Abstract
Most α-herpesviruses are pantropic, neuroinvasive pathogens that establish a reactivateable, latent infection in the PNS of their natural hosts. Various manifestations of herpes disease rely on extent and direction of the spread of infection between the surface epithelia and the nervous system components that innervate that surface. One aspect of such controlled spread of infection is the capacity for synaptically defined, transneuronal spread, a property that makes α-herpesviruses useful tools for determining the connectivity of neural circuits. The current understanding of intra-axonal transport and transneuronal spread of α-herpesviruses is reviewed, focusing on work with herpes simplex virus and pseudorabies virus, the available in vitro technology used to study viral transport and spread is evaluated and how certain viral mutants can be used to examine neural circuit architecture is described in this article.
Keywords: α-herpesvirus, axonal sorting, axonal transport, circuit tracing, herpes simplex virus, pseudorabies virus
Infection & neuroinvasion
α-herpesviruses
The α-herpesvirus subfamily consists of related dsDNA viruses, many of which have the unique capacity to establish a latent infection in the PNS ganglia of their natural hosts. These viruses include the well-studied human pathogens, herpes simplex virus (HSV)-1 and -2, as well as varicellazoster virus (VZV). For these viruses, humans are the only reservoir. The molecular biology of agricultural pathogens in the α-herpesvirus subfamily, including bovine herpesvirus, equine herpes virus and pseudorabies virus (PRV), is also understood in some detail. In this article, we focus on HSV-1 and PRV, which have provided in vivo and in vitro models for neural invasion and spread. Comparative virology of these viruses is interesting, both for the differences as well as the similarities that are revealed. While both are α-herpesviruses, well known differences exist between PRV and HSV-1 in host range (PRV infects essentially all mammals except higher primates, while the natural host range of HSV-1 is restricted to humans) and genome content. The genome of PRV is largely colinear with that of HSV-1 and other α-herpesviruses, except for a large internal inversion in the UL region situated between UL46 and UL26.5 [1]. PRV genes ORF1.2, and UL3.5 are not found in HSV-1, while at least 16 HSV-1 genes are not present in PRV [2]. Despite these differences, the structure of all herpesvirus virions is unique in virology and is remarkably similar. For all α-herpesviruses, the viral genome is enclosed in an icosahedral nucleocapsid, which is surrounded by a layer of viral and cellular proteins, collectively termed the tegument. The nucleocapsid and tegument are enveloped in a host-derived membrane that contains viral proteins, most of which are glycosylated [3].
While serious complications following primary herpesvirus infection or reactivation from latency occur only rarely in the natural host, they can nonetheless lead to life-threatening disease. The common outcome of reactivation in the latently infected PNS ganglion is spread of infection to the mucosal epithelium innervated by that ganglion [4]. This process results in recurrent lesions (cold sores) in HSV-1 infection, or in shingles after VZV reactivation. Less commonly, infection spreads from the peripheral to the CNS, resulting in encephalitis [5]. Unlike infection of natural hosts, infection of non-natural hosts often leads to CNS infection. One such example for humans is when the herpes B virus (Cercopithecine herpesvirus 1), an endemic simplex virus of macaque monkeys, infects humans. Non-natural host infections by PRV occur frequently in nature amongst rodents, cattle, dogs, cats and many other species [1]. In these cases, the infected animals experience trans-neuronal viral spread from the peripheral to the CNS, and succumb to infection. Therefore, one critical step in the development of herpes disease is the spread of infection from a ganglion to the epithelial or neuronal cells it contacts. We examine the process of neuroinvasion (spread from the PNS to the CNS) and transneuronal spread of α-herpesviruses with a focus on HSV-1 and PRV, two of the best-studied members of the subfamily.
Neuroinvasion & latency
Initial infection by an α-herpesvirus typically occurs at a mucosal epithelium. Viral glycoproteins mediate attachment and fusion of the viral envelope and the plasma membrane, resulting in release of the tegument and nucleocapsid into the cytoplasm.
The viral-attachment glycoprotein gD mediates strong interactions with cell-surface receptors. Subsequently, the viral-fusion machinery (gB and gH/gL) promotes fusion between the viral and cellular membranes [6]. Several cellular gD receptors have been characterized: herpesvirus entry mediator (HVEM), nectin-1 and -2, as well as modifications of heparan sulfate introduced by 3-O-sulfotransferases [7–9]. Murine nectin-2 is used for PRV entry, but not for HSV entry [10]. HVEM and nectin-1 are the principal gD-binding entry receptors allowing PRV entry into mouse cells, and allowing both serotypes of HSV entry into human cells. In addition, nectin-1 is the mediator of HSV entry into cultured primary rat and mouse sensory neurons [11]. Recently, a putative gB receptor, paired immunoglobulin-like receptor-α, has been reported [12].
Following amplification of infection in the epithelial cells, virions invade the nerve terminals innervating the site of infection, and spread by retrograde transport to the cell bodies, where a lifelong latent infection is established [13]. It was recently determined that the deubiquitinase domain of the viral tegument protein UL36 is required for spread of infection from the periphery to the innervating axons [14]. Sensory neurons are readily infected, but if deeper layers of the epithelium are exposed, efferent and autonomic nerve terminals can also be infected. Upon entering the axon termini of PNS neurons, nucleocapsids and their inner tegument components are released and engage the retrograde-directed, microtubule-associated cellular motor dynein for delivery to the neuronal cell body [15,16]. Studies of fluorescently tagged PRV capsids in cultured chick dorsal root ganglia (DRG) neurons reveal that the retrograde transport of the nucleocapsid is accomplished at the average velocity of 1.17 ± 0.03 μm/s. The movement was bidirectional and saltatory, with average retrograde run lengths of 7.38 ± 0.47 μm [17].
The structure of the PRV particle engaged in retrograde transport, as revealed by transmission electron microscopy, has no membrane surrounding it [18,19]. Furthermore, only the inner tegument proteins remain associated with the capsid during retrograde transport, while the outer tegument is released upon entry [20–22]. Therefore, viral proteins that are likely to interact with the dynein motor complex include capsid components and the inner tegument, as has been shown by in vitro motility assays for HSV [23]. While several herpesvirus proteins are capable of binding dynein subunits in vitro, these proteins either are absent from the retrograde-trafficking particle, or play no role in retrograde transport, as determined by live-cell imaging of fluorescently labeled capsids and other techniques [24,25]. Our current understanding is that the essential innermost tegument protein, UL36, engages the retrograde transport machinery. However, this hypothesis has proven exceedingly difficult to test because UL36 is essential for particle assembly [24].
Following arrival at the neuronal cell body, the capsid (with inner tegument proteins) docks at nuclear pores and delivers the viral DNA to the nucleus. Viral replication may ensue with subsequent death of the cell, or a quiescent, latent infection may be established. The number of neurons in a peripheral ganglion that can be infected varies substantially, depending on many variables (e.g., virus strain, animal species, site of infection and dose of virus). Latency has been well studied for HSV and poorly studied for PRV. Interestingly, at least for HSV-1, the copy number of quiescent genomes in latently infected mouse neurons ranges from one to 1000 or more. In murine systems, following experimentally induced stress, reactivation of productive infection does not occur in all latently infected neurons. As few as one to five of the latently infected neurons will reactivate while the others remain quiescent [26]. An obvious question asks why so few neurons are activated at any given time, even when all neurons should receive the activation signal? One recent study suggests that stochastic derepression of the viral VP16 promoter leads to gradual accumulation of the VP16 tegument protein [26]. This trans-activator forms a complex with two cellular gene products, the transcription factor Oct-1, and the cell-cycle regulator HCF-1, which initiates transcription of viral immediate–early genes [27,28]. After sufficient quantities of VP16 are synthesized, the VP16-induced complex binds to a particular sequence in the immediate–early promoters (ICP0 and ICP4 genes) to initiate the viral gene-expression cascade and mediate exit from latency [26]. Interestingly, the majority of the VP16 protein does not remain associated with capsids during retrograde transport in neurons [21], and the same is true for the HSV VP16 protein [Smith GA, Pers. Comm.]. Therefore, the absence of this transactivator in the cell body may favor the establishment of latency.
While no viral proteins are synthesized during latency, the viral genome is transcriptionally active. The latency-associated transcripts function in inhibiting apoptosis of the infected cell, and maintaining viral latency (reviewed in [29]). The identification of latency-associated transcript-derived miRNA and an additional virally encoded miRNA was recently described [30]. These molecules appear to function in targeted degradation of mRNA of the viral transactivators ICP0 and ICP4, thereby preventing the transcriptional cascade required for exit from latency [30]. Suffice it to say that the mechanisms of establishment and reactivation of the latent infection are active subjects of research and debate [31].
