Abstract
Fibrillin-1 and fibrillin-2 are large cysteine-rich glycoproteins that serve two key physiological functions: as supporting structures that impart tissue integrity and as regulators of signaling events that instruct cell performance. The structural role of fibrillins is exerted through the temporal and hierarchical assembly of microfibrils and elastic fibers, whereas the instructive role reflects the ability of fibrillins to sequester transforming growth factor β (TGFβ) and bone morphogenetic protein (BMP) complexes in the extracellular matrix. Characterization of fibrillin mutations in human patients and in genetically engineered mice has demonstrated that perturbation of either function manifests in disease. More generally, these studies have indicated that fibrillins are integral components of a broader biological network of extracellular, cell surface, and signaling molecules that orchestrate morphogenetic and homeostatic programs in multiple organ systems. They have also suggested that the relative composition of fibrillin-rich microfibrils imparts contextual specificity to TGFβ and BMP signaling by concentrating the ligands locally so as to regulate cell differentiation within a spatial context during organ formation (positive regulation) and by restricting their bioavailability so as to modulate cell performance in a timely fashion during tissue remodeling/ repair (negative regulation). Correlative evidence suggests functional coupling of the cell-directed assembly of micro-fibrils and targeting of TGFβ and BMP complexes to fibrillins. Hence, the emerging view is that fibrillin-rich microfibrils are molecular integrators of structural and instructive signals, with TGFβ and BMPs as the nodal points that convert extracellular inputs into discrete and context-dependent cellular responses.
Keywords: Elastic fibers, Extracellular matrix, Fibrillin, Marfan syndrome, TGFß
Introduction
Elastic fibers are key architectural elements of connective tissues that are normally subject to stretch and expansile forces, because they provide the mechanical basis of numerous body functions, such as phonation, respiration, and maintenance of vascular tone. Our understanding of the elastic fiber system has evolved throughout the past 50 years concomitantly with advances in protein biochemistry, imaging, gene cloning, and human and mouse genetics. During the original purification of elastin, Hall (1951) noted that an elastase-resistant mucoprotein-containing outercoat was tightly bound to the elastic fibers. At about the same time, Karrer (1958) showed ultrastructural images of small fibrils (~10 nm in diameter), which lay around and within amorphous elastin, and which Ross and Bornstein (1969) later termed “elastic fiber microfibrils” to refine Low's broader definition of “20-nm-diameter fibers without characteristic collagen banding” (Low 1962). Parallel histochemical studies by Fullmer and Lillie (1958) and by Gawlik (1965) identified microfibrils without and with small amounts of elastin; these were named oxytalan (acid-resistant) and elaunin (stretchable) fibers, respectively. The early observations thus established the notion that the 10-nm-diameter microfibrils were distinct extracellular assemblies that could exist either as individual structures or in association with various amounts of elastin in elastic fibers. Furthermore, visual evidence that microfibrils were deposited into the matrix before tropoelastin was widely interpreted to indicate a role in guiding elastic fiber formation.
Several years later, Sakai et al. (1986) identified fibrillin-1 as the major structural component of the 10-nm micro-fibrils, which Hollister et al. (1990) reported to be significantly reduced in tissues and cultured cells from patients with Marfan syndrome (MFS; OMIM 154700). Dietz et al. (1991) corroborated this last observation by demonstrating that fibrillin-1 mutations cause MFS, whereas cloning work by Lee et al. (1991) led to the discovery of a second fibrillin protein (fibrillin-2) genetically linked with congenital contractural arachnodactyly (CCA; OMIM 121050). By analogy to collagenopathies, Aoyama et al. (1993) argued that heterozygous fibrillin-1 mutations in MFS might adversely affect the deposition, assembly, and/ or function of wild-type molecules. Subsequent mouse studies by Pereira et al. (1997, 1999) refined the dominant-negative model of MFS pathogenesis with the observation that disease onset and progression depends on threshold levels of functional microfibrils that trigger secondary cellular events. Additional mouse work by Neptune et al. (2003) later demonstrated that perturbed transforming growth factor β (TGFβ) activation is a critical determinant of MFS pathogenesis. A significant role of promiscuous TGFβ signaling in driving MFS manifestations was consistent with early work by Dallas et al. (1995) who localized latent TGFβ-binding protein-1 (LTBP-1) to non-collagenous fibrils in cultured cells and with concurrent studies by Isogai et al. (2003) who demonstrated that fibrillin-1 binds LTBP-1 and LTBP-4 in vitro. Based on gene expression patterns and the distinct phenotypes of MFS and CCA, Zhang et al. (1995) originally proposed that fibrillin-1 and fibrillin-2 might contribute to different steps in elastic tissue formation and homeostasis. In addition to these roles, mouse work by Arteaga-Solis et al. (2001) later documented the unique contribution of fibrillin-2 to bone morphogenetic protein-7 (BMP7)-driven digit formation, a finding more recently substantiated by Gregory et al. (2005) and by Sengle et al. (2008a) who reported in vivo co-localization of BMPs and microfibrils and in vitro interaction between BMP pro-domains and fibrillins. Thus, a new view has emerged whereby fibrillin-rich microfibrils perform both structural and instructive roles in organismal physiology, and that perturbation of these functions manifests in disease. The scope of the present review is to outline the current understanding of microfibril assembly and function, while pointing out old and new unresolved issues that are likely to constitute the focus of future investigations.
Fibrillin structure
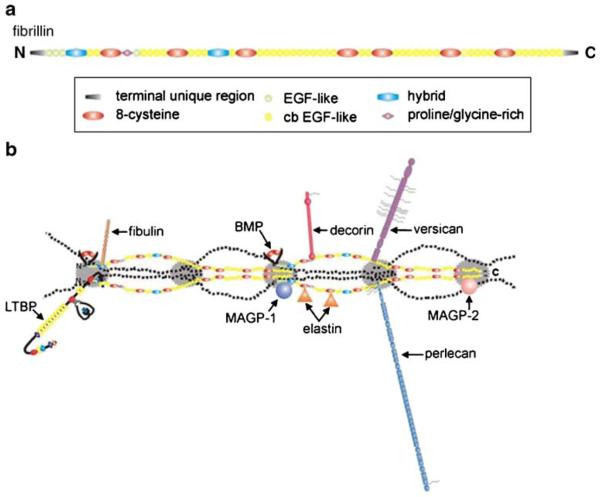
Fibrillins are large (~350-kDa) glycoproteins that are grouped together with LTBPs and fibulins into a structurally related family of extracellular matrix (ECM) proteins (Kielty et al. 2005; Hubmacher et al. 2006; Wagensell and Mecham 2007; Ramirez et al. 2007). Three functional fibrillin genes have been identified in all metazoan genomes sequenced thus far; the sole exception is the rodent fibrillin-3 gene that is inactivated by a chromosomal rearrangement (Corson et al. 2004). The function of fibrillin-3 is yet to be determined, and therefore this protein will not be discussed in subsequent sections of this review. All three fibrillins display superimposable modular structures that consist of 46/47 epidermal growth factor (EGF)-like domains (42/43 of which are of the calcium-binding type; cb-EGF) interspersed with seven 8-cysteine containing modules (TB/8-Cys; Fig. 1a). Whereas cbEGF-like domains are found in numerous other proteins, TB/8-Cys modules are unique to fibrillins and LTBPs. Additionally, fibrillins contain two hybrid domains composed of TB/8-Cys and cb-EGF sequences, and N- and C-terminal regions with sequence homologies with the respective segments of LTBPs and fibulins. The only major sequence differences between the fibrillins reside in an internal domain, which is enriched in either proline residues (fibrillin-1) or glycine residues (fibrillin-2) or both proline and glycine residues (fibrillin-3), and in the number of integrin-binding Arg-Gly-Asp (RGD) sequences and putative glycosylation sites (Kielty et al. 2005; Hubmacher et al. 2006; Ramirez et al. 2007).
