Abstract
Purpose
DNA adduct levels may be influenced by metabolic activity, DNA repair capabilities, and genomic integrity, all of which play a role in cancer progression.
Experimental Design
To determine if elevated DNA adducts are a marker for prostate cancer progression, we measured polycyclic aromatic hydrocarbon - DNA adducts by immunohistochemistry in prostate cells of 368 surgical prostate cancer patients treated at the Henry Ford Hospital in Detroit, Michigan, between September 1999 and July 2004. Patients were followed up to 5 years after surgery with relative risk for biochemical recurrence (BCR) estimated with a Cox proportional hazards model that adjusted for standard clinical risk factors.
Results
At 1 year of follow-up, patients with adduct levels above the median in tumor cells [hazard ratio (HR), 2.40; 95% confidence interval (95% CI), 1.10–5.27] and nontumor cells (HR, 3.22; 95% CI,1.40–7.39) had significant increased risk of BCR, but these HRs decreased to 1.12 (95% CI, 0.68–1.83) and 1.46 (95% CI, 0.89–2.41) in tumor and nontumor cells at 5 years postsurgery. When we restricted our analysis to patients with advanced-stage (III+) disease, those with high adduct levels in either tumor (53.5% versus 30.2%; P = 0.07) or nontumor (55.2% versus 28.6%; P = 0.02) cells had BCR rates almost 2-fold higher. In race-stratified analyses, the greatest risk of BCR associated with high adduct levels (in nontumor cells) was for African American patients younger than 60 years old (HR, 3.79; 95% CI,1.01–14.30).
Conclusions
High polycyclic aromatic hydrocarbon - DNA adduct levels in nontumor prostate cells are most strongly associated with BCR between 1 and 2 years after surgery and in patient subsets defined by younger age, advanced tumor stage, and African American race.
Polycyclic aromatic hydrocarbons (PAH) result from incomplete combustion processes, are ubiquitous environmental contaminants, and are known carcinogens (1). PAH derive their carcinogenic properties through their ability to form PAH-DNA adducts (2, 3). In vitro experiments have detected DNA adducts in human prostate after exposure to benzo(a)pyrene (4, 5), a known carcinogenic PAH, and have shown that exposure levels of benzo(a)pyrene are positively correlated with DNA damage as measured by the comet assay (6). In men diagnosed with prostate cancer who underwent radical prostatectomy, we found that levels of PAH-DNA adducts in prostate epithelial cells were inversely related to tumor grade (7), and more recently have also shown that the effects of underlying genetic variation in PAH-metabolizing enzymes and cigarette smoke exposure leading to PAH-DNA adduct formation in the prostate may be different by race (8).
Retrospective epidemiologic studies support a link between occupational PAH exposure and prostate cancer risk (9–11), but a recent prospective cohort study was unable to replicate this association (12). Occupational PAH exposure may need to reach a threshold level before having an effect on cancer risk (13, 14), and genetic susceptibility likely also plays a role (13, 15). Other environmental sources of PAH include diet (16) and cigarette smoke (17). Whether an increased prostate cancer risk is associated with cigarette smoke is unclear (18–20), although several recent studies suggest that cigarette smoke exposure in combination with genetic risk factors for bulky PAH-DNA adduct formation may increase prostate cancer risk (13, 15, 21). Dietary intake of PAH is primarily through consumption of well-done meats, but epidemiologic evidence for an association between meat consumption and increased risk for prostate cancer is equivocal (22). Only one epidemiologic study has examined dietary intake of benzo(a)pyrene, the primary PAH in well-done meats, and prostate cancer risk, but it had null results (23).
Whereas prior examination of PAH exposures on prostate cancer risk has predominantly relied on self-reported measures, PAH-DNA adducts may serve as a marker of the biologically effective dose of all types of PAH exposure that is less prone to information bias. For prostate cancer patients, predicting who will have recurrent disease after primary treatment has traditionally relied on clinical and pathologic variables such as Gleason grade and tumor stage. More recently, molecular approaches to predicting prostate cancer recurrence using proteomic and expression array technologies have expanded the potential markers of poor disease outcome (24), but biomarkers currently under investigation lack information about the prostate cell DNA integrity and capacity to metabolize and clear carcinogens. In addition to an individual’s PAH exposures, PAH-DNA adduct levels in the prostate reflect metabolic capacity to activate PAH compounds for DNA binding, PAH detoxification capacity, and DNA repair capacity, biological variables that may also be related to cancer prognosis. For instance, the same metabolic enzymes that activate PAH adduct–forming compounds, such as CYP1B1, may also stimulate cancer cell growth and division (25, 26).
If PAH-DNA adducts in prostate cells are indicative of the overall metabolic activity, DNA repair, and genomic integrity of the prostate, then they may be related to prostate cancer progression. To test this hypothesis, we measured PAH-DNA adduct levels in tumor and adjacent nontumor prostate cells of men that had a radical prostatectomy and then followed these men for prostate-specific antigen (PSA) failure to determine whether adduct levels could predict recurrent disease.
Materials and Methods
Study sample ascertainment and follow-up
Between July 1, 2001, and December 31, 2004, we attempted to enroll 863 men that had a prostate cancer diagnosis within the last 2 years at the Henry Ford Health System in Detroit, Michigan, as part of a prostate cancer-case control study (13), and 668 agreed to participate (77%). During the course of enrollment, 8 cases were found ineligible and 23 cases did not complete the study protocol, resulting in final study participation percentages of 75% (637 of 855). Of these 637 cases, 419 (66%) underwent radical prostatectomy. Tissue specimens with sufficient areas of tumor and nontumor cells were available for 392 (94%) of these patients such that immunohistochemical studies for PAH-DNA adduct determination could be done. For these 392 patients, whose dates of prostatectomy occurred between September 1, 1999, and December 27, 2004, we then electronically retrieved all PSA tests from the date of surgery forward. A total of 3,413 test results were retrieved, with the men in this sample having a median of eight PSA tests and the number of tests ranging from 0 to 46 tests. We excluded the 2 men who had no PSA tests, 8 men who had only one PSA test following surgery, and 14 men who also had hormone treatment. The remaining 368 men comprised the analytic study sample. All protocols used in this study were reviewed and approved by the Henry Ford Hospital Institutional Review board and all study participants signed an informed consent before participating.
Pathology
H&E-stained slides of study cases were reviewed by the study pathologist (A.T.S.) to confirm the diagnosis and identify a paraffin block with sufficient tumor and nontumor prostatic tissue for staining. For each patient sample, consecutive sections (5-µm-thick) were cut from the tissue block. One slide was H&E stained and examined by the study pathologist who circled two separate areas of tumor and nontumor cell populations to be used for adduct scoring. Tumors were characterized according to lymph node involvement, primary and secondary grade (i.e., Gleason score), lobe involvement, extraprostatic extension, and seminal vesicle involvement.
