Abstract
Objective
We used longitudinal data to extend previous cross-sectional research on factors associated with perceived cancer susceptibility and to examine the temporal relations between variables. Additionally, we explored whether predictors differed depending on how perceived susceptibility was measured.
Design
Annual self-report surveys were completed by US women veterans (N = 3758) aged 52 years and older who were participating in a repeat mammography intervention trial. To examine individual- and group-level change in perceived susceptibility to breast cancer, we conducted multivariable non-linear mixed model analyses.
Main Outcome Measures
We examined predictors of three single-item measures of perceived susceptibility to breast cancer (percent risk, ordinal risk, and comparative risk likelihood) and changes over time. Predictors included demographic, health status, health behavior, affect, knowledge, and subjective norm variables.
Results
Breast symptoms and greater cancer worry increased perceived susceptibility to breast cancer for all three dependent measures. Other predictors varied by dependent measure. Random change, indicating individual variability, was observed with only the percent risk measure.
Conclusion
Despite small model effect sizes, breast symptoms and cancer worry appear to be consistent predictors and thus may be good targets for future interventions designed to influence women’s perceived susceptibility to breast cancer. Researchers attempting to measure change in risk perceptions may benefit from using measures with larger response scales, but additional measurement research is needed. Combining indicators of perceived susceptibility may be undesirable when different predictors are associated with different measures.
Keywords: attitude to health, perception, mammography, prospective studies, questionnaires
The construct of perceived risk is included in many health behavior models (e.g., Health Belief Model, (Janz & Becker, 1984) Precaution Adoption Process Model, (Weinstein, 1988) and Protection Motivation Theory (Rogers & Prentice-Dunn, 1997)) and is used to inform the development and evaluation of interventions designed to increase cancer screening. The Health Belief Model delineated separate constructs for perceptions of risk susceptibility and risk severity; however, risk severity has been less useful in explaining cancer prevention behaviors due to overwhelming agreement that cancer is very severe, negative, and undesirable (Janz & Becker, 1984). Therefore, perceived risk in this paper refers to individuals’ perceptions of risk susceptibility, which Weinstein (1989) defined as “the likelihood of experiencing personal harm if no action is taken” (p. 144).
Increased awareness and perceived susceptibility are expected to increase preventive health behaviors, such as cancer screening (Weinstein, 1988). Although perceptions of susceptibility are not sufficient to change behavior, people rarely adopt precautions when they do not believe they are at risk (Weinstein, 1989). Despite some concerns that very high perceived susceptibility would decrease mammography use, most of the evidence supports a positive linear association (McCaul, Branstetter, Schroeder, & Glasgow, 1996). Small but significant direct effects of perceived susceptibility to breast cancer on mammography screening behavior ranged from r = .10 to r = .21; stronger associations were observed when measures of lifetime mammogram use or cross-sectional data were used (McCaul et al., 1996). Perceived susceptibility may be more accurately characterized as a distal predictor of behavior whose direct effect is largely mediated by other factors such as perceived benefits, norms, and self-efficacy (Aiken, West, Woodward, Reno, & Reynolds, 1994; Fishbein, 2000; McQueen, Vernon, Myers, & Tilley, 2006).
Because perceived susceptibility is expected to begin the process of precaution adoption, it is important to understand what factors predict or determine such risk perceptions. The positive correlates of perceived susceptibility to breast cancer identified in a recent meta-analysis of mostly cross-sectional studies included: younger age, less education, White race/ethnicity, personal or family history of breast problems, mammography adherence, and breast cancer concern or worry (Katapodi, Lee, Facione, & Dodd, 2004).
Better understanding of what promotes changes in perceived susceptibility over time would help researchers to develop more effective health behavior interventions. However, longitudinal research examining predictors of perceived susceptibility has been scarce. Only one study used prospective data to examine predictors of perceived susceptibility to breast cancer (Aiken, Fenaughty, West, Johnson, & Luckett, 1995), but the only predictor examined was past mammography use, which was positively associated with perceived susceptibility. Another longitudinal study examined cross-sectional and prospective associations with perceived susceptibility of colorectal cancer (CRC) among male autoworkers (Vernon, Myers, Tilley, & Li, 2001). Several variables including family history, cancer worry, intent to be screened, screening history, and family support for CRC test use were significant correlates as well as predictors, but other variables were only significant in cross-sectional analysis. These findings suggest that relying on cross-sectional associations of perceived susceptibility in the design of interventions to increase cancer screening may not be useful if the associations are not stable over time or predictive of changes in perceived susceptibility. Thus, it is important to examine previously identified correlates of perceived susceptibility to breast cancer in prospective analyses.
Because there is no consensus on the best way to measure perceived susceptibility (Vernon, 1999), multiple measures should be examined whenever possible. Although absolute and comparative measures of perceived susceptibility have been shown to be strongly associated and factor together (Gerend, Aiken, West, & Erchull, 2004; Lipkus et al., 2000), other studies have reported different results for the two measures (Blalock, DeVellis, Afifi, & Sandler, 1990; Lipkus & Klein, 2006; Lipkus, Klein, Skinner, & Rimer, 2005; Weinstein et al., 2004). We conducted a secondary data analysis using three waves of survey data collected as part of Project H.O.M.E. (Healthy Outlook on the Mammography Experience), a randomized behavioral intervention trial to increase regular mammography screening in a national population of women veterans age 52 years and older. The specific aims of this study were to 1) examine whether correlates of perceived susceptibility to breast cancer reported in the literature were prospectively associated with perceived susceptibility to breast cancer, as well as with changes in perceived susceptibility over time, 2) examine additional variables that have been previously associated with perceived susceptibility for other cancer types for their prospective association with perceived susceptibility to breast cancer and changes over time, and 3) explore potential differences in the predictors of perceived susceptibility using three different dependent measures.
Method
Project H.O.M.E. Study Design
The target population was women veterans aged 52 years and over as of June 6, 2000, listed in the U.S. National Registry of Women Veterans (NRWV), who had no history of breast cancer, and had contact information available through the Internal Revenue Service or Experian, a credit tracing agency. A total of 21,449 names were randomly selected from the NRWV. Registrants for whom contact information was available (n = 16,341) were mailed an eligibility survey between September 2000 and July 2001 to assess their age, military status, and breast cancer and mammography history, as well as invite their participation in Project H.O.M.E.
Eligible respondents and non-respondents to the eligibility form were randomized to one of five groups; three of which are included in this report. Each group received a baseline survey and follow-up surveys at year 1 and year 2. Group 1 received targeted and tailored intervention materials following both the baseline and year 1 surveys. Group 2 received targeted (but not tailored) intervention materials following both the baseline and year 1 surveys. Group 3 served as an assessment-only control group and received only the three surveys over the study period.