Fig. 1.
a Representation of fibrillin domain structure. All three fibrillins are composed of the same types of domains arranged in the same sequential order. A region of unique sequence is proline-rich in fibrillin-1, glycine-rich in fibrillin-2, and proline- and glycine-rich in fibrillin-3. This region lies between the first 8-cysteine module and the fourth epidermal growth factor (EGF)-like domain. b Representation of fibrillin ligands as they might appear when bound to microfibrils (LTBP latent TGFβ-binding protein-1, BMP bone morphogenetic protein, MAGP microfibril-associated glycoprotein). Ligands and their approximate binding sites are positioned according to published data, using the model for microfibrils proposed by Kuo et al. (2007)
Calcium binding has been shown to stabilize contiguous cb-EGF domains into a rigid linear structure that is thought to be necessary for the assembly of microfibrils and interaction with proteins and for protection from proteolysis (Downing et al. 1996; Reinhardt et al. 1997a, 1997b). The unique RGD sequence in the TB/8-Cys4 module of fibrillin-1 (and by extrapolation the corresponding RGD sequence of fibrillin-2) resides within a flexible loop that apparently favors binding to integrin receptors α5β1, αvβ3, and αvβ6 (Sakamoto et al 1996; Pfaff et al. 1996; Bax et al. 2003; Jovanovic et al 2007). Cell culture experiments have suggested that the RGD sequence in the TB/8-Cys3 module of fibrillin-2 and the common RGD in the TB/8-Cys4 module of fibrillin-1 and -2 contribute differently to platelet-derived growth-factor-induced migration of lung fibroblasts (McGowan et al 2008). Other in vitro investigations have implicated discrete sequences of fibrillin-1 in the interaction with molecules that modify structural properties of microfibril networks, such as elastin, proteoglycans (PGs), fibulins, and microfibril-associated proteins (MAPGs), and that confer instructive properties to micro-fibrils, such as the LTBPs and BMPs (Fig. 1b; Isogai et al 2003; Gregory et al 2005; Sengle et al. 2008a, 2008b; Kielty et al. 2005; Hubmacher et al. 2006; Wagensell and Mecham 2007; Ramirez et al. 2007). Binding with microfibril-associated glycoprotein (MAGPs), LTBPs, and BMPs has also been reported for fibrillin-2 (Isogai et al. 2003; Sengle et al. 2008a; Penner et al. 2002; Werneck et al. 2004; Hanssen et al. 2004). Whereas genetic studies have validated the physiological significance of fibrillin interactions with TGFβ and BMP complexes, similar results have not been revealed for molecules that have been implicated in microfibril assembly. However, a recent study of mice in which Magp-1 was inactivated indicated phenotypes that might be related to improper TGFβ signaling (Weinbaum et al. 2008).
Fibrillin assemblies
Microfibrils and elastic fibers display a large variety of tissue-specific architectures that fulfill the mechanical requirements of individual organ systems (Kielty et al. 2005; Ramirez et al. 2007). Illustrative examples include: (1) the long continuous elastic fibers of the perichondrium/ periosteum, which participate in imparting physeal constraint and bone elasticity, (2) the parallel bundles of microfibrils in the ocular suspensory ligament, which anchors the lens to the ciliary body, (3) the concentric rings of elastic fibers in the arterial wall, which normalize blood flow throughout the cardiac cycle, and (4) the loose meshwork of microfibrils and elastic fibers in the dermis, which contribute to skin pliability. Assembly of fibrillin monomers into microfibrils and microfibrils into elastic fibers are complex multistep processes that are yet to be fully delineated. As previously mentioned, ultrastructural evidence originally suggested that microfibrils provide the extracellular scaffold that guides tropoelastin alignment and cross-linking (Kielty et al. 2005; Hubmacher et al. 2006; Wagensell and Mecham 2007; Ramirez et al. 2007). In vitro analyses have implicated MAGP-1 and fibulin-5 in this process as bridging molecules between tropoelastin and fibrillins and between them and integrin receptors, respectively (Jensen et al. 2001; Yanagisawa et al. 2002; Nakamura et al. 2002; Rock et al. 2004; Freeman et al. 2005; Lomas et al. 2007; El-Hallous et al. 2007). However, data from mutant mice indicate that elastin cross-linking proceeds normally in the absence of either fibrillin-1 or fibrillin-2 (Carta et al. 2006), and that elastic fiber morphology is apparently unaffected by the loss of MAGP-1 production (Weinbaum et al. 2008). These findings can be explained by invoking functional redundancy between the fibrillins in guiding elastin assembly and maturation (a point that has not been addressed by the study of doubly null Fbn mice) and between MAGPs and other microfibril-associated molecules (a point that may be resolved by the characterization of mice with graded deficiencies of elastic fiber components).
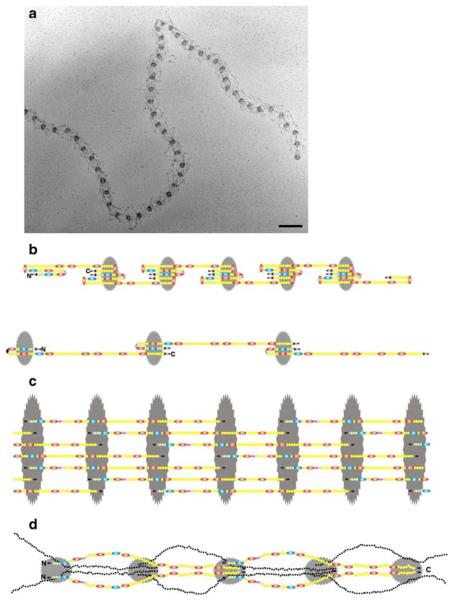
Fibrillins polymerize into microfibrils in which individual molecules are organized in a head-to-tail arrangement and also associate laterally (Sakai et al. 1991; Reinhardt et al. 1996). Rotary-shadowing electron microscopy has visualized fibrillin polymers as multiple strings with regularly spaced beads (Fig. 2a), the periodicity of which can extend from ~50 to ~150 nm depending on the tissue source and extraction procedure (Keene et al. 1991; Ren et al. 1991). Whereas intermolecular disulfide bonding is widely accepted as an important initial step in microfibril assembly (Reinhardt et al. 2000), the precise sequence of events leading to the formation of the beads-on-a-string structure remains controversial. Some investigators have argued that N-terminally mediated formation of homo-dimers potentially drive fibrillin polymerization, even though they disagree on whether this step occurs intracellularly or extracellularly (Trask et al. 1999; Ashworth et al. 1999). Other investigators have more recently demonstrated that C-terminally promoted interactions are required first for fibrillin-1 oligomerization into bead-like structures and subsequently for their incorporation of N-terminal sequences (Hubmacher et al. 2008). Similarly, early investigations have suggested that C-terminal processing of fibrillin-1 by furin/PACE enzymes is a precondition for microfibril formation, without however resolving whether cleavage occurs in the secretory pathway or pericellularly after precursor molecules somehow escape processing in the trans-Golgi network (Ritty et al. 1999; Raghunath et al. 1999; Wallis et al. 2003). Furthermore, proteomic analyses have recently detected unprocessed fibrillin-1 peptides in native microfibrils (Cain et al. 2006).
Fig. 2.
a Rotary-shadowed image of a fibrillin microfibril extracted from connective tissue. The bead-to-bead periodicity is approximately 55 nm. Bar 100 nm. b The intramolecular pleating model. This model allows for the extreme extensibility of extracted microfibrils to periodicities of 150 nm (bottom), while also accommodating a 150-nm fibrillin monomer within a 55-nm period (top). c The 1/3-staggered model. This model is based on molecular studies indicating limited flexibility of interdomain regions in fibrillin. It accommodates crosslinks that suggested a 1/3 stagger. d The half-staggered model with N-terminal halves on the surface of the microfibril. This model is based on antibody epitope mapping by using extracted microfibrils and on the identification of crude collagenase cleavage sites in fibrillin-1. It accommodates the findings of ligand-binding sites to N-terminal halves of fibrillin
Several cell culture experiments have implicated heparin/heparan sulfate proteoglycans (HSPGs) and fibronectin in the driving of the initial pericellular steps of microfibril assembly in vitro. Some of the studies have shown that a heparin-binding site of fibrillin-1 (conceivably recognized by cell surface HSPGs) synergizes with the proximate RGD sequence to support α5β1-mediated focal adhesion of dermal fibroblasts, and that inhibition of heparan sulfate attachment to core proteins or glycosaminoglycan sulfation disrupts microfibril assembly (Tiedemann et al. 2001; Ritty et al. 2003; Cain et al. 2005; Bax et al. 2007; Cain et al. 2008). Similar in vitro analyses have demonstrated that fibronectin fibrils are absolutely required to drive microfibril formation (Kinsey et al. 2008; Sabatier et al. 2009). Interestingly, one of the studies has reported that fibronectin fibrils interact with the same C-terminally assembled fibrillin oligomers that have been shown to drive the initial formation of microfibrils (Sabatier et al. 2009). This and the additional finding that HSPGs and fibronectin fibrils also support LTBP assembly raise the intriguing possibility that these molecules coordinate the pericellular targeting of latent TGFβ complexes to the nascent microfibril scaffold (Dallas et al. 2005; Chen et al. 2007; Kantola et al. 2008). Indeed, dynamic imaging analyses have recently revealed the coupling of cell-mediated assembly and the reorganization of fibronectin and LTBP fibrils during the formation of the provisional matrix (Sivakumar et al. 2006).
