Abstract
BACKGROUND
Recent studies demonstrate that glucocorticoids (GCs) have both supportive (stimulatory) and suppressive effects on immune responses, depending upon the GC concentration. Since some GC effects on inflammation are stimulatory, we hypothesized that acute in vivo GC depletion would decrease inflammatory responses of human monocytes.
METHODS
Monocytes were isolated from healthy volunteer participants before and after in vivo treatment with; 1) IV saline, 2) IV high dose hydrocortisone (8 μg · kg−1 · min−1) followed by oral hydrocortisone overnight, and 3) oral RU486 (200 mg at 0400 and 1600 h) to block the intracellular GC receptor and IV etomidate (1.5 mg · kg−1 · h−1) for 12 h to prevent compensatory adrenal cortisol synthesis. Plasma adrenocorticotropic hormone, plasma, and salivary cortisol were measured serially. Monocytes were tested for; 1) cytokine responses, 2) expression of CD163, CD119, and CD54, and 3) mRNA levels of GC-responsive inflammatory mediators. All measurements were made with and without in vitro stimulation of monocytes by lipopolysaccharide.
RESULTS
Cortisol and adrenocorticotropic hormone measurements demonstrated effective manipulation of in vivo cortisol. In vivo hypercortisolemia and in vivo GC depletion had reciprocal effects on monocyte mRNA levels of 4 important GC-responsive molecules: 1) GC receptor, CD163, interleukin-10, and suppressor of the cytokine synthesis-3. Monocyte cytokine responses and protein expression were not affected by GC depletion. CD163 expression was increased by hypercortisolemia.
CONCLUSIONS
Short-term GC depletion affects mRNA levels of GC-responsive molecules but does not affect monocyte protein expression or cytokine responses.
In 1949, Hench et al. surprised most of the medical community by reporting that glucocorticoids (GCs) suppress inflammation in humans.1 Their report, and a subsequent half century of research, effectively eclipsed decades of previous work by Selye and others who observed, in animals, stimulatory effects from GCs.2 Given the range of symptoms and pathophysiologic events that are caused by inflammation, it is not surprising that clinicians and researchers have chosen to search primarily for the antiinflammatory effect of GCs.
Recently, human inflammatory responses have gained new levels of clinical significance with the emergence of the Systemic Inflammatory Response Syndrome (SIRS) and related conditions as a leading cause of death in hospitalized patients.3 No widely accepted treatment for this highly lethal condition is available, and overall mortality from SIRS continues to increase.4 Recent studies suggest that some patients may benefit from GC therapy of SIRS if they are in a (patho)physiologic state termed “relative adrenocortical insufficiency” but these studies have not been confirmed and it is still unclear which patients may benefit from GC treatment and whether the benefit is due to suppression of inflammation. Interestingly, Selye used the term “relative adrenocortical insufficiency” as early as 19405 in a conceptual framework in which cortisol was understood to stimulate the resistance of an organism to the effects of injury.
Studies completed within the past 15 yr have used modern laboratory methods to reveal, at the cellular and molecular level, and often in a dose-dependent manner, stimulatory as well as suppressive effects of GCs on immune activity (Sapolsky et al. for recent review and definitions6). Perhaps most importantly, several studies report biphasic effects of GCs on the release of inflammatory mediators such as tumor necrosis factor-α (TNF-a), interleukin-6 (IL-6),7 acute phase proteins,8 and plasma macrophage migration inhibitory factor in vivo.9 These studies raise an important clinical question: Do all in vivo concentrations of cortisol have suppressive effects on human innate immune responses or do GC effects on immunity vary depending upon GC concentration? In this study we hypothesized that diurnal concentrations of cortisol support innate immune responses and that acute in vivo GC depletion would consequently act to decrease inflammatory responsiveness of circulating immune effector cells. Experimentally, we first either increased or decreased in vivo cortisol concentrations. Peripheral blood monocytes were then examined for their responses to the in vivo treatments and to further stimulation in vitro.
METHODS
This study was approved by the Dartmouth College Committee for the Protection of Human Subjects (IRB) and informed, written consent was obtained from all participants.
Participants
Participants (ages 18–50) yr were prescreened to confirm that they were healthy, taking no chronic medications, and had no recent illness (90 days). Female participants had either a prior tubal ligation or a hysterectomy to preclude the possibility of undiagnosed pregnancy.
Treatments
Each participant underwent three separate treatment interventions with each intervention taking place over a 3-day period with a washout interval of at least 45 days between each 3-day treatment: Control. Participants received a peripheral IV infusion of normal saline at 50 mL/h from 0900 h to 1700 h. High Cortisol. Participants received a peripheral IV infusion of hydrocortisone at 8 μg · kg−1 ·min−1 from 0900 h to 1700 h followed by 2 oral doses of hydrocortisone (3 mg/kg) at 2000 h and 2400 h. Low Cortisol. Participants received 2 oral doses of RU486 (Mifeprex®; Danco Laboratories, NY) 200 mg at 0400 h and 1600 h and, on the same day, a 12-h IV infusion of etomidate, 1.5 mg · kg−1 · h−1 from 0900 h to 2100 h.