Immunohistochemistry
The immunohistochemical assay for PAH-DNA adducts was carried out as described previously (27, 28). This chemical assay uses the monoclonal 5D11 antibody, which in cell culture studies has been shown to produce strongly correlated staining levels (r = 0.99; P = 0.011) with the treatment dose of benzo(a)pyrene diol epoxide (29, 30). Consistent with our previous study (7) and other prior studies (27, 31) using immunohistochemical assays to measure PAH-DNA adducts, we report our results in absorbance units, which provide a measure of the relative intensity of staining. For each prostate specimen, two technicians independently scored 50 epithelial cells (five fields with 10 cells per field scored) in the two areas (tumor and nontumor) circumscribed by the study pathologist. Scored cells were selected to be representative, in terms of intensity, of the cells in the field and the mean of the two technicians’ scores was used. The dual scoring technique has proven to yield a high test-retest reliability in prostate cells (7). PAH-DNA adduct data were standardized across experiments using a series of two “control” slides cut from two separate nonstudy prostate specimens that were run across all batches.
Statistical analyses
A biochemical recurrence (BCR) event was defined as having two consecutive detectable (>0.2 ng/mL) increasing PSA levels 4 weeks or more after surgery (32, 33). Time to event was the duration between the dates of surgery and the second PSA test that defined the recurrence event or censored at the last postoperative PSA test for men that did not recur. HRs for BCR were estimated with Cox proportional hazards models using PROC PHREG in the Statistical Analysis Software package (34). Differences in survival curves were tested using the Wilcoxon rank test in PROC LIFETEST. In addition to adduct levels measured in absorbance units, multivariable models included age, race, pack-years of cigarette smoking, tumor stage, Gleason grade, and preoperative PSA level.
Results
PAH-DNA adduct levels in the prostate tumor and nontumor cells of 368 study participants did not vary significantly by age, race, dietary PAH intake, body size, family history of prostate cancer, PSA at surgery, or advanced tumor grade (Table 1). Tumor stage was significantly inversely associated with PAH-DNA adduct levels in tumor and nontumor cells, and current smokers had a suggestive, albeit nonsignificant, association with higher PAH-DNA adduct levels in both nontumor and tumor cells. A BCR event was experienced by 67 (18.2%) men in the analytic sample that had a median time to recurrence of 14 months with recurrence times ranging between 1.5 and 60 months. Men without a BCR event had follow-up ranging from 2 to 81 months with a median follow-up time of 38 months. For the purposes of analysis and presentation of survival data, all follow-up was censored at 60 months. Men that experienced BCR were more likely to have tumors with advanced Gleason grade, advanced tumor stage, and higher PSA levels at diagnosis (Table 2). Age, race, and smoking status were not associated with BCR nor were mean PAH-DNA adduct levels in either tumor or nontumor prostate cells. Quantifying smoking exposure by pack-years showed that men that experienced prostate cancer recurrence had a marginally higher exposure level to cigarette smoke compared with those that did not recur (24.3 ± 8.2 versus 17.8 ± 22.9 pack-years; P = 0.08).
Table 1.
PAH-DNA adduct levels in tumor and nontumor prostate cells of 368 prostate cancer cases by selected characteristics
| Characteristic | Tumor cells | P | Nontumor cells | P |
|---|---|---|---|---|
| Age | ||||
| <60 | 0.152 ± 0.004 | 0.31 | 0.247 ± 0.006 | 0.92 |
| 60+ | 0.147 ± 0.004 | 0.246 ± 0.005 | ||
| Race | ||||
| African American (n = 162) | 0.150 ± 0.004 | 0.67 | 0.246 ± 0.006 | 0.99 |
| Caucasian or other*(n = 206) | 0.148 ± 0.004 | 0.246 ± 0.005 | ||
| Cigarette smoking status | ||||
| Never (n = 136) | 0.144 ± 0.005 | 0.07 | 0.241 ± 0.007 | 0.31 |
| Former (n = 194) | 0.149 ± 0.004 | 0.246 ± 0.006 | ||
| Current (n = 38) | 0.166 ± 0.009 | 0.263 ± 0.013 | ||
| Dietary PAH intake | ||||
| Below median (<; n = 184) | 0.153 ± 0.004 | 0.17 | 0.250 ± 0.006 | 0.30 |
| Above median (>; n = 184) | 0.145 ± 0.004 | 0.242 ± 0.006 | ||
| Body size | ||||
| Normal (BMI <25 kg/m2; n = 80) | 0.145 ± 0.006 | 0.65 | 0.235 ± 0.009 | 0.37 |
| Overweight (BMI 25–29.9 kg/m2; n = 190) | 0.151 ± 0.004 | 0.249 ± 0.006 | ||
| Obese (BMI 30+; n = 98) | 0.148 ± 0.005 | 0.250 ± 0.008 | ||
| Family history† | ||||
| Negative (n = 269) | 0.149 ± 0.003 | 0.50 | 0.246 ± 0.005 | 0.50 |
| Positive (n = 90) | 0.144 ± 0.005 | 0.239 ± 0.008 | ||
| PSA at surgery (ng/mL) | ||||
| <4 (n = 65) | 0.153 ± 0.007 | 0.72 | 0.252 ± 0.010 | 0.23 |
| 4–10 (n = 245) | 0.147 ± 0.003 | 0.241 ± 0.005 | ||
| >10 (n = 58) | 0.151 ± 0.007 | 0.259 ± 0.010 | ||
| Pathologic tumor stage | ||||
| 2 (n = 297) | 0.153 ± 0.003 | 0.007 | 0.252 ± 0.005 | 0.006 |
| 3 or 4 (n = 71) | 0.134 ± 0.006 | 0.246 ± 0.005 | ||
| Advanced tumor grade‡ | ||||
| No (n = 259) | 0.150 ± 0.003 | 0.27 | 0.246 ± 0.005 | 0.46 |
| Yes (n = 109) | 0.146 ± 0.005 | 0.247 ± 0.008 |
NOTE: PAH-DNA adduct levels are measured in absorbance units.
Abbreviation: BMI, body mass index.
“Other” includes one Asian and two Hispanic cases.
Positive family history is defined as having a brother or father diagnosed with prostate cancer; nine had unknown family history.
Advanced tumor grade is defined as total Gleason grade of 8 or higher or total Gleason grade of 7 and primary Gleason grade of 4 or higher.
Table 2.