Gene expression data indicate that fibrillin-2 is generally produced during organ development and tissue remodeling, but consistently in lower amounts than fibrillin-1, which continues to be produced throughout postnatal growth and in the adult organism (Zhang et al. 1995; Quondamatteo et al. 2002; Kelleher et al. 2004). Studies in chicken, frog, and zebrafish, in particular, have traced the onset of fibrillin gene expression to the beginning of gastrulation (and thus earlier than elastin production), in addition to showing restricted fibrillin production in mesenchymal tissues (such as notochord, heart, blood vessels, somites, lungs, and limbs) later in development (Gallagher et al. 1993; Skoglund et al. 2006; Miao et al. 2007; Gansner et al. 2008). Comparable tissue-specific patterns have been observed during fetal development of higher vertebrates (Zhang et al. 1995; Quondamatteo et al. 2002; Kelleher et al. 2004). Additional work with recombinant polypeptides has documented that the N- and C-termini of human fibrillin-1 and -2 can interact with each other to form both homotypic and heterotypic macro-aggregates (Lin et al. 2002; Charbonneau et al. 2003). However, in spite of sharing similar expression patterns and identical macromolecular assemblies, the discrete phenotypes of MFS and CCA patients and of Fbn1-null and Fbn2-null mice indicate that the two microfibril subunits play both unique and overlapping roles in organ formation and function (Arteaga-Solis et al. 2001; Ramirez et al. 2007; Carta et al. 2006). Although the significance of these phenotypic differences is yet to be fully understood, preliminary evidence suggests that they might reflect the relative location of fibrillin-1 and fibrillin-2 within a given macro-aggregate and/or fibrillin-specific interactions with cells and signaling molecules.
The organization of fibrillin molecules within microfibrils and microfibril elasticity remains controversial. On one hand, there is general agreement from epitope-mapping studies that fibrillins are organized in a head-to-tail arrangement in which the beads correspond to the positions at which the N- and C-termini of contiguous parallel monomers reside (Maddox et al. 1989; Reinhardt et al. 1996). On the other hand, three different models have been proposed to explain the way that such an arrangement could afford microfibril extensibility while preserving the multiple intermolecular interactions of fibrillins (Fig. 2; Kielty et al. 2005; Hubmacher et al. 2006; Ramirez et al. 2007). The intramolecular pleating model envisions that un-staggered fibrillin monomers progressively fold into the ~50-nm microfibril period and unfold into longer periods when microfibrils are extended (Fig. 2b; Baldock et al. 2001; Wang et al. 2009). The 1/3-staggered model proposes that each fibrillin molecule is in a 1/3-staggered configuration that accommodates putative trans-glutaminase cross-links (Fig. 2c; Qian and Glanville 1997; Lee et al. 2004). The half-staggered model postulates that fibrillins are half-staggered with the N-terminal halves forming an outer surface on the microfibrils that mediates binding to multiple proteins (Fig. 2d; Kuo et al. 2007). Whereas the intramolecular pleating model seeks to explain the highly extended 150-nm microfibril period (Baldock et al. 2001; Wang et al. 2009), the half-staggered model is based on non-enzymatically extracted microfibrils whose periods are only rarely extended to 80 nm (Kuo et al. 2007). Even in populations of collagenase-digested microfibrils, most periods are between 46 and 66 nm, and only 10% of the population exceeds 70 nm (Wang et al. 2009). The question of microfibril elasticity and the contribution of microfibrils to the elasticity of elastic fibers have been directly addressed by Koenders et al. (2009), but these studies have also been hampered by the difficulties in separating elastin fibers from microfibrils.
Control of TGFβ and BMP bioavailability by fibrillin
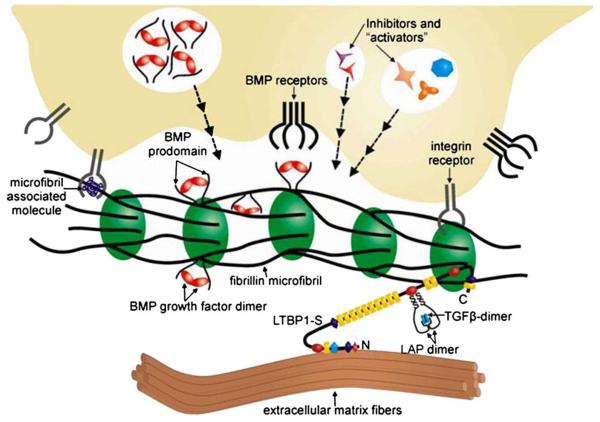
Multiple mechanisms that operate within and outside the cell and at the cell surface regulate TGFβ and BMP signaling in a context-specific temporal (developmental) and spatial (tissue-specific) manner (Derynck and Miyazono 2008). Fibrillin-rich microfibrils contribute to the extracellular regulation of endogenous TGFβ activity by providing a structural platform that controls the diffusion, storage, presentation, and release (i.e., bioavailability) of the ligands. TGFβ-1, –2, and –3 (hereafter collectively referred to as TGFβ unless otherwise indicated) are each secreted either as a small latent complex (SLC) that consists of the bioactive homodimer non-covalently associated with its pro-peptide (latency-associated protein; LAP) or as a large latent complex (LLC) in which the SLC is covalently bound to LTBP–1, –3, or –4 (Fig. 3; Rifkin 2005). Whereas LTBP–1 and –3 can associate with all TGFβ isotypes, LTBP-4 binds only to TGFβ-1 and at a lower affinity than the other LTBPs. With the notable exception of osteoblastic cells (Dallas et al. 1994), the SLC is usually secreted as part of the larger LTBP-bound complex (Rifkin 2005). Recent mouse work has validated the earlier hypothesis that LTBPs might also have a structural role in the ECM in agreement with the finding that only a fraction of them are bound to the SLC (Dallas et al. 1995; Dabovic et al. 2009). Association of TGFβ with LAP blocks interaction with its receptors, whereas association with LTBPs promotes the targeting of latent TGFβ to fibronectin or fibrillin assemblies (Rifkin 2005). With regard to the latter structures, in vitro data indicate that fibrillin-1 and –2 bind LTBP–1 and –4 (Isogai et al. 2003). Several extracellular molecules (including some that are associated with fibrillin-rich microfibrils, such as small leucine-rich PGs, fibulin-4, MAGP-1, emilin-1, and lysyl oxidase) have been implicated in modulating TGFβ bioavailability and signaling (Weinbaum et al. 2008;Schaefer and Iozzo 2008; Zacchigna et al. 2006; Hanada et al. 2007; Atsawasuwan et al. 2008). Additionally, integrins αVβ6 and αVβ8 have been shown to bind the RGD sequence of LAPs for TGFβ–1 and –3 and promote LLC activation through non-proteolytic and proteolytic mechanisms, respectively (Wipff and Hinz 2008).
Fig. 3.
Representation of cellular and molecular interactions with fibrillin microfibrils that work in concert to control growth factor signaling. The large latent TGFβ complex is associated with fibrillin microfibrils through interactions between LTBPs and fibrillin. BMP complexes are targeted to fibrillin microfibrils through direct interactions with fibrillin. Cells receive positional information through integrin interactions with fibrillin or with microfibril-associated proteins and can activate ECM-sequestered growth factors or inhibit activated growth factors as required by the tissue or developmental context. Defects in fibrillin microfibril structure, perceived by the cell, can result in the secretion of activators of growth factor signaling, leading to MFS and related disorders of the connective tissue
BMPs are also stored in the ECM and synthesized as complexes containing pro-domains associated with bioactive dimers (Koenders et al. 2009). Albeit less well understood, the extracellular control of BMP bioavailability differs from the mechanisms that modulate endogenous TGFβ signaling because of two main features. First, BMPs can be targeted directly to microfibrils through non-covalent interactions between their pro-domains and the N-terminal regions of fibrillin-1 and –2 (Fig. 3; Sengle et al. 2008a). Pro-BMP tethering to microfibrils is probably uncoupled from the process of fibronectin-directed fibrillin assembly or fibronectin-mediated sequestration of latent TGFβ complexes. Second, BMP signaling can be activated simply through the competitive displacement of the pro-domain by type II receptors (Sengle et al. 2008b). Hence, soluble antagonists and their modulators might be the only extracellular molecules required to control the activity of free (ECM-unbound) BMP complexes. We can however reasonably predict that microfibril-bound BMPs require proteolytic or non-proteolytic release from fibrillins in order to signal.
In general, several variables appear to influence fibrillin-directed control of endogenous TGFβ and BMP signaling. These include the heterogeneous and differential binding affinities of LTBPs for TGFβ and for fibrillins (including a role for LTBP-2, which cannot escort TGFβ but nevertheless may compete with other LTBPs for binding to fibrillin-1; Hirani et al. 2007), the spatiotemporal expression patterns of activators and inhibitors of matrix-bound and free ligands, and the molecular mechanisms that target TGFβ and BMP complexes to selected fibrillin molecules.
Fibrillins in organ development
The transient appearance of an elastic scaffold that demarcates prospective chondrogenic sites in the developing chick limb was originally interpreted to indicate a role of elastic macro-aggregates in tissue compartmentalization (Hurle et al. 1994). In support of such a notion, the disruption and reorganization of the elastic scaffold was noted to precede the experimentally induced formation of ectopic digits in the chick embryo (Hurle and Colombatti 1996). Subsequent analyses of fibrillin mutant mice have corroborated and extended the concept that elastic macro-aggregates define physically discrete tissue compartments and/or guide functionally distinct developmental programs (Ramirez et al. 2007). More recent genetic perturbations in frog and zebrafish embryos have demonstrated the phylogenetic importance of microfibrils in vertebrate development, in addition to reiterating the organ-specific roles of fibrillin proteins (Skoglund and Keller 2007; Gansner et al. 2008).