Measurements
Peripheral blood was drawn into heparinized syringes at 8:30 am on the morning before treatment (day 1 [sample 1]), on the day of treatment at 8:30 am (sample 2), at 1230 (sample 3) and at 4:30 pm (sample 4), and on the morning after treatment at 8:30 am (day 3, [sample 5]). Monocyte isolation. Mononuclear cells were isolated by ficoll-hypaque density gradient (Histopaque 1077, Sigma-Aldrich, St. Louis, MO). Monocytes were positively selected from mononuclear cells with anti-CD14 magnetic beads (Miltenyi Biotec, Auburn, CA); purity was always more than 95%. Purified monocytes were cultured in 96-well flat bottomed plates in 5% CO2 at 37°C in complete medium consisting of HEPES-buffered RPMI 1640 (Hazelton Biologicals, Lenexa, KS), 20 mg/mL gentamicin sulfate (Elkins-Sinn, Cherry Hill, NJ), 5 × 10−5 M mercaptoethanol and 10% fetal bovine serum (Hyclone Laboratories, Logan, UT). Treatments and their duration were as noted in the Results. Cytokine analysis of monocyte cultures. Supernatants were collected and frozen at −80°C for batched measurement of TNFa, IL-6 and IL-10 concentrations. TNFa was measured using a TNFa sandwich enzyme-linked immunosorbent assay (ELISA) (paired antibodies, BD Biosciences Pharmingen, San Diego, CA). IL-6 levels were determined using an IL-6 ELISA kit (Peprotech, Rocky Hill, NJ). IL-10 was measured using an IL-10 ELISA kit (Biosource, Camarillo, CA). Cell staining and flow cytometry. After culture and treatments, washed monocytes were stained for surface expression of CD54, CD119, and CD163 (FITC-labeled anti-CD54 and FITC-labeled anti-CD119, R&D Systems, Minneapolis, MN; PE-labeled anti-CD163, Trillium Diagnostics, Bangor, ME). Cells were stained for flow cytometry directly in the 96-well plates on a bed of ice, causing adherent cells to detach. Cells were analyzed by flow cytometry on a FACSCAN flow cytometer and monocytes were gated on forward/side scatter to eliminate dead cells (<5%) from the analysis. Mean fluorescent intensity was determined by the geometric mean of the fluorescence of gated monocytes. Messenger RNA (mRNA) isolation and quantification. Adequate cell numbers for mRNA analysis were available after all 3 treatments (day 3, sample 5) for 5 participants (“n” = 5/group for all mRNA results). After 3 h treatment with or without lipopolysaccharide (LPS), total RNA was extracted from peripheral blood monocytes using RNeasy mini columns (Qiagen). RNA samples were treated with RNase-free DNase before amplification. RNA integrity and concentration were determined with RNA6000 Nano LabChip kit (Agilent). Using 500 ng of RNA as template, first-strand cDNA was synthesized using random hexamers and SuperScript II Moloney murine leukemia virus reverse transcriptase (Invitrogen). Expression of mRNA was quantified by Taqman polymerase chain reaction (PCR). cDNA (0.5 μL/well) was transferred into 96-well format plates, and Taqman Master Mix (Applied Biosystems) was added. Taqman-validated primers and Taqman MGB probes (labeled with fluorescent reporter dye 6FAM) were used for amplification of genes. To amplify GRalpha, Taqman primers that distinguish between GRalpha and βisoforms were used. Input cDNA was normalized with a validated predeveloped assay reagent β-actin primer probe pair (Applied Biosystems) as an internal control. Amplification was performed using an Applied Biosystems 7300 Real-Time PCR system with an optical unit that permits real-time monitoring of increased PCR product. Threshold cycle number was determined with Opticon software, and mRNA expression levels were normalized to β-actin levels with the formula 2−(Et−Rt), where Rt is the mean threshold cycle for the reference gene (β-actin), and Et is the mean threshold cycle for the experimental gene. Relative fluorescence units were assigned to these values and these data were used to generate the expression profiles reported. The expression of β-actin was consistent across samples when equal starting amounts of RNA were used, indicating that it was a good endogenous control for our material. No changes in β-actin expression were observed with GC or LPS treatment, excluding this as a source of bias in the analyses. The expression values of experimental genes indicated in the text were, therefore, normalized to the respective β-actin values for each sample. Cycling conditions for Taqman PCR consisted of 2 min at 50°C and 10 min at 95°C, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Amplicon accumulation was measured during the extension phase. All reactions were performed in triplicate. Data were analyzed using ABI 7300 quantification software (Applied Biosystems) and are expressed as relative mRNA. Cortisol. Total plasma cortisol was measured on an Immulite® analyzer using a DPC Cortisol kit (Los Angelos, CA). Salivary free cortisol, as a measure of active free cortisol at in vivo effector cell sites, was measured by Salimetrics® Expanded Range High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit (State College, PA). Adrenocorticotropic hormone (ACTH). Plasma ACTH was measured by solid-phase chemiluminscent assay (Immulite, Diagnostic Products Corporation). Statistical Analysis. Because participants were assayed over multiple days, statistical models incorporated the correlated nature of the data. We used generalized estimating equations to model the measurements with an exchangeable correlation matrix and Gaussian link. Data were analyzed on the log scale because the data were more normally distributed. All models incorporated an interaction term for time and assay conditions. For each measurement, differences between sample times were compared between treatment protocols as noted. A P value <0.05 was taken to indicate statistical significance without adjustment for multiple comparisons.10
RESULTS
Participants
Eight subjects (ages 19–47) yr completed all 3 treatment protocols; 6 were male and 2 were female. No clinical symptoms or signs were observed during or after any treatment intervention.
Cortisol and ACTH
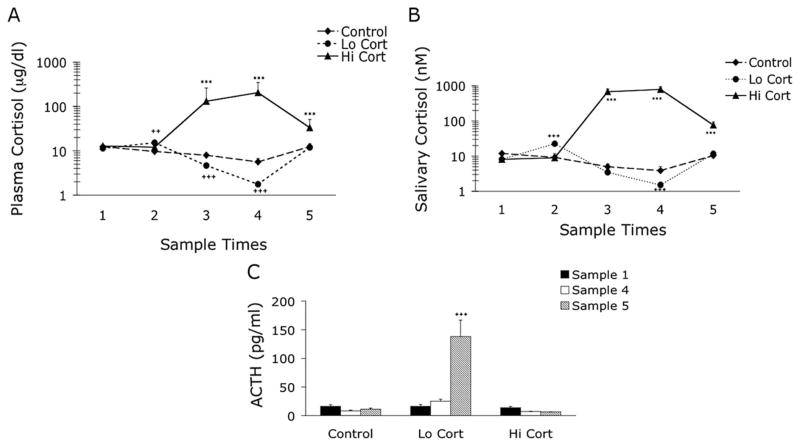
Total plasma cortisol, salivary free cortisol, and ACTH values are shown in Figures 1A–C. Plasma and salivary cortisol values increased slightly 4 h after the first RU486 dose on day 2 of the Low Cortisol treatment, just before the etomidate infusion (Figs. 1A and B). Although cortisol concentrations in plasma are expected to decrease during the day of the Control treatment (to reflect normal diurnal variations), etomidate caused a steeper decline in cortisol concentration during Low Cortisol treatment compared to Control treatment (Figs. 1A and B). Plasma and salivary free cortisol values were significantly increased during High Cortisol treatment at all time points after the start of treatment. Plasma ACTH values increased substantially on day 3 after Low Cortisol treatment (Fig. 1C).
