Abstract
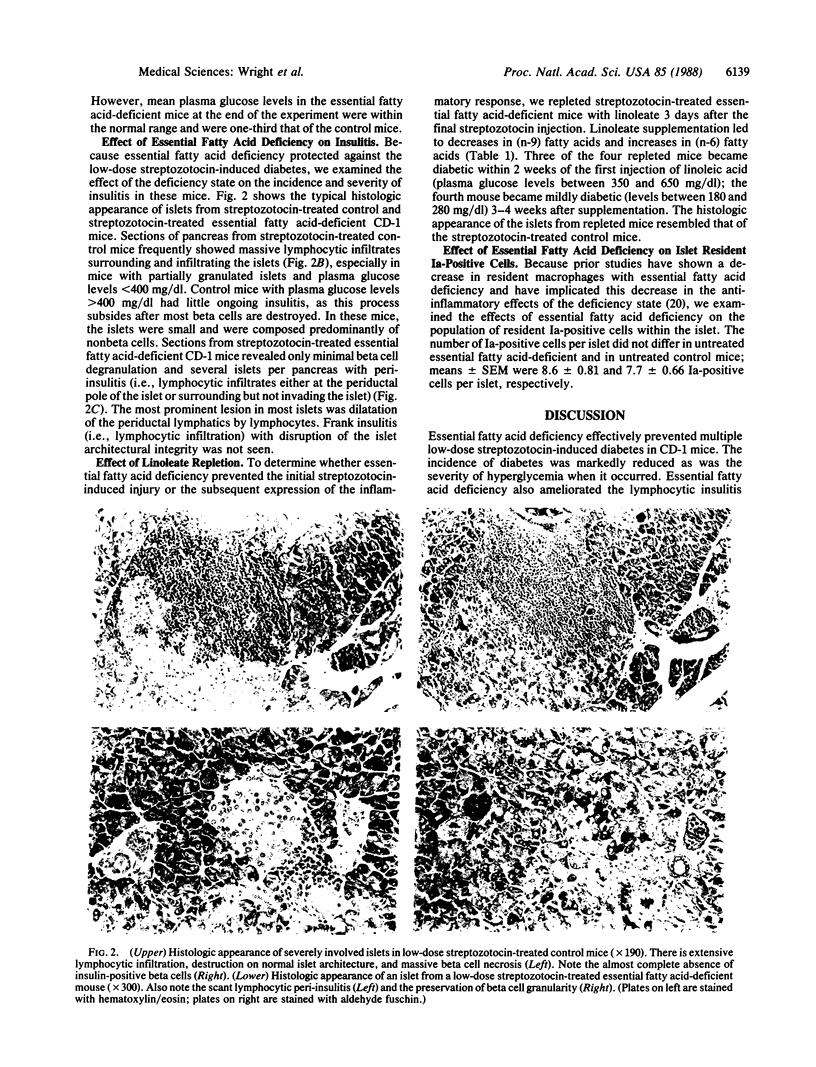
Multiple i.p. injections of low-dose streptozotocin (40 mg/kg) produce insulitis, beta cell destruction, and diabetes in male CD-1 mice. Recent data also suggest that macrophages figure in the low-dose streptozotocin model. Because other recent studies have shown that essential fatty acid deficiency prevents autoimmune nephritis in mice, decreases the number of resident Ia-positive glomerular macrophages, and decreases the elicitation of macrophages into the glomerulus in inflammation, we examined the effect of essential fatty acid deficiency on the incidence and severity of insulitis and diabetes in CD-1 mice treated with low-dose streptozotocin. Streptozotocin-treated mice on the control diet uniformly developed diabetes (19/19). Essential fatty acid-deficient mice treated with streptozotocin did not develop diabetes (1/13). Mean plasma glucose levels for the control and essential fatty acid-deficient mice were 384.5 +/- 23.6 and 129.1 +/- 15.5 mg/dl, respectively, at the end of 1 month. To discern whether essential fatty acid deficiency prevented the streptozotocin-induced beta cell injury or the inflammatory response to injured beta cells, mice were repleted with daily injections of 99% pure methyl linoleate beginning 3 days after the last streptozotocin injection. These mice also quickly developed severe (3/4) or mild (1/4) diabetes. Histologic examination of the pancreata of control mice or repleted mice showed marked insulitis and beta cell destruction; in contrast, the pancreata of essential fatty acid-deficient mice showed preservation of beta cells and only focal mild peri-insulitis. Essential fatty acid deficiency thus prevents the insulitis and resultant diabetes in low-dose streptozotocin-treated CD-1 mice, suggesting a central role for macrophages and lipid mediators in this autoimmunity model.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrechtsen D., Helgerud P., Rugstad H. E. Cyclosporine in blood and thoracic duct lymph in rat. Transplant Proc. 1986 Dec;18(6 Suppl 5):77–78. [PubMed] [Google Scholar]
- Alexander N. J., Smythe N. L., Jokinen M. P. The type of dietary fat affects the severity of autoimmune disease in NZB/NZW mice. Am J Pathol. 1987 Apr;127(1):106–121. [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Eisenbarth G. S. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med. 1986 May 22;314(21):1360–1368. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- HOLMAN R. T. The ratio of trienoic: tetraenoic acids in tissue lipids as a measure of essential fatty acid requirement. J Nutr. 1960 Mar;70:405–410. doi: 10.1093/jn/70.3.405. [DOI] [PubMed] [Google Scholar]
- Hurd E. R., Johnston J. M., Okita J. R., MacDonald P. C., Ziff M., Gilliam J. W. Prevention of glomerulonephritis and prolonged survival in New Zealand Black/New Zealand White F1 hybrid mice fed an essential fatty acid-deficient diet. J Clin Invest. 1981 Feb;67(2):476–485. doi: 10.1172/JCI110056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley V. E., Ferretti A., Izui S., Strom T. B. A fish oil diet rich in eicosapentaenoic acid reduces cyclooxygenase metabolites, and suppresses lupus in MRL-lpr mice. J Immunol. 1985 Mar;134(3):1914–1919. [PubMed] [Google Scholar]
- Kolb-Bachofen V., Epstein S., Kiesel U., Kolb H. Low-dose streptozocin-induced diabetes in mice. Electron microscopy reveals single-cell insulitis before diabetes onset. Diabetes. 1988 Jan;37(1):21–27. doi: 10.2337/diab.37.1.21. [DOI] [PubMed] [Google Scholar]
- Kolb H. Mouse models of insulin dependent diabetes: low-dose streptozocin-induced diabetes and nonobese diabetic (NOD) mice. Diabetes Metab Rev. 1987 Jul;3(3):751–778. doi: 10.1002/dmr.5610030308. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Davie J. M. Transplantation of pancreatic islets. Annu Rev Immunol. 1984;2:183–198. doi: 10.1146/annurev.iy.02.040184.001151. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Lefkowith J. B. Essential fatty acid deficiency inhibits the in vivo generation of leukotriene B4 and suppresses levels of resident and elicited leukocytes in acute inflammation. J Immunol. 1988 Jan 1;140(1):228–233. [PubMed] [Google Scholar]