Anterograde transport & spread of α-herpesviruses
After reactivation, new virions are produced and transported from the peripheral ganglion to the epithelial tissue, which was the original site of infection. Such spread involves sorting of virion components into axons followed by long-distance, microtubule-based anterograde transport of these components toward sites of egress. The nature of this directed, long-distance movement of infection via axons remains contentious – one important question is ‘what is being targeted to axons and transported to distal sites?’
Data from PRV and HSV infections have provided two different answers. Electron- and confocal-microscopy studies are consistent with the idea that mature virions produced from cell bodies move through the axons in transport vesicles [13,18,32–42]. However, other studies provide data that are more consistent with the concept that virions are not sorted fully assembled into axons, but rather enter and move as separate structural components, or subassemblies [43–46]. The static images demonstrated capsids with no surrounding membranes in axons, and no obvious colocalization of capsid and glycoprotein signals using antibody techniques [44–48]. In addition, electron microscopy demonstrated accumulations of enveloped capsids only at axonal varicosities and termini, the suggested sites of virion egress [49,50]. In this scenario, unenveloped capsids and viral glycoproteins are independently transported in axons, and secondary envelopment of these subassemblies occurs in distal sites of the axon and axon terminals [51]. Currently, the former model (fully assembled virions in vesicles move into axons for transport) is favored for PRV, while the latter (separate capsids and envelopes move into axons for independent transport) is favored for HSV.
The differences observed in the PRV and HSV data suggest that the two viruses use different mechanisms for axonal transport of newly made virions and distant egress. If true, it will be important to understand the basis of different evolutionary adaptation to such a fundamental process as axonal sorting and transport. However, other explanations should be explored as well. For example, many of the studies are not directly comparable. Differences in viral strains, protocols and preparations of neuronal tissue and cells (explants vs dissociated neurons, vs cell lines) all provide important variables that need to be reconciled. Recent work by Smith and Antinone, where HSV-1 and PRV were compared directly in chick DRG neurons, supports the idea that both viruses have much in common with respect to axonal sorting and anterograde transport [Smith GA, Pers. Comm.]. Certainly some important differences are obvious when one compares neuronal infections by HSV and PRV. For example, during PRV and HSV infection of cultured rodent PNS neurons, many more virions and virion components are observed in axons of PRV- than in HSV-infected neurons; moreover, in direct comparisons, HSV structural proteins enter axons substantially later than do the PRV structural proteins [Mettenleiter TC, Pers. Comm.] [Smith GA, Pers. Comm.] [46,48,51]. Consequently, the time of observation of infected axons coupled with different particle abundance in axons make it difficult to compare and interpret data from both viruses, particularly using the electron microscope.
Our own experience demonstrates some of the important confounding factors in analysis. Initially, we reported that axons of PRV-infected PNS neurons in culture contained capsids with no envelopes [43,52]. However, it became clear that at least two issues complicated and confounded these experiments (and any experiments that use dissociated cultures of neurons or neuron-like cell lines). First, it was difficult, if not impossible, to distinguish input virions from newly replicated virions in neurons. Virions adsorb to cultured neurons not only at cell bodies and axon terminals, but also along the axon shafts. Second, axons in dissociated neuronal cultures form synaptic connections with other cell bodies, as well as axons, such that a single axon can support both entering particles (retrograde movement) and egressing particles (anterograde movement) [53]. In fact, as time progresses, it becomes impossible to deduce by looking at fixed images of axons if the particles or subassemblies observed are ‘coming or going’.
The use of live-cell imaging to study PRV particle dynamics, as well as compartmented neuronal cultures to physically isolate the site of infection from the site of imaging, have been particularly useful in solving several problems. These techniques help define the direction of movement in axons (toward or away from the cell body), and reduce or eliminate the ambiguity between input inoculum and newly made virions. Obviously, imaging techniques rely on the use of recombinant viruses that produce fluorescent fusion proteins that assemble into virions. Such recombinants are, by definition, mutants and it is essential to demonstrate that the fusion proteins have wild-type function. This condition holds for many, but not all PRV recombinants that express GFP- and RFP-tagged virion proteins in neurons. Not as many studies have been reported with HSV-1 recombinants, primarily because many grow poorly in neurons for reasons that are not entirely well understood.
Electron microscopy of PRV-infected PNS neurons in compartmented cultures (where cell bodies are physically separated from the imaged axons) demonstrated that the vast majority of anterograde-trafficking PRV nucleocapsids in axons are enclosed in a double membrane – the viral envelope and, presumably, the trans-Golgi network (TGN) vesicle from which it is derived [35,54]. Similar results were obtained by examining axons in PRV-infected rat superior cervical ganglion explants [Mettenleiter TC, Pers. Comm.]. By tagging different virion components with fluorescent proteins (envelope, tegument and capsid), Antinone et al. demonstrated that the newly made PRV capsid fluorescent puncta undergoing anterograde movement are associated with viral membrane proteins [32]. By contrast, during retrograde movement, the fluorescent capsid puncta do not exhibit colocalization with viral membrane proteins signals. These results were recapitulated by Feierbach et al. in a compartmented neuronal culture system, where viral inoculum was applied to cell bodies and images were obtained of physically isolated axons, and by Liu et al. in fluidically isolated axons [54,55]. Coller and Smith first revealed another source of so-called ‘naked capsids’ in axons of infected neurons [22]. They proposed that partially enveloped virions were sorted into axons and moved in the anterograde direction. However, these structures are moderately unstable and can dissociate into capsid- and membrane-vesicle components. When this dissociation occurs, the capsid immediately moves back to the cell body (retrograde motion), while the glycoprotein-tagged membrane vesicle (sans capsid) continues in the anterograde direction. This finding was also supported by data from Feierbach et al. [54]. Coller and Smith demonstrated that the function of the two PRV-encoded protein kinases, products of the Us3 and UL13 genes, are important in the stability of these partially assembled complexes [22]. This phenomenon of dramatic reversals of anterograde moving capsid puncta was first reported by Smith et al. [17], and later documented further in a second paper [56]. Using a microfluidic chamber system, Liu et al. quantified the number of retrograde and anterograde moving particles in axons after sorting [55]. They recapitulated the observations that fluorescent puncta exhibiting capsid and tegument signals travel predominantly in the net anterograde direction, while nearly all puncta exhibiting only capsid fluorescence travel in the retrograde direction [55]. Therefore, acquisition of a viral envelope appears to dictate the direction of viral transport: enveloped capsids engage anterograde-directed motors, while naked capsids engage the retrograde-directed motor dynein [32]. These observations offer an explanation for the presence of occasional ‘naked’ capsids in axons of PRV-infected neurons, and highlight the importance of temporal dynamics in the study of anterograde trafficking.
In the aforementioned studies, PRV-infected rodent superior cervical ganglion and chicken DRG axons were observed to contain a substantial number of vesicles with no capsid signal, but had glycoprotein or outer-tegument protein signals (often both). Some of these vesicles may be secretory vesicles for membrane proteins. Others may be L-particles (light particles, virion envelopes and outer-tegument proteins without capsids), which can be produced in PRV and HSV infections [57]. These nonvirion structures can be sorted into axons of PRV-infected neurons and moved in the anterograde direction. However, capsid-free vesicles bearing viral tegument or membrane proteins have never been observed to move back to the cell body in PRV-infected axons [54,55]. The activity of L-particles in neuronal infections has not been studied, but the fact that they are rich in biologically active membrane and tegument proteins has potential implications for biological effects at sites of cell–cell interactions.
Axonal sorting
Neurons are highly polarized cells with distinct axonal, cell body and dendritic compartments. This unique morphology relates to function and, accordingly, cellular cargoes must be correctly appropriated to the somatodendritic or the axonal compartments. This complex process bares some similarities to the basolateral versus apical partitioning of cellular cargoes in polarized epithelial cells [58,59]. Cargoes are delivered to the appropriate membrane via distinct transport carriers, or are preferentially retained in specific cellular domains [60]. For anterograde transmission of viral infection, viral cargoes must be sorted into the axon compartment and moved long distances to sites of egress. Without axonal sorting, there can be no spread of infection from the presynaptic to the postsynaptic neuron or epithelial cell (during reactivation of latent infection). Electron microscopy of compartmented neuronal cultures has shown that in axons, newly replicated PRV particles are contained in a double membrane [33,35]. This observation is consistent with the hypothesis that fully assembled virions enclosed within a transport vesicle are sorted into the axon for anterograde transport to distant sites [61].
