Abstract
Animals and humans are able to predict and synchronize their daily activity to signals present in their environments. Environmental cues are most often associated with signaling the beginning or the end of a daily activity cycle but they can also be used to time the presentation or availability of scarce resources. If the signal occurs consistently, animals can begin to anticipate its arrival and ultimately become entrained to its presence. While many stimuli can produce anticipation for a daily event, these events rarely lead to changes in activity patterns during the rest of the circadian cycle. We demonstrate that performance of a task requiring sustained attention not only produces entrainment, but produces a robust modification in the animals’ activity throughout the entire circadian cycle. In particular, normally nocturnal rats, when trained during the light phase (ZT 4) adopted a significant and reversible diurnal activity pattern. Importantly, control experiments demonstrated that this entrainment could not be attributed to the non-cognitive components of task performance, such as handling, water deprivation, access to water used as a reward, or animal activity associated with operant training. These findings additionally indicate that levels of cognitive performance are modulated by the circadian cycle and that such activity can act as a highly effective entrainment signal. These results form the basis for future research on the role of neuronal systems mediating interactions between cognitive activity and circadian rhythms.
Keywords: Entrain, phase shift, non-photic, sustained attention, cognition
Introduction
A variety of daily photic and non-photic environmental signals are able to synchronize (entrain) animals to a 24 hour period. Entrainment to these cues is characterized by daily activity that occurs coincident with or in advance of the daily cue and extends well beyond the actual presentation of the cue. If the activity persists in the absence of the cue and absence of all external cues, including the light/dark (LD) cycle, the activity synchronized by the signal is considered to be entrained. Non-photic cues are thought to entrain activity best in the absence of a LD cycle, however some daily events are significant enough that they cause entrainment of activity in the presence of a LD cycle. For example, novel wheel access in hamsters (Gorman & Lee, 2001) and palatable food access in rats (R. Mistlberger & Rusak, 1987) are both strong entraining signals in the presence of a LD cycle. Other non-photic cues are less effective at entrainment during the LD cycle, including forced treadmill running in mice (Marchant & Mistlberger, 1996) and daily timed water access in rats (R. E. Mistlberger & Rechtschaffen, 1985).
The primary circadian pacemaker in mammals resides in the suprachiasmatic nucleus (SCN) of the hypothalamus (Moore, 1983). The SCN produces endogenous biological rhythms, with a period of approximately 24 hours in duration, that are self-sustained in the absence of environmental input (Moore, 1989). Direct light information reaches the SCN via the retinohypothalamic tract (RHT) which projects directly from the retina to the SCN (Card & Moore, 1984; Moore & Lenn, 1972). This input provides the strongest signal influencing circadian activity patterns and is considered the preeminent zeitgeber or universal time-giver. However, the SCN also receives several other non-photic inputs that act to influence circadian patterns. For example, in the hamster non-photic information is thought to be conveyed via the intergeniculate leaflet (IGL) which receives neural input from various brain regions, including the visual system, the prefrontal cortex, and the ascending neuromodulatory systems (Vrang, Mrosovsky, & Mikkelsen, 2003). In addition, oscillators outside of the SCN sensitive to non-photic zeitgebers (e.g., food entrainable oscillator) are thought to project to the SCN to influence entrainment (Guilding & Piggins, 2007).
Other non-retinal inputs to the SCN presumed to influence circadian rhythms include pyramidal projection neurons from neocortical regions involved in action selection and executive function (Hurley, Herbert, Moga, & Saper, 1991; Vertes, 2004), serotonergic neurons originating in the dorsal raphe nucleus (Edgar, Miller, Prosser, Dean, & Dement, 1993; Medanic & Gillette, 1992; Meyer-Bernstein & Morin, 1996; Ying & Rusak, 1994), and cholinergic projections from midbrain and basal forebrain nuclei (Bina & Rusak, 1996; Bina, Rusak, & Semba, 1993, 1997; Erhardt et al., 2004; Gillette et al., 2001). The role of cholinergic inputs, particularly from the basal forebrain, has remained unclear, although experimental in vivo and in vitro data suggest that acetylcholine (ACh) can significantly alter circadian rhythm expression. Injections of carbachol, a non-specific acetylcholine receptor (AChR) agonist, into either the lateral ventricle or SCN produces a phase-dependent shift of activity patterns (Bina & Rusak, 1996). In vitro electrophysiological data from the SCN also demonstrate that stimulation of AChRs by carbachol can modify the time at which endogenous peak firing rate occurs in SCN slices (Gillette et al., 2001). These physiological and anatomical data suggest that ACh can have major effects on circadian rhythms; however, the in vivo conditions and importance of endogenous ACh release at the SCN have yet to be explored.
One condition under which ACh release from the basal forebrain is elevated is during performance of tasks of sustained attention; sustained attention is characterized by a subject’s readiness to detect an infrequent and unpredictable signal(s) over a prolonged period of time, to discriminate this signal from non-signal events or “noise” and to report the presence or absence of such a signal. Normal sustained attention performance, above chance levels, is dependent upon the integrity of the basal forebrain cholinergic projections to the cortex. Sustained attention performance robustly increases cortical cholinergic transmission; ACh release is further augmented under challenging conditions (e.g. distracter presentation or fatigue) that require top-down optimization of input processing (Kozak, Bruno, & Sarter, 2006; Parikh, Kozak, Martinez, & Sarter, 2007; Sarter, Gehring, & Kozak, 2006; Sarter, Hasselmo, Bruno, & Givens, 2005).
Conversely, the SCN is likely to influence the cognitive and motivational requirements of attentional processing via modulation of circadian sleep/wake/arousal states. The SCN acts to modulate attention networks via projections to the dorsomedial hypothalamic nucleus (DMN), locus coeruleus (LC), and orexin/hypocretin neurons in the hypothalamus, which in turn project to the basal forebrain (Gabbott, Warner, Jays, & Bacon, 2003; Hajszan & Zaborszky, 2002; Lee, Kim, & Waterhouse, 2005; Novak, Harris, Smale, & Nunez, 2000; Novak & Nunez, 2000; Salazar-Juarez, Escobar, & Aguilar-Roblero, 2002). Overall, this anatomical organization suggests that the forebrain structures involved in attention and the SCN might interact in a bi-directional manner.
The present study was designed to examine the effects of cognitive training, using a task known to activate the basal forebrain cholinergic system, on circadian activity. Although nocturnal rats are naturally more alert during the dark phase, experimenters typically train and test animals during the light phase. Given this natural paradox we chose to look at how daily training on a cognitive task during the light phase influences circadian rhythmicity. We reasoned that such training would provide a non-photic signal, much like food (R. Mistlberger & Rusak, 1987) or exposure to novel wheel running (Gorman & Lee, 2001). It is important to note that water alone provides only a weak non-photic signal (R. E. Mistlberger & Rechtschaffen, 1985) and thus was chosen over food as the conditional reward for correct responses during training. Our findings demonstrate that sustained attention performance in rats produced a powerful and reversible entraining influence on circadian activity. Control experiments indicate that the elements of training and task performance outside of the requirement for attentional up-regulation were insufficient to drive the changes seen in circadian phase. Collectively, the effects of attention on circadian entrainment could provide a useful model for exploring cognitive therapies for circadian disorders as well as ameliorating the effects of nightshift work on circadian rhythms in humans.
Methods
Subjects
Twenty-four male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 350 g at the start of testing were housed individually in opaque single standard cages (27.7cm × 20.3cm). Cages were lined with corn cob bedding and kept in a humidity- and temperature-controlled environment. Animals were allowed to acclimate for two weeks and were then mildly water deprived to ~95% of their free feeding weight and had ad libitum access to food (Purina 5001; supplier: Frontier, Oxford, MI). Animals were allowed access to water for the twelve-hour lighted period beginning on day one and gradually over the next 6 days were titrated down to a single one hour water access period from ZT 4 to ZT 5. Rats acclimated for two weeks prior to experimentation and were subsequently divided into two groups designated to either undergo operant training (i.e. task-performing group N=16) or to undergo handling procedures without operant training (i.e. non-performing group N=8). All task-performing animals were given free access to water for twenty minutes following daily experimentation in addition to the quantity of water (~5 ml) obtained as reward during operant testing. Non-performing animals were given one hour of water access daily (ZT 4). Standard housing conditions for all animals followed a 12:12 LD cycle with lights on at 1200 h. The task performing group consisted of two subsets of animals: a control group that underwent operant training on a simple reaction time task (SRTT) and an experimental group trained on a task designed to require and test sustained attentional (SAT) performance. Locomotor activity during training was recorded using either infrared (IR) motion detectors placed above the home cages (Slimline PIR; SmartHome, Irvine, CA) or via running wheel activity (Minimitter, Bend, OR). Activity data were collected in 10-min bins using Vitalview software (Minimitter, Bend, OR). Cages were cleaned and animals were weighed once-weekly during the light phase. All procedures were in accordance with protocols approved by the University Committee for the Care and Use of Animals at the University of Michigan.
Experimental timeline: SAT Group
Following the two-week acclimation period and gradual water deprivation, task-performing rats began operant training procedures to facilitate shaping on a task that measures sustained attention (SAT). Upon achieving a performance criteria (described below), rats underwent additional operant testing in the presence of a visual distracter (i.e a flashing house light). Distracter presentation occurred for a total of 10 sessions. Five sessions occurred under standard lighting conditions and five were given following a 6 hour photic phase advance. The two sets of distracter sessions were separated by two weeks of daily training on the standard task version. This order of events was reversed for half the rats, such that the first exposure to the visual distracter occurred during the phase shift and the second exposure occurred under conditions of stable circadian entrainment. Following the final distracter session animals continued to train daily until all animals had recovered stable entrainment on the new light/dark cycle. The operant component of the experiment was then terminated. On the last day of training the animals entered constant dark conditions (DD) for a period of 9 days to determine whether free-running rhythms would originate from the diurnal phase.
