Abstract
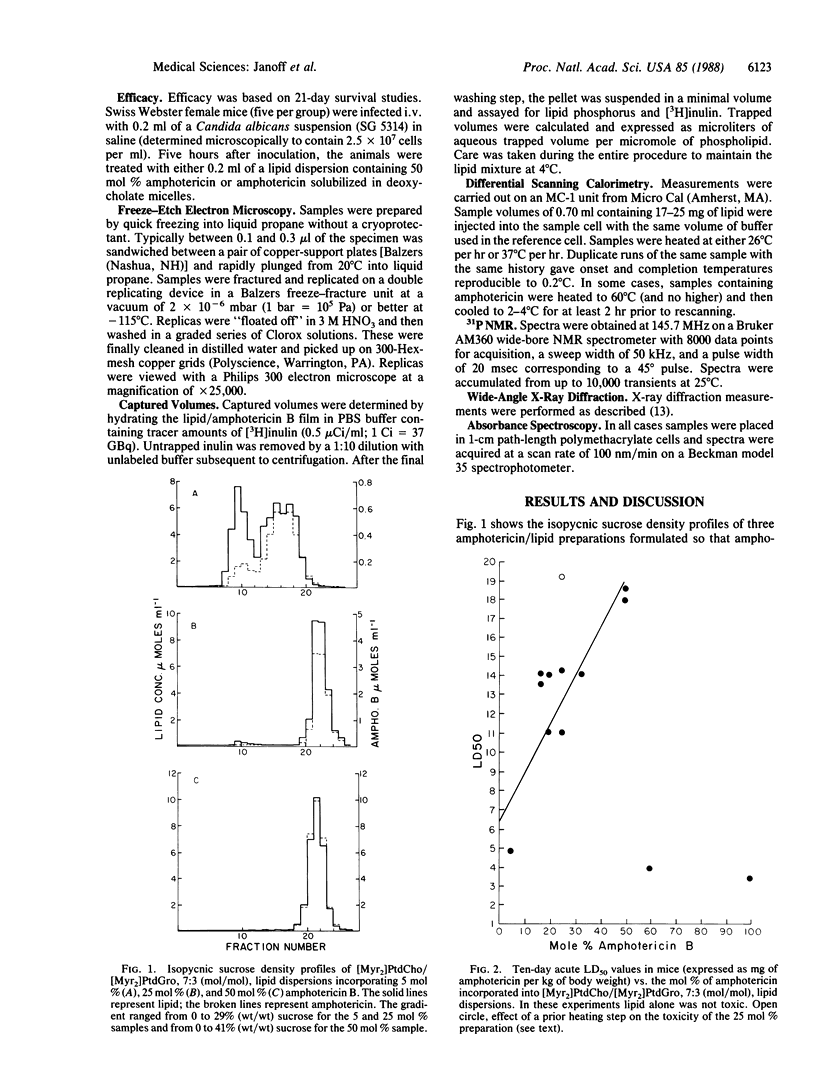
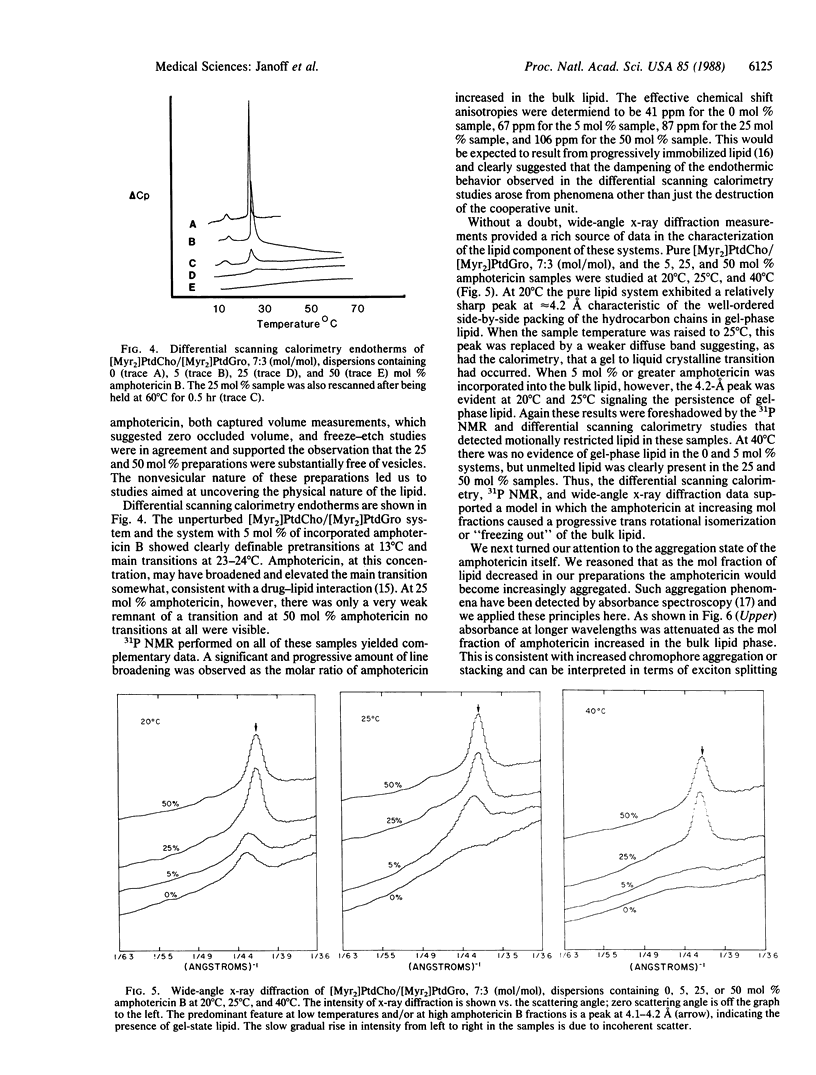
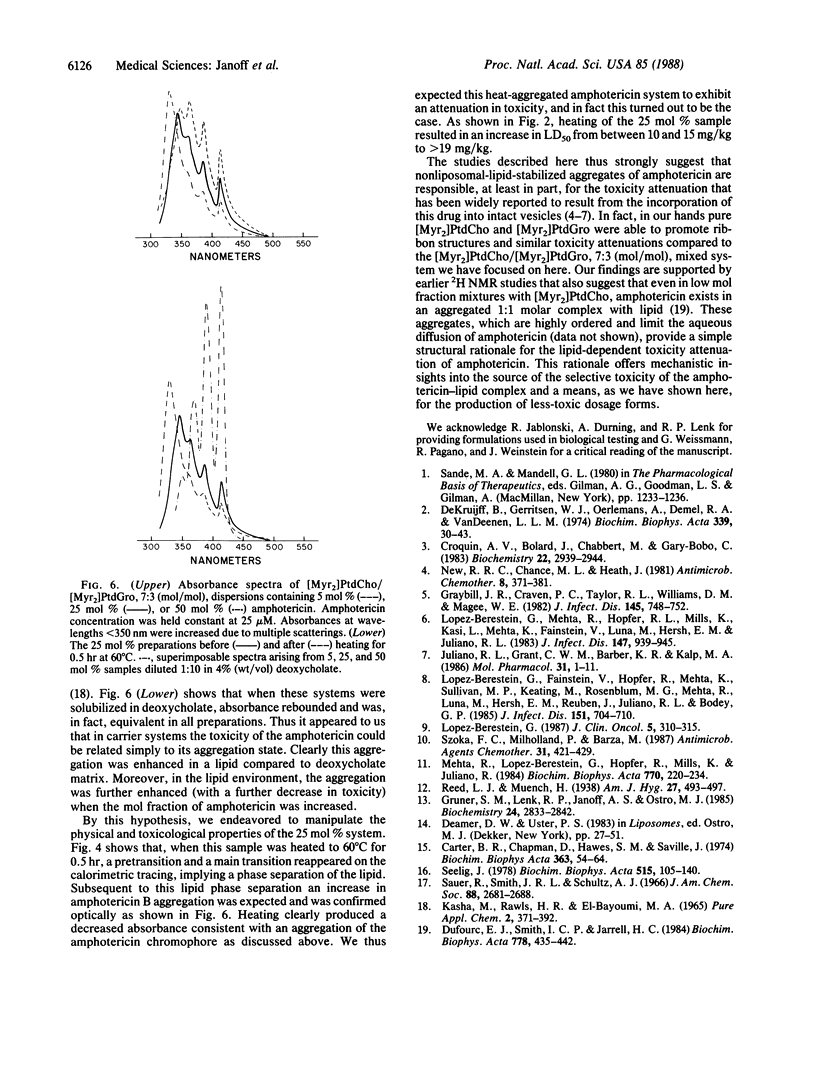
Ribbon-like structures result when amphotericin B interacts with lipid in an aqueous environment. At high ratios of amphotericin to lipid these structures, which are lipid-stabilized amphotericin aggregates, become prevalent resulting in a dramatic attenuation of amphotericin-mediated mammalian cell, but not fungal cell, toxicity. Studies utilizing freeze-etch electron microscopy, differential scanning calorimetry, 31P NMR, x-ray diffraction, and optical spectroscopy revealed that this toxicity attenuation is related to the macromolecular structure of the complexes in a definable fashion. It is likely that amphotericin in this specific form will have a much improved therapeutic utility.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cater B. R., Chapman D., Hawes S. M., Saville J. Lipid phase transitions and drug interactions. Biochim Biophys Acta. 1974 Aug 21;363(1):54–69. doi: 10.1016/0005-2736(74)90006-6. [DOI] [PubMed] [Google Scholar]
- Dufourc E. J., Smith I. C., Jarrell H. C. Interaction of amphotericin B with membrane lipids as viewed by 2H-NMR. Biochim Biophys Acta. 1984 Dec 19;778(3):435–442. doi: 10.1016/0005-2736(84)90391-2. [DOI] [PubMed] [Google Scholar]
- Graybill J. R., Craven P. C., Taylor R. L., Williams D. M., Magee W. E. Treatment of murine cryptococcosis with liposome-associated amphotericin B. J Infect Dis. 1982 May;145(5):748–752. doi: 10.1093/infdis/145.2.748. [DOI] [PubMed] [Google Scholar]
