Abstract
Non-Hodgkin lymphoma (NHL) represents a group of heterogeneous diseases that significantly vary in their causes, molecular profiles, and natural progression. In 2006, there will be ~59,000 newly diagnosed NHL cases in the U.S. and over 300,000 cases worldwide. While new therapeutic regimens are minimizing the number of deaths related to NHL, causes for the majority of lymphomas remain undetermined. Recent studies suggest that dietary factors may contribute to the rising rates of NHL. This review will summarize epidemiological reports that have studied the relationship between obesity, physical activity, diet and risk of NHL.
Based on a number of case-control and prospective cohort studies, overweight/obesity likely increases the risk of NHL; whereas, moderate physical activity may reduce risk. Several studies support an inverse association between intakes of vegetables and NHL risk, particularly for the consumption of cruciferous vegetables. This may relate to the induction of apoptosis and growth arrest in pre-neoplastic and neoplastic cells, two important actions of isothiocyanates found in cruciferous vegetables. Studies also suggest that fish intake may be inversely associated with risk of NHL, though findings have not been entirely consistent. This may relate to the high organochlorine content in some fish that could override a protective effect. High consumption of fats, meat and dairy products also may increase lymphoma risk. The accumulated scientific evidence concerning the associations between obesity, diet, and NHL suggests several identified modifiable risk factors that might be recommended to decrease lymphoma risk.
Introduction
Worldwide, longer life spans have led to increases in morbidity and mortality as a result of chronic, lifestyle-influenced diseases that may include cancers such as non-Hodgkin lymphoma (NHL). There has been a global rise in NHL over the past several decades, though reasons for this increase are unclear. Factors that enhance proliferation and survival of B-cells such as autoimmune disease and infection have been associated with lymphoma risk. Recent studies also suggest that obesity and the diet may contribute to these rising rates. Obesity results in pathological states of inflammation and altered immune responses, and has been associated with several cancers. Further, the diet may influence cancer risk through exposure to dietary carcinogens or through its effects on hormonal and metabolic responses to cell growth and survival. Particularly relevant to lymphoma, the diet imposes substantial antigenic challenges to lymphoid tissue in the gastrointestinal tract that can alter immune system responses. This review will summarize epidemiological reports that have studied the relationship between obesity, physical activity, diet and risk of NHL.
Role of obesity and risk of lymphoma
Several case-control and prospective cohort studies have examined the role of obesity and risk of NHL [Table 1, references online)] and found fairly consistent evidence that obesity [body mass index (BMI), weight (kg)/height (m2) >30] was associated with associated with elevated risks of NHL, diffuse large B-cell lymphoma (DLCL), follicular lymphoma (FL) and chronic lymphocytic leukemia. Pan et al. also reported that high caloric intakes increased risks for FL, small lymphocytic lymphoma and other subtypes, but not for DLBCL. Two other large case-control studies found increased risks of DLCL associated with morbid obesity (BMI >35). Further, one large cohort study of black and white men in the U.S. found no associations between BMI and NHL, but reported positive associations between BMI and CLL. However, an Italian hospital-based case-control study found no associations between obesity and NHL risk. A large prospective study of >900,000 U.S. adults also found that obesity was positively associated with risk of NHL mortality in men (RR=1.56, 95% CI=1.29-1.87) and in women (RR=1.95, 95% CI=1.39-2.72) (1). In general, these studies support a role for obesity in NHL-related morbidity.
Associations also were reported between NHL and polymorphisms in obesity-related genes such as leptin (LEP) and leptin receptor, (2, 3), key regulators of energy balance and immune function. Of note, polymorphisms in the LEP gene (-2548G>A, 19A>G), associated with high circulating leptin and an obese phenotype, were identified as susceptibility loci for NHL in two independent studies (2, 3). Obesity promotes a state of low-grade, chronic inflammation and increased production of proinflammatory cytokines such as IL-6, TNF-α, IL-1b and leptin. These cytokines can deregulate T- and B-cell responses and enhance B-cell proliferation and survival, factors that may provide a milieu that favors lymphomagenesis.
