzo S, Fiaschi A, Manganotti P. Modulation of motor cortex excitability in the left hemisphere during action observation: a single- and paired-pulse transcranial magnetic stimulation study of self- and non-self-action observation. Neuropsychologia. 2003;41:1272–8. doi: 10.1016/s0028-3932(02)00293-2. [DOI] [PubMed] [Google Scholar]
- 25.Leonard G, Tremblay F. Corticomotor facilitation associated with observation, imagery and imitation of hand actions: a comparative study in young and old adults. Exp Brain Res. 2007 Feb;177:167–75. doi: 10.1007/s00221-006-0657-6. [DOI] [PubMed] [Google Scholar]
- 26.Lewis JW, Van Essen DC. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol. 2000 Dec 4;428:112–37. doi: 10.1002/1096-9861(20001204)428:1<112::aid-cne8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 27.Stepniewska I, Fang PC, Kaas JH. Microstimulation reveals specialized subregions for different complex movements in posterior parietal cortex of prosimian galagos. Proc Natl Acad Sci U S A. 2005 Mar 29;102:4878–83. doi: 10.1073/pnas.0501048102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis SJ, Slabosz A, Robbins TW, Barker RA, Owen AM. Dopaminergic basis for deficits in working memory but not attentional set-shifting in Parkinson’s disease. Neuropsychologia. 2005;43:823–32. doi: 10.1016/j.neuropsychologia.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Lewis JW, Van Essen DC. Mapping of architectonic subdivisions in the macaque monkey, with emphasis on parieto-occipital cortex. J Comp Neurol. 2000 Dec 4;428:79–111. doi: 10.1002/1096-9861(20001204)428:1<79::aid-cne7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 30.Dum RP, Strick PL. Frontal lobe inputs to the digit representations of the motor areas on the lateral surface of the hemisphere. J Neurosci. 2005 Feb 9;25:1375–86. doi: 10.1523/JNEUROSCI.3902-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang PC, Stepniewska I, Kaas JH. Ipsilateral cortical connections of motor, premotor, frontal eye, and posterior parietal fields in a prosimian primate, Otolemur garnetti. J Comp Neurol. 2005 Sep 26;490:305–33. doi: 10.1002/cne.20665. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell BD, Cauller LJ. Corticocortical and thalamocortical projections to layer I of the frontal neocortex in rats. Brain Res. 2001 Dec 7;921:68–77. doi: 10.1016/s0006-8993(01)03084-0. [DOI] [PubMed] [Google Scholar]
- 33.Graziano MS, Gross CG. Visual responses with and without fixation: neurons in premotor cortex encode spatial locations independently of eye position. Exp Brain Res. 1998 Feb;118:373–80. doi: 10.1007/s002210050291. [DOI] [PubMed] [Google Scholar]
- 34.Graziano MS, Gandhi S. Location of the polysensory zone in the precentral gyrus of anesthetized monkeys. Exp Brain Res. 2000 Nov;135:259–66. doi: 10.1007/s002210000518. [DOI] [PubMed] [Google Scholar]
- 35.Kakei S, Hoffman DS, Strick PL. Sensorimotor transformations in cortical motor areas. Neurosci Res. 2003 May;46:1–10. doi: 10.1016/s0168-0102(03)00031-2. [DOI] [PubMed] [Google Scholar]
- 36.Graziano MS, Yap GS, Gross CG. Coding of visual space by premotor neurons. Science. 1994 Nov 11;266:1054–7. doi: 10.1126/science.7973661. [DOI] [PubMed] [Google Scholar]