Mice lacking fibrillin-2 gene (Fbn2) expression display a limb-patterning defect (syndactyly) that is not observed in Fbn1-null mice, even though both proteins are abundantly deposited in the ECM of the forming autopods (Arteaga-Solis et al. 2001; Penner et al. 2002). Digit formation is the combined result of chondrogenic outgrowth and interdigital cell death, which are under the control of several signaling molecules, including BMPs (Dahn and Fallon 2000). Syndactyly in Fbn2-null mice is accounted for by the impaired commitment of mesenchymal cells in the prospective interdigital tissue to undergo apoptosis, because of failed activation of BMP7-induced cell death determinants (Arteaga-Solis et al. 2001). Consistent with the latter point, syndactyly and polydactyly is also observed in Bmp7-null mice (Luo et al. 1995) but not in mice haploinsufficient for either Fbn2 or Bmp7; however, combined Fbn2 and Bmp7 haploinsufficiency yields syndactyly and polydactyly in the absence of additional manifestations in other organ systems (Arteaga-Solis et al. 2001). These findings demonstrate that, in the developing autopod, the predominant effect of fibrillin-2 on BMP-7 signaling is positive regulation, and that, in spite of robust expression, fibrillin-1 cannot compensate for the loss of fibrillin-2 in the interdigital space of the forming autopod. Ongoing studies of muscle development and bone formation in microfibril-deficient mice support the notion that fibrillin-1 and –2 differentially regulate signaling by TGFβ and BMPs depending on temporal and spatial contexts.
The thoracic aorta is another example of a tissue in which fibrillins play organ-specific roles. The development of the aorta involves the change of vascular smooth muscle cells (VSMCs) from a biosynthetic to a contractile phenotype concomitantly with ECM assembly and maturation (Kelleher et al. 2004). VSMCs can however revert to a biosynthetic phenotype in response to tissue injury. At about mid-gestation, VSMCs begin to deposit and organize fibrillins and tropoelastin into elastic fibers. Whereas fibrillin-2 is largely synthesized during the fetal period, the production of fibrillin-1 increases steadily until neonatal life parallel to the gradual growth of elastic fibers into mature elastic lamellae that separate parallel layers of quiescent VSMCs (Kelleher et al. 2004). The resulting organization of the tunica media into a multilayered structure of alternating VSMCs and elastic lamellae (a.k. a.: the lamellar unit) is the functional determinant of aortic compliance. Impaired maturation of the aortic matrix accounts for dissecting aneurysm and neonatal death of Fbn1-null mice (Arteaga-Solis et al. 2001); by contrast, the loss of fibrillin-2 has no impact on vessel maturation and, consequently, on post-natal survival and fitness (Carta et al. 2006). However, mice lacking both fibrillins die at mid-gestation, significantly earlier than either of the parental strains, and exhibit a poorly developed aortic media, implying functional cooperation between fibrillins in promoting elastic matrix assembly (Carta et al. 2006). Furthermore, in contrast to Fbn1−/−;Fbn2+/− mice, which survive through neonatal and early postnatal life, half of the Fbn1+/−;Fbn2−/− embryos die in utero suggesting either that a threshold amount of microfibrils is required to drive aortic matrix formation, and that fibrillins contribute differentially to the total amount of fetal microfibrils, or that the fibrillins play functionally distinct roles in vessel morphogenesis (Carta et al. 2006). Collectively, the above studies indicate that the structural and instructive roles of microfibrils are stage-specific, tissue-specific, and fibrillin-specific.
Fibrillins in disease processes
Heterozygous mutations that affect the structure or decrease the expression of fibrillin–1 are responsible for the pleiotropic manifestations of MFS; these principally involve the ocular, skeletal, and cardiovascular systems (Ramirez et al. 2007). A number of MFS patients also display chronic obstructive lung disease and predisposition for pneumothorax, manifestations that were originally equated with destructive emphysema caused by impaired tissue integrity. The finding that newborn Fbn1-deficient mice exhibit developmental emphysema without signs of inflammation or tissue destruction later questioned this interpretation of the lung phenotype (Neptune et al. 2003). Indeed, the mouse studies have revealed that impaired lung development is associated with constitutive Smad2/3 signaling and heightened apoptosis, and that systemic administration of TGFβ-neutralizing antibodies can rescue this phenotype (Neptune et al. 2003). The causal association between fibrillin-1 mutations and dysregulated TGFβ signaling was subsequently extended to the progression of mitral valve prolapse, muscle hypoplasia, and aortic aneurysm (Ng et al. 2004; Habashi et al. 2006; Cohn et al. 2007). A new model of MFS pathogenesis has therefore emerged whereby FBN1 mutations are believed to preclude or decrease the matrix sequestration of latent TGFβ complexes, thus rendering them more prone to or accessible for activation, and this in turn leads to unproductive tissue remodeling, as evidenced by improper cell behavior and the loss of matrix integrity. An important corollary to this model is that the amount of substrate (LLC) available to activators is the limiting factor in MFS pathogenesis, or stated differently, that physiological levels of LLC activators are sufficient to drive disease progression.
Additional events have been identified that exacerbate TGFβ-driven aneurysm progression in MFS. Some investigators have reported that a C-terminal third fragment of fibrillin-1 can apparently displace LTBPs from microfibrils, thus contributing to LLC release from the ECM (Chaudhry et al. 2007). Others have shown that synthetic fibrillin-1 peptides or protein extracts from Fbn1 mutant aortas can stimulate metalloproteinase production and macrophage chemotaxis, thus increasing TGFβ activation (Booms et al. 2005; Guo et al. 2006). In accordance with these last findings, systemic administration of doxycycline to Fbn1 mutant mice has been reported to improve aortic wall architecture and to delay aneurysm rupture (Chung et al. 2008; Xiong et al. 2008). Analysis of Fbn1-null mice (which only exhibit molecular markers of tissue remodeling because of the catastrophic collapse of the microfibril-deficient aortic matrix) has implicated p38 MAPK (mitogen-activated protein kinase) as an early determinant of promiscuous Smad2/3 signaling (Carta et al. 2009). This study has however left unresolved whether stimulation of p38 MAPK in the Fbn1-null state is secondary to improper TGFβ activation or occurs in response to a structurally abnormal matrix, and whether this and/or other MAPKs may be dysregulated in progressively severe mouse models of MFS.
The realization that improper TGFβ signaling is central to MFS pathogenesis has prompted the idea to test, in Fbn1 mutant mice, the therapeutic efficacy of losartan, an angiotensin II type 1 receptor antagonist that also blunts TGFβ signaling. The results document the ability of the drug to mitigate histological signs of aortic aneurysm and to improve alveolar septation and muscle hypoplasia (Habashi et al. 2006). Although the precise mechanism of action remains to be elucidated, therapy with losartan has subsequently been shown significantly to reduce the rate of aortic growth in a small cohort of children affected by a particularly severe and rapidly progressive form of MFS (Brooke et al. 2008). Whereas these findings are extremely encouraging, both theoretical and practical limitations are apparent that justify pursuing the identification of additional biological targets that may prove amenable to combinatorial therapies in MFS patients who may be refractive or resistant to losartan treatment. Characterization of the precise mechanisms responsible for improper latent TGFβ activation in MFS tissues may yield these biological targets.
Conclusions and perspectives
Since the initial purification of fibrillin-1, extraordinary progress has been made in our understanding of the biogenesis and function of extracellular microfibrils. Fibrillins are now widely accepted to participate in the assembly of specialized matrices that confer structural properties to connective tissues, and they provide contextual specificity for TGFβ and BMP signals, which regulate matrix formation and remodeling. A new paradigm has thus emerged whereby the fibrillins are integral components of a broader biological network of extracellular, cell surface, and signaling molecules that orchestrate morphogenetic and homeostatic programs in multiple organ systems (Figs. 1b, 3). In this view, fibrillin-rich microfibrils are molecular integrators of structural and instructive signals, with TGFβ and BMPs as the nodal points that convert extracellular inputs into discrete and context-dependent cellular responses. It follows that a temporally regulated hierarchical assembly of fibrillin-rich macro-aggregates might form tissue-specific gradients of effector molecules that are released and activated to different extents during physiological (productive) and pathological (unproductive) tissue remodeling.
We have previously discussed some of the unresolved issues that are likely to direct future research effort. Additional new questions that have emerged include the molecular and cellular mechanisms responsible for targeting TGFβ and BMP complexes to selected fibrillin molecules, the physiological significance of these diverse interactions for tissue morphogenesis and homeostasis, and the role of other fibrillin-interacting proteins in specifying structural and instructive properties of microfibrils. Relevant to the second point, preliminary evidence strongly suggests that fibrillin-1 and fibrilin-2 regulate TGFβ and BMP signaling differently depending on the tissue and developmental stage, and by either concentrating the ligands at sites of intended function (positive regulation) or inhibiting their bioavailability (negative regulation). The finding that mutations in FBN1 or ADAMTS10 and FBN1 or ADAMTSL4 can cause Weill-Marchesani syndrome or isolated ectopia lentis, respectively, raises the possibility of potentially important interactions between fibrillin-1 and the two related families of extracellular proteins (Faivre et al. 2003; Dagoneau et al. 2004; Kainulainen et al. 1994; Ahram et al. 2009). Similarly, the heightened TGFβ signaling in geleophysic dysplasia resulting from ADAMTSL2 mutations (Le Goff et al. 2008) supports the conclusion that these families of related proteins perform significant roles in modulating or specifying the functional properties of fibrillin-rich microfibrils.