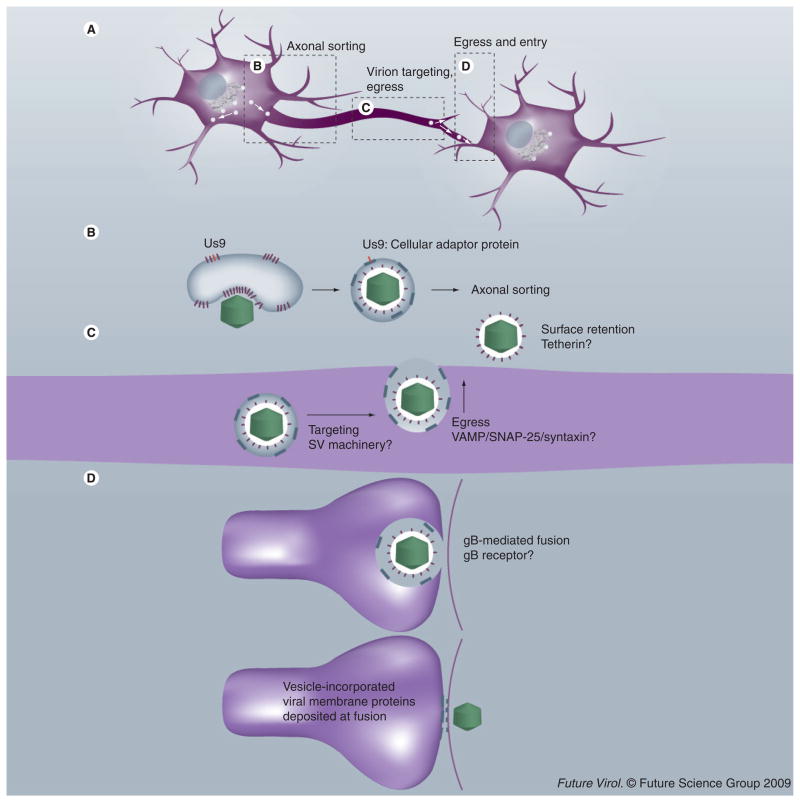
Lyman and colleagues discovered that the viral protein Us9 mediates targeting of PRV virions to axons [62]. This 98-amino acid type II membrane protein has a short (~two amino acid) ectodomain, and a relatively large 68-amino acid cytoplasmic tail [63]. Like other viral membrane proteins, Us9 localizes to the TGN-derived membrane that is the site of viral envelope acquisition. The predicted topology of viral membrane proteins in the TGN vesicle is opposite to that found in the viral envelope: the short ectodomain of the vesicle-incorporated type II Us9 protein faces the enclosed virion, while its tail domain is in the cytoplasm, well positioned for establishing interactions with cellular proteins. A recent study by Lyman et al. revealed that Us9 must associate with lipid-raft microdomains in order to mediate axonal sorting of PRV structural proteins [53]. In addition, putative phosphorylation sites in the Us9 tail, an acidic cluster region and two dityrosine motifs, are required for axonal sorting of PRV [53]. The current hypothesis is that the PRV Us9 protein localizes to lipid microdomains in TGN-derived vesicles, where its phosphorylated form engages the appropriate cell machinery to enable targeting of the virion-containing vesicle to the axon (Figures 1A & B). It is still unclear if Us9 is involved only in sorting to the axonal compartment or in movement of cytoplasmic vesicles to and through the cytoskeleton-rich proximal axonal segment.
Figure 1. Model for transneuronal pseudorabies virus spread.
(A) Anterograde transmission of pseudorabies virus (PRV) requires axonal sorting of newly synthesized virions, movement in axons via microtubule based motor systems, proper delivery of virions to presynaptic sites for egress and subsequent penetration by virions of the postsynaptic membrane. Note that the images (B–D) are detailed examples of events illustrated in (A). (B) PRV virions are sorted into axons within trans-Golgi network-derived vesicles that are the site of secondary envelopment. The phosphorylated cytoplasmic tail of Us9 (highlighted in red) in lipid rafts (thicker lines) recruits the appropriate cellular adaptor protein that mediates axonal sorting of the PRV-containing vesicle. (C) The vesicle is targeted to varicosities and axon termini in a process that may depend on SV-targeting machinery. Egress of virions from axons occurs in a gB-independent fashion, and may utilize cellular soluble N-ethylmaleimide-sensitive factor attachment protein receptors. Released virions remain associated to the axonal surface, an event that may be mediated by cellular proteins such as tetherin, known to retain HIV virions and other enveloped virus-like particles on the surface of infected cells [104]. (D) Upon egress at a synapse, virion-incorporated gB (in conjunction with the fusion proteins gH–gL) mediates entry into the postsynaptic cell. In the process, vesicle-incorporated viral membrane proteins are deposited in the presynaptic membrane, and virion-incorporated viral membrane proteins are deposited in the postsynaptic membrane. gB (in conjunction with fusion proteins gH–gL) at these locations may mediate fusion pore formation, a process that has no bearing on the efficiency of spread, but which may alter the electrophysiology of infected neurons [100,105].
SV: Synaptic vesicle.
The viral proteins gE and gI, which function as heterodimers, work with Us9 to facilitate axonal sorting of PRV virions. PRV mutants that do not express either of these proteins exhibit reduced anterograde spread of infection in vivo and in vitro [35,64,65]. Currently, the idea is that the PRV gE–gI complex may stabilize interactions between Us9 and cellular adaptor proteins, thereby facilitating the process of axonal targeting of viral cargo [53].
Studies of the HSV-1 Us9 homolog implicate the protein in axonal targeting of viral particles and anterograde spread of the virus [47,66,67]. However, the contribution of Us9 to axonal sorting of HSV may not be as significant as that seen in PRV infection. Recent in vitro and in vivo work in mice indicates that HSV Us9 mutants have a modest effect on anterograde spread of infection, while the gE–gI complex was essential [68]. This conclusion is in contrast to the findings in PRV, where mutation of the genes encoding gE and gI results in a leaky phenotype (incomplete block of anterograde sorting and transmission of infection) [35]. Interestingly, when Us9 is deleted from the PRV genome, the HSV or VZV Us9 homolog cannot mediate axonal sorting of PRV, while the bovine herpesvirus-1 and equine herpes virus-1 homologs can [69]. Together, these results suggest that the function of Us9 in the targeting of newly synthesized virions to axons is different between PRV and the human viruses, HSV and VZV. It appears that the role of Us9 and the gE–gI complex between these two viruses may be reversed.
Egress & spread
In cultured epithelial cells, egress of PRV is proposed to occur via exocytosis of vesicles carrying single or multiple virions. The release of virions into the media enables infection of the neighboring cells. However, another route for transmission of infection is direct cell–cell spread where free virions are not involved. This mode of spread requires the viral fusion proteins gB, gH and gL. These fusion proteins are not required for exocytosis, but the mutant particles so released are completely noninfectious [70–74]. The viral attachment protein gD is required for virion infection for both PRV and HSV-1. Interestingly, gD is not required for cell–cell spread of PRV infection, but is essential for cell–cell spread of HSV-1 infection [75,76].
The presence of ‘viral highways’ – virus particles arranged in a linear fashion – has been noted on the surface of VZV-infected non-neuronal cells. These were observed on actin-rich filopodia via scanning electron microscopy [77,78]. While this arrangement of particles is so far unique to VZV among the α-herpesviruses subfamily [77], HSV-1 was recently proposed to induce filopodia in differentiated P19 cells, and ‘surf’ on the extracellular surface in a manner that facilitates viral spread [79]. It remains to be conclusively shown whether the proposed surfing particles are on the outside or the inside of the cell. However, viral surfing may represent another mode of α-herpesvirus transmission.
Similar to cell–cell spread in epithelial cells, transneuronal spread of PRV does not require gD, but does require the viral fusion machinery, gB and gH/gL [35,75,76,80,81]. Several experiments suggest that viral egress and subsequent spread to neighboring cells occurs at specific axonal locations. Using an in vitro co-culture system with swine sensory neurons and epithelial cells, De Regge et al. demonstrated that spread of PRV from an infected neuron to epithelial cells occurs primarily at axonal varicosities [82]. Other in vivo and in vitro studies also report that spread of PRV from an axon to the surrounding cells occurs at scattered sites along the axon, and at neuronal termini [35,83]. Similar conclusions have been made for HSV spread. For example, electron microscopy of human fetal DRG infected with HSV demonstrates accumulations of mature virions at varicosities and growth cones [49]. Therefore, it may be that both HSV and PRV virions are targeted to specific locations within the axon for egress. However, the mechanisms underlying virion targeting and neuronal egress remain uncharacterized (Figure 1C).