- Gruner S. M., Lenk R. P., Janoff A. S., Ostro M. J. Novel multilayered lipid vesicles: comparison of physical characteristics of multilamellar liposomes and stable plurilamellar vesicles. Biochemistry. 1985 Jun 4;24(12):2833–2842. doi: 10.1021/bi00333a004. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Grant C. W., Barber K. R., Kalp M. A. Mechanism of the selective toxicity of amphotericin B incorporated into liposomes. Mol Pharmacol. 1987 Jan;31(1):1–11. [PubMed] [Google Scholar]
- Lopez-Berestein G., Bodey G. P., Frankel L. S., Mehta K. Treatment of hepatosplenic candidiasis with liposomal-amphotericin B. J Clin Oncol. 1987 Feb;5(2):310–317. doi: 10.1200/JCO.1987.5.2.310. [DOI] [PubMed] [Google Scholar]
- Lopez-Berestein G., Fainstein V., Hopfer R., Mehta K., Sullivan M. P., Keating M., Rosenblum M. G., Mehta R., Luna M., Hersh E. M. Liposomal amphotericin B for the treatment of systemic fungal infections in patients with cancer: a preliminary study. J Infect Dis. 1985 Apr;151(4):704–710. doi: 10.1093/infdis/151.4.704. [DOI] [PubMed] [Google Scholar]
- Lopez-Berestein G., Mehta R., Hopfer R. L., Mills K., Kasi L., Mehta K., Fainstein V., Luna M., Hersh E. M., Juliano R. Treatment and prophylaxis of disseminated infection due to Candida albicans in mice with liposome-encapsulated amphotericin B. J Infect Dis. 1983 May;147(5):939–945. doi: 10.1093/infdis/147.5.939. [DOI] [PubMed] [Google Scholar]
- Mehta R., Lopez-Berestein G., Hopfer R., Mills K., Juliano R. L. Liposomal amphotericin B is toxic to fungal cells but not to mammalian cells. Biochim Biophys Acta. 1984 Mar 14;770(2):230–234. doi: 10.1016/0005-2736(84)90135-4. [DOI] [PubMed] [Google Scholar]