In summary, three questions relevant to the physiology and pathophysiology of the elastic fiber system remain unanswered. (1) How do tissue- and stage-specific interactions between fibrillins, other ECM molecules, and cell surface receptors translate into morphologically distinct macro-aggregates and matrices with discrete mechanical properties? (2) How does the temporal hierarchical assembly of microfibril macro-aggregates influence the proper sequestration and release of TGFβ and BMP ligands during organ formation and tissue remodeling/repair? (3) How do fibrillin mutations lead to dysregulated TGFβ and BMP signaling and do these mutations also promote additional cellular responses that sustain and/or exacerbate disease onset and progression in different tissues? Answering these questions will significantly advance our knowledge of the roles of microfibrils (and, more generally, of the ECM) in organ formation and function, in addition to improving the clinical management of congenital and acquired disorders of the connective tissue.
Acknowledgements
We are indebted to Karen Johnson for organizing the manuscript and to Noe Charbonneau for preparing the illustrations.
The described studies from the authors' laboratories were supported by grants from the National Institutes of Health (AR-049698 and AR-42044) and the Shriners Hospital for Children.
Contributor Information
Francesco Ramirez, Department of Pharmacology and Systems Therapeutics, Mount Sinai School of Medicine, New York, N.Y., USA.
Lynn Y. Sakai, L. Y. Sakai Department of Biochemistry and Molecular Biology, Shriners Hospital for Children, Oregon Health & Science University, Portland, Ore., USA
References
- Ahram D, Sato TS, Kohilan A, Tayeh M, Chen S, Leal S, Al-Salem M, El-Shanti H. A homozygous mutation in ADAMTSL4 causes autosomal-recessive isolated ectopia lentis. Am J Hum Genet. 2009;84:274–278. doi: 10.1016/j.ajhg.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama T, Tynan K, Dietz HC, Francke U, Furthmayr H. Missense mutations impair intracellular processing of fibrillin and microfibril assembly in Marfan syndrome. Hum Mol Genet. 1993;2:2135–2140. doi: 10.1093/hmg/2.12.2135. [DOI] [PubMed] [Google Scholar]
- Arteaga-Solis E, Gayraud B, Lee SY, Shum L, Sakai L, Ramirez F. Regulation of limb patterning by extracellular micro-fibrils. J Cell Biol. 2001;154:275–281. doi: 10.1083/jcb.200105046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth JL, Kelly V, Wilson R, Shuttleworth CA, Kielty CM. Fibrillin assembly: dimer formation mediated by amino-terminal sequences. J Cell Sci. 1999;112:3549–3558. doi: 10.1242/jcs.112.20.3549. [DOI] [PubMed] [Google Scholar]
- Atsawasuwan P, Mochida Y, Katafuchi M, Kaku M, Fong KS, Csiszar K, Yamauchi M. Lysyl oxidase binds transforming growth factor-β and regulates its signaling via amine oxidase activity. J Biol Chem. 2008;283:34229–34240. doi: 10.1074/jbc.M803142200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldock C, Koster AJ, Ziese U, Rock MJ, Sherratt MJ, Kadler SCA, Kielty CM. The supramolecular organization of fibrillin-rich microfibrils. J Cell Biol. 2001;152:1045–1056. doi: 10.1083/jcb.152.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bax DV, Bernard SE, Lomas A, Morgan A, Humphries J, Shuttleworth CA, Humphries MJ. Cell adhesion to fibrillin-rich molecules and microfibrils is mediated by α5β1and αvβ3integrins. J Biol Chem. 2003;278:34605–34616. doi: 10.1074/jbc.M303159200. [DOI] [PubMed] [Google Scholar]
- Bax DV, Mahalingam Y, Cain S, Mellody K, Freeman L, Younger K, Shuttleworth CA, Humphries MJ, Couchman JR, Kielty CM. Cell adheshion to fibrillin-1: identification of an Arg-Gly-Asp-dependent synergy region and a heparin-binding site that regulates focal adhesion formation. J Cell Sci. 2007;120:1383–1392. doi: 10.1242/jcs.003954. [DOI] [PubMed] [Google Scholar]
- Booms P, Pregla R, Ney A, Barthel F, Reinhardt DP, Pletschacher A, Mundlow S, Robinson PN. RGD-containing fibrillin-1 fragments upregulate matrix metalloproteinase expression in cell culture: a potential factor in the pathogenesis of the Marfan syndrome. Hum Genet. 2005;116:51–61. doi: 10.1007/s00439-004-1194-7. [DOI] [PubMed] [Google Scholar]
- Brooke BS, Habashi JP, Judge D, Patel N, Loeys B, Dietz HC. Angiotensin II blockade and aortic-root dilation in Marfan's syndrome. N Engl J Med. 2008;358:2787–2795. doi: 10.1056/NEJMoa0706585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain SA, Baldock C, Gallagher J, Morgan A, Bax DV, Weiss AS, Shuttleworth CA, Kielty CM. Fibrillin-1 interactions with heparin. J Biol Chem. 2005;280:30526–30537. doi: 10.1074/jbc.M501390200. [DOI] [PubMed] [Google Scholar]
- Cain SA, Morgan A, Sherratt MJ, Ball SG, Shuttleworth CA, Kielty CM. Proteomic analysis of fibrillin-rich microfibrils. Proteomics. 2006;6:111–122. doi: 10.1002/pmic.200401340. [DOI] [PubMed] [Google Scholar]
- Cain SA, Baldwin AK, Mahalingam Y, Raynal B, Jowitt TA, Shuttleworth CA, Couchman JR, Kielty CM. Heparan sulfate regulates fibrillin-1 and C-terminal interactions. J Biol Chem. 2008;283:27017–27027. doi: 10.1074/jbc.M803373200. [DOI] [PubMed] [Google Scholar]
- Carta L, Pereira L, Arteaga-Solis E, Lee-Arteaga SY, Lenart B, Starcher B, Merkel CA, Sukoyan M, Kerkis A, Hazeki N, Keene DR, Sakai LY, Ramirez F. Fibrillins 1 and 2 perform partially overlapping functions during aortic development. J Biol Chem. 2006;281:8016–8023. doi: 10.1074/jbc.M511599200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta L, Smaldone S, Zilberberg L, Loch D, Dietz HC, Rifkin DB, Ramirez F. p38 MAPK is an early determinant of promiscuous Smad2/3 signaling in the aortas of fibrillin-1 (Fbn1)-null mice. J Biol Chem. 2009;284:5630–5636. doi: 10.1074/jbc.M806962200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau NL, Dzamba BJ, Ono RN, Keene DR, Corson GM, Reinhardt DP, Sakai LY. Fibrillins can co-assemble in fibrils, but fibrillin fibril composition displays cell-specific differences. J Biol Chem. 2003;278:2740–2749. doi: 10.1074/jbc.M209201200. [DOI] [PubMed] [Google Scholar]
- Chaudhry SS, Cain SA, Morgan A, Dallas SL, Shuttleworth CA, Kielty CM. Fibrillin-1 regulates the bioavailability of TGFβ1. J Cell Biol. 2007;176:355–367. doi: 10.1083/jcb.200608167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Sivakumar P, Barley C, Peters DM, Gomes RR, Farach-Carson MC, Dallas SL. Potential role for heparan sulfate proteoglycans in regulation of transforming growth factor-β (TGF-β) by modulating assembly of latent TGF-β-binding protein-1. J Biol Chem. 2007;282:26418–26430. doi: 10.1074/jbc.M703341200. [DOI] [PubMed] [Google Scholar]
- Chung AWY, Yang HHC, Radomski MW, van Breemen C. Long-term doxycycline is more effective than atenolol to prevent thoracic aortic aneurysm in Marfan syndrome through the inhibition of matrix metalloproteinase-2 and -9. Circ Res. 2008;102:e73–e85. doi: 10.1161/CIRCRESAHA.108.174367. [DOI] [PubMed] [Google Scholar]
- Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, Gamradt M, Rhys CM, Holm TM, Loeys BL, Ramirez F, Judge DP, Ward C, Dietz HC. Angiotensin II type 1 receptor blockade prevents TGFβ-induced failure of muscle regeneration in multiple myopathic states. Nat Med. 2007;13:204–210. doi: 10.1038/nm1536. ap. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corson GM, Charbonneau NL, Keene DR, Sakai LY. Differential expression of fibrillin-3 adds to microfibril variety in human and avian, but not rodent, connective tissues. Genomics. 2004;83:461–472. doi: 10.1016/j.ygeno.2003.08.023. [DOI] [PubMed] [Google Scholar]