Recent work from our laboratory suggests that PRV egress from axons of cultured PNS neurons does not require the viral fusion machinery, and that many of the newly released particles remain associated with the axonal membrane [84]. However, in this study, entry into the postsynaptic cell was mediated by viral fusion proteins found on the released, but tethered virions. We proposed that entry of released virions involves fusion of viral envelope and cellular membranes, but that these lipid bilayers must be in close apposition. This condition only occurs at synaptic contacts or other sites of cell–cell communication. The very close apposition of membranes at synapses would explain the restriction of viral spread to synaptically connected cells (Figure 1D).
Neural circuit tracing
The broad host range and circuit-specific directional spread of α-herpesviruses has utility for revealing the connectivity of neural circuits and the synaptic architecture of the nervous system. A variety of recombinant viruses have been constructed for expressing labels and activity probes in neurons [85–87]. Virions are injected into peripheral sites or CNS structures in vivo, and the extent of transneuronal spread of infection is determined by visualizing viral gene expression or reporter expression in anatomical sections.
Attenuated virus strains with directional spread phenotypes have been particularly useful as circuit tracers. The PRV vaccine strain Bartha exhibits significantly lower virulence in most animal species compared with wild-type strains. The infected animal survives longer, allowing deeper viral spread and more extensive labeling of the nervous system [88]. Importantly, the PRV Bartha strain is a directional spread mutant in a neuronal circuit; it only spreads in the retrograde direction (from the postsynaptic cell to the presynaptic cells and not vice versa) [84,89]. This phenotype arises because the Bartha genome harbors a small deletion that removes the gE, gI and Us9 genes – all required for sorting virions into axons.
The PRV dsDNA genome is easily manipulated to carry reporter genes, as well as precise lesions that attenuate virulence or restrict viral replication and transneuronal spread. Many tracing strains now exist with unique properties. For example, PRV derivatives of virulent strains that are deleted for gE and gI are utilized as retrograde tracers [87]. Another example is the PRV strain Bartha2001, a retrograde-restricted tracer with a conditional replication phenotype [90]. The utility of the strain is that its replication in nonmitotic cells depends on expression of the Cre recombinase. The strain does not express the viral thymidine kinase and will not replicate in nonmitotic cells (e.g., neurons). However, after exposure to Cre, a tau-green fluorescent protein reporter and a viral thymidine kinase are expressed and viral replication ensues. The virions produced in the Cre-expressing neuron will only spread transneuronally to those neurons projecting axons to the infected cell, and will replicate in these neurons even if they do not express the Cre recombinase. Therefore, transgenic mice expressing Cre from specific neuronal promoters enable tracing of synaptic connections to these precisely defined neuronal populations.
Herpes simplex virus tracing strains are also well known, but the HSV-1 strain H129 bears particular mention. The HSV strain H129 is a clinical isolate from the brain of a patient suffering from herpes simplex encephalitis [91]. H129 exhibits anterograde-restricted spread, and is used as a unidirectional tracer [92,93]. At present, the mechanisms responsible for this phenotype have not been identified.
Challenges ahead
Mechanisms of anterograde spread
Anterograde spread (from pre- to postsynaptic neuron) requires sorting of viral structural proteins to the axon and transport to sites of egress. Our understanding of the virally encoded determinants for axonal sorting and transport of α-herpesvirus virions or virion components is progressing. However, the cellular proteins that are involved have not been identified. Cellular cargoes targeted to the axon must engage the appropriate adaptor proteins for correct localization. The cellular proteins that enable axonal targeting of virion components are not known, but their discovery would undoubtedly lead to interesting mechanistic revelations and potential inhibitors of viral spread. A fundamental question is: do all α-herpesviruses engage similar sorting transport machinery during anterograde transneuronal spread?
Newly synthesized virions or virion components that are sorted into the axon must spread to cells in contact with the infected axon at varicosities and axon termini. This assertion implies that virions are targeted to specific egress locations within an axon. What are the mechanisms of targeting of virion components? Neurosecretory vesicles localize to sites of synaptic contact for efficient signal transduction between connected neurons. Proper targeting of synaptic vesicles, which carry neurotransmitters, and dense core granules, which carry neuropeptides, relies on a number of proteins [94,95]. The subsequent exocytosis of these vesicles depends on cellular soluble N-ethylmaleimide-sensitive factor attachment protein receptors [96], proteins that bring cellular membranes in close apposition and mediate subsequent fusion of the lipid bilayers. At present, we cannot say whether the cellular machinery involved in targeting and exocytosis of these vesicles is also used in targeting α-herpesviruses to sites of egress.
The mechanisms of egress of infectious herpes-virus virions from any cell, including neurons, are poorly understood. We do know that the viral fusion machinery is not required for egress of PRV from axons [97], suggesting that cellular processes may carry out virion egress. Immunoelectron-microscopy studies in HSV-infected neurons were particularly revealing. Miranda-Saksena et al. demonstrated that the neurosecretion proteins, SNAP-25, Rab3A and GAP-43, colocalize with HSV-1 antigens in the axons of human fetal DRG, a finding that supports the involvement of cellular-secretion machinery in the release of α-herpesviruses from axons [98]. The challenge is to verify that these proteins play a direct role in viral egress and transneuronal spread, and if they are used for PRV egress and spread.
Mechanisms of retrograde spread
Retrograde spread of infection occurs when virions pass from a neuronal cell body to pre-synaptic axon termini. It should be determined whether virions are targeted specifically to post-synaptic sites in the infected neuron (directed egress and entry) or if virions exit randomly, and only those virions that are released at or near synapses are capable of infecting the pre-synaptic second-order neuron. It may be that cellular machinery is usurped to direct egressing virions to postsynaptic sites in the infected cell body. Such a mechanism would impart high efficiency to the process of retrograde viral spread. In uninfected neurons, cell proteins are sorted specifically to these somatodendritic sites. For example, the neurotransmission machinery localizes to sites of synaptic contact for efficient signal transduction [99]. It may be that the newly enveloped virion is targeted to the appropriate sites in the somatodendritic compartment via the same cellular machinery responsible for organizing postsynaptic sites. An alternative mechanism is that egressing virions emerge randomly from the cell body and dendrites of the infected neuron, and only a few of these released virions have the opportunity to engage the presynaptic axon. In this model, only those external virions that emerge at or near synapses would be able to infect the presynaptic terminal. Those that do not infect are held fast on the neuronal surface, perhaps by specific cellular proteins. We have some evidence to support his model [100]. At a first glance, random egress would not seem to be an efficient means to ensure trans-synaptic spread. However, given that hundreds, if not thousands, of virions are assembled in an infected neuron, transneuronal spread of infection may require only one transmission event. Our observations of fluorescently tagged virus particles suggest that small numbers of particles mediate retrograde spread of infection in cultured sympathetic neurons (see supplemental videos S3 and S4 in [53]). In that study, we documented that only a small number of particles pass from postsynaptic to presynaptic neurons in culture. In the example illustrated in [53], we counted seven capsid puncta moving into the presynaptic axon over a 14-min imaging session. Over the same period, there were hundreds of single green puncta moving around the infected cell body, with no other evidence of egress.
The quantitative aspects of the spread of infection in neural circuits have not been established. How many projecting neurons are labeled by spread from one neuron, and what parameters influence this number? Unfortunately, mutations that affect retrograde transneuronal spread for PRV or for HSV are rare, so addressing these fundamental questions has been difficult. We do know that viral gene products that reduce the number of particles produced per cell or have reduced cell–cell spread kinetics, reduce efficiency of retrograde transneuronal spread [97,101]. For example, we know that all PRV Bartha tracing strains carry a mutated UL21 locus that reduces efficiency of retrograde transneuronal spread. When a wild-type UL21 locus was restored in the PRV Bartha genome, retrograde spread in neural circuitry increased with more extensive penetration into a circuit [97]. We found that infectious yield was reduced in the presence of the mutant UL21 protein and some aberrant capsid structures were seen in the nucleus. We speculated that the capsids produced in the presence of the mutant UL21 protein were also less competent for secondary envelopment and egress. It may be that decreased retrograde spread efficiency in our experiments with PRV Bartha reflects the transfer of fewer particles per synapse or transfer of infection through a smaller number of synapses from a single cell.