Participants in Group 1 received tailored feedback about their objective risk for breast cancer calculated using the Gail model (Gail et al., 1989), their perceived percent risk likelihood of developing breast cancer, and a tailored message trying to better align the two risk estimates. Group 2 participants received only targeted materials, which included generic, stage-based booklets that addressed risk factors for breast cancer and population incidence rates for breast cancer, but no specific attention was paid to women’s perceived susceptibility and personalized objective risk estimates. Other constructs used to create tailored messages included stage of change, decisional balance, specific pros and cons, processes of change, and self-efficacy.
Participants and Procedures
Eligible women veterans (n = 8,444) were mailed a baseline survey and were randomized to Groups 1 – 3. After excluding women who were diagnosed with breast cancer, physically unable to complete a survey by mail or phone, or untraceable 5,500 women remained in Groups 1 – 3. Survey non-respondents were sent a reminder postcard after three weeks, a second survey after seven weeks, and were called up to six times after 11 weeks to complete a shorter version over the phone. A third survey was mailed to participants who refused the phone survey, but stated that they were willing to complete a mailed survey. Similar procedures were repeated for the follow-up surveys. Survey data for Groups 1 - 3 were collected between November, 2000 and October, 2004. Of the 5,500 eligible women, 3,414 participants (62.1%) completed baseline surveys and 2,129 completed all three surveys. Of the 3,414 participants who completed the baseline survey, 15% did so by phone. Less than 4% of follow-up surveys were completed by phone. Descriptive statistics of baseline respondents are presented in Table 1.
Table 1. Descriptive statistics for baseline survey respondents (n = 3,414).
| Variable | Frequency | % | Mean | SD |
|---|---|---|---|---|
| Intervention condition | 3414 | |||
| Tailored | 1116 | 32.7 | ||
| Targeted | 1169 | 34.2 | ||
| Survey only | 1129 | 33.1 | ||
| Age | 3414 | 62.7 | 10.1 | |
| Race/ethnicity | 3360 | |||
| White | 2991 | 87.6 | ||
| Other | 369 | 10.8 | ||
| Education | 3307 | 3.48 | 1.04 | |
| < High school | 37 | 1.1 | ||
| High school | 459 | 13.4 | ||
| College/tech/nursing | 1500 | 43.9 | ||
| College graduate | 494 | 14.5 | ||
| Post graduate work | 817 | 23.9 | ||
| General health | 3377 | 3.41 | 1.03 | |
| Poor | 129 | 3.8 | ||
| Fair | 502 | 14.7 | ||
| Good | 1092 | 32.0 | ||
| Very good | 1165 | 34.1 | ||
| Excellent | 489 | 14.3 | ||
| Family history | 3206 | 0.20 | 0.58 | |
| None | 2671 | 72.8 | ||
| 1 relative | 453 | 13.3 | ||
| 2 relatives | 66 | 1.9 | ||
| 3+ relatives | 16 | 0.5 | ||
| Breast symptoms | 3334 | 0.36 | 0.67 | |
| 0 | 2458 | 72.0 | ||
| 1 | 562 | 16.5 | ||
| 2 | 294 | 8.6 | ||
| 3+ | 20 | 0.6 | ||
| Smoking status | 3385 | |||
| Never | 1416 | 41.5 | ||
| Former | 1388 | 40.7 | ||
| Current | 581 | 17.0 | ||
| Mammogram | 3294 | |||
| Not in past 2 years | 441 | 12.9 | ||
| ≤ 2 years ago | 2853 | 83.6 | ||
| Cancer worry | 2820 | 2.58 | 1.03 | |
| Strongly disagree | 295 | 8.6 | ||
| Disagree | 1032 | 30.3 | ||
| Undecided | 803 | 23.5 | ||
| Agree | 503 | 14.7 | ||
| Strongly agree | 187 | 5.5 | ||
| Anxiety | 2823 | 2.09 | 0.79 | |
| Knowledge score | 3411 | 2.66 | 1.50 | |
| 0 | 567 | 16.6 | ||
| 1 | 101 | 3.0 | ||
| 2 | 581 | 17.0 | ||
| 3 | 1123 | 32.9 | ||
| 4 | 737 | 21.6 | ||
| 5 | 302 | 8.8 | ||
| Subjective Norms | 2682 | 0 | 0.92 | |
| Percent risk | ||||
| Baseline | 3053 | 21.78 | 22.62 | |
| Year 1 | 2343 | 19.93 | 21.92 | |
| Year 2 | 2048 | 19.62 | 21.39 | |
| Ordinal risk | ||||
| Baseline | 3270 | 1.04 | 0.57 | |
| Year 1 | 2400 | 0.96 | 0.48 | |
| Year 2 | 2109 | 0.96 | 0.48 | |
| Comparative risk | ||||
| Baseline | 2806 | 2.38 | 1.05 | |
| Year 1 | 2408 | 2.40 | 1.02 | |
| Year 2 | 2113 | 2.40 | 1.02 |
SD = standard deviation
Measures
Dependent Variables
Because no consensus exists regarding the measurement of perceived susceptibility (Vernon, 1999; Weinstein, 1999), three commonly used measures were assessed on all three surveys. Absolute perceived susceptibility of breast cancer in the next five years was assessed with both a 0-100% response scale (percent risk likelihood) and a four-point ordinal scale ranging from definitely will not (0) to definitely will (3) get breast cancer (ordinal risk likelihood). Respondents also assessed their own risk compared with other women their age on a five-point scale ranging from much lower to much higher risk (comparative risk likelihood).
Independent Variables
Study variables
Because one component of the tailored intervention condition was to provide participants with personalized feedback concerning their perceived susceptibility to breast cancer in relation to their objective risk, intervention condition was included as a categorical predictor of perceived susceptibility to breast cancer. Additionally, a variable identifying participants who completed any phone surveys from those who completed only mailed surveys was included as a predictor to explore whether mode of survey administration and shortened phone surveys had an impact on perceived breast cancer susceptibility.
All other independent variables were assessed in the baseline survey. Previously identified correlates of perceived susceptibility to breast cancer were examined as predictors in this study including demographics, family history, breast symptoms, health behaviors, affect, and knowledge variables (Katapodi et al., 2004; Lipkus, Iden, Terrenoire, & Feaganes, 1999; Vernon, Vogel, Halabi, & Bondy, 1993). Other variables including general health and social influence have been examined as correlates of perceived susceptibility to colorectal cancer and were included to assess their potential association with perceived susceptibility to breast cancer (Robb, Miles, & Wardle, 2004; Vernon et al., 2001). Table 2 reports the bivariate correlations of all independent variables with the three measures of perceived susceptibility across the three time points.