SAT Operant Methods
Operant training took place 7 days per week. Training and testing was conducted in individual operant chambers (MedAssociates, St. Albans, Vermont) each equipped with two retractable levers, a central panel white light (2.8 W), a ceiling mounted white house-light (2.8 W) and a water dispenser (located on the same wall as the panel lights and levers). Operant chambers were housed within individual sound-attenuating cabinets. Animals were transported from their home cages to the operant chambers and then placed in the unlit chambers for 3 minutes prior to task onset. Animals were first trained to press a lever for a water reward in accordance with a modified fixed-ratio 1 schedule of reinforcement. During this phase of training every lever press results in the delivery of a water reward. In most instances, animals show no side bias with regard to which lever is pressed; however, if more than 5 presses occured on any one lever in a row, the FR1 schedule is modified to require the animal to press the opposite lever before the next reward can be obtained. Rats were next trained to detect signals and discriminate between signal events and non-signal events (i.e. illumination of the central panel light for 1 s vs. non-illumination of the light). Two seconds following a signal or non-signal event, both levers extended into the operant chamber and remained active for four seconds or until a lever press occurred. If the animal failed to respond within 4 seconds, the levers were retracted and an omission was scored. Immediately following a response (either correct or incorrect), both levers were retracted and the variable inter-trial interval or ITI (12±3 s) was reset. During signal trials, a left-lever press indicated a correct response and was scored as a hit whereas a right-lever press indicated an incorrect response was scored as a miss. Conversely, during non-signal trials a left-lever press indicated an incorrect response and was scored as a false-alarm and a right-lever press indicated a correct response and was scored as a correct rejection. Half the animals were trained in the opposite pattern to address the possibility of any selection bias. Animals received water rewards only for correct responses (30 µL for each hit and correct rejection) whereas incorrect responses (misses and false alarms) were not rewarded. During this phase of shaping incorrect responses resulted in the trial being repeated up to three times in the form of correction trials. If the animal responded incorrectly to three consecutive correction trials, a forced-choice trial was initiated. A forced-choice trial consisted of a signal or non-signal event followed by extension of only the correct lever into the operant chamber for 90 s or until a lever press occurred. In the event that the forced-choice trial was a signal trial, the signal light remained illuminated for as long as the lever was extended. The house light was off during this shaping phase. Behavioral sessions consisted of 162 trials per session. Animals progressed to the subsequent step of shaping if they responded correctly to ≥ 59% of both signal- and non-signal trials for three consecutive days.
During the third phase of shaping, signal durations were shortened to 500, 50, or 25 ms (27 trials per signal duration) and the ITI was reduced to 9±3 s. Correction and forced-choice trials were also eliminated. Sessions were divided into three blocks of 54 trials each with all signal durations occurring randomly 9 times per block. Animals were advanced to the final stage of shaping when their performance met or exceeded a performance criterion of 70% hits to the 500 ms signal trials, 70% correct rejections and fewer than 20 omitted trials per session.
Throughout the final stage of testing (referred to as the ‘SAT’), the house-light was illuminated throughout the entire session. The addition of the illuminated house-light represents a crucial element of testing sustained attention as it requires the animal to constrain its behavior and focus on the central panel light during task performance. Acquisition of the final stage of training is considered complete once animals reach the final criterion performance of ≥ 70% correct responses to the 500 ms signal trials, ≥ 70% correct responses to non-signal trials and fewer than that 25 omissions per sessions for a minimum of 3 consecutive sessions. Animals then advanced to the visual distracter phase of training (described below). The latency to reach final performance criteria varied between animals, but was achieved in less than three months for all animals. One animal was ultimately excluded from all performance analysis due to a high level of inconsistency across repeated days independent of condition.
After reaching performance criteria, rats were exposed to a total of ten sessions that included the presentation of a visual distracter as a performance challenge (0.5 Hz flashing house light, referred to as ‘dSAT’ sessions; (Kozak et al., 2006; Nuechterlein, Luck, Lustig, & Sarter, 2009). dSAT sessions were broken into two separate sets of 5 continuous daily sessions; each set was separated by two weeks of standard task performance. During distracter sessions, a visual distracter (i.e. a house-light flashing at 0.5 Hz) was presented during the second block of trials (middle 54 trials). Distracter presentation during task performance typically results in reduced correct rejection rates for non-signal trials. Performing during distracter presentation is thought to necessitate the implementation of top-down mechanisms required for the successful detection of signals and the filtering of extraneous stimuli. All task-performing animals received 5 distracter sessions under conditions of stable circadian entrainment and 5 sessions beginning on the first day of a 6-hour phase-advance.
Phase Shift
Circadian data collection (described above) began as animals approached performance criteria on the SAT. Once at criteria performance, the LD cycle was advanced 6 h (lights on at 0600 h from 1200 h). The actual timing of task performance in the 24 hour day was not shifted with the LD cycle and therefore now occurred at ZT 10 (2 hours before lights off) following the phase advance. The number of days required to recover stable entrained activity following the phase shift of the light cycle was determined as previously described (Goel & Lee, 1996). Briefly, animals were considered stable when the phase (ψ) of activity onset and offset relative to the light cycle and daily SAT training remained unchanged for at least 3 consecutive days after the LD shift. The period of reentrainment was defined as latency to achieve ψ stability following the phase shift. In most studies employing a phase shift, reentrainment is considered complete only when the animals also recover the same ψ that they had prior to the LD shift. However, this criteria is insufficient in this experiment due to the influence of SAT training now occurring at ZT 10. Because of task influence on entrainment, many animals never returned to the same ψ, rather they developed a new stable relationship to the LD cycle associated with the new time of SAT training.
Entrained Phase or Masking
After animals regained stable entrainment, the possibility of masking (expression of an activity rhythm that is not controlled by entrainment of the SCN) rather than entrainment by the SAT was tested by releasing animals into conditions with no circadian time signals: constant darkness (DD) with no daily SAT training. DD conditions facilitated the assessment of the circadian entrainment underlying the observed patterns of activity. Animals were placed under DD conditions for 9 days. During the initial 48 h, animals underwent complete water deprivation to eliminate the time of water access as a potential circadian cue. During the subsequent 7 days rats were given ad libitum access to water. Dim red lights were used in order to assess animal health and to supply food and water at random times of day during the 7 day period.
Attention Data Analysis
SAT performance yielded measures of hits (H), misses (M), false alarms (FA), correct rejections (CR) and omissions. Statistical analyses were carried out on the relative number of hits (%H=H/H+M), the relative number of correct rejections (%CR=CR/CR+FA), and the number of omissions. Additionally, a Vigilance Index (VI) was also calculated as an overall measure of sustained performance. VI is calculated using the formula VI= (%H−%FA)/[2(%H+%FA) − (%H+%FA)2]. This calculation is similar to the Sensitivity Index (Frey & Colliver, 1973) except that omitted trials are excluded from the calculation. VI values can range from −1 to +1, with +1 indicating that all trials were either hits or correct rejections, 0 being a complete lack of ability to discriminate between signal and non-signal events, and −1 being all trials scored as misses or false alarms. Prior to statistical analyses all percentage data underwent arcsine transformation (Zar, 1974). Multiple within-subjects analyses of variance (ANOVAs) were used to determine the effects of distracter on task performance, and to determine if diurnality attenuates the deleterious effects of the visual distracter. Mixed analyses tested the main effects and interactions of entrainment conditions (entrained vs. phase-shifted), signal duration (where applicable: 500, 50 and 25 ms) and distracter/trial block (3 blocks of 54 trials: blocks 1, 2 and 3) on the relative number of hits (%H), correct rejections (%CR), overall vigilance index (VI), and percent omissions (%O). During a distracter session, distracter presentation occurred during the second block of trials, and is thus represented in subsequent analyses as the factor ‘trial-block’. Post hoc analyses for within subjects comparisons were carried out using the Least Significant Difference test (LSD). All analyses were performed using SPSS V16.
Control Treatment: Entrainment to Restricted Water Access
A second group of rats (n=8) was used to determine whether the altered circadian activity produced by SAT training could occur in animals undergoing all procedures except training in the operant chamber. Daily restricted access to water typically acts as a weak zeitgeber for rats (R. E. Mistlberger & Rechtschaffen, 1985), and daily handling can also act as a zeitgeber (Meixner et al., unpublished findings). We hypothesized that together the two cues might be sufficient to cause the circadian reorganization detected in animals undergoing SAT training during the light phase. Experimental animals were housed and circadian rhythms monitored as described previously.
Control Water Restriction and Handling Procedures
Water deprived rats are known to anticipate water availability during restricted water access and daily handling by experimenters (R. E. Mistlberger & Rechtschaffen, 1985). In order to test the hypothesis that task-performing rats would entrain to a task of sustained attention, a separate group of non-performing rats was used to ensure that the circadian entrainment observed in task-performing rats could not be attributed to the handling procedures or restricted water access which accompanied task training. Animals in the control treatment group underwent water deprivation and handling procedures identical to those of the task-performing group; however these rats were never trained to perform the sustained attention task. Control procedures were carried out in two phases: First, animals remained in the home cage environment and received one hour of daily water access from ZT 4 to ZT 5. Animals were maintained on this daily schedule for 3 weeks. Entrainment was assessed by releasing the animals into free-running conditions (total darkness) and measuring circadian activity for a period of 10 days. Animals resumed a 12:12 LD cycle with daily 1 hour water access from ZT 4 to ZT 5 for a period of 2 weeks followed by 2 weeks of handling procedures consistent with those used with task-performing animals: non-performing rats were removed from their home cages and placed into transport tubs (an opaque 50.8 × 50.8 cm cage) at ZT 4. Animals were transported on a rolling cart from the cage-room to the testing chambers over a period of ~3 minutes after which they were returned to their home cages and given access to water for 1 h. Following 2 weeks of daily handling and water restriction, animals were released into free-running conditions with ad libitum water access for a period of 10 days to assess the level of circadian entrainment produced by the control treatment.