Figure 1.
Cortisol and ACTH Responses. Total plasma cortisol in μg/dL (A), salivary free cortisol in nM (B) and plasma adrenocorticotropic hormone (ACTH) in pg/mL (C) on days 1–3 of each treatment protocol. Samples times were: 1) at 8:30 am on day 1, 2) 8:30 am on day 2 (4.5 h after first RU486 dose and before all other treatments), 3) 12:30 pm on day 2, 4) 4:30 pm on day 2, 5) 8:30 am on day 3. Lo Cort = low cortisol; Hi Cort = high cortisol. ++ = P < 0.01 Control versus Lo Cort; +++ = P < 0.001 Control versus Lo Cort; *** = P < 0.001 Control versus Hi Cort.
Monocyte mRNA Quantification
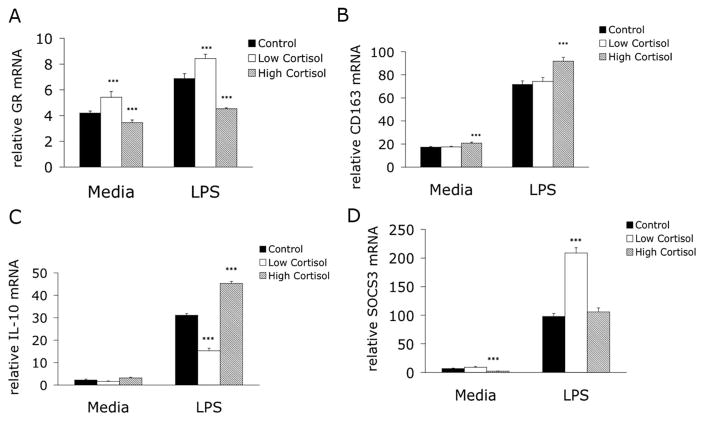
Monocytes were isolated after each treatment (day 3, sample 5) and incubated with or without 1 ng/mL LPS for 3 h. After Control treatment, in vitro stimulation of monocytes increased mRNA of the GC receptor (GR), CD163, IL-10, and Suppressor of Cytokine Synthesis 3 (SOCS3) (Figs. 2A–D). Compared to Control, High Cortisol treatment decreased GR mRNA levels in both unstimulated and stimulated monocytes while Low Cortisol treatment increased GR mRNA in both samples (Fig. 2A). CD163 mRNA levels increased after High Cortisol treatment in both unstimulated and LPS-stimulated cells, while Low Cortisol treatment did not affect CD163 mRNA (Fig. 2B). LPS stimulation increased IL-10 mRNA after all treatments. High Cortisol treatment further increased whereas Low Cortisol decreased LPS-induced IL-10 expression (Fig. 2C). Finally, High Cortisol treatment decreased SOCS3 mRNA in unstimulated monocytes whereas Low Cortisol treatment markedly increased SOCS3 mRNA in stimulated monocytes (Fig. 2D).
Figure 2.
High and Low Cortisol Treatment Effects on mRNA in Unstimulated and lipopolysaccharide (LPS)-stimulated Monocytes. After each of 3 in vivo treatments, monocytes were isolated from peripheral blood on day 3 (sample 5) and incubated for 3 h in either media alone or media supplemented with 1 ng/mL LPS. Messenger RNA levels were then determined for the glucocorticoid receptor (GR), CD163, interluekin-10 (IL-10) and Suppressor of Cytokine Synthesis-3 (SOCS3) as described in materials and methods section. GR mRNA levels (A) were increased significantly by in vivo Low Cortisol and decreased by in vivo High Cortisol treatment under both conditions. CD163 mRNA (B) was significantly increased by High Cortisol treatment. IL-10 mRNA (C) increased significantly in LPS-stimulated monocytes after High Cortisol treatment and decreased after Low Cortisol treatment. Finally, SOCS3 mRNA levels (D) were decreased in unstimulated cells after High Cortisol treatment and increased significantly after Low Cortisol treatment in stimulated cells. *** = P < 0.001 versus control cells under similar conditions.
Monocyte Cytokine Release and Protein Expression After LPS Stimulation
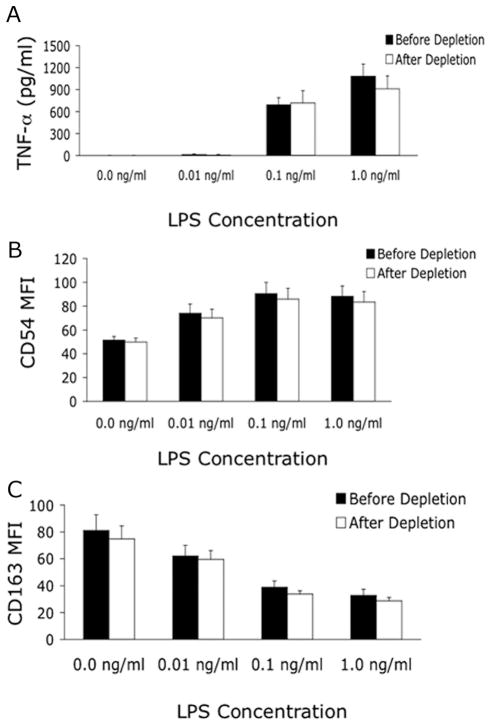
Monocytes were stimulated for 3 h without or with varying LPS concentrations (0.01, 0.1, 1.0 ng/mL) or for 24 h without or with 1 ng/mL LPS for comparisons of before and after Low Cortisol treatment. There was no evidence that the positive selection isolation procedure activated monocytes since there was no release of TNF-a into supernatant or CD163 shedding of untreated monocytes. Monocytes from all subjects released TNF-a after the 3-h incubation with LPS. TNF-a release was not different before and after Low Cortisol treatment and showed the expected dose-response relationship (Fig. 3A). IL-6 and IL-10 were detected after 3-h of LPS stimulation in only a few samples, while after 24-h incubation substantial amounts of all 3 cytokines were measured and were not different when comparing before and after Low Cortisol treatment values (data not shown). LPS stimulation led to a concentration-dependent increase in monocyte CD54 expression (Fig. 3B) and to a concentration-dependent decrease in CD163 expression (Fig. 3C), neither of which was affected by Low Cortisol treatment.