Table 2. Bivariate correlations between baseline predictors and all three measures of perceived susc eptibility.
| BL percent |
Y1 percent |
Y2 percent |
BL ordinal |
Y1 ordinal |
Y2 ordinal |
BL compare |
Y1 compare |
Y2 compare |
|
|---|---|---|---|---|---|---|---|---|---|
| Baseline percent risk | -- | ||||||||
| Y1 percent risk | .51** | -- | |||||||
| Y2 percent risk | .48** | .49** | -- | ||||||
| Baseline ordinal risk | .39** | .27** | .25** | -- | |||||
| Y1 ordinal risk | .32** | .37** | .33** | .37** | -- | ||||
| Y2 ordinal risk | .30** | .28** | .36** | .34** | .38** | -- | |||
| Baseline comparative risk | .53** | .42** | .38** | .40** | .33** | .31** | -- | ||
| Y1 comparative risk | .39** | .47** | .36** | .25** | .39** | .26** | .55** | -- | |
| Y2 comparative risk | .40** | .38** | .46** | .29** | .32** | .35** | .55** | .51** | -- |
| Intervention condition | −.01 | −.04 | −.01 | −.02 | −.02 | −.01 | <−.01 | <−.01 | −.01 |
| Age | −.08** | −.06** | −.08** | .02 | −.03 | −.02 | −.13** | −.12** | −.10** |
| Race/ethnicity | −.02 | −.02 | −.04 | <−.01 | −.03 | −.03 | −.02 | −.05* | −.02 |
| Education | −.04* | −.04 | −.02 | −.07** | −.01 | .06** | .03 | .04 | .03 |
| General health | −.12** | −.10** | −.11** | −.13** | −.07** | −.11** | −.11** | −.08** | −.11** |
| Family history | .17** | .13** | .13** | .10** | .10** | .09** | .19** | .16** | .15** |
| Breast symptoms | .10** | .12** | .04 | .08** | .09** | .06** | .14** | .13** | .13** |
| Smoking status | .04* | .04* | .04 | .03* | .05* | .03 | .05** | .07** | .05* |
| Mammogram use | .10** | .07** | .09** | .09** | .10** | .05* | .12** | .16** | .08** |
| Cancer worry | .35** | .31** | .25** | .29** | .28** | .22** | .32** | .27** | .28** |
| Anxiety | .15** | .12** | .13** | .15** | .10** | .12** | .14** | .14** | .14** |
| Knowledge | .05* | .06** | .04 | −.18** | .06** | .08** | .15** | .11** | .11** |
| Subjective norms | .11** | .10** | .08** | .12** | .11** | .09** | .09** | .08** | .09** |
BL = baseline, Y1 = year 1, Y2 = year 2
Demographics
Age was analyzed as a continuous variable and race/ethnicity was dichotomized (white, non-white). Women’s highest level of education completed ranged from less than high school to post graduate work (i.e., medical or legal degree) (see table 1).
Health Status variables included self-reported general health (poor to excellent; see table 1), family history (number of first-degree relatives with diagnosed breast cancer), and a sum score of personal benign breast symptoms for breast cancer (number of previous breast biopsies and any presence of atypical hyperplasia in a biopsy specimen).
Health Behaviors
The smoking status variable combined responses to two survey items creating categories of never smokers (< 100 cigarettes in their lifetime), former smokers (≥ 100 cigarettes in lifetime but none currently), and current smokers. A single item assessed whether women had at least one mammogram in the two years prior to the study (yes/no).
Affect
Breast cancer worry was assessed by the mean of two items “I often worry about getting breast cancer” and “I am worried about having an abnormal mammogram” with response options ranging from 1 = strongly disagree to 5 = strongly agree. These worry items were adapted from measures used in The Next Step Trial, a behavioral intervention designed to increase colorectal cancer screening (Vernon, Myers, & Tilley, 1997). Psychometric data from this study sample have been reported elsewhere (Tiro et al., 2005). General mood and anxiety during the past month was assessed by a five-item mental health index (Stewart, Hays, & Ware, Jr., 1988). Sample items include “How much of the time have you been a happy person?” and “Have you felt so down in the dumps that nothing could cheer you up?” Responses ranged on a five-point scale from none of the time to all of the time. After items were reverse coded, mean scores were calculated and higher scores reflected more positive mood.
Knowledge was measured with a sum score of correct responses to five questions regarding the utility of mammograms, the recommended interval between screenings, non-reliance on symptoms to detect cancer, odds of developing breast cancer in one’s lifetime, and age range of highest risk for being diagnosed with breast cancer. Scores ranged from 0-5; higher scores indicated greater knowledge. Cronbach’s alpha was 0.72.
Subjective norms for having regular mammograms were assessed with two questions for each referent (family members, friends): “[Members of my immediate family] think I should have regular mammograms” and “I want to do what [immediate family members] think I should do about getting regular mammograms”. Scores ranged from 1 = strongly disagree to 5 = strongly agree; higher scores reflected higher perceived subjective norms for mammography use. In a previous examination of the items, all four loaded on a single factor (Tiro et al., 2005); therefore, the factor score was used as the independent variable.
Data Analysis
The examination of means and standard deviations for the dependent variables over time showed little change at the group level (Table 1); however, it is possible for individual scores to change while the means remain constant. Therefore, a mixed-modeling approach was used to examine individual level change (Littell, Milliken, Stroup, & Wolfinger, 1996). The benefit of using a mixed model is that we can estimate the variance of individual changes in the dependent variable across time and model these changes as functions of other variables. The mixed model allows for an estimation of model effect size and can accommodate missing data. The separate models include an assessment of perceived susceptibility at a specific time point (intercept parameter), as well as a rate of within-person change in perceived susceptibility at that same time point (slope parameter), and nonlinear changes in the rate of growth; the rate at which the linear slope is changing (curvature parameter). A mixed model simultaneously models individual participant change parameters as random effects and group means or correlates of those parameters as fixed effects. Therefore, a random effect for any growth parameter (intercept, slope, curvature) indicates individual variation across participants for that parameter. If, however, there is insufficient variance in an individual parameter, it may be fixed to indicate that there is no individual variation, only group variation. Correlates are included in the model if their relation to the parameters differs significantly from zero, which indicates a change in the shape of the growth curve (Bryk & Raudenbush, 1992).