Control Treatment: Entrainment to Restricted Water Access with Operant Conditioning
A third group of rats (n=4) served as a control to test whether the altered circadian activity produced by SAT training was a result of activity associated with operant task performance. The physical activity associated with timed treadmill running in mice (Marchant & Mistlberger, 1996) and the physical activity/rewarding stimuli associated with novel running wheel access in hamsters (Gorman & Lee, 2001) is sufficient to produce entrainment in the presence of a LD cycle. Therefore, we chose a task that mimics the SAT in number of trials, physical activity, and amount of reward, but requires a minimal level of cognitive vigilance.
Control Operant Methods - SRTT group
Operant training mirrored the training of animals in the SAT group. All animals were trained 7 days per week on a simple reaction time task (SRTT) in the same operant chambers used for the sustained attention task. After a two-week period of acclimation to the laboratory, animals experienced a week of gradual water deprivation consistent with animals trained on the SAT. Once animals reached one hour of daily access between ZT4 and ZT5, training on the SRTT was initiated. Controls were first trained to press levers for a water reward on the same fixed-ratio 1 schedule of reinforcement as animals trained on the SAT. After 3 days of shaping on the FR1 schedule, animals were advanced to training on the simple reaction time task. The SRTT is designed to mimic the final version of the SAT, albeit without the attentional demand required of the sustained attention task. This version of the task has been previously shown to produce only modest increases in cortical ACh release compared to training on the SAT (Arnold, Burk, Hodgson, Sarter, & Bruno, 2002). The SRTT differs from the SAT in only one way – that is, on every trial only the correct lever extends into the operant chamber. Animals are not forced to attend to the signal cue in order to maximize reward on the task. On each trial, as long as a lever press occurs, animals gain access to the same amount of reward as animals performing the SAT. All other conditions, including lighting, number of trials, signal cue duration, and the number of signal to non-signal trials remains consistent between the two versions of the task. Training continued on the operant task for a period of 20 days before the control experiment was terminated.
Circadian Analysis
The phases (ψ) of onset and offset of activity relative to dark onset and SAT training onset were determined prior to the phase advance and after reentrainment. The activity onset was defined as 3 or more 10-minute bins of activity that was more than 10% of daily mean activity and was followed by a period of sustained activity. The phase of the onset of free-running activity was described relative to the previous phase of training (ZT 4 or ZT 10) and the previous phase of lights off (ZT 12). One animal was excluded from analysis because sensor sensitivity was too weak for circadian rhythms to be scored. Repeated measures, multi-variate analysis of variance (MANOVA; Systat V10, Systat Software, Inc., San Jose, CA) was used to assess the effect of SAT time (ZT 4 vs. ZT 10) on onset and offset to phase of dark onset, onset and offset to phase of SAT onset, and ratio of locomotor activity between the light and dark phases. Activity ratios (LD Ratio) were determined using counts of the total number of 10 min bins in which activity was recorded during the light and dark period, rather than absolute number of movements during the light or dark periods to reduce the variability in amount of activity between animals and within animals across the circadian day. LD ratio values greater than one indicate diurnal activity pattern, whereas levels less than one indicate nocturnal activity pattern. Post hoc Tukey tests was used to compare SAT time effects on specific variables when there was an overall effect on entrainment to LD or SAT. Paired t-tests were also used to assess significance between paired comparisons of repeated measures on the SAT and control groups.
Correlations
Correlations between circadian measures of entrainment at ZT 4 and ZT 10 and attention performance data were performed for hits (%H), correct rejections (%CR), vigilance index (VI) and percent omissions (% O). The possibility of correlations between performance variables altered during the phase shift were tested against four circadian measures: activity phase to SAT time (ZT 4 and ZT 10), activity phase to dark onset, activity phase to rate of re-entrainment, and activity phase to overall LD ratio. Significance of all correlations was tested using a Bartlett chi-square test with Bonferroni-adjusted probabilities.
Comparison between Controls and SAT trained animals
LD ratio comparisons for within subjects tests between SAT(ZT4) and SAT(ZT10) and between H20 and H20+handling were made using a paired t-test. In addition, the entrained activity phase to the LD cycle and non-photic zeitgebers of daily SAT training at ZT 4, daily 1 h water access at ZT 4, daily handling with 1 h water access at ZT 4, and daily SRTT training at ZT 4 were compared across groups with MANOVA. Post hoc Tukey tests used to determine significant group differences between treatments for variables with significant overall effects.
Results
Performance at baseline
Baseline levels of performance were calculated using data from the final five sessions prior to experimental manipulations (distracter presentation with or without phase-shift). Hit rates were signal duration-dependent (F(2,20)= 73.55, p<0.001; 500 ms: 76.9±4.5%, 50 ms: 49.9±0.3%, 25 ms: 38.8±4.3%) and animals responded correctly to 81.31±2.3% of all non-signal trials. Animals omitted an average of 7.34±2.7% of trials per session.
Correlations Between Circadian Entrainment and Baseline Performance
The level of diurnal entrainment, as measured by LD activity ratio, was positively correlated with SAT performance as measured by vigilance index for the 5 day baseline period measured prior to the beginning of distracter training (Bartlett Chi-square = 3.983, p = 0.046; data not shown). This finding indicates that animals with a more robust diurnal entrainment level during the final stages of operant training at ZT 4 showed better overall performance on the SAT.
Effects of distracter presentation during conditions of stable entrainment
During conditions of stable circadian entrainment, animals received 5 consecutive distracter sessions. Performance during distracter presentation (block 2) was contrasted with performance of standard trials from the same session (blocks 1 and 3). Distracter presentation disrupted animals’ performance on non-signal trials (F(2,20)=26.28, p<0.001). Post hoc analyses determined that correct rejection rates were impaired during the distracter block relative to the two blocks of standard trials (block 1 vs. distracter: LSD=0.34, p≤0.001; distracter vs. block 3: LSD=0.471, p≤0.001). Performance of signal trials was dependent on signal duration (F(2,20)=81.15, p≤0.001). Hit rates were not affected during the block of distracter trials, but were reduced during trial block 3 following exposure to the distracter (F(2,20)=6.56, p=0.006; block 1 vs. block 3: LSD=0.205, p=0.025; distracter vs. block 3: LSD=0.252, p=0.003; block 1 vs. distracter: LSD=0.048, p=0.556). Block 3 consists of the final 54 trials of a daily session and measures the ability of the animal to recover from the deleterious effects of distracter trials presented during block 2. Distracter presentation did not affect the number of omitted trials.
Correlations Between Circadian Entrainment and Distracter Performance
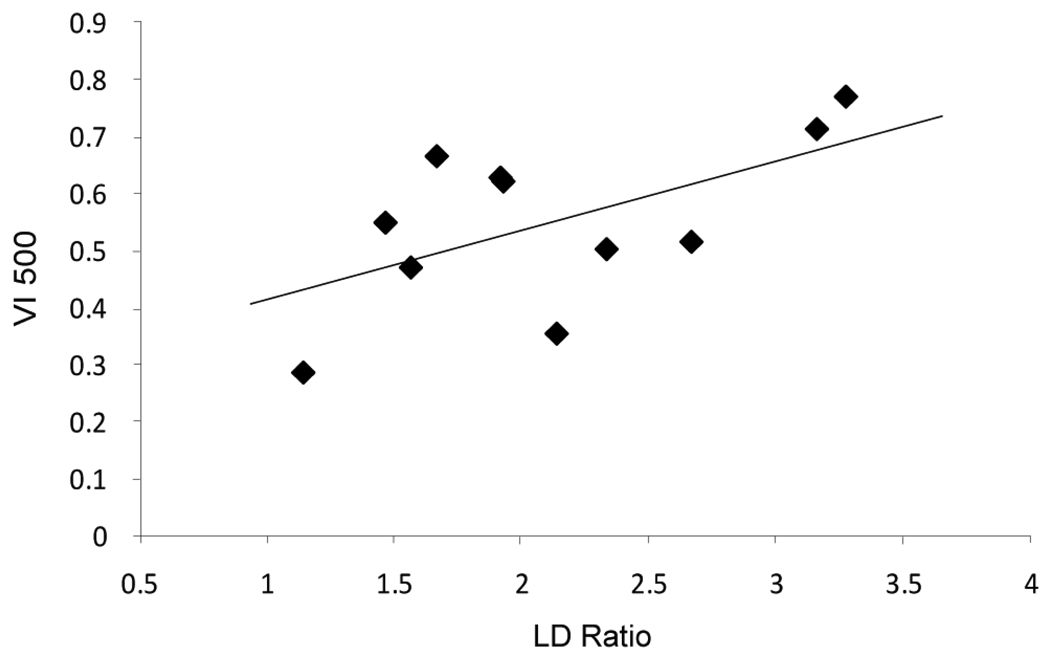
Performance during the 5 continuous days of distracter presentation was compared with the level of diurnal entrainment during the same period. Although distracter presentation impaired performance in all animals, diurnal behavior was a reliable predictor of performance during the recovery phase of distracter sessions. Performance during blocks 1 and 2 were not significantly correlated with entrainment; however, during block 3, the recovery block, performance on the composite measure of vigilance index was correlated with LD ratio. Figure 1 represents the correlation between LD ratio and vigilance index for the longest signal duration (VI500) and suggests that animals with a more diurnal activity pattern during task training show enhanced performance recovery in the trials following the distracter block (Bartlett Chi-square = 3.875, p= 0.048; Fig. 1).