Characteristics of 368 prostate cancer cases by BCR status after surgery
| Characteristic | No recurrence (n = 301) | Recurrence (n = 67) | P |
|---|---|---|---|
| Age | 61.0 ± 6.8 | 60.9 ±6.1 | 0.84 |
| Percent African American | 44.2 | 43.3 | 0.89 |
| Observation time (mo)* | 51.0 ±16.1 | 54.5 ±17.6 | 0.13 |
| PSA at diagnosis (ng/mL) | 6.0 ±4.2 | 11.1 ±10.1 | 0.0001 |
| Advanced Gleason grade† | 16.9 | 49.3 | <0.0001 |
| Advanced tumor stage (III or IV) | 14.3 | 41.8 | <0.0001 |
| Cigarette smoking status | |||
| Never | 38.2 | 31.3 | 0.56 |
| Former | 51.5 | 58.2 | |
| Current | 10.3 | 10.5 | |
| PAH-DNA adduct level in tumor cells‡ | 0.149 ±0.053 | 0.149 ±0.053 | 0.96 |
| PAH-DNA adduct level in nontumor cells‡ | 0.245 ±0.078 | 0.252 ±0.079 | 0.49 |
Time from study entry to date of last PSA test for the entire cohort.
Gleason sum of 8 or higher or primary Gleason grade 4 or higher.
Expressed in absorbance units.
To determine whether PAH-DNA adduct levels were associated with BCR in prostate cancer in a nonlinear fashion, we investigated associations between time to BCR and adduct levels by quartile and median in tumor and nontumor prostate cells. There was no evidence for a trend by quartile in either tumor (P = 0.78) or nontumor (P = 0.26) cells. In tumor cells, the hazard ratio (HR) associated with PAH-DNA adduct levels above the median was slightly elevated, but not statistically significant [HR, 1.18; 95% confidence interval (95% CI), 0.72–1.94; P = 0.51]. In nontumor cells, a larger HR was observed, but it did not reach statistical significance (HR, 1.56; 95% CI, 0.94–2.59; P = 0.08).
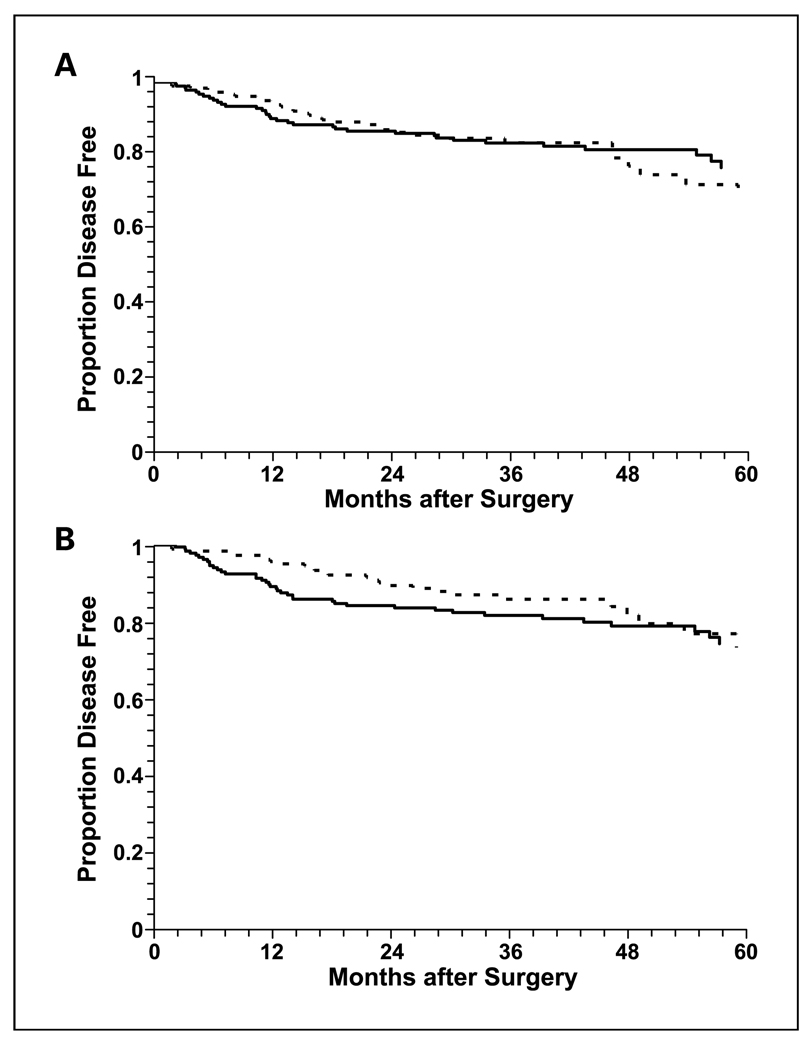
The unadjusted BCR distributions stratified according to high and low PAH-DNA adduct levels in tumor (Fig.1A) and nontumor (Fig.1B) prostate cells were not significantly different (P = 0.88 in tumor cells; P = 0.11 in nontumor cells). Although the survival curves tended to move toward each other as follow-up time increased, at earlier follow-up durations (up to 2 years for tumor cells and 4 years in nontumor cells), higher adduct levels were associated with a higher event rate. To quantify the association of high PAH-DNA adduct levels and BCR by follow-up time, we recalculated the HRs for follow-up times ranging from 1 to 3 years (Table 3). In tumor cells, the strongest association with higher PAH-DNA adduct levels was at 1 year of follow-up. In the third and fourth highest quartiles of adduct levels, HRs were both greater than 3. The test for trend for increasing risk across the four quartiles was statistically significant (P = 0.03) and the HR for PAH-DNA adduct levels above the median was 2.41 (95% CI, 1.10–5.29). In nontumor cells, the strongest association with higher PAH-DNA adduct levels was also observed at 1 year of follow-up. In the third and fourth highest quartiles of adduct levels, HRs were 3.83 and 3.45, respectively. The test for trend for increasing risk across the four quartiles was statistically significant (P = 0.01) and the HR for PAH-DNA adduct levels above the median was 3.24 (95% CI, 1.41–7.42). The HRs for PAH-DNA adduct levels above the median in nontumor cells were significant for follow-up periods up to 3 years and were consistently higher than comparable HRs for high PAH-DNA adduct levels in tumor cells.
Fig. 1.
Kaplan-Meier survival curves for BCR in prostate cancer patients stratified by low (below median, broken line) and high (above median, solid line) PAH-DNA adduct levels in tumor (A) and nontumor (B) cells.
Table 3.