- Dabovic B, Chen Y, Choi J, Vassallo M, Dietz HC, Ramirez F, von Melchner H, Davis CC, Rifkin DB. Dual functions for LTBP in lung development: LTBP-4 independently modulates elastogenesis and TGF-β activity. J Cell Physiol. 2009;219:14–22. doi: 10.1002/jcp.21643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagoneau N, Benoist-Lasselin C, Huber C, Faivre L, Mégarbané A, Alswaid A, Dollfus H, Alembik Y, Munnich A, Legeai-Mallet L, Cormier-Daire V. ADAMTS10 mutations in autosomal recessive Weill-Marchesani syndrome. Am J Hum Genet. 2004;75:801–806. doi: 10.1086/425231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahn RD, Fallon JF. Interdigital regulation of digit identity and homeotic transformation by modulated BMP signaling. Science. 2000;289:438–441. doi: 10.1126/science.289.5478.438. [DOI] [PubMed] [Google Scholar]
- Dallas SL, Park-Snyder S, Miyazono K, Twardzik D, Mundy GR, Bonewald LF. Characterization and autoregulation of latent transforming growth factor β (TGF β) complexes in osteoblast-like cell lines. Production of a latent complex lacking the latent TGF β-binding protein. J Biol Chem. 1994;269:6815–6821. [PubMed] [Google Scholar]
- Dallas SL, Miyazono K, Skerry TM, Mundy GR, Bonewald LF. Dual role for the latent transforming growth factor-β binding protein in storage of latent TGF-β in the extracellular matrix and as a structural matrix protein. J Cell Biol. 1995;131:539–549. doi: 10.1083/jcb.131.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas SL, Sivakumar P, Jones CJP, Chen Q, Peters DM, Mosher DF, Humphries MJ, Kielty CM. Fibronectin regulates latent transforming growth factor-β (TGFβ) by controlling matrix assembly of latent TGFβ binding protein-1. J Biol Chem. 2005;280:18871–18880. doi: 10.1074/jbc.M410762200. [DOI] [PubMed] [Google Scholar]
- Derynck R, Miyazono K. The TGFβ family. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 2008. [Google Scholar]
- Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM, Puffenberger EG, Hamosh A, Nanthakumar EJ, Curristin SM, Stetten G, Meyers DA, Francomano CA. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- Downing AK, Knott V, Werner JM, Cardy CM, Campbell ID, Handford PA. Solution structure of a pair of calcium-binding epidermal growth factor-like domains: implications for the Marfan syndrome and other genetic disorders. Cell. 1996;85:597–605. doi: 10.1016/s0092-8674(00)81259-3. [DOI] [PubMed] [Google Scholar]
- El-Hallous E, Sasaki T, Hubmacher D, Getie M, Tiedemann K, Brinckmann J, Batge B, Davis EC, Reinhardt DP. Fibrillin-1 interactions with fibulins depend on the first hybrid domain and provide an adaptor function to tropoelastin. J Biol Chem. 2007;282:8935–8946. doi: 10.1074/jbc.M608204200. [DOI] [PubMed] [Google Scholar]
- Faivre L, Gorlin RJ, Wirtz MK, Godfrey M, Dagoneau N, Samples JR, Le Merrer M, Collod-Beroud G, Boileau C, Munnich A, Cormier-Daire V. In frame fibrillin-1 gene deletion in autosomal dominant Weill-Marchesani syndrome. J Med Genet. 2003;40:34–36. doi: 10.1136/jmg.40.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman LJ, Lomas A, Hodson N, Sherratt MJ, Mellody KT, Weiss AS, Shuttleworth A, Kielty CM. Fibulin-5 interacts with fibrillin-1 molecules and microfibrils. Biochem J. 2005;388:1–5. doi: 10.1042/BJ20050368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullmer HM, Lillie RD. The oxytalan fiber: a previously undescribed connective tissue fiber. J Histochem Cytochem. 1958;6:425–430. doi: 10.1177/6.6.425. [DOI] [PubMed] [Google Scholar]
- Gallagher BC, Sakai LY, Little CD. Fibrillin delineates the primary axis of the early avian embryo. Dev Dyn. 1993;196:70–78. doi: 10.1002/aja.1001960109. [DOI] [PubMed] [Google Scholar]
- Gansner JM, Madsen EC, Mecham RP, Gitlin JD. Essential role for fibrillin-2 in zebrafish notochord and vascular morphogenesis. Dev Dyn. 2008;237:2844–2861. doi: 10.1002/dvdy.21705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawlik Z. Morphological and morphochemical properties of the elastic system in the motor organ of man. Folia Histochem Cytochem. 1965;3:233–251. [PubMed] [Google Scholar]
- Gregory KE, Ono RN, Charbonneau NL, Kuo C-L, Keene DR, Bachinger HP, Sakai LY. The prodomain of BMP-7 targets the BMP-7 complex to the extracellular matrix. J Biol Chem. 2005;280:27979–27980. doi: 10.1074/jbc.M504270200. [DOI] [PubMed] [Google Scholar]
- Guo G, Booms P, Halushka M, Dietz HC, Ney A, Stricker S, Hecht J, Mundlos S, Robinson PN. Induction of macrophage chemotaxis by aortic extracts of the mgR Marfan mouse model and a GxxPG-containing fibrillin-1 fragment. Circulation. 2006;114:1855–1862. doi: 10.1161/CIRCULATIONAHA.105.601674. [DOI] [PubMed] [Google Scholar]
- Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Garielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DA. Elastin from human tissue and from ox ligament. Nature. 1951;168:513. doi: 10.1038/168513a0. [DOI] [PubMed] [Google Scholar]
- Hanada K, Vermeij M, Garinis GA, de Waard MC, Kunen MGS, Myers L, Maas A, Duncker DJ, Meijers C, Dietz HC, Kanaar R, Essers J. Perturbations of vascular homeostasis and aortic valve abnormalities in fibulin-4 deficient mice. Circ Res. 2007;100:738–746. doi: 10.1161/01.RES.0000260181.19449.95. [DOI] [PubMed] [Google Scholar]
- Hanssen E, Hew FH, Moore E, Gibson MA. MAGP-2 has multiple binding regions on fibrillins and has covalent periodic association with fibrillin-containing microfibrils. J Biol Chem. 2004;279:29185–29194. doi: 10.1074/jbc.M313672200. [DOI] [PubMed] [Google Scholar]
- Hirani R, Hanssen E, Gibson MA. LTBP-2 specifically interacts with the amino-terminal region of fibrillin-1 and competes with LTBP-1 for binding to this microfibrillar protein. Matrix Biol. 2007;26:213–223. doi: 10.1016/j.matbio.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Hollister DW, Godfrey M, Sakai LY, Pyeritz RE. Immunohistologic abnormalities of the microfibrillar-fiber system in the Marfan syndrome. N Engl J Med. 1990;323:152–159. doi: 10.1056/NEJM199007193230303. [DOI] [PubMed] [Google Scholar]
- Hubmacher D, Tiedemann K, Reinhardt DP. Fibrillins: from biogenesis of microfibrils to signaling functions. Curr Top Dev Biol. 2006;75:93–123. doi: 10.1016/S0070-2153(06)75004-9. [DOI] [PubMed] [Google Scholar]
- Hubmacher D, El-Hallous EI, Nelea V, Kaartinen MT, Lee ER, Reinhardt DP. Biogenesis of extracellular microfibrils: multimerization of the fibrillin-1 C terminus into bead-like structures enables self assembly. Proc Natl Acad Sci USA. 2008;105:6548–6553. doi: 10.1073/pnas.0706335105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurle JM, Colombatti A. Extracellular matrix modifications in the interdigital spaces of the chick embryo leg bud during the formation of ectopic digits. Anat Embryol. 1996;193:355–364. doi: 10.1007/BF00186692. [DOI] [PubMed] [Google Scholar]
- Hurle JM, Corson G, Daniels K, Reiter RS, Sakai LY, Solursh M. Elastin exhibits a distinctive temporal and spatial pattern of distribution in the developing chick limb in association with the establishment of the cartilaginous skeleton. J Cell Sci. 1994;107:2623–2634. doi: 10.1242/jcs.107.9.2623. [DOI] [PubMed] [Google Scholar]
- Isogai Z, Ono RN, Ushiro S, Keene DR, Chen Y, Mazzieri R, Charbonneau NL, Reinhardt DP, Rifkin DB, Sakai LY. Latent transforming growth factor β-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J Biol Chem. 2003;278:2750–2757. doi: 10.1074/jbc.M209256200. [DOI] [PubMed] [Google Scholar]
- Jensen SA, Reinhardt DP, Gibson MA, Weiss AS. Protein interaction studies of MAGP-1 with tropoelastin and fibrillin-1. J Biol Chem. 2001;276:39661–39666. doi: 10.1074/jbc.M104533200. [DOI] [PubMed] [Google Scholar]
- Jovanovic J, Takagi J, Choulier L, Abrescia NG, Stuart DI, van der Merwe PA, Mardon HJ, Handford PA. αvβ6 is a novel receptor for human fibrillin-1: comparative studies of molecular determinants underlying integrin-RGD affinity and specificity. J Biol Chem. 2007;282:6743–6751. doi: 10.1074/jbc.M607008200. [DOI] [PubMed] [Google Scholar]