In animal infection models, the HSV strain H129 undergoes retrograde transneuronal spread only to first-order neurons, but undergoes efficient anterograde transneuronal spread [102,103]. This phenotype is unique among all α-herpesviruses examined to date, and has been exploited to conduct anterograde tracing of neural circuits [92,93]. Importantly, the strain may offer insight into the mechanisms of retrograde spread of infection. The efficient anterograde spread of H129 implies that the mechanisms of retrograde and anterograde spread are determined by different viral loci. Study of this strain may elucidate the steps that constitute the process of retrograde viral spread, as well as the viral and cellular proteins involved.
Conclusion
The overall objective in the field is to come to a comprehensive understanding of the molecular mechanisms of transneuronal spread of herpesviruses. In this article, we focused on recent progress in determining how HSV and PRV virion components move inside and between neurons. It is important to stress that the majority of our insight into the cell biology of anterograde spread has come from in vitro studies with primary cultures of neurons or neuronal cell lines. To determine if the basic principles so obtained hold true in vivo, imaging technology that will reveal the temporal kinetics of individual herpesvirus particles in a living animal needs to be developed. In vitro studies with both viruses have provided fundamental technology and insight into the processes of entry, assembly, axonal sorting, transport and egress. The similarities and differences shown for PRV and HSV are stimulating considerable research and debate, and many questions remain unanswered. Further research is required, not only to obtain a better understanding of herpesvirus pathogenesis, but also to provide the means to reveal aspects of neuronal cell biology and neural circuitry that are crucial for viral invasion and spread.
Future perspective
What are the molecular mechanisms by which neuroinvasive α-herpesviruses invade and spread in the mammalian nervous system? We can all agree that by understanding these mechanisms of axonal and dendritic sorting, transport, assembly, release and uptake, our understanding of viral spread will substantially expand and potential sites of antiviral intervention can be identified. Despite modern medicine and antiviral drugs, viral infections of the nervous system are devastating and exceedingly difficult to manage. Understanding the host and viral interactions involved in neuroinvasion and resulting pathogenesis has relevance to human health by revealing new targets for prevention and therapy. By contrast, the same properties of neuroinvasion provide opportunities to understand the organization of the nervous system by using viruses as tracers of neural circuitry.
Executive summary
α-herpesviruses
The α-herpesvirus subfamily of Herpesviridae includes important human and veterinary pathogens that establish a reactivatable latent infection in the peripheral ganglia of their natural hosts that may spread to the CNS.
The herpesviral dsDNA genome is easily manipulated to introduce desired mutations or reporter genes.
Neuroinvasion & latency
Intracellular and transneuronal trafficking of α-herpesviruses is a critical aspect for the development of various manifestations of herpesvirus pathogenesis.
Following replication in epithelial cells, α-herpesviruses spread to the peripheral ganglia innervating the site of infection.
Viral latency persists in the peripheral ganglia for the lifetime of the natural host.
Occasional reactivation from latency results in viral spread from the PNS to the original site of infection, resulting in recurrent epithelial lesions.
While spread of infection to the CNS is rare in the natural host, non-natural hosts necessarily experience a lethal CNS infection.
Transneuronal spread of infection & neural circuit tracing
During anterograde transmission of infection, α-herpesvirus virions and virion components are sorted into the axonal compartment and transported by cellular molecular motors toward the epithelial or postsynaptic cell along microtubule tracks.
Different models exist for axonal sorting of pseudorabies virus versus herpes simplex virus virions and virion components.
In retrograde transmission of infection, the unenveloped, tegumented capsid engages the minus-end directed cellular motor dynein for transport to the soma.
Attenuated strains of α-herpesviruses with restricted invasiveness can be used to study neural circuit organization.
Future perspective
The cellular machinery that mediates axonal sorting of virions and virion components should be identified.
The mechanisms of virion release from axons should be determined.
It should be established whether virions are specifically targeted to postsynaptic sites in soma during retrograde transmission of infection and, if so, by what mechanism.
Neural circuit tracing viruses that encode reporter proteins and spread in a controlled manner should be developed to study nervous system connectivity and activity.
Acknowledgments
The authors thank members of the Enquist laboratory for advice and encouragement. GA Smith provided critical comments. Anonymous reviewers helped improve the review.
Financial & competing interests disclosure
The authors acknowledge support from NIH grants R37 NS 033506, R01 NS060699, P40 RR 018604, AI 033063 and P50 GM071508. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Contributor Information
D Curanovic, Department of Pharmacology, Weill Medical College, Cornell University, New York, NY 10065, USA.
LW Enquist, Email: lenquist@princeton.edu, Department of Molecular Biology, Princeton University, Princeton, NJ 08544, USA, Tel.: +1 609 258 2415, Fax: +1 609 258 1035.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1▪.Pomeranz LE, Reynolds AE, Hengartner CJ. Molecular biology of pseudorabies virus: impact on neurovirology and veterinary medicine. Microbiol Mol Biol Rev. 2005;69(3):462–500. doi: 10.1128/MMBR.69.3.462-500.2005. Detailed review of pseudorabies virus (PRV) biology, as well as its use in circuit tracing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klupp BG, Hengartner CJ, Mettenleiter TC, Enquist LW. Complete, annotated sequence of the pseudorabies virus genome. J Virol. 2004;78(1):424–440. doi: 10.1128/JVI.78.1.424-440.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mettenleiter TC, Klupp BG, Granzow H. Herpesvirus assembly: an update. Virus Res. 2009;143(2):222–234. doi: 10.1016/j.virusres.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Roizman B, Knipe DM. Herpes simplex viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. Lippincott Williams & Wilkins; PA, USA: 2001. pp. 2399–2459. [Google Scholar]
- 5.Whitley RJ, Kimberlin DW, Roizman B. Herpes simplex viruses. Clin Infect Dis. 1998;26(3):541–553. doi: 10.1086/514600. quiz 554–545. [DOI] [PubMed] [Google Scholar]
- 6.Spear PG, Longnecker R. Herpesvirus entry: An update. J Virol. 2003;77(19):10179–10185. doi: 10.1128/JVI.77.19.10179-10185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spear PG, Eisenberg RJ, Cohen GH. Three classes of cell surface receptors for αherpesvirus entry. Virology. 2000;275(1):1–8. doi: 10.1006/viro.2000.0529. [DOI] [PubMed] [Google Scholar]
- 8.Warner MS, Geraghty RJ, Martinez WM, et al. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology. 1998;246(1):179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- 9.Krummenacher C, Baribaud F, Ponce de Leon M, et al. Comparative usage of herpesvirus entry mediator A and nectin-1 by laboratory strains and clinical isolates of herpes simplex virus. Virology. 2004;322(2):286–299. doi: 10.1016/j.virol.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Shukla D, Rowe CL, Dong Y, Racaniello VR, Spear PG. The murine homolog (Mph) of human herpesvirus entry protein B (HveB) mediates entry of pseudorabies virus but not herpes simplex virus types 1 and 2. J Virol. 1999;73(5):4493–4497. doi: 10.1128/jvi.73.5.4493-4497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richart SM, Simpson SA, Krummenacher C, et al. Entry of herpes simplex virus type 1 into primary sensory neurons in vitro is mediated by nectin-1/HveC. J Virol. 2003;77(5):3307–3311. doi: 10.1128/JVI.77.5.3307-3311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satoh T, Arii J, Suenaga T, et al. Pilrα is a herpes simplex virus-1 entry coreceptor that associates with glycoprotein B. Cell. 2008;132(6):935–944. doi: 10.1016/j.cell.2008.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill TJ, Field HJ. The interaction of herpes simplex virus with cultures of peripheral nervous tissue: an electron microscopic study. J Gen Virol. 1973;21:123–133. doi: 10.1099/0022-1317-21-1-123. [DOI] [PubMed] [Google Scholar]