Risk of BCR after prostatectomy in 368 prostate cancer cases at different lengths of follow-up associated with PAH-DNA adduct levels in prostate cells adjusting for clinical risk factors
| Cell type | Follow-up period | |||
|---|---|---|---|---|
| Model variable | 1 y | 18 mo | 2 y | 3 y |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Tumor adduct level | ||||
| 2nd Quartile | 1.79 (0.49–6.52) | 1.06 (0.42–2.67) | 0.99 (0.42–2.34) | 0.79 (0.36–1.76) |
| 3rd Quartile | 3.43 (1.09–10.77) | 1.71 (0.75–3.90) | 1.54 (0.71–3.34) | 1.37 (0.68–2.74) |
| 4th Quartile | 3.05 (0.82–11.38) | 1.31 (0.47–3.61) | 1.39 (0.56–3.46) | 1.12 (0.49–2.54) |
| Linear trend | 1.50 (1.04–2.17) | 1.17 (0.87–1.57) | 1.17 (0.88–1.54) | 1.10 (0.86–1.42) |
| Above median | 2.41 (1.10–5.29) | 1.52 (0.80–2.87) | 1.48 (0.82–2.70) | 1.41 (0.82–2.43) |
| Nontumor adduct level | ||||
| 2nd Quartile | 1.29 (0.31–5.35) | 0.96 (0.32–2.84) | 0.89 (0.33–2.40) | 0.80 (0.34–1.91) |
| 3rd Quartile | 3.83 (1.20–12.27) | 3.15 (1.32–7.48) | 2.66 (1.20–5.91) | 1.89 (0.92–3.92) |
| 4th Quartile | 3.45 (1.04–11.43) | 1.82 (0.69–4.82) | 1.77 (0.73–4.27) | 1.50 (0.69–3.28) |
| Linear trend | 1.57 (1.11–2.22) | 1.34 (1.01–1.78) | 1.32 (1.01–1.71) | 1.23 (0.96–1.57) |
| Above median | 3.24 (1.41–7.42) | 2.56 (1.32–4.99) | 2.35 (1.27–4.34) | 1.89 (1.09–3.28) |
| No. events | 28 | 40 | 46 | 55 |
NOTE: Clinical risk factors include PSA, tumor grade, and tumor stage.
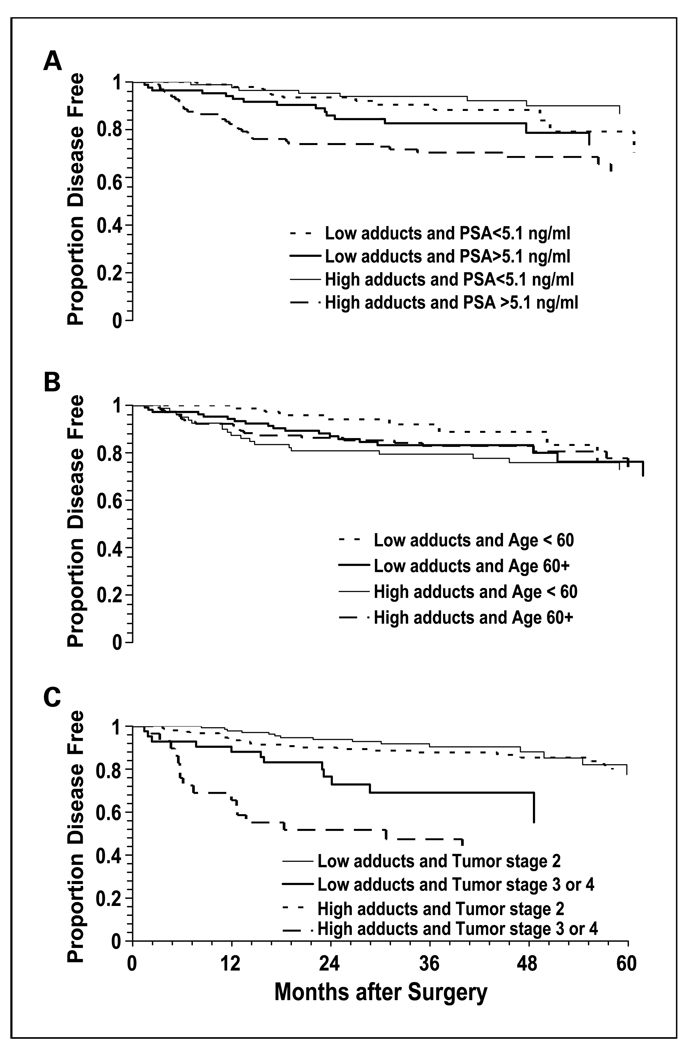
We next investigated whether high PAH-DNA adduct levels in tumor and nontumor prostate cells might have stronger associations with BCR in patient subsets defined by known clinical risk factors such as high PSA level at diagnosis, advanced tumor stage, and advanced Gleason grade. We also examined patients dichotomized by race (Caucasian, African American) and age (<60, ≥60 years) with age categories based largely on the distribution of patients in the study sample, but also driven by several studies that suggest prostate cancer patients younger than 60 years old have worse outcomes (35–37). Of these five factors, tumor stage was the strongest modifying factor of the association between high PAH-DNA adduct levels in tumor cells and BCR. In patients with tumor stage III or IV, those with high PAH-DNA adducts had almost a 2-fold greater BCR rate over 5 years (53.5% versus 30.2%; P = 0.07). In nontumor cells, age and PSA level as well as tumor stage had differential associations with BCR (Fig.2A–C). In separate analyses of the higher risk groups for these three factors, the BCR rates for high PAH-DNA adduct levels were significantly different in patients with tumor stage III or IV (55.2% versus 28.6%; P = 0.02), younger than 60 years old (23.8% versus 10.4%; P = 0.02), and with PSA levels above the median (31.6% versus 17.6%; P = 0.03).
Fig. 2.
Kaplan-Meier survival curves for BCR in prostate cancer patients stratified by PAH-DNA adduct levels (above vs below median) in nontumor cells and (A) PSA at diagnosis; (B) age at diagnosis; and (C) tumor stage.
To determine how BCR associations with high adduct levels varied with follow-up time within patient subsets, we calculated HRs associated with high PAH-DNA adduct levels in tumor and nontumor cells for patient subsets defined by tumor stage, age at surgery, and PSA level at diagnosis at 2, 3, and 5 years of follow-up (Table 4). In tumor cells, the HR for high PAH-DNA adduct levels in patients with advanced tumor stage was consistently elevated in the range of 1.86 to 1.93 between 2 and 5 years of follow-up. In nontumor cells, high PAH-DNA adduct levels were associated with the greatest risk for BCR in patients younger than 60 years old after 2 years of follow-up (HR, 4.62; 95% CI, 1.49–14.35). The elevated risk in the younger age group dissipated as follow-up times were extended, but at 5 years of follow-up high PAH-DNA adduct levels still conferred a risk of BCR greater than 2 (HR, 2.21; 95% CI, 0.94–5.26) in the younger patient group. Patients with PSA levels above the median (≥5.1 ng/mL) had significantly increased risk of BCR associated with high adduct levels across all three follow-up intervals, ranging from a HR of 2.41 at 2 years of follow-up to 1.91 at 5 years of follow-up. For patients with advanced tumor stage (III or IV), the risk of BCR was greatest at 2 years of follow-up (HR, 2.53; 95% CI, 1.07–5.99), and remained elevated through 5 years of follow-up.
Table 4.