Because the dependent measures were not normally distributed, we used a non-linear mixed modeling approach to obtain more accurate estimates of the p-values. Non-linear mixed models allow non-linear relations between the parameters and the actual measures. This is done by assuming a particular distribution for the dependent variable and using a link function to relate that variable to an underlying latent variable eta (η). We specified a Poisson distribution to account for the positive skew in our variables and used a log link function. Analyses were conducted using SAS version 8.2 and the GLIMMIX macro.
Although many people interpret the size of beta weights in linear regression as being stronger or weaker in comparison to other predictors in the model, due to differences in measurement error across predictors, this practice is not advised (Soofi, Retzer, & Yasai-Ardekani, 2000). In our analyses, the interpretation of the coefficients, which reflect the association between predictors and eta (not the original dependent variable itself), is difficult, especially for non-dichotomous variables. Additionally, there is currently no accepted procedure for estimating effect sizes of individual effects in non-linear mixed models. Therefore, the interpretation of the results focused on the identification of statistically significant predictors of perceived susceptibility and not on the magnitude of effects.
Three time points of data were collected in this study; therefore, only the intercept and slope could be estimated for every individual (random effects). We tested for possible curvature in the model by including a quadratic parameter (time2) as a fixed effect. Using the restricted maximum-likelihood estimation method with an unstructured covariance structure, a series of mixed models was examined. First we compared models with fixed and random slopes to select the best fitting model for each dependent variable according to Akaike’s Information Criterion (AIC) and z-tests. A random slope indicates individual variation; however, a fixed slope may still vary by a specific group and is evidenced by significant interactions with time. Additionally, we plotted perceived susceptibility over time for each of the three dependent variables to determine the nature of the functional form for change over time. Then a full model including all independent variables and their interactions with time and time2 was tested. For each dependent measure, the first reduced model excluded non-significant predictors (p > .20). A final reduced model of significant predictors (p < .05) is presented for each dependent variable. If an independent variable significantly interacted with time or time2, all three terms were retained in the final model, regardless of significance level. All independent variables were measured at baseline and treated as time invariant predictors of perceived susceptibility. Time was centered at year 1 follow-up in order to examine whether baseline variables predicted subsequent levels of and change in perceived susceptibility at that time point.
The initial and final models were reanalyzed using a full maximum likelihood estimation method to calculate model effect sizes, which reflect the amount of variance explained by each model. To calculate an effect size for each model, the estimated variance associated with the intercept in the final model was added to the total variance and divided by the estimated intercept variance of the initial model added to the total variance, then subtracted from 1.00 (Snijders T. & Bosker, 1999). When applicable, the same calculation was applied to the slope variance.
Results
For the absolute perceived susceptibility dependent variable measured as percent risk likelihood, the model included a random intercept, random slope, and fixed curvature. The variance associated with the intercept and slope was statistically significant, as was the covariance between the intercept and slope (Table 3), indicating that those with higher levels of percent risk likelihood were more likely to increase that level over time. The significant variables in the final multivariable model predicting percent risk likelihood at year 1 follow-up (intercept) were: being exposed to either intervention compared with the survey only control, age, general health, family history of cancer, breast symptoms, cancer worry, and knowledge (Table 3). Most variables were positively associated with percent risk likelihood, except for age and general health status which were negatively associated.
Table 3. Raw (unstandardized) coefficients, standard error (SE), and t-tests for the multivariable mixed model predicting level and change in the percent risk likelihood of breast cancer.
| Fixed effects | Estimate | SE | DF | t | p |
|---|---|---|---|---|---|
| Intercept | 2.136 | 0.215 | 2488 | 9.93 | <.0001 |
| Tailored intervention | 0.151 | 0.057 | 1449 | 2.64 | 0.0083 |
| Targeted intervention | 0.112 | 0.056 | 1449 | 1.98 | 0.0483 |
| Survey only control | Referent | ||||
| Age | −0.007 | 0.003 | 1449 | −2.68 | 0.0075 |
| General health | −0.103 | 0.020 | 1449 | −5.18 | <.0001 |
| Family history | 0.181 | 0.030 | 1449 | 6.03 | <.0001 |
| Breast symptoms | 0.123 | 0.033 | 1449 | 3.73 | 0.0002 |
| Cancer worry | 0.336 | 0.022 | 1449 | 14.99 | <.0001 |
| Knowledge | 0.098 | 0.019 | 1449 | 5.23 | <.0001 |
| Linear slope (time) | 0.237 | 0.102 | 2021 | 2.33 | 0.0200 |
| Quadratic slope (time2) | 0.341 | 0.145 | 2021 | 2.35 | 0.0188 |
| Tailored*time | −0.025 | 0.032 | 1449 | −0.77 | 0.4426 |
| Tailored*time2 | −0.134 | 0.046 | 1449 | −2.89 | 0.0038 |
| Targeted*time | 0.020 | 0.031 | 1449 | 0.64 | 0.5199 |
| Targeted*time2 | −0.081 | 0.045 | 1449 | −1.78 | 0.0753 |
| Survey only control*time | Referent | ||||
| Survey only control*time2 | Referent | ||||
| Age*time | −0.004 | 0.001 | 1449 | −2.36 | 0.0184 |
| Age*time2 | −0.002 | 0.002 | 1449 | −1.09 | 0.2766 |
| Breast symptoms*time | −0.035 | 0.018 | 1449 | −1.92 | 0.0556 |