- 14.Lee JI, Sollars PJ, Baver SB, Pickard GE, Leelawong M, Smith GA. A herpesvirus encoded deubiquitinase is a novel neuroinvasive determinant. PLoS Pathog. 2009;5(4):e1000387. doi: 10.1371/journal.ppat.1000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dohner K, Nagel CH, Sodeik B. Viral stop-and-go along microtubules: taking a ride with dynein and kinesins. Trends Microbiol. 2005;13(7):320–327. doi: 10.1016/j.tim.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Sodeik B. Mechanisms of viral transport in the cytoplasm. Trends Microbiol. 2000;8(10):465–472. doi: 10.1016/s0966-842x(00)01824-2. [DOI] [PubMed] [Google Scholar]
- 17▪▪.Smith GA, Gross SP, Enquist LW. Herpesviruses use bidirectional fast-axonal transport to spread in sensory neurons. Proc Natl Acad Sci USA. 2001;98(6):3466–3470. doi: 10.1073/pnas.061029798. The authors fluorescently tag PRV capsids and describe the nature of capsid dynamics in axons of chicken dorsal root ganglia neurons via live-cell imaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18▪▪.Lycke E, Hamark B, Johansson M, Krotochwil A, Lycke J, Svennerholm B. Herpes simplex virus infection of the human sensory neuron. An electron microscopy study. Arch Virol. 1988;101(1–2):87–104. doi: 10.1007/BF01314654. One example of the classic, prescient studies of herpes simplex virus (HSV) infection in neurons. [DOI] [PubMed] [Google Scholar]
- 19.Lycke E, Kristensson K, Svennerholm B, Vahlne A, Ziegler R. Uptake and transport of herpes simplex virus in neurites of rat dorsal root ganglia cells in culture. J Gen Virol. 1984;65(Pt 1):55–64. doi: 10.1099/0022-1317-65-1-55. [DOI] [PubMed] [Google Scholar]
- 20.Granzow H, Klupp BG, Mettenleiter TC. Entry of pseudorabies virus: an immunogold-labeling study. J Virol. 2005;79(5):3200–3205. doi: 10.1128/JVI.79.5.3200-3205.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luxton GWG, Haverlock S, Coller KE, Antinone SE, Pincetic A, Smith GA. Targeting of herpesvirus capsid transport in axons is coupled to association with specific sets of tegument proteins. Proc Natl Acad Sci USA. 2005;102(16):5832–5837. doi: 10.1073/pnas.0500803102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coller KE, Smith GA. Two viral kinases are required for sustained long distance axon transport of a neuroinvasive herpesvirus. Traffic. 2008;9(9):1458–1470. doi: 10.1111/j.1600-0854.2008.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23▪.Wolfstein A, Nagel CH, Radtke K, Dohner K, Allan VJ, Sodeik B. The inner tegument promotes herpes simplex virus capsid motility along microtubules in vitro. Traffic. 2006;7(2):227–237. doi: 10.1111/j.1600-0854.2005.00379.x. Example of a clever use of cell biology, biochemistry and virology to study how HSV capsids engage and move on microtubules. [DOI] [PubMed] [Google Scholar]
- 24.Antinone SE, Shubeita GT, Coller KE, et al. The herpesvirus capsid surface protein, VP26, and the majority of the tegument proteins are dispensable for capsid transport toward the nucleus. J Virol. 2006;80(11):5494–5498. doi: 10.1128/JVI.00026-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dohner K, Radtke K, Schmidt S, Sodeik B. Eclipse phase of herpes simplex virus type 1 infection: efficient dynein-mediated capsid transport without the small capsid protein VP26. J Virol. 2006;80(16):8211–8224. doi: 10.1128/JVI.02528-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26▪.Thompson RL, Preston CM, Sawtell NM. De novo synthesis of VP16 coordinates the exit from HSV latency in vivo. PLoS Pathog. 2009;5(3):e1000352. doi: 10.1371/journal.ppat.1000352. Addresses the mechanisms of HSV reactivation in vivo using a detailed analysis of individual infected ganglia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stern S, Tanaka M, Herr W. The Oct-1 homoeodomain directs formation of a multiprotein–DNA complex with the HSV transactivator VP16. Nature. 1989;341(6243):624–630. doi: 10.1038/341624a0. [DOI] [PubMed] [Google Scholar]
- 28.Preston CM, Frame MC, Campbell ME. A complex formed between cell components and an HSV structural polypeptide binds to a viral immediate early gene regulatory DNA sequence. Cell. 1988;52(3):425–434. doi: 10.1016/s0092-8674(88)80035-7. [DOI] [PubMed] [Google Scholar]
- 29.Kent JR, Kang W, Miller CG, Fraser NW. Herpes simplex virus latency-associated transcript gene function. J Neurovirol. 2003;9(3):285–290. doi: 10.1080/13550280390200994. [DOI] [PubMed] [Google Scholar]
- 30.Umbach JL, Kramer MF, Jurak I, Karnowski HW, Coen DM, Cullen BR. MicroRNAs expressed by herpes simplex virus 1 during latent infection regulate viral mRNAs. Nature. 2008;454(7205):780–783. doi: 10.1038/nature07103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawtell NM, Thompson RL. Comparison of herpes simplex virus reactivation in ganglia in vivo and in explants demonstrates quantitative and qualitative differences. J Virol. 2004;78(14):7784–7794. doi: 10.1128/JVI.78.14.7784-7794.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32▪.Antinone SE, Smith GA. Two modes of herpesvirus trafficking in neurons: membrane acquisition directs motion. J Virol. 2006;80(22):11235–11240. doi: 10.1128/JVI.01441-06. The authors propose a mechanism that directs PRV capsids in the retrograde or anterograde direction; these findings help resolve some of the controversies surrounding the structure and components of the virion sorted into the axon during anterograde transport. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.del Rio T, Ch’ng TH, Flood EA, Gross SP, Enquist LW. Heterogeneity of a fluorescent tegument component in single pseudorabies virus virions and enveloped axonal assemblies. J Virol. 2005;79(7):3903–3919. doi: 10.1128/JVI.79.7.3903-3919.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ch’ng TH, Enquist LW. An in vitro system to study trans-neuronal spread of pseudorabies virus infection. Vet Microbiol. 2006;113(3–4):193–197. doi: 10.1016/j.vetmic.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Ch’ng TH, Enquist LW. Neuron-to-cell spread of pseudorabies virus in a compartmented neuronal culture system. J Virol. 2005;79(17):10875–10889. doi: 10.1128/JVI.79.17.10875-10889.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cook ML, Stevens JG. Pathogenesis of herpetic neuritis and ganglionitis in mice: evidence for intra-axonal transport of infection. Infect Immun. 1973;7(2):272–288. doi: 10.1128/iai.7.2.272-288.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hill TJ, Field HJ, Roome AP. Intra-axonal location of herpes simplex virus particles. J Gen Virol. 1972;15(3):233–235. doi: 10.1099/0022-1317-15-3-253. [DOI] [PubMed] [Google Scholar]
- 38.Kristensson K, Ghetti B, Wisniewski HM. Study on the propagation of herpes simplex virus (type 2) into the brain after intraocular injection. Brain Res. 1974;69:189–201. doi: 10.1016/0006-8993(74)90001-8. [DOI] [PubMed] [Google Scholar]
- 39.Kristensson K, Sheppard RD, Bornstein MB. Observations on uptake of herpes simplex virus in organized cultures of mammalian nervous tissue. Acta Neuropathol (Berl) 1974;28(1):37–44. doi: 10.1007/BF00687516. [DOI] [PubMed] [Google Scholar]
- 40.LaVail JH, Topp KS, Giblin PA, Garner JA. Factors that contribute to the transneuronal spread of herpes simplex virus. J Neurosci. 1997;49:485–496. [PubMed] [Google Scholar]
- 41.Ohara PT, Chin MS, LaVail JH. The spread of herpes simplex virus type 1 from trigeminal neurons to the murine cornea: an immunoelectron microscopy study. J Virol. 2000;74(10):4776–4786. doi: 10.1128/jvi.74.10.4776-4786.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamamoto T, Otani S, Shiraki H. Ultrastructure of herpes simplex virus infection of the nervous system of mice. Acta Neuropathol (Berl) 1973;26(4):285–299. doi: 10.1007/BF00688077. [DOI] [PubMed] [Google Scholar]