Figure 1.
Regression analysis demonstrates positive correlation between diurnality, as measured by LD ratio, and VI500 performance in the recovery block following distracter training (dSAT). Individual points represent each animals (N=11) average performance from block 3 (final 54 trials representing recovery from distracter) over the 5 day period of distracter presentation plotted against LD Ratio from the same 5 day period. Slope = 0.138; r2= 0.366; p= 0.048. Performance during blocks 1 and 2 (before and during distracter, respectively) were not significantly correlated with entrainment (p = 0.13 and p = 0.17).
Effects of the phase-shift on SAT performance
The phase advance did not significantly affect animals’ SAT performance for the variables measured. Animals’ ability to respond correctly to signal or non-signal events was unchanged relative to baseline (hits: F(1,10)=2.48, p=0.14; correct rejections: F(1,10)=0.932, p=0.357). Performance on signal trials remained signal duration dependent (F(2,20)=38.93, p≤0.001; 500 ms: 82.5±5.4%, 50 ms: 54.8±7.9%, 25 ms: 41.1±6.6%). Similarly, errors of omission were not affected by the phase shift (p>0.05). Following the shift, animals responded correctly to 82.9±3.1% of all non-signal trials and omitted only 4.8±1.5% of all trials per session.
Effect of SAT Training and Control Treatment on Circadian Rhythms
Circadian analysis revealed a robust effect of daily SAT training on locomotor timing. All animals (n = 11) exhibited diurnal behavior when the training period was at ZT 4 with the average amount of activity during the light phase, divided by that during the dark phase (LD ratio) being 1.69 ± 0.23 and no animals having an LD ratio below 1.0, which would indicate a nocturnal activity pattern (Fig. 2, Fig. 3). When the daily test sessions occurred at ZT 10 and circadian activity rhythms regained stable entrainment, robust diurnal activity was still present, and animals became more robustly diurnal as measured by LD ratio (Fig. 2, Fig. 3, Fig. 4). The increased preference for diurnal activity when SAT was shifted to ZT 10 was reflected in a significant advance in daily activity onset relative to the SAT training time (F(1,16)= 5.633; p = 0.03) but not relative to the LD cycle (Fig. 5). Additionally, the LD ratio with SAT at ZT4 correlated strongly with the LD ratio with SAT at ZT10 (r = 0.961, Chi-square = 21.760, p < 0.001) demonstrating a consistent impact of SAT on circadian entrainment during the light phase both before and after the phase shift.
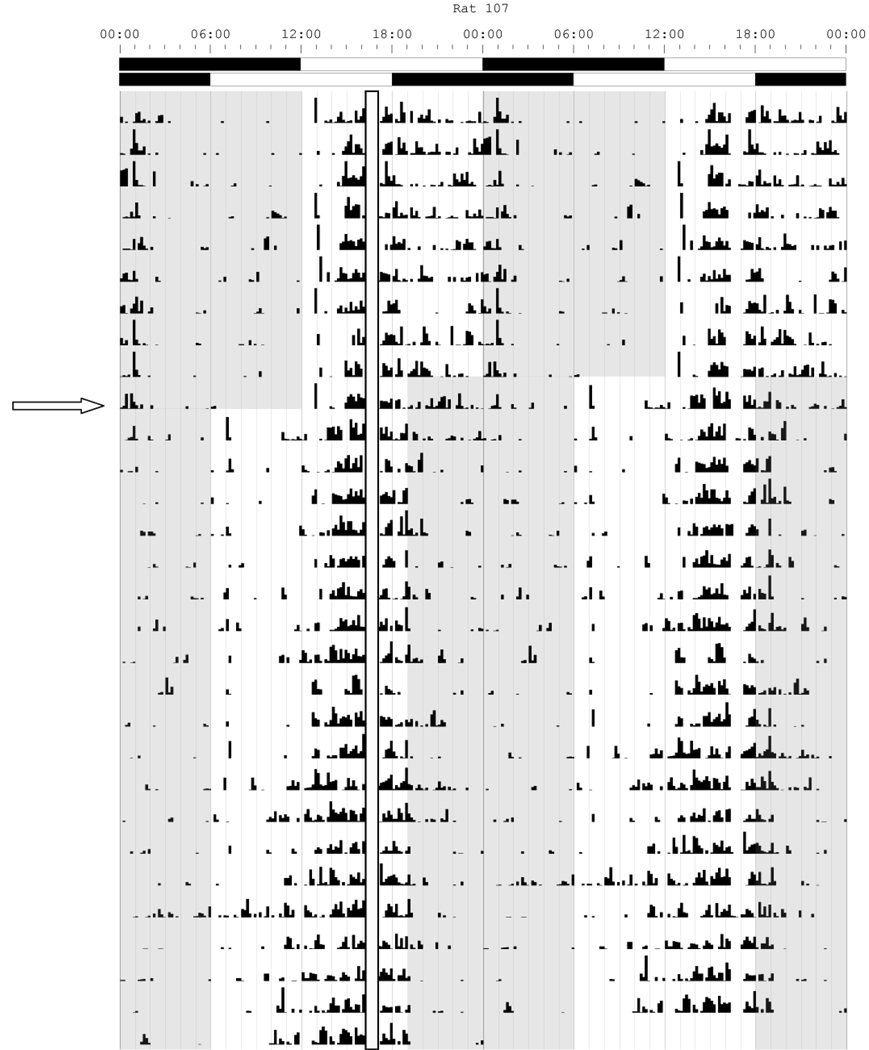
Figure 2.
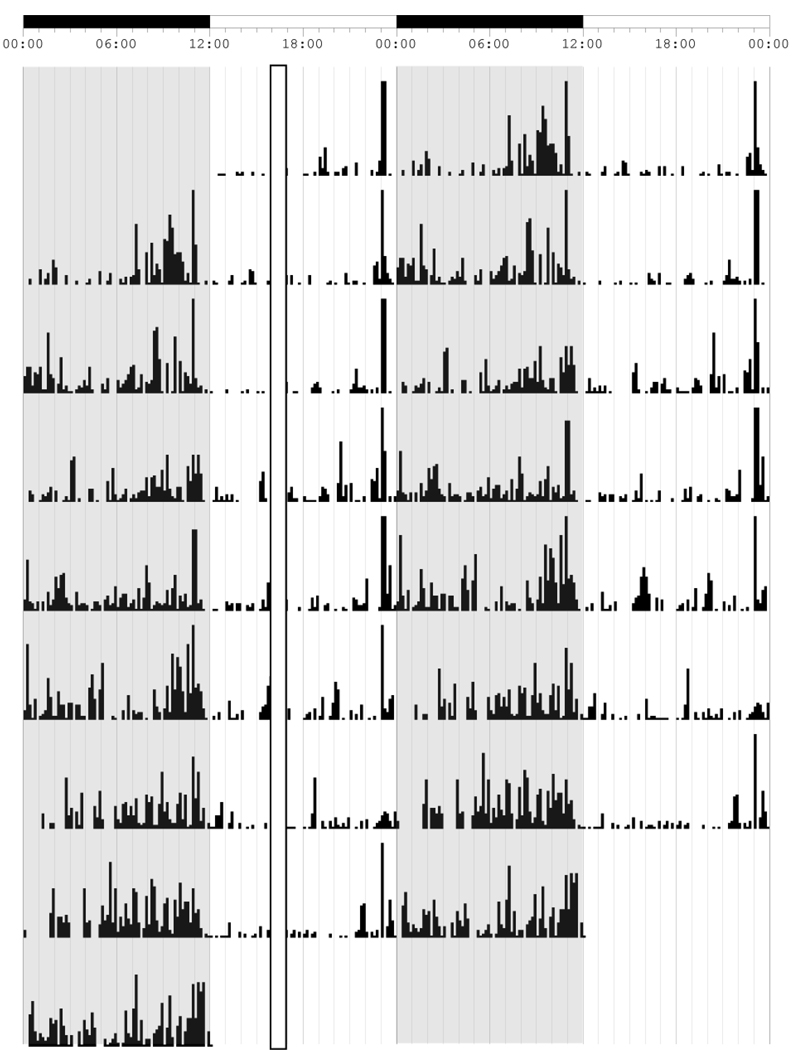
Double-plotted actogram for locomotor activity collected with infrared motion detector with 48 hours per line. The actogram shows SAT training at ZT 4 relative to the topmost LD bar (where dark bar = lights off) and the open column represents the approximate 40 min SAT training session in which animals are absent from their home cages. Dark phases each day are represented as shaded regions on the figure. The phase shift occurs on the 10th day of the actogram as indicated by the arrow on the left hand side. Following the 6 h phase advance, SAT training remains unchanged but now occurs at ZT 10. Re-entrainment to lights-off stabilizes by the 6th day post shift; however, the animal continues to advance its daily onset of activity until day 12 following the shift. This rate of re-entrainment represents the average rate of recovery for all animals in this study.