| Breast symptoms*time2 | −0.057 | 0.025 | 1449 | −2.26 | 0.0242 |
| Cancer worry*time | −0.031 | 0.013 | 1449 | −2.48 | 0.0133 |
| Cancer worry*time2 | −0.035 | 0.018 | 1449 | −1.95 | 0.0517 |
| Random effects | |||||
| Variance of intercept | 0.623 | 0.030 | 20.92 | <.0001 | |
| Variance of slope | 0.071 | 0.012 | 5.98 | <.0001 | |
| Covariance of intercept and slope | 0.039 | 0.013 | 3.02 | 0.0025 | |
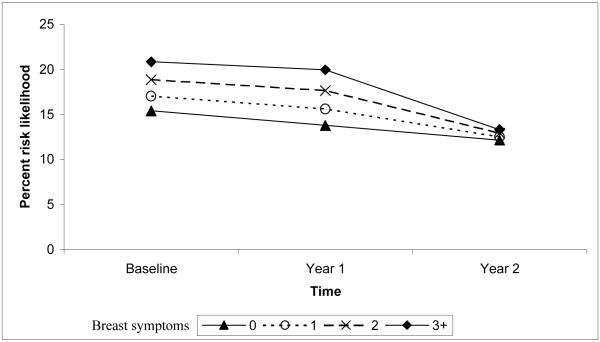
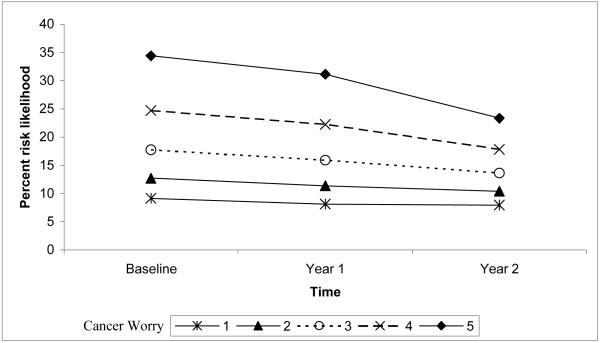
The change in percent risk likelihood over time was associated with intervention exposure, age, breast symptoms, and cancer worry. Women in the control group decreased their percent risk likelihood from baseline to year 1 more than women exposed to the tailored intervention. Women of all ages decreased their percent risk likelihood from baseline to year 1. However, from year 1 to year 2, percent risk likelihood continued to decline among older women, but increased among younger women. Women who reported more breast symptoms maintained a higher percent risk likelihood of developing breast cancer from baseline to year 1, but declined more from year 1 to year 2. Women with fewer breast symptoms decreased percent risk likelihood from baseline to year 1, but increased from year 1 to year 2. Thus, while there is a difference in percent risk likelihood between women with the lowest and highest number of reported breast symptoms, there were no differences at year 2 (Figure 1). Women reporting the highest level of breast cancer worry at baseline maintained a higher level of percent risk likelihood for developing breast cancer, despite a decrease from baseline levels to years 1 and 2 (Figure 2). Greater cancer worry at baseline predicted greater percent risk likelihood at year 1, but less difference in percent risk likelihood by levels of worry was observed at year 2. The final model explained 6.5% of the variance associated with the percent risk likelihood intercept and 5.0% of the slope variance.
Figure 1.
Quadratic interaction of breast symptoms and time predicting the level and change in percent risk likelihood.
Note. The scale range is reduced from 0-100 to better illustrate the small, but statistically significant interaction.
Figure 2.
Quadratic interaction of cancer worry and time predicting the level and change in percent risk likelihood.
Note. The scale range is reduced from 0-100 to better illustrate the small, but statistically significant interaction.
For the absolute perceived susceptibility dependent variable measured with an ordinal scale, only the intercept was included as a random effect, because no random slope variance was detected. Similar to the percent risk likelihood measure, breast symptoms, cancer worry, and knowledge were significant predictors of ordinal risk likelihood (Table 4). Prior mammography use, higher anxiety, and current smoking were also associated with greater ordinal risk likelihood. Changes in ordinal risk likelihood over time were predicted by prior mammography use and knowledge. Women with higher knowledge scores maintained a higher ordinal risk likelihood to breast cancer over time, whereas women with lower knowledge scores showed decreased ordinal risk likelihood over time. Ordinal risk likelihood was similar for women who did not have a recent mammogram compared with women who did until year 2 when those with a prior mammogram reported lower ordinal risk likelihood. The final model explained 13.3% of the variance associated with the ordinal risk likelihood intercept.
Table 4. Raw (unstandardized) coefficients, standard error (SE), and t-tests for the multivariable mixed model predicting the level and change of ordinal risk likelihood of breast cancer.
| Fixed effects | Estimate | SE | DF | t | p |
|---|---|---|---|---|---|
| Intercept | −0.604 | 0.056 | 2633 | −10.75 | <.0001 |
| Breast symptoms | 0.029 | 0.012 | 3780 | 2.43 | 0.0153 |
| Never smoker | −0.049 | 0.024 | 3780 | −2.09 | 0.0363 |
| Former smoker | −0.027 | 0.023 | 3780 | −1.17 | 0.2429 |
| Current smoker | Referent | ||||
| Prior mammography use | 0.117 | 0.039 | 3780 | 2.99 | 0.0028 |
| Cancer worry | 0.121 | 0.008 | 3780 | 14.91 | <.0001 |
| Anxiety | 0.041 | 0.011 | 3780 | 3.81 | <.0001 |
| Knowledge | 0.018 | 0.011 | 3780 | 1.64 | 0.1017 |
| Linear slope (time) | −0.008 | 0.028 | 3780 | −0.28 | 0.7762 |
| Quadratic slope (time2) | −0.040 | 0.049 | 3780 | −0.80 | 0.4227 |
| Prior mammogram*time | −0.042 | 0.023 | 3780 | −1.84 | 0.0659 |
| Prior mammogram*time2 | −0.055 | 0.041 | 3780 | −1.36 | 0.1748 |
| Knowledge*time | 0.011 | 0.007 | 3780 | 1.71 | 0.0875 |
| Knowledge*time2 | 0.026 | 0.012 | 3780 | 2.27 | 0.0234 |
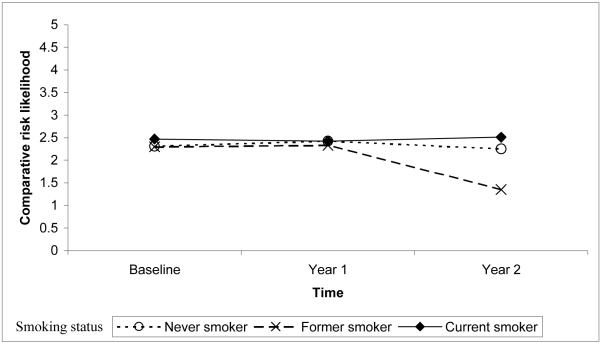
For the comparative perceived susceptibility dependent variable, only the intercept was included as a random effect; the slope and curvature terms were fixed. All significant predictors of comparative risk likelihood (Table 5) also were associated with absolute perceived susceptibility measures (Tables 3 - 5). However, unlike the two absolute measures of perceived susceptibility, knowledge was not a significant predictor of comparative risk likelihood. The change in comparative risk likelihood over time was predicted by family history, smoking status, and prior mammography use. Although having more relatives with a history of breast cancer was consistently associated with higher comparative risk likelihood, the differences became smaller over time. Comparative risk likelihood did not differ by smoking status at baseline, but decreased between year 1 and 2 among former smokers (Figure 3). Although the association between mammography status and comparative risk likelihood was similar at baseline and year 2, women who did not report having a mammogram within the two years before baseline showed a slight decrease in comparative risk likelihood at year 1. The final model explained 11.0% of the variance associated with the comparative risk likelihood intercept.