- 43.Tomishima MJ, Enquist LW. A conserved α-herpesvirus protein necessary for axonal localization of viral membrane proteins. J Cell Biol. 2001;154(4):741–752. doi: 10.1083/jcb.200011146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miranda-Saksena M, Armati P, Boadle RA, Holland DJ, Cunningham AL. Anterograde transport of herpes simplex virus type 1 in cultured, dissociated human and rat dorsal root ganglion neurons. J Virol. 2000;74(4):1827–1839. doi: 10.1128/jvi.74.4.1827-1839.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45▪▪.Penfold ME, Armati P, Cunningham AL. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc Natl Acad Sci USA. 1994;91(14):6529–6533. doi: 10.1073/pnas.91.14.6529. This seminal paper set-up the current research and debate on ‘what is being sorted and transported in axons’. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Snyder A, Wisner TW, Johnson DC. Herpes simplex virus capsids are transported in neuronal axons without an envelope containing the viral glycoproteins. J Virol. 2006;80(22):11165–11177. doi: 10.1128/JVI.01107-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snyder A, Polcicova K, Johnson DC. Herpes simplex virus gE/gI and US9 proteins promote transport of both capsids and virion glycoproteins in neuronal axons. J Virol. 2008;82(21):10613–10624. doi: 10.1128/JVI.01241-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Snyder A, Bruun B, Browne HM, Johnson DC. A herpes simplex virus gD–YFP fusion glycoprotein is transported separately from viral capsids in neuronal axons. J Virol. 2007;81(15):8337–8340. doi: 10.1128/JVI.00520-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saksena MM, Wakisaka H, Tijono B, et al. Herpes simplex virus type 1 accumulation, envelopment, and exit in growth cones and varicosities in mid-distal regions of axons. J Virol. 2006;80(7):3592–3606. doi: 10.1128/JVI.80.7.3592-3606.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holland DJ, Miranda-Saksena M, Boadle RA, Armati P, Cunningham AL. Anterograde transport of herpes simplex virus proteins in axons of peripheral human fetal neurons: an immunoelectron microscopy study. J Virol. 1999;73(10):8503–8511. doi: 10.1128/jvi.73.10.8503-8511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diefenbach RJ, Miranda-Saksena M, Douglas MW, Cunningham AL. Transport and egress of herpes simplex virus in neurons. Rev Med Virol. 2008;18(1):35–51. doi: 10.1002/rmv.560. [DOI] [PubMed] [Google Scholar]
- 52.Enquist LW, Tomishima MJ, Gross S, Smith GA. Directional spread of an α-herpesvirus in the nervous system. Vet Microbiol. 2002;86(1–2):5–16. doi: 10.1016/s0378-1135(01)00486-2. [DOI] [PubMed] [Google Scholar]
- 53▪▪.Lyman MG, Curanovic D, Enquist LW. Targeting of pseudorabies virus structural proteins to axons requires association of the viral US9 protein with lipid rafts. PLoS Pathog. 2008;4(5):e1000065. doi: 10.1371/journal.ppat.1000065. Uses a variety of techniques to reveal that the PRV membrane protein Us9 must associate with lipid-raft domains to mediate axonal sorting of virions, and offers a model for how PRV structural proteins can be targetted to the axon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feierbach B, Bisher M, Goodhouse J, Enquist LW. In vitro analysis of transneuronal spread of an αherpesvirus infection in peripheral nervous system neurons. J Virol. 2007;81(13):6846–6857. doi: 10.1128/JVI.00069-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu WW, Goodhouse J, Jeon NL, Enquist LW. A microfluidic chamber for analysis of neuron-to-cell spread and axonal transport of an α-herpesvirus. PLoS ONE. 2008;3(6):e2382. doi: 10.1371/journal.pone.0002382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith G, Pomerantz L, Gross SP, Enquist LW. Local modulation of plus-end transport targets herpesvirus entry and egress in sensory axons. Proc Natl Acad Sci USA. 2004;101(45):16034–16039. doi: 10.1073/pnas.0404686101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rixon FJ, Addison C, McLauchlan J. Assembly of enveloped tegument structures (L particles) can occur independently of virion maturation in herpes simplex virus type 1-infected cells. J Gen Virol. 1992;73(Pt 2):277–284. doi: 10.1099/0022-1317-73-2-277. [DOI] [PubMed] [Google Scholar]
- 58.Pietrini G, Suh YJ, Edelmann L, Rudnick G, Caplan MJ. The axonal γ-aminobutyric acid transporter Gat-1 is sorted to the apical membranes of polarized epithelial cells. J Biol Chem. 1994;269(6):4668–4674. [PubMed] [Google Scholar]
- 59.de Hoop M, von Poser C, Lange C, Ikonen E, Hunziker W, Dotti CG. Intracellular routing of wild-type and mutated polymeric immunoglobulin receptor in hippocampal neurons in culture. J Cell Biol. 1995;130(6):1447–1459. doi: 10.1083/jcb.130.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sampo B, Kaech S, Kunz S, Banker G. Two distinct mechanisms target membrane proteins to the axonal surface. Neuron. 2003;37(4):611–624. doi: 10.1016/s0896-6273(03)00058-8. [DOI] [PubMed] [Google Scholar]
- 61▪▪.Granzow H, Klupp BG, Fuchs W, Veits J, Osterrieder N, Mettenleiter TC. Egress of αherpesviruses: comparative ultrastructural study. J Virol. 2001;75(8):3675–3684. doi: 10.1128/JVI.75.8.3675-3684.2001. Offers a detailed ultrastructural analysis of several α-herpesviruses in different cell types during egress, and shows that the envelopment–de-envelopment process at the nuclear membrane is common to the different viruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lyman MG, Feierbach B, Curanovic D, Bisher M, Enquist LW. Pseudorabies virus US9 directs axonal sorting of viral capsids. J Virol. 2007;81(20):11363–11371. doi: 10.1128/JVI.01281-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brideau AD, Banfield BW, Enquist LW. The us9 gene product of pseudorabies virus, an αherpesvirus, is a phosphorylated, tail-anchored type II membrane protein. J Virol. 1998;72(6):4560–4570. doi: 10.1128/jvi.72.6.4560-4570.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tirabassi RS, Townley RA, Eldridge MG, Enquist LW. Molecular mechanisms of neurotropic herpesvirus invasion and spread in the CNS. Neurosci Biobehav Rev. 1998;22(6):709–720. doi: 10.1016/s0149-7634(98)00009-8. [DOI] [PubMed] [Google Scholar]
- 65.Ch’ng TH, Enquist LW. Efficient axonal localization of αherpesvirus structural proteins in cultured sympathetic neurons requires viral glycoprotein E. J Virol. 2005;79(14):8835–8846. doi: 10.1128/JVI.79.14.8835-8846.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.LaVail JH, Tauscher AN, Sucher A, Harrabi O, Brandimarti R. Viral regulation of the long distance axonal transport of herpes simplex virus nucleocapsid. Neuroscience. 2007;146(3):974–985. doi: 10.1016/j.neuroscience.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Polcicova K, Biswas PS, Banerjee K, Wisner TW, Rouse BT, Johnson DC. Herpes keratitis in the absence of anterograde transport of virus from sensory ganglia to the cornea. Proc Natl Acad Sci USA. 2005;102(32):11462–11467. doi: 10.1073/pnas.0503230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McGraw HM, Awasthi S, Wojcechowskyj JA, Friedman HM. Anterograde spread of herpes simplex virus type 1 requires glycoprotein E and glycoprotein I but not US9. J Virol. 2009;83(17):8315–8326. doi: 10.1128/JVI.00633-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lyman MG, Kemp CD, Taylor MP, Enquist LW. Comparison of the pseudorabies virus US9 protein with homologs from other veterinary and human αherpesviruses. J Virol. 2009;83(14):6978–6986. doi: 10.1128/JVI.00598-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cai WH, Gu B, Person S. Role of glycoprotein b of herpes simplex virus type 1 in viral entry and cell fusion. J Virol. 1988;62(8):2596–2604. doi: 10.1128/jvi.62.8.2596-2604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Desai PJ, Schaffer PA, Minson AC. Excretion of non-infectious virus particles lacking glycoprotein H by a temperature-sensitive mutant of herpes simplex virus type 1: evidence that gH is essential for virion infectivity. J Gen Virol. 1988;69:1147–1156. doi: 10.1099/0022-1317-69-6-1147. [DOI] [PubMed] [Google Scholar]