Physical activity and risk of lymphoma
Few studies have examined the role of exercise relative to lymphoma risk. In a cohort study of women in the U.S., low physical activity was associated with an increased risk for FL (OR=1.8, 95% CI=0.9-3.6), consistent with two large case-control studies based in the U.S. and Canada. The U.S. study found that vigorous leisure-time physical activity was associated with a reduced risk of NHL (OR=0.79, 95% CI=0.60-1.04), particularly DLBCL (OR=0.60, 95% CI=0.40-0.88) (4). In the Canadian study, moderate physical activity was associated with reduced risk of NHL, with a more protective effect for FL and SLL than for DLBCL (5). Overall, these studies suggest that low physical activity increases the risk of NHL and that moderate exercise may reduce risk.
Diet and risk of lymphoma
Dairy
International correlation studies show a positive association between consumption of the non-fat portion of milk and NHL mortality, consistent with reports of increased lymphoma risk associated with milk consumption in studies from Norway, the U.S. and Italy (6-9). Positive associations also have been reported between NHL and intakes of butter or margarine, cream soups, ice cream or milkshakes (9), cheese (10, 11), and dairy products (12). However, the Canadian, Swedish and another U.S. study (13) found no association with milk consumption.
Mechanisms remain to be resolved for the link between dairy consumption and NHL. One potential mechanism could involve inhibition of 1,25(OH)2D production (the biologically active form of vitamin D) by the calcium in dairy products. 1,25(OH)2D is considered an anti-carcinogen since it promotes differentiation and apoptosis and inhibits cell growth in pre-neoplastic and neoplastic cells. Inverse associations between vitamin D intake (14), ultraviolet sunlight exposure (15) and NHL risk lend further credence to the hypothesis that vitamin D might protect against NHL, though more evidence is needed to establish a causal role of vitamin D deficiency.
Dairy fat contains significant levels of organochlorines such as dioxins and polychlorinated biphenyls, known human carcinogens and immunotoxins that can alter normal B-cell responses. Positive associations between organochlorines and NHL suggest a role of dairy fat in lymphomagenesis. Finally, bovine leukemia virus (BLV) associated with lymphosarcoma in cattle may be transmitted through milk to humans, though there is no clear evidence of human infection. Further studies are warranted that include examination of calcium and dairy fat intakes, and the potential relevance of BLV infection in risk of NHL.
Meat
Several studies reported associations with red meat or meat protein consumption in risk of NHL [reviewed in (16)]. More recently, the Nurses’ Health Study (17) and several case-control studies reported positive associations between red meat (18), processed meat (11), fried red meat (12) and animal protein (9) intake and NHL risk. Three case-control studies, two from the U.S. (8, 19) and one from Japan (18), found no associations with red meat intake and NHL, though Cross et al. found a marginally elevated risk for NHL associated with broiled meat (OR=1.32, 95% CI=0.99-1.77). Further, they reported that animal protein was inversely associated with NHL (OR=0.39, 95% CI=0.22-0.70). While it is suggestive that a link exists between meat consumption and risk of NHL, more data are needed to clarify whether a true association exists.
Fish
Reports of fish consumption and risk of lymphoma have varied. A number of studies (13, 20, 21) found non-significant decreased risks for NHL associated with high fish consumption. Further, Fritschi et al. found that those in the highest versus lowest quartile from percentage of fat from fresh fish and those that had worked in the fishing industry had significant reductions in NHL, leukemia and multiple myeloma. A Japanese study reported a protective effect of fish intake for NHL in women (OR=0.6, 95% CI 0.46-0.99) (18), consistent with findings from a U.S.-based case-control study (9). However, two large case-control studies did not support associations between fish consumption and risk for NHL, though odds ratios were not reported by sex or for high versus low percent fat intake from fish (11, 12). Further, the Nurses’ Health Study found no association between fish n-3 fatty acid intake and risk of NHL, though the number of cases in quintiles was small (17). Overall, the evidence is inconclusive but is suggestive that an inverse association between fish consumption and NHL exists. Possible reasons for discrepancies across studies may reflect varying levels of organochlorine pesticides and PCBs, compounds that have been associated with NHL. Thus, adverse health effects related to their high content in some fish may diminish the otherwise protective effects conferred by fish consumption.