Table 5. Raw (unstandardized) coefficients, standard error (SE), and t-tests for the multivariable mixed model predicting the level and change of comparative risk likelihood of breast cancer.
| Fixed effects | Estimate | SE | DF | t | p |
|---|---|---|---|---|---|
| Intercept | 0.900 | 0.072 | 2522 | 12.56 | <.0001 |
| Age | −0.005 | 0.001 | 3652 | −6.83 | <.0001 |
| General health | −0.043 | 0.007 | 3652 | −5.82 | <.0001 |
| Family history | 0.075 | 0.015 | 3652 | 5.11 | <.0001 |
| Breast symptoms | 0.059 | 0.010 | 3652 | 5.70 | <.0001 |
| Never smoker | −0.072 | 0.027 | 3652 | −2.64 | 0.0083 |
| Former smoker | −0.040 | 0.027 | 3652 | −1.47 | 0.1410 |
| Current smoker | Referent | ||||
| Prior mammography use | 0.168 | 0.032 | 3652 | 5.27 | <.0001 |
| Cancer worry | 0.108 | 0.007 | 3652 | 15.41 | <.0001 |
| Linear slope (time) | −0.005 | 0.019 | 3652 | −0.29 | 0.7732 |
| Quadratic slope (time2) | 0.025 | 0.033 | 3652 | 0.76 | 0.4456 |
| Family history*time | −0.014 | 0.007 | 3652 | −1.97 | 0.0489 |
| Family history*time2 | 0.014 | 0.013 | 3652 | 1.10 | 0.2697 |
| Never smoker*time | 0.011 | 0.015 | 3652 | 0.77 | 0.4406 |
| Never smoker*time2 | 0.044 | 0.025 | 3652 | 1.77 | 0.0764 |
| Former smoker*time | 0.025 | 0.014 | 3652 | 1.76 | 0.0790 |
| Former smoker*time2 | 0.059 | 0.025 | 3652 | 2.42 | 0.0156 |
| Current smoker*time | Referent | ||||
| Current smoker*time2 | Referent | ||||
| Prior mammography use*time | −0.008 | 0.017 | 3652 | −0.47 | 0.6360 |
| Prior mammography use*time2 | −0.087 | 0.030 | 3652 | −2.86 | 0.0042 |
Figure 3.
Quadratic interaction between smoking status and time predicting the level and change in comparative risk likelihood.
Discussion
One contribution of this study is the extension of previous work by examining whether cross-sectional correlates of perceived cancer susceptibility identified in the literature were significant predictors of perceived susceptibility to breast cancer in prospective analyses. Additionally, our inclusion of three different measures of perceived susceptibility allowed us to examine the consistency of predictors across dependent measures. Only breast symptoms and cancer worry predicted greater perceived breast cancer susceptibility for all three dependent measures in our study. The consistency of these associations in the literature and for our three measures in prospective analyses may support the investigation of these variables as potential targets for intervention messages designed to influence women’s absolute and comparative perceived susceptibility of breast cancer.
Our results suggest differences in the predictors of perceived susceptibility to breast cancer depending on how it was measured. However, several variables were significant predictors of both the absolute percent and comparative risk likelihood measures and deserve further examination. For example, the negative association between general health and risk likelihood measures is consistent with studies assessing perceived susceptibility of any cancer (Helzlsouer, Ford, Hayward, & Midzenski, 1994) and colorectal cancer (Robb et al., 2004). Individuals who report worse health may feel more pessimistic and vulnerable to other health problems or diseases, whereas healthy individuals may be more likely to display unrealistic optimism for their future health. For percent and comparative risk likelihood measures, women at increased risk for breast cancer due to a family history of the disease were more likely to acknowledge their risk, whereas women at increased risk for breast cancer due to older age were less likely to acknowledge their risk, which supports previous findings (Katapodi et al., 2004). In another study, 25% of women believed they were too old to develop breast cancer (Grunfeld, Ramirez, Hunter, & Richards, 2002) and provided explanations such as “I think if I was going to develop it I would have had it by now” (p. 1374). These findings suggest that it is important for physicians and public health campaigns to educate older women that breast cancer risk increases with age and that regular mammography is recommended unless otherwise contraindicated.
Our data suggest that perceived susceptibility did not substantially change over time – at either the group or individual level. Our finding that intervention participants maintained their percent risk likelihood over time compared to the slight decrease observed among control group participants is similar to the small intervention effects on perceived susceptibility reported in the literature. Individuals’ perceived susceptibility to developing cancer has resisted change, even when interventions attempted to directly modify risk perceptions (Klein, 1996; Lipkus et al., 2005; Lipkus, Klein, & Rimer, 2001). Studies presenting individuals with objective risk information have found only moderate changes in perceived susceptibility among a subset of intervention participants (Davis, Stewart, & Bloom, 2004; Quilin, Fries, McClish, deParedes, & Bodurtha, 2004), as well as a return to baseline levels of perceived susceptibility within six months (Lipkus et al., 2001). Individuals may be motivated to maintain a particular level of perceived susceptibility, and interventions may have only a temporary impact. Greater understanding of what influences changes in perceptions of susceptibility over time is needed.
We found a small but significant random slope variance only for percent risk likelihood, possibly due to the wider range of response options (0-100). Using measures with few response options restricts the variance and decreases the likelihood of observing random variability, which may be the reason we did not find random slope variance for two of our perceived susceptibility measures (the two with 4- and 5-point response scales). However, group-level changes were observed for all three dependent measures. For percent risk likelihood, women who reported benign breast symptoms at baseline had greater perceived susceptibility to developing breast cancer, but only at year 1, which suggests a leveling off or temporary effect of objective risk on perceived susceptibility. Recent evidence supports benign breast disease as a risk factor for breast cancer (Hartmann et al., 2005; Wang et al., 2004). Thus, it may be especially important for women with a history of breast symptoms to recognize the importance of receiving regular mammograms. Future research also should examine the prevalence, motivations, and mammography use of women at higher objective risk who perceive lower absolute risk than average-risk women. For absolute (ordinal) and comparative risk likelihood, women without a recent mammogram temporarily decreased their perceived risk for breast cancer compared with women who had a recent mammogram, which may suggest some cognitive dissonance or defensive information processing among women who are not completing regular mammograms. Similarly, in a study of smokers in cessation clinics, changes in risk perceptions were not found to precede or predict relapse; however, risk perceptions and commitment to quit decreased following relapse (Gibbons, Eggleston, & Benthin, 1997). For comparative risk likelihood, our findings regarding the decrease in risk perceptions over time among former smokers may suggest optimistic beliefs regarding the reduction in breast cancer risk after quitting smoking. For ordinal risk likelihood, our results suggest that breast cancer susceptibility is more salient and enduring for women with greater breast cancer awareness and knowledge. In our study, education and breast cancer knowledge were positively associated (r = 0.23, p < .001).