- Kainulainen K, Karttunen L, Puhakka L, Sakai LY, Peltonen L. Mutations in the fibrillin gene responsible for dominant ectopia lentis and neonatal Marfan syndrome. Nat Genet. 1994;6:64–69. doi: 10.1038/ng0194-64. [DOI] [PubMed] [Google Scholar]
- Kantola AK, Keski-Oja J, Koli K. Fibronectin and heparin binding domains of latent TGF-β binding protein (LTBP)-4 mediate matrix targeting and cell adhesion. Exp Cell Res. 2008;314:2488–2500. doi: 10.1016/j.yexcr.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Karrer HE. The fine structure of connective tissue in the tunica propria of bronchioles. J Ultrastruct Res. 1958;2:96–121. doi: 10.1016/s0022-5320(58)90049-2. [DOI] [PubMed] [Google Scholar]
- Keene DR, Maddox BK, Kuo HJ, Sakai LY, Glanville RW. Extraction of extendable beaded structures and their identification as fibrillin-containing extracellular matrix microfibrils. J Histochem Cytochem. 1991;39:441–449. doi: 10.1177/39.4.2005373. [DOI] [PubMed] [Google Scholar]
- Kelleher CM, McLean SE, Mecham RP. Vascular extracellular matrix and aortic development. Curr Top Dev Biol. 2004;62:153–188. doi: 10.1016/S0070-2153(04)62006-0. [DOI] [PubMed] [Google Scholar]
- Kielty CM, Sherratt JM, Marson A, Baldock C. Fibrillin microfibrils. Adv Protein Chem. 2005;70:405–436. doi: 10.1016/S0065-3233(05)70012-7. [DOI] [PubMed] [Google Scholar]
- Kinsey R, Willamson MR, Chaudhry S, Mellody KT, McGovern A, Takahashi S, Shuttleworth CA, Kielty CM. Fibrillin-1 microfibril deposition is dependent on fibronectin assembly. J Cell Sci. 2008;121:2696–2704. doi: 10.1242/jcs.029819. [DOI] [PubMed] [Google Scholar]
- Koenders MM, Yang L, Wismans RG, van der Werf KO, Reinhardt DP, Daamen W, Bennink ML, Dijkstra PJ, van Kuppevelt TH, Feijen J. Microscale mechanical properties of single elastic fibers: the role of fibrillin-microfibrils. Biomaterials. 2009;30:2425–2432. doi: 10.1016/j.biomaterials.2009.01.038. [DOI] [PubMed] [Google Scholar]
- Kuo CL, Isogai Z, Keene DR, Hazeki K, Ono RN, Bachinger HP, Sakai LY. Effects of fibrillin-1 degradation on microfibril structure. J Biol Chem. 2007;282:4007–4020. doi: 10.1074/jbc.M606370200. [DOI] [PubMed] [Google Scholar]
- Le Goff C, Morice-Picard F, Dagoneau N, Wang LW, Perrot C, Crow YJ, Bauer F, Flori E, Prost-Squarcioni C, Krakow D, Ge G, Greenspan DS, Bonnet D, Le Merrer M, Munnich A, Apte SS, Cormier-Daire V. ADAMTSL2mutations in geleophysic dysplasia demonstrate a role for ADAMTS-like proteins in TGF-β bioavailability regulation. Nat Genet. 2008;40:1119–1123. doi: 10.1038/ng.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Godfrey M, Vitale E, Hori H, Mattei MG, Sarfarazi M, Tsipouras P, Ramirez F, Hollister DW. Linkage of Marfan syndrome and a phenotypically related disorder to two different fibrillin genes. Nature. 1991;352:330–334. doi: 10.1038/352330a0. [DOI] [PubMed] [Google Scholar]
- Lee SS, Knott V, Jovanovic J, Harlos K, Grimes JM, Choulier L, Mardon HJ, Stuart DI, Handford P. Structure of the integrin binding fragment from fibrillin-1 gives new insights into microfibril organization. Structure. 2004;12:717–729. doi: 10.1016/j.str.2004.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Tiedemann K, Vollbrandt T, Peters H, Batge B, Brinckmann J, Reinhardt DP. Homo- and heterotypic fibrillin-1 and -2 interactions constitute the basis for the assembly of microfibrils. J Biol Chem. 2002;277:50795–50804. doi: 10.1074/jbc.M210611200. [DOI] [PubMed] [Google Scholar]
- Lomas AC, Mellody KT, Freeman LJ, Bax DV, Shuttleworth CA, Kielty CM. Fibulin-5 binds human smooth-muscle cells through α5β1 and α4β1 integrins, but does not support receptor activation. Biochem J. 2007;405:417–428. doi: 10.1042/BJ20070400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low FN. Microfibrils: fine filamentous components of the tissue space. Anat Rec. 1962;142:131–137. doi: 10.1002/ar.1091420205. [DOI] [PubMed] [Google Scholar]
- Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 1995;9:2808–2820. doi: 10.1101/gad.9.22.2808. [DOI] [PubMed] [Google Scholar]
- Maddox BK, Sakai LY, Keene DR, Glanville RW. Connective tissue microfibrils. Isolation and characterization of three large pepsin-resistant domains of fibrillin. J Biol Chem. 1989;264:21381–21385. [PubMed] [Google Scholar]
- McGowan SE, Holmes AJ, Mecham RP, Ritty TM. Arg-Gly-Asp-containing domains of fibrillin-1 and -2 distinctly regulate lung fibroblast migration. Am J Respir Cell Mol Biol. 2008;38:435–445. doi: 10.1165/rcmb.2007-0281OC. [DOI] [PubMed] [Google Scholar]
- Miao M, Bruce AE, Bhanji T, Davis EC, Keeley FW. Differential expression of two tropoelastin genes in zebrafish. Matrix Biol. 2007;26:115–124. doi: 10.1016/j.matbio.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Lozano PR, Ikeda Y, Iwanaga Y, Hinek A, Minamisawa S, Cheng CF, Kobuke K, Dalton N, Takada Y, Tashiro K, Ross J, Jr, Honjo T, Chien KR. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415:171–175. doi: 10.1038/415171a. [DOI] [PubMed] [Google Scholar]
- Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-β activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- Ng CM, Cheng A, Myers LA, Martinez-Murillo F, Jie C, Bedja D, Gabrielson KL, Hausladen JM, Mecham RP, Judge DP, Dietz HC. TGF-β-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J Clin Invest. 2004;114:1586–1592. doi: 10.1172/JCI22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner AS, Rock MJ, Kielty CM, Shipley JM. Microfibril-associated glycoprotein-2 interacts with fibrillin-1 and fibrillin-2 suggesting a role for MAGP-2 in elastic fiber assembly. J Biol Chem. 2002;277:35044–35049. doi: 10.1074/jbc.M206363200. [DOI] [PubMed] [Google Scholar]
- Pereira L, Andrikopoulos K, Tian J, Lee SY, Keene DR, Ono R, Reinhardt DP, Sakai LY, Jensen-Biery N, Bunton T, Dietz HC, Ramirez F. Targeting of the gene coding fibrillin-1 recapitulates the vascular phenotype of Marfan syndrome in the mouse. Nat Genet. 1997;17:218–222. doi: 10.1038/ng1097-218. [DOI] [PubMed] [Google Scholar]
- Pereira L, Lee SY, Gayraud B, Andrikopoulos K, Shapiro SD, Bunton T, Jensen-Biery N, Dietz HC, Sakai LY, Ramirez F. Pathogenetic sequence for aneurysm revealed in mice under-expressing fibrillin-1. Proc Natl Acad Sci USA. 1999;96:3819–3823. doi: 10.1073/pnas.96.7.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff M, Reinhardt DP, Sakai LY, Timpl R. Cell adheshion and integrin binding to recombinant human fibrillin-1. FEBS Lett. 1996;384:247–250. doi: 10.1016/0014-5793(96)00325-0. [DOI] [PubMed] [Google Scholar]
- Qian RQ, Glanville RW. Alignment of fibrillin molecules in elastic microfibrils is defined by transglutaminase-derived cross links. Biochemistry. 1997;36:15841–15847. doi: 10.1021/bi971036f. [DOI] [PubMed] [Google Scholar]
- Quondamatteo F, Reinhardt DP, Charbonneau NL, Pophal G, Sakai LY, Herken R. Fibrillin-1 and fibrillin-2 in human embryonic and early fetal development. Matrix Biol. 2002;21:637–646. doi: 10.1016/s0945-053x(02)00100-2. [DOI] [PubMed] [Google Scholar]
- Raghunath M, Putnam EA, Ritty T, Hamstra D, Park ES, Tschödrich-Rotter M, Peters R, Rehemtulla A, Milewicz DM. Carboxy-terminal conversion of profibrillin to fibrillin at a basic site by PACE/furin-like activity required for incorporation in the matrix. J Cell Sci. 1999;112:1093–1100. doi: 10.1242/jcs.112.7.1093. [DOI] [PubMed] [Google Scholar]
- Ramirez F, Sakai LY, Rifkin DB, Dietz HC. Extracellular microfibrils in development and disease. Cell Mol Life Sci. 2007;64:2437–2446. doi: 10.1007/s00018-007-7166-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt DP, Keene DR, Corson GM, Pöschl E, Bächinger HP, Gambee JE, Sakai LY. Fibrillin-1: organization in micro-fibrils and structural properties. J Mol Biol. 1996;258:104–116. doi: 10.1006/jmbi.1996.0237. [DOI] [PubMed] [Google Scholar]