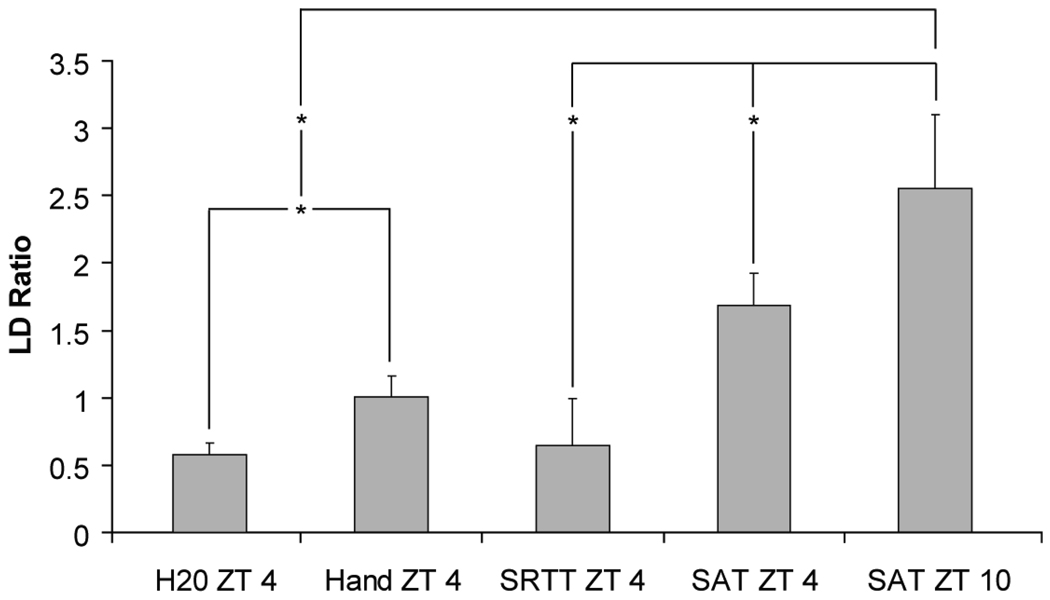
Figure 3.
Light:Dark ratio of activity from animals with stable entrainment from before and after phase shifting (SAT at ZT 4 and SAT at ZT 10) as well as three control conditions (water access-ZT 4, handling + water access-ZT 4, and SRTT-ZT4). Both SAT groups and non-task performing control groups show significant differences from one another, but only SAT-ZT 10 significantly differs from all the control groups (* indicates a significant difference between bracketed bars with a p < 0.05).
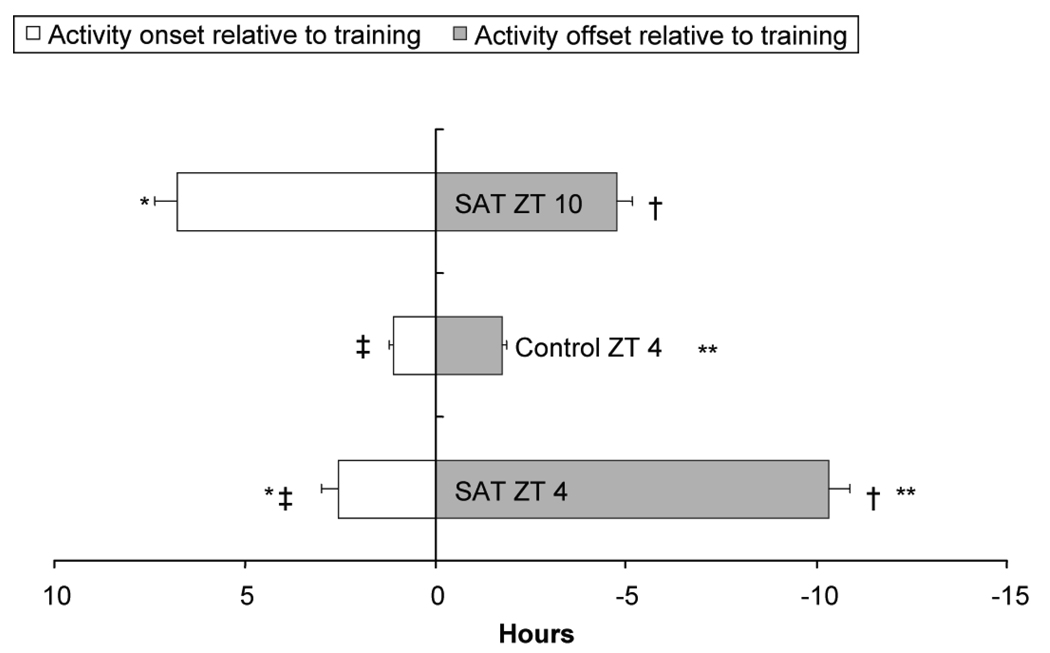
Figure 4.
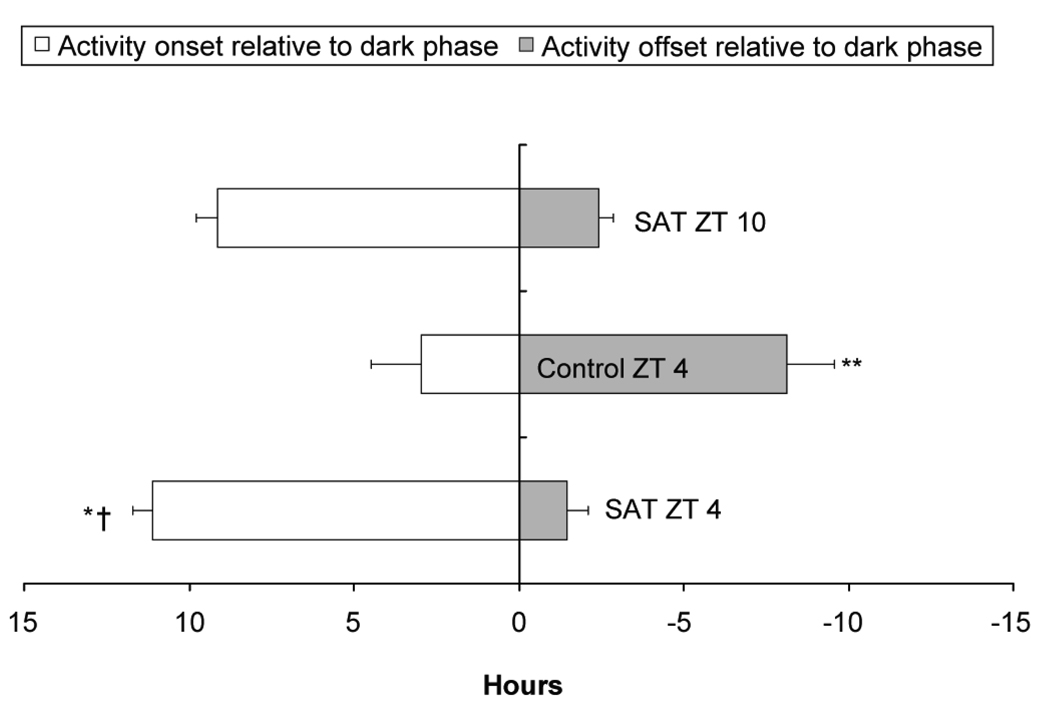
Mean phase relationship between time of diurnal locomotor activity and time of SAT training. Activity began and ended significantly earlier, relative to training, for animals with SAT at ZT 10 than SAT at ZT 4. The control animals that were handled and provided with water at ZT 4 differ significantly from both SAT groups for activity onset, cessation and duration (* p < 0.001, SAT ZT10 and SAT ZT 4 differ for activity onset; ** p < 0.001, Control and SAT ZT 4 differ for activity onset; † p< 0.001, SAT ZT 10 and SAT ZT 4 differ for activity offset; ‡ p < 0.05, Control and SAT ZT 4 differ for activity offset).
Figure 5.
Mean phase relationship between time of locomotor activity and lights-off. The animals experiencing SAT at ZT 4 and ZT 10 began and ended activity significantly earlier than the control animals that were handled and provided with water at ZT 4. SAT ZT 4 animals also began activity earlier than animals with SAT at ZT 10, but they did not end activity earlier after lights off (* p < 0.05, activity onset before dark differs between SAT ZT 10 and SAT ZT 4, † p < 0.001; activity onset before dark differs between Control and SAT at ZT 4; ** p < 0.001, activity offset differs between Control and SAT at ZT 4).
The free running data collected in constant conditions following SAT training at ZT 10 indicated that, while all subjects displayed robust diurnal behavior, a varying amount of diurnal entrainment was evident after release into DD. The range of responses is demonstrated in figure 6. Activity from Rat 110 represents the entrainment pattern most commonly presented by animals released into constant conditions. Ten of the eleven animals scored, including Rat 110, expressed two clear activity components: one that free ran from the onset of light or the onset of a sustained diurnal activity and one from the high amplitude activity around the ZT10 SAT session. Rat 110 also demonstrates the reemergence of a nocturnal activity phase component soon after release into DD, suggesting that some negative masking has occurred as a result of SAT training. Rat 113 shows a robust level of diurnal entrainment that free ran from the period of lights on and continues until the end of the actogram. This animal also demonstrates very little, if any, nocturnal masking. In some animals (n= 4) significant behavior was also immediately apparent during early subjective night, suggesting that the SAT session may also reduce activity during the dark phase (i.e., negative masking). Rat 106 represents the opposite end of the spectrum, showing the least robust level of diurnal entrainment and a significant shift to a nocturnal activity presence shortly after being released into DD. However, it is important to note that on the first day of DD and no water access, all animals demonstrated a robust period of activity during what would be the subjective day. Overall, the free running behavior following the phase shift to ZT10 training, suggests that circadian activity was entrained by light onset, onset of the SAT training period, and to a lesser extent, light offset.
Figure 6.