Risk of BCR after prostatectomy associated with high PAH-DNA adduct levels in prostate cells adjusting for clinical risk factors at different lengths of follow-up in selected subsets of prostate cancer cases
| Cell type follow-up period | HR (95% CI) | HR (95% CI) | P* |
|---|---|---|---|
| Tumor | Age <60 (n = 157) | Age ≥ 60 (n = 211) | |
| 2 y | 2.54 (0.93–6.92) | 0.97 (0.44–2.12) | 0.13 |
| 3 y | 1.75 (0.71–4.32) | 1.11 (0.55–2.24) | 0.35 |
| 5 y | 1.46 (0.64–3.33) | 0.98 (0.51–1.87) | 0.41 |
| Nontumor | |||
| 2 y | 4.62 (1.49–14.35) | 1.53 (0.70–3.33) | 0.12 |
| 3 y | 2.86 (1.09–7.52) | 1.38 (0.69–2.78) | 0.20 |
| 5 y | 2.22 (0.94–5.26) | 1.18 (0.62–2.25) | 0.27 |
| PSA < median† (n = 185) | PSA ≥median† (n = 183) | ||
| Tumor | |||
| 2 y | 1.01 (0.27–3.69) | 1.37 (0.70–2.68) | 0.59 |
| 3 y | 1.02 (0.34–3.03) | 1.34 (0.72–2.51) | 0.63 |
| 5 y | 0.70 (0.27–1.82) | 1.25 (0.69–2.27) | 0.35 |
| Nontumor | |||
| 2 y | 1.21 (0.30–4.86) | 2.41 (1.18–4.94) | 0.25 |
| 3 y | 0.84 (0.26–2.78) | 2.18 (1.12–4.25) | 0.13 |
| 5 y | 0.78 (0.28–2.16) | 1.91 (1.02–3.57) | 0.14 |
| Stage II (n = 298) | Stage III or IV (n = 70) | ||
| Tumor | |||
| 2 y | 1.17 (0.49–2.78) | 1.86 (0.80–4.31) | 0.33 |
| 3 y | 1.02 (0.48–.18) | 1.93 (0.88–28) | 0.20 |
| 5 y | 0.79 (0.41–.54) | 1.87 (0.87–00) | 0.08 |
| Nontumor | |||
| 2 y | 2.22 (0.88–.66) | 2.53 (1.07–99) | 0.62 |
| 3 y | 1.66 (0.75–.67) | 2.14 (1.06–47) | 0.53 |
| 5 y | 1.32 (0.67–.62) | 1.96 (0.91–25) | 0.47 |
NOTE: Clinical risk factors include PSA, tumor grade, and tumor stage except when stratified on one of these factors.
P value for significant difference in HR between strata.
Median PSA level was 5.1 ng/mL.
In the full sample, high DNA adduct levels had similar associations with BCR in African Americans and Caucasians; however, in the stratified clinical subsets in which high DNA adduct levels had the strongest association with BCR, HRs for African Americans tended to be greater. In tumor cells, elevated DNA adduct levels were associated with higher HRs in younger (2.02 versus 1.26) and advanced-stage (2.15 versus 1.50) African Americans compared with Caucasian patients. In nontumor cells, the highest HR associated with elevated DNA adduct levels was observed for younger African-American patients (HR, 3.79; 95% CI, 1.01–14.30), whereas the comparable HR for Caucasians was only 1.72 (95% CI, 0.51–5.69). Because cigarette smoking can be considered an antecedent variable in the putative adduct-prostate carcinogenesis pathway, it was not included in our multivariable analyses of PAH-DNA adduct levels. However, given the marginal association of cigarette smoking with BCR, and the possibility that this association may not be fully explained by adduct formation, we reran all multivariable models including a covariate for pack-years of smoking. The resulting β estimates for PAH-DNA adduct levels were only nominally (<10%) changed in all circumstances.
Discussion
The current paradigm of DNA adduct formation associates adducts with the initiation phase of carcinogenesis in which an activated xenobiotic compound binds and damages a DNA molecule. In reality, DNA adducts may be relevant to all stages of carcinogenesis as biomarkers of underlying risk related to an individual’s ability to both metabolize carcinogens and repair damaged DNA. In the present study, we have shown that PAH-DNA adduct levels in prostate at time of diagnosis may be a biomarker of increased risk for early BCR. We also found that PAH-DNA adducts were inversely associated with tumor stage, but not tumor grade, which is in contrast to our previous reports (7, 8). It should be noted, however, that the eligibility criteria for the present study were different than that of the two previous studies. Further, in the present study, we defined tumor grade and stage in a dichotomous fashion to facilitate survival analyses, which was different than how we defined these variables in our original report (7).
The association between PAH-DNA adducts and BCR risk was greater for adduct levels in nontumor cells, which may better reflect inherited genetic capabilities of xenobiotic metabolism and DNA repair rather than adduct levels in tumor cells where changes in the genetic background have occurred due to somatic mutations. Consistent with what we have previously reported (7, 8), adduct levels were higher in nontumor cells compared with tumor cells. Several studies of other tissues that measured PAH-DNA adducts in both tumor and adjacent nontumor cells, including lung (38), laryngeal (39), pancreas (40), and liver (41), have also reported higher adduct levels in adjacent nontumor cells. To confirm that total DNA adduct burden in the prostate did not afford more information about BCR, we calculated a composite total score of PAH-DNA adducts based on a tumor volume–weighted average of PAH-DNA adducts in both tumor and nontumor cells, but found no associations with BCR (data not shown). Combining separate PAH-DNA adduct level measures in tumor and nontumor prostate cells into a composite score may dilute information in the adduct measure unique to each, particularly if the function of key genes in the adduct formation and repair pathways changes during malignant transformation (42, 43).
Nuclear accumulation of the p53 protein in prostate tumor cells has been associated with poor disease prognosis (44, 45). The diol epoxide metabolites of benzo(a)pyrene diol epoxide preferentially bind to the most frequently mutated guanine nucleotides within p53 codons (46) and other forms of PAH also bind to p53 mutational hotspots (47). Therefore, increased PAH-DNA adduct level in prostate leading to increased p53 mutations may be a possible mechanism by which higher PAH-DNA adduct levels affect increased BCR in the short term. Another explanation for the association between higher PAH-DNA adduct levels and increased short-term BCR may lie in the metabolic environment of the premalignant cell. Cytochrome P450 phase I enzymes CYP1A1 and CYP1B1 activate PAH parent compounds. Allelic variants of CYP1A1 and CYP1B1, which may exhibit different catalytic capabilities toward PAH parent compounds (48), have been linked to aggressive prostate cancer (26). Furthermore, CYP1B1 is also overexpressed in prostate tumors due to hypomethylation (49).