Fat
Positive associations between saturated fat consumption and NHL were reported in two large case-control studies (9, 11) and two cohort studies (13, 17), while another case-control study found positive associations between NHL and oil, mainly polyunsaturated (6). Studies that stratified by subtype found that this association was particularly evident for DLBCL and not FL (9, 11). Transunsaturated fats (17) and animal fat (13, 19) also were associated with increased risks. Furthermore, two studies found positive associations with monounsaturated fats (11, 13), while two studies found protective effects for NHL and DLBCL for high consumption of polyunsaturated fats (9, 14). These studies provide fairly strong evidence of an association between high fat intake and NHL risk, though questions remain as to whether effects of fat differ by level of saturation. Saturated fats can modulate immune function by enhancing NFκB activation and anti-apoptotic behavior in T-cells, and increasing expression of pro-inflammatory agents such as IL-6, COX-2 and iNOS. On the other hand, omega-3 fats, such as from fish oil, inhibit production of pro-inflammatory arachidonate-derived agents and up-regulate apoptosis in T-lymphocytes.
Fruits and vegetables
There is increasing evidence to suggest that high vegetable intakes reduce the risk of NHL. Specifically, NHL risk was inversely associated with vegetable intake, particularly of green leafy and cruciferous vegetables in a U.S. case-control study (22). Consistent with these findings, the U.S. Nurses’ Health Study found that vegetables, particularly cruciferous vegetables, were associated with reduced NHL risk (17), and an Italian group (10) reported inverse associations for vegetable (OR=0.49, 95% CI=0.28-0.87) and fruit intakes (OR=0.51, 95% CI=0.30-0.85). In a U.S. case-control study of women (9), high intakes of tomatoes, cruciferous vegetables, lettuce and fiber were associated with significant reductions in NHL risk. Furthermore, two Swedish and Japanese case-control studies found a variety of vegetables including cruciferous vegetables protective, though the association was limited to women (12, 18), while a U.S. case-control study found that green vegetables, carrots and citrus fruit intakes were inversely associated with NHL risk in men (8).
Overall, intakes of vegetables, particularly of cruciferous vegetables, were inversely associated with lymphoma risk. Green leafy vegetables contain high levels of lutein, a potent antioxidant that may protect cells from free radical damage. They are also rich in vitamins, particularly folate, which provides one-carbon units for normal DNA synthesis, repair and methylation processes. Folate deficiency has been associated with chromosomal damage and increased cancer risk. Cruciferous vegetables contain indole-3-carbinol (I3C) and isothiocyanates, compounds that have multiple anti-carcinogenic properties (Figure 1). Furthermore, I3C ameliorates the effects of estrogen in estrogen-dependent tissues, a factor that may be attributable to the observed sex-specific differences in disease risk in some studies.