- 72.Klupp B, Altenschmidt J, Granzow H, Fuchs W, Mettenleiter TC. Glycoproteins required for entry are not necessary for egress of pseudorabies virus. J Virol. 2008;82(13):6299–6309. doi: 10.1128/JVI.00386-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peeters B, de Wind N, Broer R, Gielkens A, Moormann R. Glycoprotein H of pseudorabies virus is essential for entry and cell-to-cell spread of the virus. J Virol. 1992;66(6):3888–3892. doi: 10.1128/jvi.66.6.3888-3892.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Favoreel HW, Van Minnebruggen G, Nauwynck HJ, Enquist LW, Pensaert MB. A tyrosine-based motif in the cytoplasmic tail of pseudorabies virus glycoprotein B is important for both antibody-induced internalization of viral glycoproteins and efficient cell-to-cell spread. J Virol. 2002;76(13):6845–6851. doi: 10.1128/JVI.76.13.6845-6851.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peeters B, de Wind N, Hooisma M, Wagenaar F, Gielkens A, Moormann R. Pseudorabies virus envelope glycoproteins gp50 and gII are essential for virus penetration, but only gII is involved in membrane fusion. J Virol. 1992;66:894–905. doi: 10.1128/jvi.66.2.894-905.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rauh I, Mettenleiter TC. Pseudorabies virus glycoproteins gII and gp50 are essential for virus penetration. J Virol. 1991;65(10):5348–5356. doi: 10.1128/jvi.65.10.5348-5356.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Padilla JA, Nii S, Grose C. Imaging of the varicella zoster virion in the viral highways: comparison with herpes simplex viruses 1 and 2, cytomegalovirus, pseudorabies virus, and human herpes viruses 6 and 7. J Med Virol. 2003;70(Suppl 1):S103–S110. doi: 10.1002/jmv.10330. [DOI] [PubMed] [Google Scholar]
- 78.Carpenter JE, Hutchinson JA, Jackson W, Grose C. Egress of light particles among filopodia on the surface of varicellazoster virus-infected cells. J Virol. 2008;82(6):2821–2835. doi: 10.1128/JVI.01821-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dixit R, Tiwari V, Shukla D. Herpes simplex virus type 1 induces filopodia in differentiated p19 neural cells to facilitate viral spread. Neurosci Lett. 2008;440(2):113–118. doi: 10.1016/j.neulet.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Klupp BG, Nixdorf R, Mettenleiter TC. Pseudorabies virus glycoprotein m inhibits membrane fusion. J Virol. 2000;74(15):6760–6768. doi: 10.1128/jvi.74.15.6760-6768.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Babic N, Mettenleiter TC, Flamand A, Ugolini G. Role of essential glycoproteins gII and gp50 in transneuronal transfer of pseudorabies virus from the hypoglossal nerves of mice. J Virol. 1993;67(7):4421–4426. doi: 10.1128/jvi.67.7.4421-4426.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82▪.De Regge N, Nauwynck HJ, Geenen K, et al. α-herpesvirus glycoprotein D interaction with sensory neurons triggers formation of varicosities that serve as virus exit sites. J Cell Biol. 2006;174(2):267–275. doi: 10.1083/jcb.200510156. One of the few publications using swine PNS neurons to study PRV infection and spread. The work reveals that infection alters neuronal cell biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tomishima MJ, Enquist LW. In vivo egress of an αherpesvirus from axons. J Virol. 2002;76(16):8310–8317. doi: 10.1128/JVI.76.16.8310-8317.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pickard GE, Smeraski CA, Tomlinson CC, et al. Intravitreal injection of the attenuated pseudorabies virus PRV Bartha results in infection of the hamster suprachiasmatic nucleus only by retrograde transsynaptic transport via autonomic circuits. J Neurosci. 2002;22(7):2701–2710. doi: 10.1523/JNEUROSCI.22-07-02701.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Card JP, Enquist LW. Transneuronal circuit analysis with pseudorabies viruses. Curr Protoc Neurosci. 2001;Chapter 1(Unit 15) doi: 10.1002/0471142301.ns0105s09. [DOI] [PubMed] [Google Scholar]
- 86.Ekstrand MI, Enquist LW, Pomeranz LE. The α-herpesviruses: molecular pathfinders in nervous system circuits. Trends Mol Med. 2008;14(3):134–140. doi: 10.1016/j.molmed.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87▪▪.Boldogkoi Z, Balint K, Awatramani GB, et al. Genetically timed, activity-sensor and rainbow transsynaptic viral tools. Nat Methods. 2009;6(2):127–130. doi: 10.1038/nmeth.1292. Investigates neuronal activity and connectivity in the brain by engineering two modifications into a retrograde-restricted PRV strain, a ratiometric calcium sensor and two fluorophores that are expressed at different times postinfection. [DOI] [PubMed] [Google Scholar]
- 88.Brittle EE, Reynolds AE, Enquist LW. Two modes of pseudorabies virus neuroinvasion and lethality in mice. J Virol. 2004;78(23):12951–12963. doi: 10.1128/JVI.78.23.12951-12963.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Enquist LW. Exploiting circuit-specific spread of pseudorabies virus in the central nervous system: Insights to pathogenesis and circuit tracers. J Infect Dis. 2002;186(Suppl 2):S209–S214. doi: 10.1086/344278. [DOI] [PubMed] [Google Scholar]
- 90.DeFalco J, Tomishima M, Liu H, et al. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291(5513):2608–2613. doi: 10.1126/science.1056602. [DOI] [PubMed] [Google Scholar]
- 91.Dix RD, McKendall RR, Baringer JR. Comparative neurovirulence of herpes simplex virus type 1 strains after peripheral or intracerebral inoculation of BALB/c mice. Infect Immun. 1983;40(1):103–112. doi: 10.1128/iai.40.1.103-112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barnett EM, Evans GD, Sun N, Perlman S, Cassell MD. Anterograde tracing of trigeminal afferent pathways from the murine tooth pulp to cortex using herpes simplex virus type 1. J Neurosci. 1995;15(4):2972–2984. doi: 10.1523/JNEUROSCI.15-04-02972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun N, Cassell MD, Perlman S. Anterograde, transneuronal transport of herpes simplex virus type 1 strain h129 in the murine visual system. J Virol. 1996;70(8):5405–5413. doi: 10.1128/jvi.70.8.5405-5413.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pfeffer SR. Transport-vesicle targeting: tethers before SNAREs. Nat Cell Biol. 1999;1(1):E17–E22. doi: 10.1038/8967. [DOI] [PubMed] [Google Scholar]
- 95.Goldstein AY, Wang X, Schwarz TL. Axonal transport and the delivery of pre-synaptic components. Curr Opin Neurobiol. 2008;18(5):495–503. doi: 10.1016/j.conb.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scalettar BA. How neurosecretory vesicles release their cargo. Neuroscientist. 2006;12(2):164–176. doi: 10.1177/1073858405284258. [DOI] [PubMed] [Google Scholar]
- 97.Curanovic D, Lyman MG, Bou-Abboud C, Card JP, Enquist LW. Repair of the ul21 locus in pseudorabies virus Bartha enhances the kinetics of retrograde, transneuronal infection in vitro and in vivo. J Virol. 2009;83(3):1173–1183. doi: 10.1128/JVI.02102-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miranda-Saksena M, Boadle RA, Aggarwal A, et al. Herpes simplex virus utilizes the large secretory vesicle pathway for anterograde transport of tegument and envelope proteins and for viral exocytosis from growth cones of human fetal axons. J Virol. 2009;83(7):3187–3199. doi: 10.1128/JVI.01579-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sheng M. Molecular organization of the postsynaptic specialization. Proc Natl Acad Sci USA. 2001;98(13):7058–7061. doi: 10.1073/pnas.111146298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Curanovic D, Enquist LW. Virion-incorporated glycoprotein B mediates transneuronal spread of pseudorabies virus. J Virol. 2009;83(16):7796–7804. doi: 10.1128/JVI.00745-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Olsen LM, Ch’ng TH, Card JP, Enquist LW. Role of pseudorabies virus US3 protein kinase during neuronal infection. J Virol. 2006;80(13):6387–6398. doi: 10.1128/JVI.00352-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zemanick MC, Strick PL, Dix RD. Direction of transneuronal transport of herpes simplex virus 1 in the primate motor system is strain-dependent. Proc Natl Acad Sci USA. 1991;88(18):8048–8051. doi: 10.1073/pnas.88.18.8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Garner JA, LaVail JH. Differential anterograde transport of HSV type 1 viral strains in the murine optic pathway. J Neurovirol. 1999;5(2):140–150. doi: 10.3109/13550289909021996. [DOI] [PubMed] [Google Scholar]
- 104.Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 VPU. Nature. 2008;451(7177):425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 105.McCarthy K, Tank DW, Enquist LW. Pseudorabies virus infection alters neuronal activity and connectivity in vitro. PLoS Pathog. 2009 doi: 10.1371/journal.ppat.1000640. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]