Figure 3.
Low Cortisol Treatment Does Not Affect Monocyte Protein Expression and Cytokine Release. Before (sample 1) and after (sample 5) in vivo glucocorticoids (GC) depletion, isolated monocytes (MO) were stimulated for 3 h in media alone or with lipopolysaccharide (LPS) at the concentrations shown. In vitro release of tumor necrosis factor (TNF) α (A) was LPS dose-dependent and unaffected by in vivo GC depletion. Expression of CD54 (B) increased while CD163 expression (C) decreased in an LPS dose-dependent manner. None of the measurements was affected by the in vivo GC depletion protocol.
Monocyte Surface CD163 Expression After 24 h In Vitro Culture
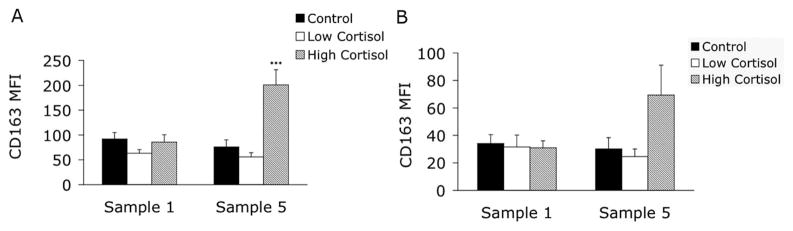
Monocytes isolated before and after each in vivo treatment were incubated overnight in medium (Fig. 4A) or in medium plus 1 ng/mL LPS (Fig. 4B). Comparison of the Low Cortisol and High Cortisol treatments to Control showed that High Cortisol increased monocyte surface expression of CD163 on unstimulated monocytes. In vitro treatment with LPS decreased monocyte CD163 expression before and after all treatments with no significant treatment effects noted (Fig. 4B). In vivo treatments did not affect monocyte CD119 expression (data not shown).
Figure 4.
High and Low Cortisol Treatment Effects on Monocyte CD163 Expression. Monocytes were isolated before (sample 1) and after (sample 5) each of the in vivo treatment protocols and incubated for 24 h in media alone (5A) or in media supplemented with 1 ng/mL lipopolysaccharide (5B). High dose glucocorticoids therapy (High Cortisol) resulted in increased CD163 expression (compared to controls) on unstimulated monocytes (*** = P < 0.001); lipopolysaccharide caused lower CD163 expression in all samples.
DISCUSSION
The first reports of lethal Addisonian crisis due to adrenal suppression by exogenous GCs appeared soon after the introduction of GCs as antiinflammatory drugs.11 The fact that humans require GCs to survive has made it difficult to investigate the in vivo effects of normal, diurnal cortisol concentrations on human responses to stress, especially the inflammatory response to injury and infection. Laue et al. reported no effect on human inflammatory immune responses after several days of GR blockade with RU486.12 However, use of RU486 alone causes rapid increases in plasma ACTH and cortisol, raising the possibility that the competitive GR blockade by RU486 is at least partially overcome by increased free cortisol at GR effector sites.13 We attempted to obviate the potentially confounding effects of increased cortisol synthesis after RU486 exposure by administering etomidate at a dose that is known to inhibit adrenal 11-β hydroxylase, and thereby suppress cortisol synthesis in humans.14 We did not administer etomidate alone because it leads to a significant increase in cortisol precursor molecules,15 leading to the possibility that cortisol concentrations could be restored to normal levels, especially in the evening hours when in vivo cortisol concentrations are very low. In order to eliminate this possibility, we used the combination of etomidate and RU486. The effectiveness of this combination for creating clinical GC “depletion” was reflected in the marked increase in ACTH concentrations that we measured after Low Cortisol treatment. ACTH, like thyroid-stimulating hormone, is widely used to determine whether or not sufficient quantities of active hormone are present in hormone-sensitive tissue.16 Prior studies of in vivo RU486 treatment alone reported an approximate doubling of plasma ACTH levels,12,13 while the combination of RU486 and etomidate in the current study resulted in an average eightfold increase in ACTH. Although both plasma and salivary free cortisol concentrations had returned to normal by the morning of day 3, the marked increase in ACTH at that time, an RU486 plasma half-life of approximately 20 h17 and etomidate-induced inhibition of cortisol synthesis that persists for up to 24 h after administration,18,19 all strongly suggest that a state of acute physiologic GC depletion was achieved by the morning of day 3 with the Low Cortisol treatment paradigm.