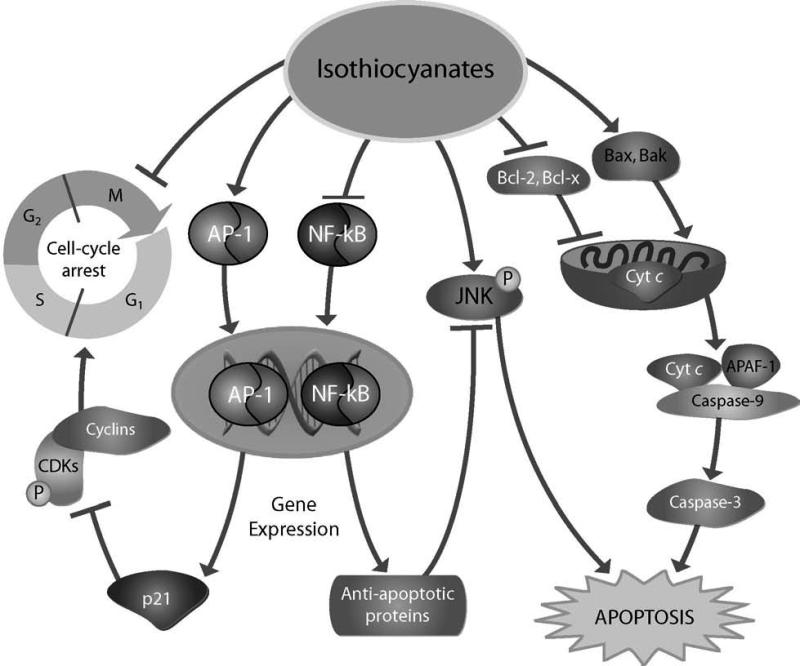
Figure 1. Mechanisms of apoptosis and growth arrest by isothiocyanates.
Isothiocyanates induce apoptosis and growth arrest involving a number of mechanisms, which may be particularly relevant in preventing lymphomagenesis and other neoplasms. Some potential mechanisms may involve their ability to inhibit the anti-apoptotic proteins, Bcl-2 and Bcl-x, and up-regulate the pro-apoptotic molecules, Bax and Bak, initiating apoptotic cell death through cytochrome c release and the subsequent activation of caspases. Isothiocyanates also can promote apoptotic cell death through the up-regulation of c-Jun N-terminal kinase (JNK) and down-regulation of nuclear factor (NF)-κB signaling pathways. These compounds also may induce cell cycle arrest by the induction of activator protein 1 (AP-1) resulting in up-regulation of the cyclin-dependent kinase (CDK) inhibitor, p21, and subsequent G(2)/M arrest.
Vitamins
Epidemiologic data relating vitamin intake to NHL risk are limited and somewhat inconsistent (8, 9, 14, 17, 21-23). This may be attributed to differences in study design or measurement error since in some studies vitamin intakes were based on food intake estimates. However, reported associations between NHL and genetic polymorphisms in folate-metabolizing genes such as 5,10-methylenetetrahydrofolate reductase, thymidylate synthase and methionine synthase (24, 25) suggest etiologic involvement of one-carbon metabolism and its related dietary exposures (Figure 1). While genetic studies suggest that folate influences risk of lymphoma, recent evidence suggests that the influence of gene variants on disease risk may be modified by folate status. Thus, studies are needed that consider interactions between folate status and folate genetic polymorphisms to establish the role of folate in lymphomagenesis.
Conclusions
In summary, there is increasing evidence based on case-control and prospective cohort studies that obesity increases NHL incidence and that moderate physical activity may reduce NHL risk. Epidemiologic studies suggest that common dietary exposures are likely to influence lymphoma risk. Cruciferous vegetable and fish intakes may reduce risk for NHL, which seems to be more evident in women than in men. However, fish with high organochlorine content could override a protective effect. There is some evidence that dairy and red meat consumption are positively associated with NHL, but these associations will need further investigation.
Based on epidemiological reports, there is growing evidence that diet plays a role in lymphomagenesis. Pooled analyses through consortia will be needed to more thoroughly investigate associations between lymphoma and dietary, lifestyle and genetic factors and to have sufficient power to examine gene-environment interactions. Mechanistic studies also will be needed to shed light on the underlying biology of how these factors may modulate disease initiation and progression. Results of these studies should substantially advance our current understanding of the relationship of diet and lymphoma risk that can be translated into prevention and treatment programs aimed at reducing the public health burden of NHL worldwide.