- Lefkowith J. B., Flippo V., Sprecher H., Needleman P. Paradoxical conservation of cardiac and renal arachidonate content in essential fatty acid deficiency. J Biol Chem. 1985 Dec 15;260(29):15736–15744. [PubMed] [Google Scholar]
- Lefkowith J. B., Jakschik B. A., Stahl P., Needleman P. Metabolic and functional alterations in macrophages induced by essential fatty acid deficiency. J Biol Chem. 1987 May 15;262(14):6668–6675. [PubMed] [Google Scholar]
- Lefkowith J. B., Schreiner G. Essential fatty acid deficiency depletes rat glomeruli of resident macrophages and inhibits angiotensin II-induced eicosanoid synthesis. J Clin Invest. 1987 Oct;80(4):947–956. doi: 10.1172/JCI113187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lernmark A. Molecular biology of type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1985 Apr;28(4):195–203. doi: 10.1007/BF00282232. [DOI] [PubMed] [Google Scholar]
- Like A. A., Rossini A. A. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science. 1976 Jul 30;193(4251):415–417. doi: 10.1126/science.180605. [DOI] [PubMed] [Google Scholar]
- Linn T., Volkmann A., Germann H., Woehrle M., Bretzel R. G., Bicker U., Federlin K. Ciamexon in the low dose streptozotocin induced diabetes of mice. Diabetes Res. 1987 Nov;6(3):113–117. [PubMed] [Google Scholar]
- Nedergaard M., Egeberg J., Kromann H. Irradiation protects against pancreatic islet degeneration and hyperglycaemia following streptozotocin treatment of mice. Diabetologia. 1983 May;24(5):382–386. doi: 10.1007/BF00251829. [DOI] [PubMed] [Google Scholar]
- Oschilewski M., Schwab E., Kiesel U., Opitz U., Stünkel K., Kolb-Bachofen V., Kolb H. Administration of silica or monoclonal antibody to Thy-1 prevents low-dose streptozotocin-induced diabetes in mice. Immunol Lett. 1986 Jun;12(5-6):289–294. doi: 10.1016/0165-2478(86)90032-5. [DOI] [PubMed] [Google Scholar]
- Prickett J. D., Robinson D. R., Steinberg A. D. Dietary enrichment with the polyunsaturated fatty acid eicosapentaenoic acid prevents proteinuria and prolongs survival in NZB x NZW F1 mice. J Clin Invest. 1981 Aug;68(2):556–559. doi: 10.1172/JCI110288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesha C. S., Pickett W. C. Platelet-activating factor and leukotriene biosynthesis is inhibited in polymorphonuclear leukocytes depleted of arachidonic acid. J Biol Chem. 1986 Jun 15;261(17):7592–7595. [PubMed] [Google Scholar]
- Rerup C. C. Drugs producing diabetes through damage of the insulin secreting cells. Pharmacol Rev. 1970 Dec;22(4):485–518. [PubMed] [Google Scholar]
- Rossini A. A., Like A. A., Chick W. L., Appel M. C., Cahill G. F., Jr Studies of streptozotocin-induced insulitis and diabetes. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2485–2489. doi: 10.1073/pnas.74.6.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini A. A., Mordes J. P., Like A. A. Immunology of insulin-dependent diabetes mellitus. Annu Rev Immunol. 1985;3:289–320. doi: 10.1146/annurev.iy.03.040185.001445. [DOI] [PubMed] [Google Scholar]
- Rossini A. A., Williams R. M., Appel M. C., Like A. A. Complete protection from low-dose streptozotocin-induced diabetes in mice. Nature. 1978 Nov 9;276(5684):182–184. doi: 10.1038/276182a0. [DOI] [PubMed] [Google Scholar]
- Scharp D. W., Kemp C. B., Knight M. J., Ballinger W. F., Lacy P. E. The use of ficoll in the preparation of viable islets of langerhans from the rat pancreas. Transplantation. 1973 Dec;16(6):686–689. doi: 10.1097/00007890-197312000-00028. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Wolf J., Lilly F., Shin S. I. The influence of genetic background on the susceptibility of inbred mice to streptozotocin-induced diabetes. Diabetes. 1984 Jun;33(6):567–571. doi: 10.2337/diab.33.6.567. [DOI] [PubMed] [Google Scholar]