The exact nature of the depletion that was induced in this study is not clear from our experiments, since it is possible that any of the results that we observed could have been due to inhibition of GR-mediated GC effects or to known non-GR GC effects,20 or both. In addition, this study did not control for effects that could have been due solely to etomidate or to RU486, ACTH, or any of the several precursor molecules that accumulate in plasma after etomidate use.21 However, we believe it unlikely that any observed effects in this study were due to etomidate alone, because the concentration of etomidate needed to suppress adrenocortical synthesis (0.2 μM) is 1/50th the sedating concentration.22 The few in vitro studies that have examined etomidate’s effects on monocyte functions have reported either no effect23,24 or modest effects, but only at the much higher, sedating concentrations of the drug.25,26
We chose to test for changes in inflammatory responsiveness of isolated peripheral blood monocytes because of the central role that this cell population plays in orchestrating both innate and acquired immune processes. The isolation procedure itself (positive selection with magnetic beads) did not seem to affect any of our determinations, since unstimulated cells did not produce cytokines and expressed normal quantities of mRNA and protein. Assays of isolated monocytes precluded the possibility of measuring any effects that are dependent upon continued presence of plasma mediators such as cortisol, or effects due to the complex interactions in vivo between monocytes and other effector cells. The timing of the measurements and the duration of cell stimulation also warrant emphasis. We tested for GC effects 24 h after the initiation of etomidate (28 h after the first dose of RU486) in the Low Cortisol group and 24 h after the initiation of hypercortisolemia in the High Cortisol group. Although genomic effects of hypercortisolemia would be expected by this time, it is possible that we may have missed measurable effects from Low Cortisol treatment by testing too soon or too late. In addition, monocytes were stimulated in vitro with LPS for either 3 or 24 h, which may also have affected results, given the known serial nature of cytokine release from stimulated monocytes and the time-dependence of monocytes protein expression for molecules such as CD163.
The most intriguing results from this study are the demonstration that in vivo GC manipulation significantly altered monocyte mRNA levels of several GC-responsive molecules in a biphasic manner. GR receptor density has been repeatedly shown to decrease after exposure to high levels of GC both in vivo.27–29 and in vitro.30,31 The decrease (in both GR mRNA and protein) reported in the above studies was measured within the 24 h timeframe of this study. Our results further support a model for direct GC control of GR density in vivo. Notably, not only did high GC levels lead to decreased GR mRNA but withdrawal of GC activity led to a significant increase in GR mRNA. This bi-directional modulation of GR density by in vivo GC manipulations persisted under conditions of in vitro LPS stimulation of monocytes, which increases GR density within 4 h.32 Increased GR density has important clinical implications since cellular responses to GCs are largely controlled by the intracellular GR concentration. An increase in GR density after diminished GC activity could confer a protective (antiinflammatory) benefit in a complementary or even synergistic interaction with the cellular GR response to LPS.
The scavenger protein, CD163, is released from monocytes/macrophages after exposure to an inflammatory stimulus. Soluble CD163 in peripheral blood is an emerging marker of inflammatory disease in humans, whereas expression of CD163 by monocytes is consistently associated with an antiinflammatory phenotype.33,34 CD163 can thus be a marker of both pro-and antiinflammatory in vivo monocyte responses. Significantly, we now show that both CD163 mRNA levels and protein expression increase in healthy humans after 24 h of in vivo exposure to high concentrations of cortisol. However, the opposite effect, a decrease in CD163 mRNA and protein after GC depletion, was not observed in this study. Possible explanations for the latter result are: (a) constitutive CD163 expression is independent of GC activity; (b) other factors (also independent of GC activity) act to maintain monocyte CD163 expression; (c) by testing monocytes after 24 h of GC depletion, we failed to capture an earlier or later change in CD163 levels; or (d) the GC depletion intervention failed to decrease total GC activity (i.e., ligand-bound GR) below a threshold value for CD163 expression. The last possibility is supported by the increase in GR mRNA that we observed after GC depletion. The finding that LPS treatment markedly increased CD163 mRNA (independent of in vivo GC status) is at odds with our previous report that LPS-induced shedding of CD163 from monocytes is accompanied by a decrease in CD163 mRNA.33 The most likely explanation for this discrepancy is the timing of the measurements. Monocytes in the current study were stimulated with LPS for only 3 h before mRNA extraction, whereas in the former study they were stimulated for 18 h. Thus, it seems that LPS stimulation of monocytes leads to an early increase in CD163 mRNA (that is not reflected in protein expression) followed by a subsequent decrease. To the extent that CD163 expression represents an antiinflammatory phenotype for monocytes,34 the lack of down-regulation of monocyte CD163 expression after GC depletion is consistent with the lack of any effects on proinflammatory cytokine release by monocytes after LPS stimulation. In both instances, the GC depletion intervention did not augment monocyte inflammatory responses, suggesting again that normal diurnal concentrations of cortisol are not antiinflammatory. Finally, we found no changes in expression of other inflammatory response molecules that respond to GCs including the γ interferon receptor CD119 and the intercellular adhesion molecule CD54 but found no effects.
IL-10 has potent antiinflammatory properties and is GC-inducible in humans after an inflammatory stimulus such as cardiac surgery35 or endotoxemia.36 An increase in LPS-induced monocyte IL-10 mRNA after High Cortisol treatment is consistent with these reports. The decrease in LPS-induced monocyte IL-10 mRNA after the Low Cortisol treatment is the first such report to our knowledge. Importantly, this effect was observed after 3 h of LPS stimulation in vitro and did not translate into reduced IL-10 protein levels after 24 h of LPS stimulation in vitro, which suggests that time may be an important factor in GC control of IL-10 responses. Mechanistically, it is possible that in a low GC environment, IL-10 mRNA is acutely shunted to the exosome for mRNA degradation, and that existing stores of IL-10 protein are sequestered subcellularly. In this model, acute deprivation of GCs could result in reduced IL-10 message and low IL-10 protein and, if GC levels remain low, these existing IL-10 stores could be secreted and assist in the resolution of inflammation. In addition, if GR protein increased coincident with increased GR mRNA, as we showed, this effect could also explain the failure of low in vivo cortisol to affect IL-10 protein release in vitro.