Supplementary Material
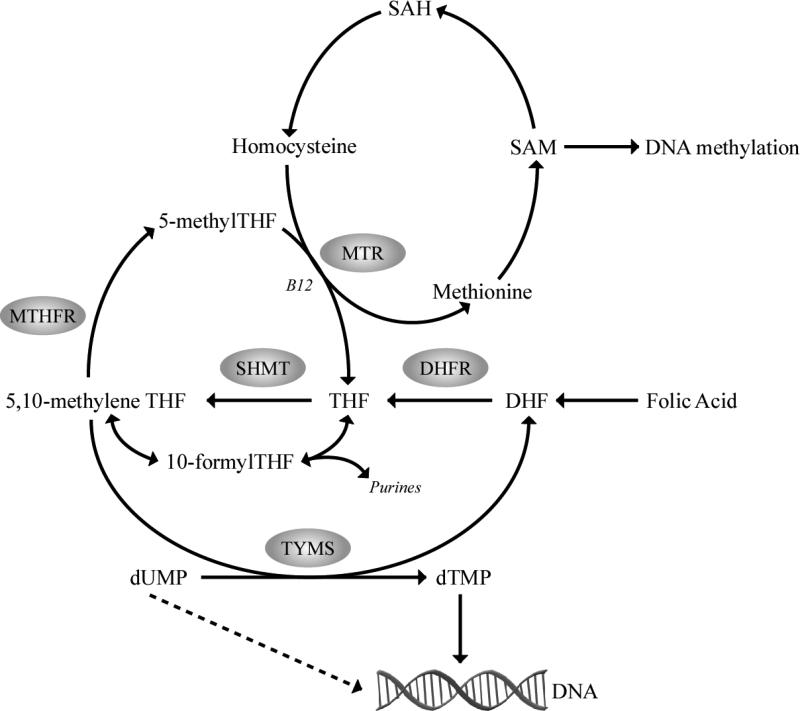
Figure 2. Overview of the folate metabolic pathway.
Abnormal one-carbon metabolism, either through folate deficiency or through polymorphisms in folate metabolizing genes, may promote lymphomagenesis through mechanisms involving aberrant DNA synthesis, repair and methylation (hypomethylation of proto-oncogenes or hypermethylation of tumor suppressor genes). A 677C>T (222Ala>Val) polymorphism in the MTHFR gene, associated with reduced MTHFR enzyme activity, may cause DNA hypomethylation while increasing the flux of one-carbon units available for purine and DNA synthesis and repair. Reduced TYMS enzyme activity may increase uracil incorporated in DNA and result in chromosome damage and fragile site induction. A 28-bp double repeat in the promoter region and a 6-bp deletion in the 3′UTR of the TYMS gene alter TYMS gene expression and mRNA stability, that can influence the rate of DNA double strand breaks and chromosomal translocations. S-adenosylmethionine (SAM); S-adenosylhomocysteine (SAH); tetrahydrofolate (THF); serine hydroxymethyltransferase (SHMT); 5,10-methylenetetrahydrofolate (5,10-methyleneTHF); 5,10-methylenetetrahydrofolate reductase (MTHFR); 5-methyltetrahydrofolate (5-methylTHF); methionine synthase (MTR); thymidylate synthase (TYMS); deoxythymidine monophosphate (dTMP); and deoxyuridine monophosphate (dUMP).