A limitation of our study was that DNA adducts were measured cross-sectionally shortly after disease diagnosis. As such, the adduct level in our analysis was a snapshot of what could potentially be a rapidly changing cellular environment. That may explain in part why after 2 years the association between PAH-DNA adducts and BCR declined precipitously. Although our results are generalizable to prostate cancer patients that undergo prostatectomy as their primary form of treatment, we cannot necessarily infer that elevated PAH-DNA adduct levels affect disease progression the same way in prostate cancer patients that receive other forms of treatment such as hormone or radiation therapy. A missing aspect of the analyses in the present study that would be of interest in terms of prevention is the source of PAH exposure(s) that lead to high adduct levels and biological modifiers that influence adduct formation and prostate cancer risk, such as inherited capacities for high metabolism of PAH (8, 15) or poor DNA repair capacity (50, 51). Although these risk factors for PAH-DNA adducts have meaning in terms of understanding the underlying reasons for interindividual variation in PAH-DNA adduct levels, because they are antecedent factors in a causal pathway they provide no further understanding of the role of adducts in BCR (52), which was the central point of this study. This was evidenced by our rerunning of multivariate models including covariates for both PAH-DNA adduct levels and pack-years of cigarette smoking, and our finding that inclusion of the latter did little to change the association of the former with the BCR outcome.
Interestingly, we found that the association of higher PAH-DNA adduct level with BCR was restricted to subsets of patients; in particular, those with advanced-stage disease, with PSA levels above the median at diagnosis, and those younger than 60 years old. Further stratified analysis also revealed that within the clinical patient subsets in which elevated DNA adduct levels were associated with BCR, African Americans were at greater risk for BCR. Both PSA and tumor stage are known risk factors for BCR and were strongly associated with BCR in our study population. Elevated PAH-DNA adducts may be a marker of a more advanced disease process involving activated phase I enzymes, which could have a greater effect in a disease progression process that has already exceeded a certain threshold as indicated by high PSA or advanced tumor stage. It is unclear why PAH-DNA adducts were a greater risk factor for BCR in men younger than 60 years old in our study. Neither age nor race was associated with BCR in our study population, but elevated DNA adduct levels had the strongest association with BCR in younger African-American cases. The combination of high adduct levels and younger age has been associated with higher risk of lung (53) and colorectal (54) cancer. Hu et al.(50) found lower nucleotide excision repair capacity was a stronger risk for prostate cancer in men younger than 60 years old and that the nucleotide excision repair capacity level was lower in younger cases, suggesting that deficient nucleotide excision repair capacity could contribute to early onset of prostate cancer. In a similar manner, high PAH-DNA adduct levels may better discriminate between aggressive and nonaggressive prostate cancer phenotypes in younger versus older cases. Few studies have examined racial differences in PAH-DNA adduct levels, but a study of smokers found that African American subjects had higher adduct levels in lymphocytes than Caucasian and Latino subjects after adjustment for gender, education, α-tocopherol and β-carotene levels, and GSTM1 status (55).
In summary, we found that higher levels of PAH-DNA adducts in prostate were associated with a transient increased risk of BCR in men with prostate cancer treated with surgery. In patient subsets defined by high PSA, advanced tumor stage, and age less than 60 years old at diagnosis, higher adduct levels conferred an increased risk of BCR that diminished less with follow-up time and was greatest in African Americans. Higher adduct levels in nontumor cells compared with tumor cells tended to be more strongly associated BCR, which may be due to the cellular environment in nontumor cells being more reflective of an individual’s innate ability to activate carcinogens and repair DNA damage. Our findings are novel and need to be replicated in independent populations. Future studies should also address whether DNA adducts in prostate cells at time of diagnosis are a harbinger of disease progression or simply a by-product of a cellular milieu already programmed for greater malignant potential.
Acknowledgments
Grant support: NIH grants R01ES011126 and R01ES011126-S1 (B.A. Rybicki). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Polycyclic aromatic hydrocarbon carcinogenesis: structure-activity relationships. Boca Raton: CRC Press; 1988. [Google Scholar]
- 2.Miller EC, Miller JA. Searches for ultimate chemical carcinogens and their reactions with cellular macro-molecules. Cancer. 1981;47:2327–2345. doi: 10.1002/1097-0142(19810515)47:10<2327::aid-cncr2820471003>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Rogan EG, Devanesan PD, RamaKrishna NV, et al. Identification and quantitation of benzo(a)pyrene-DNA adducts formed in mouse skin. Chem Res Toxicol. 1993;6:356–363. doi: 10.1021/tx00033a017. [DOI] [PubMed] [Google Scholar]
- 4.Wang CY, Debiec-Rychter M, Schut HA, et al. N-Acetyltransferase expression and DNA binding of N-hydroxyheterocyclic amines in human prostate epithelium. Carcinogenesis. 1999;20:1591–1595. doi: 10.1093/carcin/20.8.1591. [DOI] [PubMed] [Google Scholar]
- 5.Williams JA, Martin FL, Muir GH, Hewer A, Grover PL, Phillips DH. Metabolic activation of carcinogens and expression of various cytochromes P450 in human prostate tissue. Carcinogenesis. 2000;21:1683–1689. doi: 10.1093/carcin/21.9.1683. [DOI] [PubMed] [Google Scholar]