SOCS3 has emerged as another potentially important antiinflammatory mediator.37,38 Reports have shown a positive and bi-directional correlation between SOCS3 and IL-10 expression. SOCS3 acts as a mediator of IL-10 antiinflammatory effects39 and skews effector cells towards an antiinflammatory phenotype40 while IL-10 augments SOCS3 expression.41 Suppression of SOCS3 mRNA by GCs has been previously reported in cultured rat hepatocyte.42 Consistent with these studies, we show that SOCS3 mRNA expression is inhibited in unstimulated monocytes after in vivo exposure to high GCs. We also found a surprisingly robust increase in SOCS3 mRNA when monocytes taken from GC-depleted subjects were stimulated with LPS. These same cells had decreased IL-10 mRNA compared to controls. Although IL-10 supports SOCS3 expression after LPS stimulation of monocytes, this effect does not seem to be active early in the inflammatory response to LPS. Qin et al. recently reported that de novo synthesis of protein is needed to support increases in SOCS3 expression four or more hours after LPS stimulation.43 In addition, they showed that IL-10 support for SOCS3 expression after LPS stimulation does not occur before 4 h. (As noted, we measured minimal monocyte production of IL-10 after 3 h of in vitro stimulation with LPS.) Preexisting GC concentrations may, therefore, be more important for SOCS3 expression early in an inflammatory response (0–4 h) while IL-10 levels become more important later in the inflammatory response. If so, an increase in SOCS3 mRNA after GC depletion may represent a protective response against excessive inflammation similar to the upregulation of GR mRNA that we observed. Finally, it is interesting to note that a biphasic effect of GCs has been shown for other mediators of the inflammatory response. The inflammatory mediator macrophage migration inhibition factor, for example, is stimulated by low concentrations of GCs while it is inhibited by high concentrations.44 Here, we show a potentially similar, though functionally opposing effect: an increase in the antiinflammatory mediator SOCS3 with decreased GC activity and a decrease with higher GC activity.
Since the suppressive effects of high concentrations of GCs on protein expression and inflammatory cytokine production are already well documented, we looked for opposing effects that might be measured in an in vivo environment of low GC activity. There are several potential explanations for the absence of any measurable effects of in vivo GC depletion on cytokine and protein expression: First, the inflammatory response is complex at the cellular level and the use of isolated monocytes may have precluded our ability to measure changes in inflammatory responses elicited in other immune cells that interact with monocytes in vivo. For example, GCs increase the density of inflammatory cytokine receptors on many immune effector cells45 and also augment inflammatory responsiveness of tissue macrophages.46 Either of these effects would lead to enhanced monocyte responses but would not have been apparent in our in vitro testing of isolated monocytes. Second, our measurements may have been made outside a “window” of positive or negative effects due to cell re-programming, to cell trafficking out of the central circulation or, in the case of the High Cortisol treatment, effects may have been partially reversed during in vitro incubation without cortisol in the incubation medium. Third, stimulation of monocytes with LPS may have simply overwhelmed more subtle, basal effects of endogenous cortisol. Finally, prior studies showing positive effects of GCs on immune responses have added or increased cortisol concentrations in the experimental system, while we attempted to remove cortisol from a system in which cortisol was previously present. Some immune effects from GCs, once established, may not be reversible in isolated cell systems or may require a longer duration of depletion before they can be measured. The single exception to GC effects on protein expression was the observation that High Cortisol treatment increased monocyte CD163 expression. This is an expected finding, given that GCs increase monocyte CD163 expression in vitro47 and, since CD163-expressing monocytes exhibit an enhanced antiinflammatory phenotype,48 this response is also consistent with antiinflammatory actions of high in vivo concentrations of cortisol.
In conclusion, acute in vivo GC depletion did not lead to enhanced monocyte inflammatory responses in vitro. This result suggests that in vivo GC effects on innate immunity are not universally antiinflammatory, since there was no increase in monocyte responsiveness after GC depletion. The monocyte mRNA responses after acute GC depletion create new data that: (a) support prior studies that tested high GC effects on GR and IL-10 mRNA, (b) expand on these prior studies by showing a biphasic response of GR and IL-10 mRNA GC activity, and (c) provide new insights into GC control of inflammatory responses, including a substantial increase in LPS-stimulated SOCS3 mRNA after acute GC depletion. The clinical implications of these findings remain, for the moment, a matter of speculation. The findings do, however, serve as a basis for future investigations that test for both suppressive and stimulatory effects of GCs on human immune responses.
Acknowledgments
Supported by the National Institutes of Health, NIAID # AI051547 (to P.M.G.).
We thank the nurses and staff of the 1 East patient care unit at Dartmouth-Hitchcock Medical Center for their expert clinical care and assistance in the completion of this study. We also thank Tracy Wynkoop for expert editorial and administrative assistance.
Footnotes
All work completed at the Dartmouth-Hitchcock Medical Center, Lebanon, NH 03756.
Presented, in part, at the Annual Meeting of the Society of Critical Care Medicine, 2006.