- Reinhardt DP, Mechling DE, Boswell BA, Keene DR, Sakai LY, Bächinger HP. Calcium determines the shape of fibrillin. J Biol Chem. 1997a;272:7368–7373. doi: 10.1074/jbc.272.11.7368. [DOI] [PubMed] [Google Scholar]
- Reinhardt DP, Ono RN, Sakai LY. Calcium stabilizes fibrillin-1 against proteolytic degradation. J Biol Chem. 1997b;272:1231–1236. doi: 10.1074/jbc.272.2.1231. [DOI] [PubMed] [Google Scholar]
- Reinhardt DP, Gambee JE, Ono RN, Bächinger HP, Sakai LY. Initial steps in assembly of microfibrils. Formation of disulfide-cross-linked multimers containing fibrillin-1. J Biol Chem. 2000;275:2205–2210. doi: 10.1074/jbc.275.3.2205. [DOI] [PubMed] [Google Scholar]
- Ren ZX, Brewton RG, Mayne R. An analysis by rotary shadowing of the structure of the mammalian vitreous humor and zonular apparatus. J Struct Biol. 1991;106:57–63. doi: 10.1016/1047-8477(91)90062-2. [DOI] [PubMed] [Google Scholar]
- Rifkin DB. Latent transforming growth factor-β (TGF-β) binding proteins: orchestrators of TGF-β availability. J Biol Chem. 2005;280:7409–7412. doi: 10.1074/jbc.R400029200. [DOI] [PubMed] [Google Scholar]
- Ritty TM, Broekelmann T, Tisdale C, Milewicz DM, Mecham RP. Processing of the fibrillin-1 carboxyl-terminal domain. J Biol Chem. 1999;274:8933–8940. doi: 10.1074/jbc.274.13.8933. [DOI] [PubMed] [Google Scholar]
- Ritty TM, Broelmann TJ, Werneck CC, Mecham RP. Fibrillin-1 and -2 contain heparin-binding sites important for matrix deposition and that support cell attachment. Biochem J. 2003;375:425–432. doi: 10.1042/BJ20030649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock MJ, Cain SA, Freeman LJ, Morgan A, Mellody K, Marson A, Shuttleworth CA, Weiss AS, Kielty CM. Molecular basis of elastic fiber formation. Critical interactions and a tropoelastinfibrillin-1 cross-link. J Biol Chem. 2004;279:23748–23758. doi: 10.1074/jbc.M400212200. [DOI] [PubMed] [Google Scholar]
- Ross R, Bornstein P. The elastic fiber. I. The separation and partial characterization of its macromolecular components. J Cell Biol. 1969;40:366–381. doi: 10.1083/jcb.40.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier L, Chen D, Fagotto-Kaufmann C, Hubmacher D, McKee MD, Annis DS, Mosher DF, Reinhardt DP. Fibrillin assembly requires fibronectin. Mol Biol Cell. 2009;20:846–858. doi: 10.1091/mbc.E08-08-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai LY, Keene DR, Engvall E. Fibrillin, a new 350 kD glycoprotein, is a component of extracellular microfibrils. J Cell Biol. 1986;103:2499–2509. doi: 10.1083/jcb.103.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai LY, Keene DR, Glanville RW, Bächinger HP. Purification and partial characterization of fibrillin, a cysteine-rich structural component of connective tissue microfibrils. J Biol Chem. 1991;266:14763–14770. [PubMed] [Google Scholar]
- Sakamoto H, Broekelmann T, Cheresh DA, Ramirez F, Rosenbloom J, Mecham RP. Cell-type specific recognition of RGD- and non-RGD-containing cell binding domains in fibrillin-1. J Biol Chem. 1996;271:4916–4922. [PubMed] [Google Scholar]
- Schaefer L, Iozzo RV. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J Biol Chem. 2008;283:21305–21309. doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengle G, Charbonneau NL, Ono RN, Sasaki T, Alvarez J, Keene DR, Bachinger HP, Sakai LY. Targeting of bone morphogenetic protein growth factor complexes to fibrillin. J Biol Chem. 2008a;283:13874–13888. doi: 10.1074/jbc.M707820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengle G, Ono RN, Lyons KM, Bachinger HP, Sakai LY. A new model for growth factor activation: type II receptors compete with the prodomain for BMP-7. J Mol Biol. 2008b;381:1025–1039. doi: 10.1016/j.jmb.2008.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakumar P, Czirok A, Rongish BJ, Divakara VP, Wang YP, Dallas SL. New insights into extracellular matrix assembly and reorganization from dynamic imaging of extracellular matrix proteins in living osteoblasts. J Cell Sci. 2006;119:1350–1360. doi: 10.1242/jcs.02830. [DOI] [PubMed] [Google Scholar]
- Skoglund P, Keller R. Xenopus fibrillin regulates directed convergence and extension. Dev Biol. 2007;301:404–416. doi: 10.1016/j.ydbio.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoglund P, Dzamba B, Coffman CR, Harris WA, Keller R. Xenopus fibrillin is expressed in the organizer and is the earliest component of matrix at the developing notochord-somite boundary. Dev Dyn. 2006;235:1974–1983. doi: 10.1002/dvdy.20818. [DOI] [PubMed] [Google Scholar]
- Tiedemann K, Batge B, Muller PK, Reinhardt DP. Interactions of fibrillin-1 with heparin/heparin sulfate; implications for microfibrillar assembly. J Biol Chem. 2001;276:36035–36042. doi: 10.1074/jbc.M104985200. [DOI] [PubMed] [Google Scholar]
- Trask TM, Ritty TM, Broekelmann T, Tisdale C, Mecham RP. N-terminal domains of fibrillin 1 and fibrillin 2 direct the formation of homodimers: a possible first step in microfibril assembly. Biochem J. 1999;340:693–701. [PMC free article] [PubMed] [Google Scholar]
- Wagensell JE, Mecham RP. New insights into elastic fiber assembly. Birth Defects Res Part C Embryo Today. 2007;81:229–240. doi: 10.1002/bdrc.20111. [DOI] [PubMed] [Google Scholar]
- Wallis DD, Putnam EA, Cretoiu JS, Carmical SG, Cao SN, Thomas G, Milewicz DM. Profibrillin-1 maturation by human dermal fibroblasts: proteolytic processing and molecular chaperones. J Cell Biochem. 2003;90:641–652. doi: 10.1002/jcb.10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MC, Lu Y, Baldock C. Fibrillin microfibrils: a key role for the interbead region in elasticity. J Mol Biol. 2009;388:168–179. doi: 10.1016/j.jmb.2009.02.062. [DOI] [PubMed] [Google Scholar]
- Weinbaum JS, Broekelmann TJ, Pierce RA, Werneck CC, Segade F, Craft CS, Knutsen RH, Mecham RP. Deficiency in microfibril-associated glycoprotein-1 leads to complex phenotypes in multiple organ systems. J Biol Chem. 2008;283:25533–25543. doi: 10.1074/jbc.M709962200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werneck CC, Trask BC, Broekelmann TJ, Trask TM, Ritty TM, Segade F, Mecham RP. Identification of a major microfibril-associated glycoprotein-1-binding domain in fibrillin-2. J Biol Chem. 2004;279:23045–23051. doi: 10.1074/jbc.M402656200. [DOI] [PubMed] [Google Scholar]
- Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor β1—an intimate relationship. Eur J Cell Biol. 2008;87:601–615. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Xiong W, Knispel RA, Dietz HC, Ramirez F, Baxter BT. Doxycycline delays aneurysm rupture in a mouse model of Marfan syndrome. J Vasc Surg. 2008;47:166–172. doi: 10.1016/j.jvs.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa H, Davis EC, Starcher BC, Ouchi T, Yanagisawa M, Richardson JA, Olson EN. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415:168–171. doi: 10.1038/415168a. [DOI] [PubMed] [Google Scholar]
- Zacchigna L, Vecchione C, Notte A, Cordenonsi M, Dupont S, Maretto S, Cifelli G, Ferrari A, Maffei A, Fabbro C, Braghetta P, Marino G, Selvetella G, Aretinin A, Colonnese C, Bettarini U, Russo G, Soligo S, Adorno M, Bonaldo P, Volpin D, Piccolo S, Lembo G, Bressan GM. Emilin 1 links TGF-β maturation to blood pressure homeostasis. Cell. 2006;124:929–942. doi: 10.1016/j.cell.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Zhang H, Hu W, Ramirez F. Developmental expression of fibrillin genes suggests heterogeneity of extracellular micro-fibrils. J Cell Biol. 1995;129:1165–1176. doi: 10.1083/jcb.129.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]