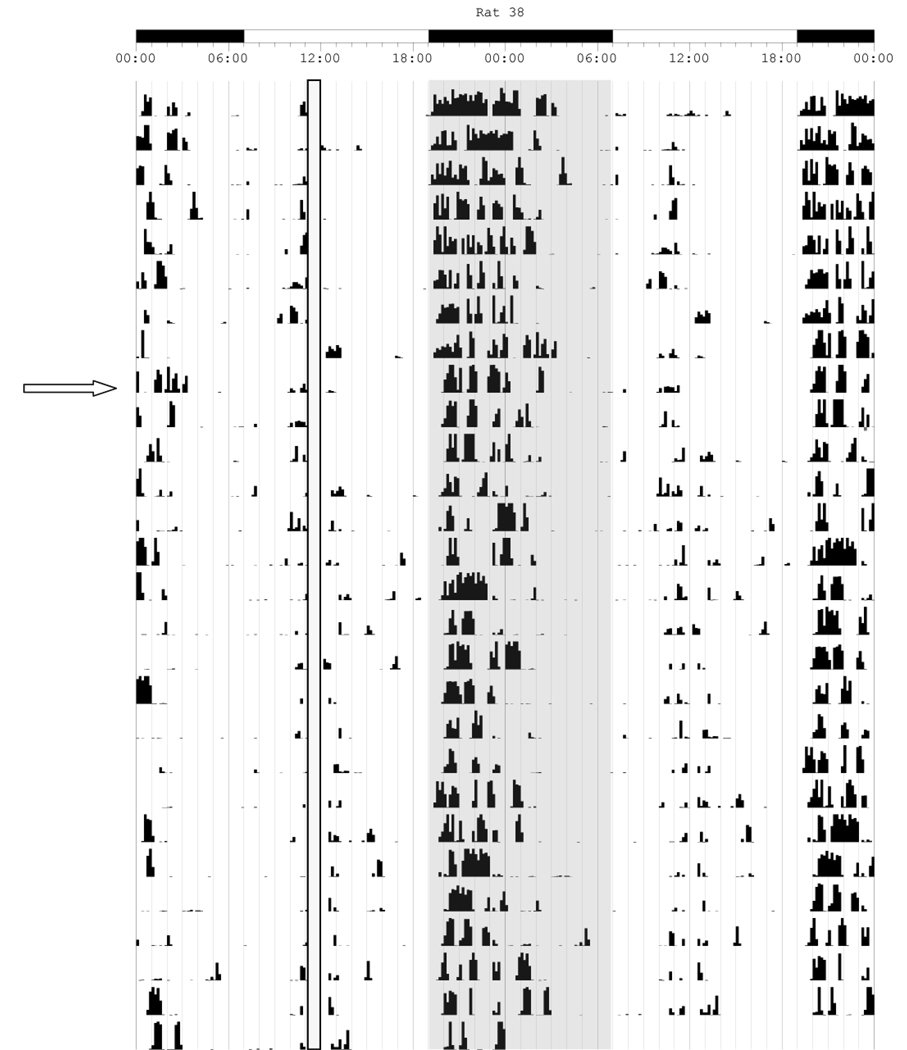
Double-plotted actogram representing the locomotor activity collected with infrared motion detectors of three animals when released into constant conditions (DD). DD begins at the day indicated by the first open arrow. The second filled arrow indicates when the LD cycle was reinstated. Shaded regions above the open arrow and below the closed arrow represent the dark phase of the 24 hour period. The shaded region below the open arrow and before the closed arrow indicates the period of time when lights-off previously occurred. All animals following training at ZT 10 show robust activity during subjective day on the first day of DD, and activity bouts on subsequent days that begin during the light phase periods prior to release into total darkness. Rat 110 demonstrates an example of diurnal activity that continues to occur at the prior phase, although some disappeared (e.g, the bout at lights-on, and activity between the onset of the main diurnal bout and the heavy bout at SAT training time) after being released into DD. While some level of nocturnal masking is apparent, at least two diurnal activity bouts also free-ran from the initial phase and are marked with superimposed solid lines demonstrating circadian changes resulting from SAT training are a product of entrainment and not masking alone. Rat 113 demonstrates a primarily diurnal level of entrainment to SAT training. This animal continues to show free-run activity from the light phase until the end of the actogram as demonstrated by the superimposed solid line with little indication of nocturnal masking. Rat 106 shows entrainment to the SAT task; however, nocturnal activity returns robustly within a few days of being released into DD, suggesting that masking may be more important for this animal than the others. Both Rats 110 and 113 continue to free-run after the LD cycle is reinstated, whereas Rat 106 is entrained with a nocturnal phase.
Interestingly, the majority of animals did not quickly re-entrain to the LD cycle when it was reinstated after the DD period. In Fig 6, only Rat 106 demonstrates a nocturnal phase pattern, although it is delayed relative to rats that have never undergone SAT training. Rats 110 and 113 continue shifting the time of activity onset throughout the duration of the actogram. Even after another 10 days in the LD cycle to which they were previously exposed, without any further operant training, they have not established a normal nocturnal phase relationship of activity onset at or just after lights off. Rat 113, for example, maintains considerable diurnal activity 20 days after SAT training has ceased.
The control treatment of one hour daily water access at ZT 4 produced no significant effect on circadian entrainment of activity. Only a few counts of binned activity occurred prior to the time of water access after 3 weeks, and no free-running activity occurred at the time of water access when animals were released into constant conditions. This control demonstrates that diurnal circadian entrainment associated with the SAT task is not caused by daily timed water access.
Weak daily entrainment occurred during the light phase when animals were handled prior to daily access to water for 1 h at ZT 4 (Fig. 7). The small diurnal entrainment effects were apparent in the few minutes of activity prior to daily handling and water access, and the few days that the effect lasted during constant conditions (2.8 ± 0.986 days - data not shown). Overall, the second control treatment produced a greater entrained diurnal activity component, but was substantially less than that seen in animals performing the SAT. All animals remained robustly nocturnal throughout the duration of the experiment as measured by the LD activity ratio (Fig. 3, Fig. 4, Fig. 5).
Figure 7.
Representative double plotted locomotor activity actogram collected by infrared motion detector of an animal experiencing both daily handling followed by 1 h of water access. The shaded regions represent the dark phase of the 24 hour period. Although there is an increase in diurnal locomotor behavior that accompanies handling, the animal remained strongly nocturnal. See Figure 3 for quantitative analysis of LD Ratio. Open rectangular column indicates the time of daily water access.
The simplified reaction time task (SRTT) serves as an essential control for understanding how cognitive activity interacts with circadian rhythms. This task controls for the critical characteristics of task performance, including physical activity and the rewarding mechanisms inherit in working for reward during operant conditioning. Even after 20 days of continuous training, all subjects (n=4) showed a robust nocturnal activity pattern as measured by LD ratio (0.67 ± 0.35), that did not differ significantly from either the water deprivation group (p=0.890) or the combined water deprivation and handling control group (p=0.550). Figure 8 shows a typical actogram for an animal training on the SRTT. The first 8 days of the actogram represent timed access to water alone occurring between ZT4 and ZT5. The arrow on day 9 indicates the first day of operant training. While no significant increase in diurnality was present, a small amount of diurnal entrainment was present based on the increase in activity in anticipation of the daily training sessions. This level of anticipation was not significantly different from animals that underwent handling and water deprivation alone.
Figure 8.
Double plotted running wheel actogram of typical animal training on a simplified low-cognitive demand operant task (SRTT – see methods for details). During the first 8 days of the actogram, the animal is receiving 1 h of daily water access from ZT4-ZT5. Operant conditioning begins on the 9th day of the actogram as indicated by the open arrow on the left hand side. The shaded region denotes the 12 hour dark phase of the experiment where the open box indicates the time of daily training. Training on the SRTT continued for 21 days with no overall shift in diurnality ratio.
When LD ratio of activity was analyzed for differences between the animals when trained at SAT-ZT 4 or SAT-ZT 10, the strength of diurnality was significantly after the shift to ZT 10 (paired t-test (1,10) = −2.427, p = 0.036; Fig. 3). Also, diurnal activity after exposure to the two non-operant control conditions, handling + water access ZT 4 and water access ZT 4, significantly differed from each other (paired t-test (1,7) t = −4.052, p = 0.007; Fig. 3). When all five groups were compared, the SAT-ZT 10 condition was significantly different from every control condition tested (p < 0.004; Fig. 3).
The phase of activity onset and offset to the SAT differed significantly between SAT-ZT 4 and SAT-ZT 10 (F(1,20)= 31.789; F(1,20)= 69.067; for both p < 0.001; Fig. 4). Significant differences were also found between the treatment and control groups (SAT-ZT 4 and handling + water control-ZT4) with respect to onset (p < 0.050) and offset (p < 0.001; Fig 4) of activity to treatment.
The two SAT treatment groups (SAT-ZT 4 and SAT-ZT 10) also differed in phase onset relative to darkness (F(1,20) = 4.743; p < 0.05; Fig. 5). Phase of activity for groups SAT-ZT 4 and handling + water control-ZT 4 significantly differed with respect to onset and offset to darkness (p < 0.001 for both; Fig. 5).
Correlations Among Circadian Measures
Entrainment to the SAT training at both ZT 4 and ZT 10 were strongly correlated with entrainment to the LD cycle. The effect was strongly driven by a single daily activity onset during the light phase and little activity during the dark phase for most animals (Fig. 2). For example, when animals were entrained to SAT training at ZT 4 there was a strong correlation between the phase of activity onset relative to time of SAT training and the activity at lights off (r = 0.845, p= 0.004; Chi-square = 43.404, p < 0.001). When animals completed re-entrainment and SAT occurred at ZT 10, the correlation was strengthened (r = 0.969, p< 0.001; Chi-square = 154.225, p< 0.001). Taken as a whole, these correlations demonstrate that animals that anticipated the SAT task most strongly also had the most diurnal activity patterns. Somewhat surprisingly, there was no correlation between the phase angle of activity to treatment at ZT 4 and ZT 10, or between the phase angle of entrainment with dark onset before and after the phase shift.