References
- 1.Hench PS, Kendall EC, Slocumb CH, Polley HF. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone: compound E) and of pituitary adrenocorticotropic hormone on rheumatoid arthritis; preliminary report. Mayo Clin Proc. 1949;24:181–97. [PubMed] [Google Scholar]
- 2.Selye H. Role of the hypophysis in the pathogenesis of the diseases of adaptation. Can Med Assoc J. 1944;50:426–33. [PMC free article] [PubMed] [Google Scholar]
- 3.Russell JA. Review article: drug therapy - management of sepsis. N Engl J Med. 2006;355:1699–713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- 4.Dombrovskiy V, Martin A, Sunderram J, Paz H. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1414–5. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 5.Selye H, Dosne C, Bassett L, Whittaker J. On the therapeutic value of adrenal cortical hormones in traumatic shock and allied conditions. Can Med Assoc J. 1940;43:1–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Sapolsky RM, Romero M, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 7.Liao J, Keiser J, Scales W, Kunkel S, Kluger M. Role of corticosterone in TNF and IL-6 production in isolated perfused rat liver. Am J Physiol. 1995;268:R699–R706. doi: 10.1152/ajpregu.1995.268.3.R699. [DOI] [PubMed] [Google Scholar]
- 8.Eastman H, Fawcett T, Udelsman R, Holbrook N. Effects of perturbations of the hypothalamic-pituitary-adrenal axis on the acute phase response: altered C/EBP and acute phase response gene expression in lipopolysaccharide-treated rats. Shock. 1996;6:286–92. doi: 10.1097/00024382-199610000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Petrovsky N, Socha L, Silva D, Grossman A, Metz C, Bucala R. Macrophage migration inhibitory factor exhibits a pronounced circadian rhythm relevant to its role as a glucocorticoid counter-regulator. Immunol Cell Biol. 2003;81:137–43. doi: 10.1046/j.0818-9641.2002.01148.x. [DOI] [PubMed] [Google Scholar]
- 10.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–60. [PubMed] [Google Scholar]
- 11.Fraser CG, Preuss FS, Bigford WD. Adrenal atrophy and irreversible shock associated with cortisone therapy. J Am Med Assoc. 1952;149:1542–3. doi: 10.1001/jama.1952.72930340001009. [DOI] [PubMed] [Google Scholar]
- 12.Laue L, Lotze M, Chrousos GP, Barnes K, Loriaux DL, Fleisher TA. Effect of chronic treatment with the glucocorticoid antagonist RU 486 in man; Toxicity, immunological, and hormonal aspects. J Clin Endocrinol Metab. 1990;71:1474–80. doi: 10.1210/jcem-71-6-1474. [DOI] [PubMed] [Google Scholar]
- 13.Bertagna X, Escourolle H, Pinquier JL, Coste J, Rauxdemay MC, Perles P, Silvestre L, Luton JP, Strauch G. Administration of RU 486 for 8 days in normal volunteers: antiglucocorticoid effect with no evidence of peripheral cortisol deprivation. J Clin Endocrinol Metab. 1994;78:375–80. doi: 10.1210/jcem.78.2.8106625. [DOI] [PubMed] [Google Scholar]
- 14.Allolio B, Schulte HM, Kaulen D, Reincke M, Jaursch-Hancke C, Winkelmann W. Nonhypnotic low-dose etomidate for rapid correction of hypercortisolaemia in Cushing’s Syndrome. Klin Wochenshr. 1988;66:361–4. doi: 10.1007/BF01735795. [DOI] [PubMed] [Google Scholar]
- 15.de Jong F, Mallios C, Jansen C, Scheck P, Lamberts S. Etomidate suppresses andrenocortical function by inhibition of 11β-hydroxylation. J Clin Endocrinol Metab. 1984;59:1143–7. doi: 10.1210/jcem-59-6-1143. [DOI] [PubMed] [Google Scholar]
- 16.Oelkers W, Diederich S, Bahr V. Diagnosis and therapy surveillance in Addison’s disease: rapid adrenocorticotropin (ACTH) test and measurement of plasma ACTH, renin activity, and aldosterone. J Clin Endocrinol Metab. 1992;75:259–64. doi: 10.1210/jcem.75.1.1320051. [DOI] [PubMed] [Google Scholar]
- 17.Liu JH, Garzo VG, Yen SS. Pharmacodyamics of the antiprogesterone RU486 in women after oral administration. Fertil Steril. 1988;50:245–9. doi: 10.1016/s0015-0282(16)60067-5. [DOI] [PubMed] [Google Scholar]
- 18.Moore RA, Allen MC, Wood PJ, Rees LH, Sear JW. Peri-operative endocrine effects of etomidate. Anaesthesia. 1985;40:124–30. doi: 10.1111/j.1365-2044.1985.tb10702.x. [DOI] [PubMed] [Google Scholar]
- 19.Duthie DJ, Fraser R, Nimmo WS. Effect of induction of anaesthesia with etomidate on corticosteroid synthesis in man. Br J Anaesth. 1985;57:156–9. doi: 10.1093/bja/57.2.156. [DOI] [PubMed] [Google Scholar]
- 20.Goulding NJ. The molecular complexity of glucocorticoid actions in inflammation - a four-ring circus. Curr Opin Pharmacol. 2004;4:629–36. doi: 10.1016/j.coph.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Preziosi P, Vacca M. Mini review: andrenocortical suppression and other endocrine effects of etomidate. Life Sci. 1988;42:477–789. doi: 10.1016/0024-3205(88)90087-2. [DOI] [PubMed] [Google Scholar]
- 22.Englelhardt D, Weber M. Therapy of Cushing’s Syndrome with steroid biosynthesis inhibitors. J Steroid Biochem Mol Biol. 1994;49:261–7. doi: 10.1016/0960-0760(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 23.Loop T, Liu Z, Humar M, Hoetzel A, Benzing A, Pahl H, Geiger K, Pannen JB. Thiopental inhibits the activation of nuclear factor kappaB. Anesthesiology. 2002;96:1202–13. doi: 10.1097/00000542-200205000-00025. [DOI] [PubMed] [Google Scholar]
- 24.Krumholz W, Reussner D, Hempelmann G. The influence of several intravenous anesthetics on the chemotaxis of human monocytes in vitro. Eur J Anaesth. 1999;16:547–9. doi: 10.1046/j.1365-2346.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- 25.Larsen B, Hoff G, Wilhelm W, Buchinger H, Wanner G, Bauer M. Effect of intravenous anesthetics on spontaneous and endotoxin-stimulated cytokine response in cultured human whole blood. Anesthesiology. 1998;89:1218–27. doi: 10.1097/00000542-199811000-00023. [DOI] [PubMed] [Google Scholar]
- 26.Kelbel I, Weiss M. Anaesthetics and immune function. [Review] Curr Opin Anaesthesiol. 2001;14:685–91. doi: 10.1097/00001503-200112000-00015. [DOI] [PubMed] [Google Scholar]