- New R. R., Chance M. L., Heath S. Antileishmanial activity of amphotericin and other antifungal agents entrapped in liposomes. J Antimicrob Chemother. 1981 Nov;8(5):371–381. doi: 10.1093/jac/8.5.371. [DOI] [PubMed] [Google Scholar]
- Seelig J. 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim Biophys Acta. 1978 Jul 31;515(2):105–140. doi: 10.1016/0304-4157(78)90001-1. [DOI] [PubMed] [Google Scholar]
- Szoka F. C., Jr, Milholland D., Barza M. Effect of lipid composition and liposome size on toxicity and in vitro fungicidal activity of liposome-intercalated amphotericin B. Antimicrob Agents Chemother. 1987 Mar;31(3):421–429. doi: 10.1128/aac.31.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertut-Croquin A., Bolard J., Chabbert M., Gary-Bobo C. Differences in the interaction of the polyene antibiotic amphotericin B with cholesterol- or ergosterol-containing phospholipid vesicles. A circular dichroism and permeability study. Biochemistry. 1983 Jun 7;22(12):2939–2944. doi: 10.1021/bi00281a024. [DOI] [PubMed] [Google Scholar]
- de Kruijff B., Gerritsen W. J., Oerlemans A., Demel R. A., van Deenen L. L. Polyene antibiotic-sterol interactions in membranes of Acholeplasma laidlawii cells and lecithin liposomes. I. Specificity of the membrane permeability changes induced by the polyene antibiotics. Biochim Biophys Acta. 1974 Feb 26;339(1):30–43. doi: 10.1016/0005-2736(74)90330-7. [DOI] [PubMed] [Google Scholar]