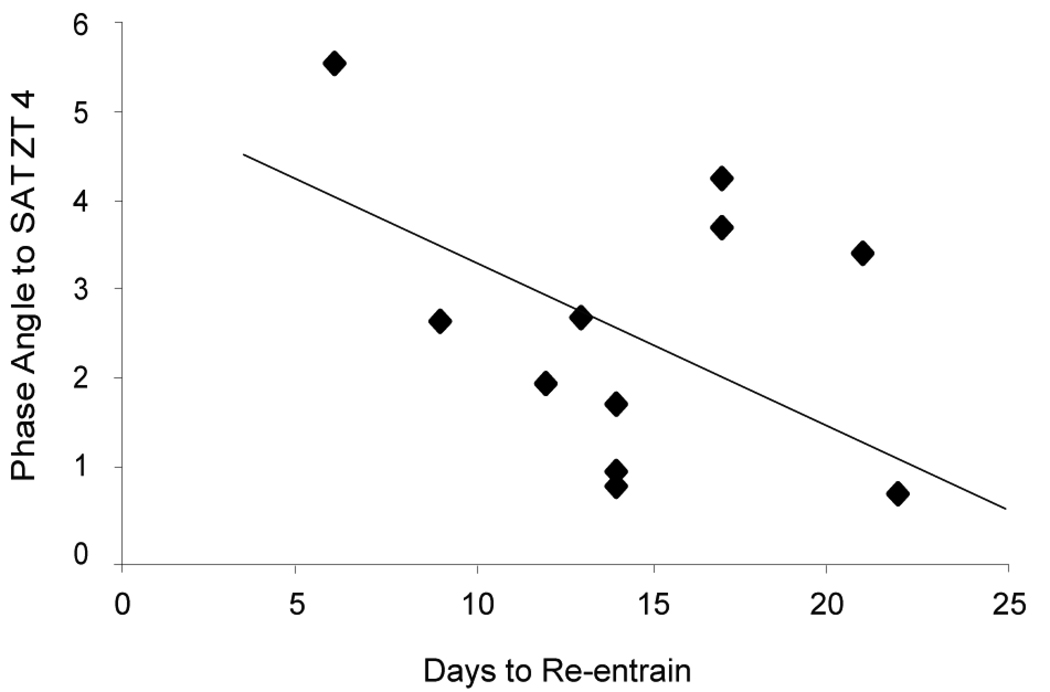
The phase of activity entrainment relative to the LD cycle during the SAT-ZT 4 treatment correlated with re-entrainment rate (r = −0.476, r = −0.577, activity onset and offset relative to dark, respectively; overall Chi-square = 12.916, p = 0.002; Fig. 9). There was also a trend for phase of activity entrainment relative to the SAT-ZT 4 training time and re-entrainment rate (r = −0.324, −0.262, activity onset and offset relative to SAT onset, respectively; overall Chi-square = 5.338, p= 0.069). There were no correlations between the post-shift entrainment of activity during the SAT-ZT 10 and re-entrainment rate.
Figure 9.
Correlation between reentrainment rate after a 6 h phase advance in the LD cycle and entrained activity onset before the phase shift when entrained with SAT at ZT 4. See text for statistical analysis.
Discussion
These data support the hypothesis that a demanding cognitive task can produce a robust and significant effect on the organization of circadian locomotor behavior. Daily SAT practice at ZT 4 produced a dramatic reversal in the activity pattern, such that all rats exhibited diurnal (predominantly light phase) activity. Additionally, this diurnal activity pattern was maintained after a 6 hour phase advance in the light cycle and additional training at ZT 10. This effect was not due to the restriction of water access, daily social contact, activity associated with operant training, or working for access to a reward. SAT performance and dSAT performance also correlated with phase of entrainment at ZT 4, suggesting that entrainment may be an important condition of performance during tasks of cognition. Also, the correlations between ZT 10 entrainment phase and performance indicate that performance during the phase advance may predict circadian entrainment patterns following re-entrainment, although this correlation may be driven entirely by the extent of diurnality produced by the SAT at ZT 4.
Future experiments are necessary to fully analyze the relationship between SAT performance and phase of circadian recovery as well as the potential for the HPA axis to interact with the attentional system during periods of phase shifting. Mohawk et al. demonstrated that depressing the stress response improved recovery time after a phase advance (Mohawk, Cashen, & Lee, 2005). Under such conditions the animals had larger changes in phase each day. This raises the interesting question of whether performance on the SAT would be worse on days individuals are making the largest changes in phase, but might have fewer days of poor performance. This hypothesis supports a condition where animals slower to change phase would perform more poorly on the SAT if the task was advanced in coincidence with the light cycle.
The robustness and size of the effect of SAT training on circadian activity were unexpected. Previous studies formed the basis for our theory that there would be entrained anticipatory locomotor activity surrounding the training period but that animals would still retain a predominantly nocturnal activity pattern (R. Mistlberger & Rusak, 1987; R. E. Mistlberger, 1992, 1993). All subjects, however, showed a robust diurnal activity pattern that extended well beyond the time immediately surrounding the training paradigm (Fig. 2). The significance of the SAT was further validated by the control treatments that demonstrated that these findings cannot be due to handling, the presence of daily timed water access, or activity associated with task performance. While all of the control groups show some level of anticipation during the light phase, each group remained primarily nocturnal. The results of these studies provide additional evidence that conclude that there is a significant impact of sustained attention practice on entrained circadian activity patterns. We suggest that the most plausible explanation for a change in circadian activity pattern is due to the activation of the cholinergic basal forebrain circuits recruited by the SAT task. This theory is bolstered by the finding that performance of a simple reinforcement schedule, known to produce only small increases in ACh levels (Arnold et al., 2002), is insufficient to produce the diurnal circadian pattern shown by the SAT animals in this study. We suggest that this modulatory input could arrive via multiple mechanisms in order to influence circadian entrainment. One possibility is that top-down cortical regulation of SCN output could occur directly via projection systems known to be involved in decision making and cognitive control. Axon tract tracing studies show that projections from these cortical regions innervate not just the SCN, but multiple hypothalamic nuclei (Hurley, Herbert, Moga, & Saper, 1991; Vertes, 2004) essential for autonomic function. Lesion studies designed to de-afferent neocortical regions essential for task performance are necessary to test the role of top-down regulation in modulation of the circadian clock. Additional studies are addressing the hypothesis that increased ACh release from the basal forebrain circuits, specifically activated with SAT training (Kozak et al., 2006), directly influence the phase of activity entrainment via the anatomical connectivity that exists between the basal forebrain and the SCN (Erhardt et al., 2004; Madeira, Pereira, Silva, Cadete-Leite, & Paula-Barbosa, 2004). These studies, in combination with microdialysis experiments in task performing animals, should offer insight into how ACh is being regulated across discrete brain regions in anticipation of daily training. Finally, the question of how cognition influences circadian activity at the level of the SCN remains to be explored. Future experiments will address if the modification of behavior is the result of changes in the expression of clock genes at the level of the SCN itself, or if the changes in phase activity seen in SAT animals occurs via regulation of SCN downstream targets.
An additional surprise in the data was the increased diurnality of animals training at ZT10 compared to ZT4. When the animals were shifted we expected to see the phase relationship shift such that activity would begin at ZT8 or 9, prior to onset of training each day, resulting in the animals being less diurnal after the shift. In other unpublished work from the laboratory (Hummer et al, unpublished), handling between ZT8 and 10 produces a phase advanced activity onset for the day, but the animals maintain a predominantly nocturnal activity pattern. This leads to the hypothesis that the history of the animal’s previous training schedule has an impact on subsequent entrainment as the timing of cognitive activity is shifted relative to the LD cycle. Experiments are needed to further explore these possibilities as they may have important consequences for humans that are shifting work schedules.
While light may be the strongest zeitgeber, the data presented here provide evidence that entrainment can be influenced substantially via mechanisms of sustained attention. Furthermore, the effects seen are not simple masking, but rather entrainment to the SAT that is clear in the absence of all external cues. The present experimental approach serves as an animal model for investigating the neuronal mechanisms that mediate the effects of cognitive performance on modulation of circadian activity. As circadian abnormalities contribute to the cognitive symptoms of major neuropsychiatric disorders (Bunney & Bunney, 2000; Monteleone & Maj, 2008; Van den Bergh, Van Calster, Pinna Puissant, & Van Huffel, 2008; Wirz-Justice & Van den Hoofdakker, 1999; Wu & Bunney, 1990), understanding the bidirectional interactions between cognitive performance and circadian control may be key to developing more conclusive neuroscientific theories of these disorders.
Acknowledgements
This research was supported by PHS Grant RO1 MH079084 (TL) and KO2 MH01072 (MS).
We would like to thank Rouba Kouzak for her technical assistance and expertise regarding collection and analysis of SAT performance data. We would additionally like to thank Hailey Hines for her assistance in animal training, data acquisition, and analysis.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne.