References
- 1.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 2.Skibola CF, Holly EA, Forrest MS, Hubbard A, Bracci PM, Skibola DR, Hegedus C, Smith MT. Body mass index, leptin and leptin receptor polymorphisms, and non-hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2004;13:779–86. [PubMed] [Google Scholar]
- 3.Willett EV, Skibola CF, Adamson P, Skibola DR, Morgan GJ, Smith MT, Roman E. Non-Hodgkin's lymphoma, obesity and energy homeostasis polymorphisms. Br J Cancer. 2005;93:811–6. doi: 10.1038/sj.bjc.6602762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cerhan JR, Bernstein L, Severson RK, Davis S, Colt JS, Blair A, Hartge P. Anthropometrics, physical activity, related medical conditions, and the risk of non-hodgkin lymphoma. Cancer Causes Control. 2005;16:1203–14. doi: 10.1007/s10552-005-0358-7. [DOI] [PubMed] [Google Scholar]
- 5.Pan SY, Mao Y, Ugnat AM. Physical activity, obesity, energy intake, and the risk of non-Hodgkin's lymphoma: a population-based case-control study. Am J Epidemiol. 2005;162:1162–73. doi: 10.1093/aje/kwi342. [DOI] [PubMed] [Google Scholar]
- 6.Franceschi S, Serraino D, Carbone A, Talamini R, La Vecchia C. Dietary factors and non-Hodgkin's lymphoma: a case-control study in the northeastern part of Italy. Nutr Cancer. 1989;12:333–41. doi: 10.1080/01635588909514034. [DOI] [PubMed] [Google Scholar]
- 7.Tavani A, Bertuccio P, Bosetti C, Talamini R, Negri E, Franceschi S, Montella M, La Vecchia C. Dietary intake of calcium, vitamin D, phosphorus and the risk of prostate cancer. Eur Urol. 2005;48:27–33. doi: 10.1016/j.eururo.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 8.Ward MH, Zahm SH, Weisenburger DD, Gridley G, Cantor KP, Saal RC, Blair A. Dietary factors and non-Hodgkin's lymphoma in Nebraska (United States). Cancer Causes Control. 1994;5:422–32. doi: 10.1007/BF01694756. [DOI] [PubMed] [Google Scholar]
- 9.Zheng T, Holford TR, Leaderer B, Zhang Y, Zahm SH, Flynn S, Tallini G, Zhang B, Zhou K, Owens PH, Lan Q, Rothman N, Boyle P. Diet and nutrient intakes and risk of non-Hodgkin's lymphoma in Connecticut women. Am J Epidemiol. 2004;159:454–66. doi: 10.1093/aje/kwh067. [DOI] [PubMed] [Google Scholar]
- 10.Talamini R, Polesel J, Montella M, Dal Maso L, Crovatto M, Crispo A, Spina M, Canzonieri V, La Vecchia C, Franceschi S. Food groups and risk of non-Hodgkin lymphoma: A multicenter, case-control study in Italy. Int J Cancer. 2006;118:2871–6. doi: 10.1002/ijc.21737. [DOI] [PubMed] [Google Scholar]
- 11.Purdue MP, Bassani DG, Klar NS, Sloan M, Kreiger N. Dietary factors and risk of non-Hodgkin lymphoma by histologic subtype: a case-control analysis. Cancer Epidemiol Biomarkers Prev. 2004;13:1665–76. [PubMed] [Google Scholar]
- 12.Chang ET, Smedby KE, Zhang SM, Hjalgrim H, Melbye M, Ost A, Glimelius B, Wolk A, Adami HO. Dietary factors and risk of non-hodgkin lymphoma in men and women. Cancer Epidemiol Biomarkers Prev. 2005;14:512–20. doi: 10.1158/1055-9965.EPI-04-0451. [DOI] [PubMed] [Google Scholar]
- 13.Chiu BC, Cerhan JR, Folsom AR, Sellers TA, Kushi LH, Wallace RB, Zheng W, Potter JD. Diet and risk of non-Hodgkin lymphoma in older women. Jama. 1996;275:1315–21. doi: 10.1001/jama.1996.03530410029029. [DOI] [PubMed] [Google Scholar]