Limitations and Future Directions
Although our study involved a population-based sample, characteristics of the sample may limit the generalizability of our results as the women veterans were predominately White, educated, and most reported regular mammography use. Our large sample size may be responsible, in part, for the statistically significant effects we observed; therefore, future research will need to confirm the findings reported here. Our models explained only a small amount of variance for each dependent variable, which is similar to other studies examining correlates of perceived susceptibility (Hay, Coups, & Ford, 2006; Katapodi et al., 2004; Oncken, McKee, Krishnan-Sarin, O’Malley, & Mazure, 2005; Wild & Cunningham, 2001) and the effects of perceived susceptibility on cancer screening behavior (Katapodi et al., 2004; McCaul et al., 1996). The small effects may be due to several factors. The use of single item measures with few response options may limit the variance available to be explained. Similarly, subjective interpretations of single-item measures of multi-faceted constructs like perceived susceptibility may increase error variance. Additional research is needed to make conceptual and psychometric improvements in the measures of perceived susceptibility (Vernon, 1999; Windschitl, 2003). Our annual waves of data collection may have decreased our ability to observe greater changes in perceived susceptibility over time. The optimal time interval between assessments of perceived susceptibility has not yet been determined, and estimation of the parameters may be improved with more time points. The mechanism by which risk perceptions change and whether this change is uni-directional or cyclical should also be examined in future research.
Even the small effects of perceived susceptibility to cancer on cancer screening may make significant reductions in morbidity and mortality (Rosenthal, 1990). The most useful targets of future health communication interventions may be the more consistent predictors of perceived susceptibility over time. Thus, more longitudinal studies are needed to inform interventions. Future research also is needed to understand perceptions of risk and why they appear to be resistant to intervention messages.
Acknowledgments
This research was supported by National Cancer Institute grant RO1CA76330 and the first author was supported by a National Cancer Institute training grant R25CA57712-11.
References
- Aiken LS, West SG, Woodward CK, Reno RR, Reynolds KD. Increasing screening mammography in asymptomatic women: Evaluation of a second-generation, theory-based program. Health Psychology. 1994;13:526–538. doi: 10.1037//0278-6133.13.6.526. [DOI] [PubMed] [Google Scholar]
- Aiken LS, Fenaughty AM, West SG, Johnson JJ, Luckett TL. Perceived determinants of risk for breast cancer and the relations among objective risk, and screening behavior over time. Women’s Health: Research on Gender, Behavior, and Policy. 1995;1:27–50. [PubMed] [Google Scholar]
- Blalock SJ, DeVellis BM, Afifi RA, Sandler RS. Risk perceptions and participation in colorectal cancer screening. Health Psychology. 1990;9:792–806. doi: 10.1037//0278-6133.9.6.792. [DOI] [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW. Hierarchical linear models: Applications and data analysis methods change. Sage; Newbury Park: 1992. [Google Scholar]
- Davis S, Stewart S, Bloom J. Increasing the accuracy of perceived breast cancer risk: Results from a randomized trail with Cancer Information Service callers. Preventive Medicine. 2004;39:64–73. doi: 10.1016/j.ypmed.2004.02.043. [DOI] [PubMed] [Google Scholar]
- Fishbein M. The role of theory in HIV prevention. AIDS Care. 2000;12:273–278. doi: 10.1080/09540120050042918. [DOI] [PubMed] [Google Scholar]
- Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. Journal of the National Cancer Institute. 1989;81:1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- Gerend MA, Aiken LS, West SG, Erchull MJ. Beyond medical risk: Investigating the psychological factors underlying women’s perceptions of susceptibility to breast cancer, heart disease, and osteoporosis. Health Psychology. 2004;23:247–258. doi: 10.1037/0278-6133.23.3.247. [DOI] [PubMed] [Google Scholar]
- Gibbons FX, Eggleston TJ, Benthin AC. Cognitive reactions to smoking relapse: The reciprocal relation between dissonance and self-esteem. Journal of Personality & Social Psychology. 1997;72:184–195. doi: 10.1037//0022-3514.72.1.184. [DOI] [PubMed] [Google Scholar]
- Grunfeld EA, Ramirez AJ, Hunter MS, Richards MA. Women’s knowledge and beliefs regarding breast cancer. British Journal of Cancer. 2002;86:1373–1378. doi: 10.1038/sj.bjc.6600260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann LC, Sellers TA, Frost MH, Lingle WL, Degnim AC, Ghosh K, et al. Benign breast disease and the risk of breast cancer. The New England Journal of Medicine. 2005;353:229–237. doi: 10.1056/NEJMoa044383. [DOI] [PubMed] [Google Scholar]
- Hay JL, Coups EJ, Ford JS. Predictors of perceived risk for colon cancer in a national probability sample in the United States. Journal of Health Communication. 2006;11:71–92. doi: 10.1080/10810730600637376. [DOI] [PubMed] [Google Scholar]
- Helzlsouer KJ, Ford DE, Hayward RSA, Midzenski M. Perceived risk of cancer and practice of cancer prevention behaviors among employees in an oncology center. Preventive Medicine. 1994;23:302–308. doi: 10.1006/pmed.1994.1042. [DOI] [PubMed] [Google Scholar]
- Janz NK, Becker MH. The health belief model: A decade later. Health Education Quarterly. 1984;11:1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- Katapodi MC, Lee KA, Facione NC, Dodd MJ. Predictors of perceived breast cancer risk and the relation between perceived risk and breast cancer screening: a meta-analytic review. Preventive Medicine. 2004;38:388–402. doi: 10.1016/j.ypmed.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Klein WM. Maintaining self-serving social comparisons: Attenuating the perceived significance of risk-increasing behaviors. Journal of Social and Clinical Psychology. 1996;15:120–142. [Google Scholar]
- Lipkus IM, Klein WMP. Effects of communicating social comparison information on risk perceptions for colorectal cancer. Journal of Health Communication. 2006;11:391–407. doi: 10.1080/10810730600671870. [DOI] [PubMed] [Google Scholar]