- 27.Shipman GF, Bloomfield CD, Gajl-Peczalska K, Munck AU, Smith KA. Glucocorticoids and lymphocytes. III. Effects of glucocorticoid administration on lymphocyte glucocorticoid receptors. Blood. 1983;61:1086–90. [PubMed] [Google Scholar]
- 28.Vachier I, Roux S, Chanez P, Loubatiere J, Terouanne B, Nicolas JC, Godard P. Glucocorticoids induced down-regulation of glucocorticoid receptor mRNA expression in asthma. Clin Exp Immunol. 1996;103:311–5. doi: 10.1046/j.1365-2249.1996.d01-628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong Y, Poellinger L, Gustafsson JA, Okret S. Regulation of glucocorticoid receptor expression: evidence for transcriptional and posttranslational mechanisms. Mol Endocrinol. 1988;2:1256–64. doi: 10.1210/mend-2-12-1256. [DOI] [PubMed] [Google Scholar]
- 30.Silva CM, Powell-Oliver FE, Jewell CM, Sar M, Allgood VE, Cidlowski JA. Regulation of the human glucocorticoid receptor by long-term and chronic treatment with glucocorticoid. Steroids. 1994;59:436–42. doi: 10.1016/0039-128x(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 31.Burnstein KL, Bellingham DL, Jewell CM, Powell-Oliver FE, Cidlowski JA. Autoregulation of glucocorticoid receptor gene expression. Steroids. 1991;56:52–8. doi: 10.1016/0039-128x(91)90124-e. [DOI] [PubMed] [Google Scholar]
- 32.Salkowski CA, Vogel S. Lipopolysaccharide increases glucocorticoid receptor expression in murine macrophages. A possible mechanism for glucocorticoid-mediated suppression of endotoxicity. J Immunol. 1992;149:4041–7. [PubMed] [Google Scholar]
- 33.Sulahian TH, Pioli PA, Wardwell K, Guyre PM. Cross-linking of FcgammaR triggers shedding of the hemoglobin-haptoglobin scavenger receptor CD163. J Leukoc Biol. 2004;76:271–7. doi: 10.1189/jlb.1003523. [DOI] [PubMed] [Google Scholar]
- 34.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJC, John S, Taams LS. CD4+ CD25+ Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104:19446–51. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeager MP, Rassias AJ, Fillinger MP, Discipio AW, Gloor KE, Gregory JA, Guyre PM. Cortisol antiinflammatory effects are maximal at postoperative plasma concentrations. Crit Care Med. 2005;33:1507–12. doi: 10.1097/01.ccm.0000164565.65986.98. [DOI] [PubMed] [Google Scholar]
- 36.van der Poll T, Barber AE, Coyle SM, Lowry SF. Hypercortisolemia increases plasma interleukin- 10 concentrations during human endotoxemia – a clinical research center study. J Clin Endocrinol Metab. 1996;81:3604–6. doi: 10.1210/jcem.81.10.8855809. [DOI] [PubMed] [Google Scholar]
- 37.Croker BA, Krebs DL, Zhang J-G, Wormald S, Willson TA, Stanley EG, Robb L, Greenhalgh CJ, Förster I, Clausen BE, Nicola NA, Metcalf D, Hilton DJ, Roberts AW, Alexander WS. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003;4:540–5. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- 38.Daewoong J, Liu D, Yao S, Collins RD, Hawiger J. Intracellular protein therapy with SOCS3 inhibits inflammation and apoptosis. Nature Med. 2005;11:892–8. doi: 10.1038/nm1269. [DOI] [PubMed] [Google Scholar]
- 39.Qin H, Wilson CA, Roberts KL, Baker BJ, Zhao X, Benveniste EN. IL-10 inhibits lipopolysaccharide-induced CD40 gene expression through induction of suppressor of cytokine signaling-3. J Immunol. 2006;177:7761–71. doi: 10.4049/jimmunol.177.11.7761. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Chu N, Rostami A, Zhang GX. Dendritic cells transduced with SOCS-3 exhibit a tolerogenic/DC2 phenotype that directs type 2 Th cell differentiation in vitro and in vivo. J Immunol. 2006;177:1679–88. doi: 10.4049/jimmunol.177.3.1679. [DOI] [PubMed] [Google Scholar]
- 41.Gasperini S, Crepaldi L, Calzetti F, Gatto L, Berlato C, Bazzoni F, Yoshimura A, Cassatella MA. Interleukin-10 and cAMP-elevating agents cooperate to induce suppressor of cytokine signaling-3 via a protein kinase A-independent signal. Eur Cytokine Netw. 2002;13:47–53. [PubMed] [Google Scholar]
- 42.Paul C, Seiliez I, Thissen JP, LeCam A. Regulation of expression of the rat SOCS-3 gene in hepatocytes by growth hormone, interleukin-6 and glucocorticoids mRNA analysis and promoter characterization. Eur J Biochem. 2000;267:5849–57. doi: 10.1046/j.1432-1327.2000.01395.x. [DOI] [PubMed] [Google Scholar]
- 43.Qin H, Roberts KL, Niyongere SA, Cong Y, Elson CO, Benveniste EN. Molecular mechanism of lipopolysaccharide-induced SOCS-3 gene expression in macrophages and microglia. J Immunol. 2007;179:5966–76. doi: 10.4049/jimmunol.179.9.5966. [DOI] [PubMed] [Google Scholar]
- 44.Calandra T, Bernhagen J, Metz CN, Splegel LA, Bacher M, Donnelly T, Cerami A, Bucala R. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995;377:68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- 45.Wiegers G, Reul J. Induction of cytokine receptors by glucocorticoids: functional and pathological significance. Trends Pharmacol Sci. 1998;19:317–21. doi: 10.1016/s0165-6147(98)01229-2. [DOI] [PubMed] [Google Scholar]
- 46.Zhang T, Daynes R. Glucocorticoid conditioning of myeloid progenitors enhances TLR4 signaling via negative regulation of the phosphatidylinositol 3-kinase-Akt pathway. J Immunol. 2007;178:2517–26. doi: 10.4049/jimmunol.178.4.2517. [DOI] [PubMed] [Google Scholar]
- 47.Sulahian TH, Hogger P, Wahner AE, Wardwell K, Goulding NJ, Sorg C, Droste A, Stehling M, Wallace PK, Morganelli PM, Guyre PM. Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine. 2000;12:1312–21. doi: 10.1006/cyto.2000.0720. [DOI] [PubMed] [Google Scholar]
- 48.Philippidis P, Mason JC, Evans BJ, Nadra I, Taylor KM, Haskard DO, Landis RC. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte- responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ Res. 2004;9:119–26. doi: 10.1161/01.RES.0000109414.78907.F9. [DOI] [PubMed] [Google Scholar]