- 14.Polesel J, Talamini R, Montella M, Parpinel M, Dal Maso L, Crispo A, Crovatto M, Spina M, La Vecchia C, Franceschi S. Linoleic acid, vitamin D and other nutrient intakes in the risk of non-Hodgkin lymphoma: an Italian case-control study. Ann Oncol. 2006;17:713–8. doi: 10.1093/annonc/mdl054. [DOI] [PubMed] [Google Scholar]
- 15.Kricker A, Armstrong B. Does sunlight have a beneficial influence on certain cancers? Prog Biophys Mol Biol. 2006 doi: 10.1016/j.pbiomolbio.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Cerhan JR. New epidemiologic leads in the etiology of non-Hodgkin lymphoma in the elderly: the role of blood transfusion and diet. Biomed Pharmacother. 1997;51:200–7. doi: 10.1016/s0753-3322(97)81596-x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang S, Hunter DJ, Rosner BA, Colditz GA, Fuchs CS, Speizer FE, Willett WC. Dietary fat and protein in relation to risk of non-Hodgkin's lymphoma among women. J Natl Cancer Inst. 1999;91:1751–8. doi: 10.1093/jnci/91.20.1751. [DOI] [PubMed] [Google Scholar]
- 18.Matsuo K, Hamajima N, Hirose K, Inoue M, Takezaki T, Kuroishi T, Tajima K. Alcohol, smoking, and dietary status and susceptibility to malignant lymphoma in Japan: results of a hospital-based case-control study at Aichi Cancer Center. Jpn J Cancer Res. 2001;92:1011–7. doi: 10.1111/j.1349-7006.2001.tb01054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cross AJ, Ward MH, Schenk M, Kulldorff M, Cozen W, Davis S, Colt JS, Hartge P, Cerhan JR, Sinha R. Meat and meat-mutagen intake and risk of non-Hodgkin lymphoma: results from a NCI-SEER case-control study. Carcinogenesis. 2006;27:293–7. doi: 10.1093/carcin/bgi212. [DOI] [PubMed] [Google Scholar]
- 20.Fritschi L, Ambrosini GL, Kliewer EV, Johnson KC. Dietary fish intake and risk of leukaemia, multiple myeloma, and non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2004;13:532–7. [PubMed] [Google Scholar]
- 21.Tavani A, Pregnolato A, Negri E, Franceschi S, Serraino D, Carbone A, La Vecchia C. Diet and risk of lymphoid neoplasms and soft tissue sarcomas. Nutr Cancer. 1997;27:256–60. doi: 10.1080/01635589709514535. [DOI] [PubMed] [Google Scholar]
- 22.Kelemen LE, Cerhan JR, Lim U, Davis S, Cozen W, Schenk M, Colt J, Hartge P, Ward MH. Vegetables, fruit, and antioxidant-related nutrients and risk of non-Hodgkin lymphoma: a National Cancer Institute-Surveillance, Epidemiology, and End Results population-based case-control study. Am J Clin Nutr. 2006;83:1401–10. doi: 10.1093/ajcn/83.6.1401. [DOI] [PubMed] [Google Scholar]
- 23.Zhang SM, Hunter DJ, Rosner BA, Giovannucci EL, Colditz GA, Speizer FE, Willett WC. Intakes of fruits, vegetables, and related nutrients and the risk of non-Hodgkin's lymphoma among women. Cancer Epidemiol Biomarkers Prev. 2000;9:477–85. [PubMed] [Google Scholar]
- 24.Lightfoot TJ, Skibola CF, Willett EV, Skibola DR, Allan JM, Coppede F, Adamson PJ, Morgan GJ, Roman E, Smith MT. Risk of non-Hodgkin lymphoma associated with polymorphisms in folate-metabolizing genes. Cancer Epidemiol Biomarkers Prev. 2005;14:2999–3003. doi: 10.1158/1055-9965.EPI-05-0515. [DOI] [PubMed] [Google Scholar]
- 25.Skibola CF, Forrest MS, Coppede F, Agana L, Hubbard A, Smith MT, Bracci PM, Holly EA. Polymorphisms and haplotypes in folate-metabolizing genes and risk of non-Hodgkin lymphoma. Blood. 2004;104:2155–62. doi: 10.1182/blood-2004-02-0557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.