- 6.Kooiman GG, Martin FL, Williams JA, Grover PL, Phillips DH, Muir GH. The influence of dietary and environmental factors on prostate cancer risk. Prostate Cancer Prostatic Dis. 2000;3:256–258. doi: 10.1038/sj.pcan.4500489. [DOI] [PubMed] [Google Scholar]
- 7.Rybicki BA, Rundle A, Savera AT, Sankey SS, Tang D. Polycyclic aromatic hydrocarbon-DNA adducts in prostate cancer. Cancer Res. 2004;64:8854–8859. doi: 10.1158/0008-5472.CAN-04-2323. [DOI] [PubMed] [Google Scholar]
- 8.Nock NL, Tang D, Rundle A, et al. Associations between smoking, polymorphisms in polycyclic aromatic hydrocarbon (PAH) metabolism and conjugation genes and PAH-DNA adducts in prostate tumors differ by race. Cancer Epidemiol Biomarkers Prev. 2007;16:1236–1245. doi: 10.1158/1055-9965.EPI-06-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krstev S, Baris D, Stewart P, et al. Occupational risk factors and prostate cancer in U.S. blacks and whites. Am J Ind Med. 1998;34:421–430. doi: 10.1002/(sici)1097-0274(199811)34:5<421::aid-ajim2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 10.Brown DA, Delzell E. Motor vehicle manufacturing and prostate cancer. Am J Ind Med. 2000;38:59–70. doi: 10.1002/1097-0274(200007)38:1<59::aid-ajim7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 11.Aronson KJ, Siemiatycki J, Dewar R, Gerin M. Occupational risk factors for prostate cancer: results from a case- control study in Montreal, Quebec, Canada. Am J Epidemiol. 1996;143:363–373. doi: 10.1093/oxfordjournals.aje.a008750. [DOI] [PubMed] [Google Scholar]
- 12.Boers D, Zeegers MP, Swaen GM, Kant I, van den Brandt PA. The influence of occupational exposure to pesticides, polycyclic aromatic hydrocarbons, diesel exhaust, metal dust, metal fumes, and mineral oil on prostate cancer: a prospective cohort study. Occup Environ Med. 2005;62:531–537. doi: 10.1136/oem.2004.018622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rybicki BA, Neslund-Dudas C, Nock NL, et al. Prostate cancer risk from occupational exposure to polycyclic aromatic hydrocarbons interacting with the GSTP1 Ile105Val polymorphism. Cancer Detect Prev. 2006;30:412–422. doi: 10.1016/j.cdp.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gammon MD, Santella RM, Neugut AI, et al. Environmental toxins and breast cancer on Long Island. I. Polycyclic aromatic hydrocarbon DNA adducts. Cancer Epidemiol Biomarkers Prev. 2002;11:677–685. [PubMed] [Google Scholar]
- 15.Nock NL, Liu X, Cicek MS, et al. Polymorphisms in polycyclic aromatic hydrocarbon metabolism and conjugation genes, interactions with smoking and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15:756–761. doi: 10.1158/1055-9965.EPI-05-0826. [DOI] [PubMed] [Google Scholar]
- 16.Phillips DH. Polycyclic aromatic hydrocarbons in the diet. Mutat Res. 1999;443:139–147. doi: 10.1016/s1383-5742(99)00016-2. [DOI] [PubMed] [Google Scholar]
- 17.Rodgman A, Smith CJ, Perfetti TA. The composition of cigarette smoke: a retrospective, with emphasis on polycyclic components. Hum Exp Toxicol. 2000;19:573–595. doi: 10.1191/096032700701546514. [DOI] [PubMed] [Google Scholar]
- 18.Hickey K, Do KA, Green A. Smoking and prostate cancer. Epidemiol Rev. 2001;23:115–125. doi: 10.1093/oxfordjournals.epirev.a000776. [DOI] [PubMed] [Google Scholar]
- 19.Plaskon LA, Penson DF, Vaughan TL, Stanford JL. Cigarette smoking and risk of prostate cancer in middle-aged men. Cancer Epidemiol Biomarkers Prev. 2003;12:604–609. [PubMed] [Google Scholar]
- 20.Giles GG, Severi G, McCredie MR, et al. Smoking and prostate cancer: findings from an Australian case-control study. Ann Oncol. 2001;12:761–765. doi: 10.1023/a:1011131105617. [DOI] [PubMed] [Google Scholar]
- 21.Mao GE, Morris G, Lu QY, et al. Glutathione S-transferase P1 Ile105Val polymorphism, cigarette smoking and prostate cancer. Cancer Detect Prev. 2004;28:368–374. doi: 10.1016/j.cdp.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Kolonel LN. Fat, meat, and prostate cancer. Epidemiol Rev. 2001;23:72–81. doi: 10.1093/oxfordjournals.epirev.a000798. [DOI] [PubMed] [Google Scholar]
- 23.Cross AJ, Peters U, Kirsh VA, et al. A prospective study of meat and meat mutagens and prostate cancer risk. Cancer Res. 2005;65:11779–11784. doi: 10.1158/0008-5472.CAN-05-2191. [DOI] [PubMed] [Google Scholar]
- 24.Kumar-Sinha C, Chinnaiyan AM. Molecular markers to identify patients at risk for recurrence after primary treatment for prostate cancer. Urology. 2003;62 Suppl 1:19–35. doi: 10.1016/j.urology.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Jana NR, Sarkar S, Ishizuka M, Yonemoto J, Tohyama C, Sone H. Comparative effects of 2,3,7,8- tetrachlorodibenzo-p-dioxin on MCF-7, RL95-2, and LNCaP cells: role of target steroid hormones in cellular responsiveness to CYP1A1 induction. Mol Cell Biol Res Commun. 2000;4:174–180. doi: 10.1006/mcbr.2001.0275. [DOI] [PubMed] [Google Scholar]
- 26.Cicek MS, Liu X, Casey G, Witte JS. Role of androgen metabolism genes CYP1B1, PSA/KLK3, and CYP11α in prostate cancer risk and aggressiveness. Cancer Epidemiol Biomarkers Prev. 2005;14:2173–2177. doi: 10.1158/1055-9965.EPI-05-0215. [DOI] [PubMed] [Google Scholar]
- 27.Rundle A, Tang D, Hibshoosh H, et al. The relationship between genetic damage from polycyclic aromatic hydrocarbons in breast tissue and breast cancer. Carcinogenesis. 2000;21:1281–1289. [PubMed] [Google Scholar]
- 28.Zhang YJ, Hsu TM, Santella R. Immunoperoxidase detection of polycyclic aromatic hydrocarbon-DNA adducts in oral mucosa cells of smokers and nonsmokers. Cancer Epidemiol Biomarkers Prev. 1995;4:133–138. [PubMed] [Google Scholar]
- 29.Zenzes MT, Puy LA, Bielecki R, Reed TE. Detection of benzo(a)pyrene diol epoxide-DNA adducts in embryos from smoking couples: evidence for transmission by spermatozoa. Mol Hum Reprod. 1999;5:125–131. doi: 10.1093/molehr/5.2.125. [DOI] [PubMed] [Google Scholar]
- 30.Zenzes MT, Puy LA, Bielecki R. Immunodetection of benzo(a)pyrene adducts in ovarian cells of women exposed to cigarette smoke. Mol Hum Reprod. 1998;4:159–165. doi: 10.1093/molehr/4.2.159. [DOI] [PubMed] [Google Scholar]
- 31.Romano G, Sgambato A, Boninsegna A, et al. Evaluation of polycyclic aromatic hydrocarbon-DNA adducts in exfoliated oral cells by an immunohistochemical assay. Cancer Epidemiol Biomarkers Prev. 1999;8:91–96. [PubMed] [Google Scholar]