- Lipkus IM, Klein WMP, Skinner CS, Rimer BK. Breast cancer risk perceptions and breast cancer worry: What predicts what? Journal of Risk Research. 2005;8:439–452. [Google Scholar]
- Lipkus IM, Klein WMP, Rimer BK. Communicating breast cancer risks to women using different formats. Cancer Epidemiology Biomarkers Prevention. 2001;10:895–898. [PubMed] [Google Scholar]
- Lipkus IM, Skinner CS, Dement J, Pompeii L, Moser B, Samsa GP, et al. Increasing colorectal cancer screening among individuals in the carpentry trade: Test of risk communication interventions. Preventive Medicine. 2005;40:489–501. doi: 10.1016/j.ypmed.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Lipkus IM, Iden D, Terrenoire J, Feaganes JR. Relationships among breast cancer concern, risk perceptions, and interest in genetic testing for breast cancer susceptibility among African-American women with and without a family history of breast cancer. Cancer Epidemiology, Biomarkers & Prevention. 1999;8:533–539. [PubMed] [Google Scholar]
- Lipkus IM, Kuchibhatla M, McBride CM, Bosworth HB, Pollack K, Siegler IC, et al. Relationships among breast cancer, perceived absolute risk, comparative risk, and worries. Cancer Epidemiology, Biomarkers & Prevention. 2000;9:973–975. [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS systems for mixed models. SAS Institute, Inc.; Cary, NC: 1996. [Google Scholar]
- McCaul KD, Branstetter AD, Schroeder DM, Glasgow RE. What is the relationship between breast cancer risk and mammography screening? A meta-analytic review. Health Psychology. 1996;15:423–429. doi: 10.1037//0278-6133.15.6.423. [DOI] [PubMed] [Google Scholar]
- McQueen A, Vernon SW, Myers RE, Tilley BC. Examining mediators of perceived susceptibility on colorectal cancer screening intention and behavior among male autoworkers; Paper presented at the 27th annual meeting for Society of Behavioral Medicine; San Francisco, CA. 2006; Manuscript under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oncken C, McKee S, Krishnan-Sarin S, O’Malley S, Mazure CM. Knowledge and perceived risk of smoking-related conditions: A survey of cigarette smokers. Preventive Medicine. 2005;40:779–784. doi: 10.1016/j.ypmed.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Quilin JM, Fries E, McClish D, deParedes ES, Bodurtha J. Gail model risk assessment and risk perceptions. Journal of Behavioral Medicine. 2004;27:205–214. doi: 10.1023/b:jobm.0000019852.53048.b3. [DOI] [PubMed] [Google Scholar]
- Robb KA, Miles A, Wardle J. Demographic and psychosocial factors associated with perceived risk for colorectal cancer. Cancer Epidemiology, Biomarkers & Prevention. 2004;13:366–372. [PubMed] [Google Scholar]
- Rogers RW, Prentice-Dunn S. Protection motivation theory. In: Gochman DS, editor. Handbook of health behavior research. Plenum Press; NY: 1997. pp. 113–132. [Google Scholar]
- Rosenthal R. How are we doing in soft psychology? American Psycholgist. 1990;45:775–777. [Google Scholar]
- Snijders T, Bosker R. Multilevel Analysis: An introduction to basic and advanced multilevel modeling. Sage; Thousand Oaks, CA: 1999. [Google Scholar]
- Soofi ES, Retzer JJ, Yasai-Ardekani M. A framework for measuring the importance of variables with applications to management research and decision models. Decision Sciences. 2000;31:595–625. [Google Scholar]
- Stewart AL, Hays RD, Ware JE., Jr. The MOS short-form general health survey: Reliability and validity in a patient population. Medical Care. 1988;26:724–735. doi: 10.1097/00005650-198807000-00007. [DOI] [PubMed] [Google Scholar]
- Tiro JA, Diamond P, Perz CA, Fernandez ME, Rakowski WA, DiClemente CC, et al. Validation of scales measuring attitudes and norms related to mammography screening in women veterans. Health Psychology. 2005;24:555–566. doi: 10.1037/0278-6133.24.6.555. [DOI] [PubMed] [Google Scholar]
- Vernon SW, Myers RE, Tilley BC, Li S. Factors associated with perceived risk in automotive employees at increased risk of colorectal cancer. Cancer Epidemiology, Biomarkers & Prevention. 2001;10:35–43. [PubMed] [Google Scholar]
- Vernon SW. Risk perception and risk communication for cancer screening behaviors: A review. Journal of the National Cancer Institute Monographs. 1999:101–119. doi: 10.1093/oxfordjournals.jncimonographs.a024184. [DOI] [PubMed] [Google Scholar]
- Vernon SW, Vogel VG, Halabi S, Bondy ML. Factors associated with perceived risk of breast cancer among women attending a screening program. Breast Cancer Research. 1993;28:137–144. doi: 10.1007/BF00666426. [DOI] [PubMed] [Google Scholar]
- Wang J, Costantino JP, Tan-Chiu E, Wickerham DL, Paik S, Wolmark N. Lower-category benign breast disease and the risk of invasive breast cancer. Journal of the National Cancer Institute. 2004;96:616–620. doi: 10.1093/jnci/djhs105. [DOI] [PubMed] [Google Scholar]
- Weinstein ND. The precaution adoption process. Health Psychology. 1988;7:355–386. doi: 10.1037//0278-6133.7.4.355. [DOI] [PubMed] [Google Scholar]
- Weinstein ND. Perceptions of personal susceptibility to harm. In: Mays VM, Albee GW, Schneider SF, editors. Primary Prevention of AIDS. Sage; Newbury Park: 1989. pp. 142–167. [Google Scholar]
- Weinstein ND. What does it mean to understand a risk? Evaluating risk comprehension. Journal of the National Cancer Institute Monographs. 1999;25:15–20. doi: 10.1093/oxfordjournals.jncimonographs.a024192. [DOI] [PubMed] [Google Scholar]
- Weinstein ND, Atwood K, Puleo E, Fletcher R, Colditz G, Emmons KM. Colon cancer: Risk perceptions and risk communication. Journal of Health Communication. 2004;9:53–65. doi: 10.1080/10810730490271647. [DOI] [PubMed] [Google Scholar]
- Wild TC, Cunningham J. Psychosocial determinants of perceived vulnerability to harm among adult drinkers. Journal of Studies on Alcohol. 2001;62:105–113. doi: 10.15288/jsa.2001.62.105. [DOI] [PubMed] [Google Scholar]
- Windschitl PD. Measuring and conceptualizing perceptions of vulnerability/likelihood; Paper presented at the Conceptualizing and Measuring Risk Perceptions Workshop; Washington, D.C.. 2003; http://dccps.nci.nih.gov/BRP/presentations/windschitl.pdf#search=‘Windschitl%20and%20perceived%20risk’. [Google Scholar]