References
- Arnold HM, Burk JA, Hodgson EM, Sarter M, Bruno JP. Differential cortical acetylcholine release in rats performing a sustained attention task versus behavioral control tasks that do not explicitly tax attention. Neuroscience. 2002;114(2):451–460. doi: 10.1016/s0306-4522(02)00292-0. [DOI] [PubMed] [Google Scholar]
- Bina KG, Rusak B. Muscarinic receptors mediate carbachol-induced phase shifts of circadian activity rhythms in Syrian hamsters. Brain Res. 1996;743(1–2):202–211. doi: 10.1016/s0006-8993(96)01043-8. [DOI] [PubMed] [Google Scholar]
- Bina KG, Rusak B, Semba K. Localization of cholinergic neurons in the forebrain and brainstem that project to the suprachiasmatic nucleus of the hypothalamus in rat. J Comp Neurol. 1993;335(2):295–307. doi: 10.1002/cne.903350212. [DOI] [PubMed] [Google Scholar]
- Bina KG, Rusak B, Semba K. Sources of p75-nerve growth factor receptor-like immunoreactivity in the rat suprachiasmatic nucleus. Neuroscience. 1997;77(2):461–472. doi: 10.1016/s0306-4522(96)00496-4. [DOI] [PubMed] [Google Scholar]
- Bunney WE, Bunney BG. Molecular clock genes in man and lower animals: possible implications for circadian abnormalities in depression. Neuropsychopharmacology. 2000;22(4):335–345. doi: 10.1016/S0893-133X(99)00145-1. [DOI] [PubMed] [Google Scholar]
- Card JP, Moore RY. The suprachiasmatic nucleus of the golden hamster: immunohistochemical analysis of cell and fiber distribution. Neuroscience. 1984;13(2):415–431. doi: 10.1016/0306-4522(84)90240-9. [DOI] [PubMed] [Google Scholar]
- Edgar DM, Miller JD, Prosser RA, Dean RR, Dement WC. Serotonin and the mammalian circadian system: II. Phase-shifting rat behavioral rhythms with serotonergic agonists. J Biol Rhythms. 1993;8(1):17–31. doi: 10.1177/074873049300800102. [DOI] [PubMed] [Google Scholar]
- Erhardt C, Galani R, Jeltsch H, Cassel JC, Klosen P, Menet JS, Pevet P, Challet E. Modulation of photic resetting in rats by lesions of projections to the suprachiasmatic nuclei expressing p75 neurotrophin receptor. Eur J Neurosci. 2004;19(7):1773–1788. doi: 10.1111/j.1460-9568.2004.03281.x. [DOI] [PubMed] [Google Scholar]
- Frey PW, Colliver JA. Sensitivity and responsivity measures for discrimination learning. Learning and Motivation. 1973;4(3):327–342. [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Bacon SJ. Areal and synaptic interconnectivity of prelimbic (area 32), infralimbic (area 25) and insular cortices in the rat. Brain Res. 2003;993(1–2):59–71. doi: 10.1016/j.brainres.2003.08.056. [DOI] [PubMed] [Google Scholar]
- Gillette MU, Buchanan GF, Artinian L, Hamilton SE, Nathanson NM, Liu C. Role of the M1 receptor in regulating circadian rhythms. Life Sci. 2001;68(22–23):2467–2472. doi: 10.1016/s0024-3205(01)01040-2. [DOI] [PubMed] [Google Scholar]
- Goel N, Lee TM. Relationship of circadian activity and social behaviors to reentrainment rates in diurnal Octodon degus (Rodentia) Physiol Behav. 1996;59(4–5):817–826. doi: 10.1016/0031-9384(95)02141-8. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Lee TM. Daily novel wheel running reorganizes and splits hamster circadian activity rhythms. J Biol Rhythms. 2001;16(6):541–551. doi: 10.1177/074873001129002231. [DOI] [PubMed] [Google Scholar]
- Guilding C, Piggins HD. Challenging the omnipotence of the suprachiasmatic timekeeper: are circadian oscillators present throughout the mammalian brain? Eur J Neurosci. 2007;25(11):3195–3216. doi: 10.1111/j.1460-9568.2007.05581.x. [DOI] [PubMed] [Google Scholar]
- Hajszan T, Zaborszky L. Direct catecholaminergic-cholinergic interactions in the basal forebrain. III. Adrenergic innervation of choline acetyltransferase-containing neurons in the rat. J Comp Neurol. 2002;449(2):141–157. doi: 10.1002/cne.10279. [DOI] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308(2):249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Kozak R, Bruno JP, Sarter M. Augmented prefrontal acetylcholine release during challenged attentional performance. Cereb Cortex. 2006;16(1):9–17. doi: 10.1093/cercor/bhi079. [DOI] [PubMed] [Google Scholar]
- Lee HS, Kim MA, Waterhouse BD. Retrograde double-labeling study of common afferent projections to the dorsal raphe and the nuclear core of the locus coeruleus in the rat. J Comp Neurol. 2005;481(2):179–193. doi: 10.1002/cne.20365. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Pereira PA, Silva SM, Cadete-Leite A, Paula-Barbosa MM. Basal forebrain neurons modulate the synthesis and expression of neuropeptides in the rat suprachiasmatic nucleus. Neuroscience. 2004;125(4):889–901. doi: 10.1016/j.neuroscience.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Marchant EG, Mistlberger RE. Entrainment and phase shifting of circadian rhythms in mice by forced treadmill running. Physiol Behav. 1996;60(2):657–663. doi: 10.1016/s0031-9384(96)80045-x. [DOI] [PubMed] [Google Scholar]
- Medanic M, Gillette MU. Serotonin regulates the phase of the rat suprachiasmatic circadian pacemaker in vitro only during the subjective day. J Physiol. 1992;450:629–642. doi: 10.1113/jphysiol.1992.sp019147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Bernstein EL, Morin LP. Differential serotonergic innervation of the suprachiasmatic nucleus and the intergeniculate leaflet and its role in circadian rhythm modulation. J Neurosci. 1996;16(6):2097–2111. doi: 10.1523/JNEUROSCI.16-06-02097.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger R, Rusak B. Palatable daily meals entrain anticipatory activity rhythms in free-feeding rats: dependence on meal size and nutrient content. Physiol Behav. 1987;41(3):219–226. doi: 10.1016/0031-9384(87)90356-8. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE. Anticipatory activity rhythms under daily schedules of water access in the rat. J Biol Rhythms. 1992;7(2):149–160. doi: 10.1177/074873049200700206. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE. Circadian properties of anticipatory activity to restricted water access in suprachiasmatic-ablated hamsters. Am J Physiol. 1993;264(1 Pt 2):R22–R29. doi: 10.1152/ajpregu.1993.264.1.R22. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Rechtschaffen A. Periodic water availability is not a potent zeitgeber for entrainment of circadian locomotor rhythms in rats. Physiol Behav. 1985;34(1):17–22. doi: 10.1016/0031-9384(85)90070-8. [DOI] [PubMed] [Google Scholar]
- Mohawk JA, Cashen K, Lee TM. Inhibiting cortisol response accelerates recovery from a photic phase shift. Am J Physiol Regul Integr Comp Physiol. 2005;288(1):R221–R228. doi: 10.1152/ajpregu.00272.2004. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Maj M. The circadian basis of mood disorders: recent developments and treatment implications. Eur Neuropsychopharmacol. 2008;18(10):701–711. doi: 10.1016/j.euroneuro.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Moore RY. Organization and function of a central nervous system circadian oscillator: the suprachiasmatic hypothalamic nucleus. Fed Proc. 1983;42(11):2783–2789. [PubMed] [Google Scholar]
- Moore RY. The geniculohypothalamic tract in monkey and man. Brain Res. 1989;486(1):190–194. doi: 10.1016/0006-8993(89)91294-8. [DOI] [PubMed] [Google Scholar]
- Moore RY, Lenn NJ. A retinohypothalamic projection in the rat. J Comp Neurol. 1972;146(1):1–14. doi: 10.1002/cne.901460102. [DOI] [PubMed] [Google Scholar]
- Novak CM, Harris JA, Smale L, Nunez AA. Suprachiasmatic nucleus projections to the paraventricular thalamic nucleus in nocturnal rats (Rattus norvegicus) and diurnal nile grass rats (Arviacanthis niloticus) Brain Res. 2000;874(2):147–157. doi: 10.1016/s0006-8993(00)02572-5. [DOI] [PubMed] [Google Scholar]
- Novak CM, Nunez AA. A sparse projection from the suprachiasmatic nucleus to the sleep active ventrolateral preoptic area in the rat. Neuroreport. 2000;11(1):93–96. doi: 10.1097/00001756-200001170-00019. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Luck SJ, Lustig C, Sarter M. CNTRICS final task selection: control of attention. Schizophr Bull. 2009;35(1):182–196. doi: 10.1093/schbul/sbn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56(1):141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Juarez A, Escobar C, Aguilar-Roblero R. Anterior paraventricular thalamus modulates light-induced phase shifts in circadian rhythmicity in rats. Am J Physiol Regul Integr Comp Physiol. 2002;283(4):R897–R904. doi: 10.1152/ajpregu.00259.2002. [DOI] [PubMed] [Google Scholar]
- Sarter M, Gehring WJ, Kozak R. More attention must be paid: the neurobiology of attentional effort. Brain Res Rev. 2006;51(2):145–160. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res Brain Res Rev. 2005;48(1):98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BR, Van Calster B, Pinna Puissant S, Van Huffel S. Self-reported symptoms of depressed mood, trait anxiety and aggressive behavior in post-pubertal adolescents: Associations with diurnal cortisol profiles. Horm Behav. 2008;54(2):253–257. doi: 10.1016/j.yhbeh.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51(1):32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vrang N, Mrosovsky N, Mikkelsen JD. Afferent projections to the hamster intergeniculate leaflet demonstrated by retrograde and anterograde tracing. Brain Res Bull. 2003;59(4):267–288. doi: 10.1016/s0361-9230(02)00875-4. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Van den Hoofdakker RH. Sleep deprivation in depression: what do we know, where do we go? Biol Psychiatry. 1999;46(4):445–453. doi: 10.1016/s0006-3223(99)00125-0. [DOI] [PubMed] [Google Scholar]
- Wu JC, Bunney WE. The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. Am J Psychiatry. 1990;147(1):14–21. doi: 10.1176/ajp.147.1.14. [DOI] [PubMed] [Google Scholar]
- Ying SW, Rusak B. Effects of serotonergic agonists on firing rates of photically responsive cells in the hamster suprachiasmatic nucleus. Brain Res. 1994;651(1–2):37–46. doi: 10.1016/0006-8993(94)90678-5. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. 2nd ed. Englewood Cliffs, NJ: Prentice Hall; 1974. [Google Scholar]