- 32.Kupelian PA, Elshaikh M, Reddy CA, Zippe C, Klein EA. Comparison of the efficacy of local therapies for localized prostate cancer in the prostate-specific antigen era: a large single-institution experience with radical prostatectomy and external-beam radiotherapy. J Clin Oncol. 2002;20:3376–3385. doi: 10.1200/JCO.2002.01.150. [DOI] [PubMed] [Google Scholar]
- 33.Freedland SJ, Sutter ME, Dorey F, Aronson WJ. Defining the ideal cutpoint for determining PSA recurrence after radical prostatectomy. Prostate-specific antigen. Urology. 2003;61:365–369. doi: 10.1016/s0090-4295(02)02268-9. [DOI] [PubMed] [Google Scholar]
- 34.SAS Institute, Inc. SAS/STAT user’s guide, version 8. vol. 3. Cary (NC): SAS Institute Inc.; 1989. pp. 4797–4828. [Google Scholar]
- 35.Wingo PA, Ries LA, Parker SL, Heath CW., Jr Long-term cancer patient survival in the United States. Cancer Epidemiol Biomarkers Prev. 1998;7:271–282. [PubMed] [Google Scholar]
- 36.Merrill RM, Bird JS. Effect of young age on prostate cancer survival: a population-based assessment (United States) Cancer Causes Control. 2002;13:435–443. doi: 10.1023/a:1015764507609. [DOI] [PubMed] [Google Scholar]
- 37.Brenner H, Arndt V. Long-term survival rates of patients with prostate cancer in the prostate-specific antigen screening era: population-based estimates for the year 2000 by period analysis. J Clin Oncol. 2005;23:441–447. doi: 10.1200/JCO.2005.11.148. [DOI] [PubMed] [Google Scholar]
- 38.Gyorffy E, Anna L, Gyori Z, et al. DNA adducts in tumour, normal peripheral lung and bronchus, and peripheral blood lymphocytes from smoking and non-smoking lung cancer patients: correlations between tissues and detection by 32P-postlabelling and immunoassay. Carcinogenesis. 2004;25:1201–1209. doi: 10.1093/carcin/bgh131. [DOI] [PubMed] [Google Scholar]
- 39.Banaszewski J, Szmeja Z, Szyfter W, Szyfter K, Baranczewski P, Moller L. Analysis of aromatic DNA adducts in laryngeal biopsies. Eur Arch Otorhinolaryngol. 2000;257:149–153. doi: 10.1007/s004050050212. [DOI] [PubMed] [Google Scholar]
- 40.Li D, Firozi PF, Zhang W, et al. DNA adducts, genetic polymorphisms, and K-ras mutation in human pancreatic cancer. Mutat Res. 2002;513:37–48. doi: 10.1016/s1383-5718(01)00291-1. [DOI] [PubMed] [Google Scholar]
- 41.Chen SY, Wang LY, Lunn RM, et al. Polycyclic aromatic hydrocarbon-DNA adducts in liver tissues of hepatocellular carcinoma patients and controls. Int J Cancer. 2002;99:14–21. doi: 10.1002/ijc.10291. [DOI] [PubMed] [Google Scholar]
- 42.Sterling KM, Jr, Cutroneo KR. Constitutive and inducible expression of cytochromes P4501A (CYP1A1 and CYP1A2) in normal prostate and prostate cancer cells. J Cell Biochem. 2004;91:423–429. doi: 10.1002/jcb.10753. [DOI] [PubMed] [Google Scholar]
- 43.Fan R, Kumaravel TS, Jalali F, Marrano P, Squire JA, Bristow RG. defective DNA strand break repair after DNA damage in prostate cancer cells: implications for genetic instability and prostate cancer progression. Cancer Res. 2004;64:8526–8533. doi: 10.1158/0008-5472.CAN-04-1601. [DOI] [PubMed] [Google Scholar]
- 44.Borre M, Stausbol-Gron B, Overgaard J. p53 accumulation associated with bcl-2, the proliferation marker MIB-1 and survival in patients with prostate cancer subjected to watchful waiting. J Urol. 2000;164:716–721. doi: 10.1097/00005392-200009010-00023. [DOI] [PubMed] [Google Scholar]
- 45.Visakorpi T, Kallioniemi OP, Heikkinen A, Koivula T, Isola J. Small subgroup of aggressive, highly proliferative prostatic carcinomas defined by p53 accumulation. J Natl Cancer Inst. 1992;84:883–887. doi: 10.1093/jnci/84.11.883. [DOI] [PubMed] [Google Scholar]
- 46.Denissenko M, Pao A, Tang M, Pfiefer G. Preferential formation of benzo(a)pyrene adducts at lung cancer mutational hotspots in p53. Science. 1996;274:430–432. doi: 10.1126/science.274.5286.430. [DOI] [PubMed] [Google Scholar]
- 47.Smith LE, Denissenko MF, Bennett WP, et al. Targeting of lung cancer mutational hotspots by polycyclic aromatic hydrocarbons. J Natl Cancer Inst. 2000;92:803–811. doi: 10.1093/jnci/92.10.803. [DOI] [PubMed] [Google Scholar]
- 48.Shimada T, Watanabe J, Inoue K, Guengerich FP, Gillam EM. Specificity of 17β-oestradiol and benzo (a)pyrene oxidation by polymorphic human cytochrome P4501B1 variants substituted at residues 48, 119 and 432. Xenobiotica. 2001;31:163–176. doi: 10.1080/00498250110043490. [DOI] [PubMed] [Google Scholar]
- 49.Tokizane T, Shiina H, Igawa M, et al. Cytochrome P450 1B1 is overexpressed and regulated by hypomethylation in prostate cancer. Clin Cancer Res. 2005;11:5793–5801. doi: 10.1158/1078-0432.CCR-04-2545. [DOI] [PubMed] [Google Scholar]
- 50.Hu JJ, Hall MC, Grossman L, et al. Deficient nucleotide excision repair capacity enhances human prostate cancer risk. Cancer Res. 2004;64:1197–1201. doi: 10.1158/0008-5472.can-03-2670. [DOI] [PubMed] [Google Scholar]
- 51.Lockett KL, Hall MC, Clark PE, et al. DNA damage levels in prostate cancer cases and controls. Carcinogenesis. 2006;27:1187–1193. doi: 10.1093/carcin/bgi288. [DOI] [PubMed] [Google Scholar]
- 52.Rundle A, Schwartz S. Issues in the epidemiological analysis and interpretation of intermediate biomarkers. Cancer Epidemiol Biomarkers Prev. 2003;12:491–496. [PubMed] [Google Scholar]
- 53.Peluso M, Munnia A, Hoek G, et al. DNA adducts and lung cancer risk: a prospective study. Cancer Res. 2005;65:8042–8048. doi: 10.1158/0008-5472.CAN-04-3488. [DOI] [PubMed] [Google Scholar]
- 54.Magagnotti C, Pastorelli R, Pozzi S, Andreoni B, Fanelli R, Airoldi L. Genetic polymorphisms and modulation of 2-amino-1-methyl-6-phenylimidazo[ 4,5-b]pyridine (PhIP)-DNA adducts in human lymphocytes. Int J Cancer. 2003;107:878–884. doi: 10.1002/ijc.11492. [DOI] [PubMed] [Google Scholar]
- 55.Weiserbs KF, Jacobson JS, Begg MD, et al. A cross-sectional study of polycyclic aromatic hydrocarbon-DNA adducts and polymorphism of glutathione S-transferases among heavy smokers by race/ethnicity. Biomarkers. 2003;8:142–155. doi: 10.1080/1354750031000086269. [DOI] [PubMed] [Google Scholar]