Abstract
Erythrocytes infected with plasmodia, including those that cause human malaria, have increased permeability to a diverse collection of organic and inorganic solutes. While these increases have been known for decades, their mechanistic basis was unclear until electrophysiological studies revealed flux through one or more ion channels on the infected erythrocyte membrane. Current debates have centered on the number of distinct ion channels, which channels mediate the transport of each solute and whether the channels represent parasite-encoded proteins or human channels activated after infection. This article reviews the identification of the plasmodial surface anion channel and other proposed channels with an emphasis on two distinct channel mutants generated through in vitro selection. These mutants implicate parasite genetic elements in the parasite-induced permeability, reveal an important new antimalarial drug resistance mechanism and provide tools for molecular studies. We also critically examine the technical issues relevant to the detection of ion channels by electrophysiological methods; these technical considerations have general applicability for interpreting studies of various ion channels proposed for the infected erythrocyte membrane.
Keywords: host-pathogen interactions, ion channels, malaria, mutant selection, patch clamp, Plasmodium falciparum
Human malaria is a leading cause of morbidity and mortality in many parts of the world [1]; it also places an astounding economic burden on developing countries [2]. As there is no approved vaccine effective against either of the two major species affecting humans, current options for control are limited to preventive measures, such as insecticide-treated bednets [3] and treatment with drugs targeting parasite-specific activities. Since available antimalarial drugs are expected to have a limited lifetime of utility owing to evolving drug resistance [4], it is generally acknowledged that new drugs, ideally targeting previously unexploited parasite activities, must be developed to effectively control malaria in the future.
An important unexploited parasite activity is the increased permeability of infected erythrocytes to a range of organic and inorganic solutes. These permeability changes were initially detected through flame photometric measurements on blood drawn from infected monkeys more than 60 years ago [5]. Subsequent studies from multiple laboratories then achieved universal consensus by confirming increased permeability with isotope uptake and qualitative osmotic lysis studies. Importantly, these studies also defined the breadth of these changes by showing that both inorganic and organic solutes of either positive or negative charge may exhibit increased permeability after infection [6–8].
These early studies also identified a number of relatively nonspecific transport inhibitors [8–10]. Since these inhibitors suppressed in vitro parasite cultures, increased erythrocyte permeability was presumed to be essential for the intracellular parasite. These inhibitors were therefore considered to be possible starting points for antimalarial drug development. However, a major limitation of these studies was that they were unable to identify the mechanism(s) responsible for these changes. Proposals included endocytosis, diffusion of solutes via membrane-delimited ducts and transmembrane flux via membrane defects, ion channels or transporters.
Since the macroscopic methods used in the previous studies could not unambiguously distinguish between these possible molecular mechanisms, our laboratory adapted patch-clamp methods to the study of the infected erythrocyte membrane. Patch-clamp methods measure the small currents resulting from transmembrane ion movement and can also detect capacitance changes resulting from changes in membrane properties or surface area [11,12]. A number of different patch-clamp configurations have been developed and are typically used to study large cells, such as muscle cells and neurons [13]. Under optimal conditions, these configurations can definitively identify the number of independent molecular entities responsible for ion flux, provide kinetic information about individual transport molecules with submillisecond resolution and determine other important information, such as copy number/cell, voltage dependence and the mechanism and site of action of inhibitors or agonists.
Owing to the significantly smaller size and unique membrane properties of human erythrocytes, several modifications to the general patch-clamp protocol were required to successfully achieve the cell-attached and whole-cell configurations in our studies of infected erythrocytes. With these modifications, described in detail below, our patch-clamp studies identified the plasmodial surface anion channel (PSAC) and proposed that this channel is the primary molecular mechanism responsible for the above parasite-induced erythrocyte permeability [14]. Subsequent studies from our group have added to this model by determining that PSAC has a number of unusual functional properties and that it may be parasite encoded. These findings have fueled interest in this channel as a drug target.
This article reviews the identification of PSAC and a number of other channels proposed through electrophysiological studies from other laboratories. Importantly, these other studies agree with the observed increase in anion conductance after infection, but they present an alternative mechanistic model based on activation or modification of human ion channels already present in the erythrocyte membrane. We emphasize the identification of two novel PSAC mutants that suggest PSAC plays a central role in uptake of diverse solutes by infected cells, implicate parasite genetic elements in the formation of this activity, provide insights into the channel’s structure and its function in parasite physiology, and raise concerns about a new antimalarial drug resistance mechanism for compounds targeting intracellular parasite activities. Finally, differences in experimental conditions utilized by each group have been a major area of discussion and may account for the discrepant conclusions reached. Hence, our technical experience on patch clamp of infected erythrocytes and our design considerations for improving detection of small conductance channels are also reviewed.
Identification of PSAC & a possible molecular mechanism for parasite-induced permeability changes
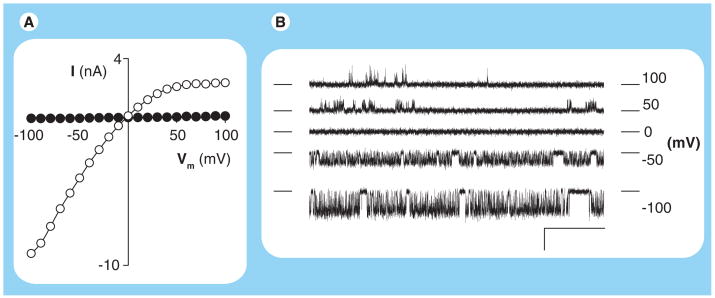
In 2000, our group reported on the identification of PSAC through the use of two patch-clamp configurations that measure currents resulting from ion movement across membranes [14]. The first of these, the whole-cell configuration, measures the total ionic permeability of individual cells. Comparison of currents from mature trophozoite-infected erythrocytes to those from uninfected cells revealed a dramatic increase in Cl− permeability (Figure 1A). Interestingly, the infected cell currents were significantly larger at negative membrane potentials (Vm) than at positive Vm despite an equal and opposite driving force for ion movement across the erythrocyte membrane; this ‘inward-rectifying’ voltage dependence has become a hallmark for infected cell currents in our hands. Furthermore, this whole-cell Cl− flux could be inhibited by furosemide, 5-nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB) and glybenclamide, known antagonists of the parasite-induced uptake of organic solutes. These experiments excluded proposals of bulk solute uptake and instead implicated transmembrane flux via one or more conductive pathways.
Figure 1. Plasmodial surface anion channel activity at a range of membrane potentials detected with patch clamp.
(A) Current–voltage profile from whole-cell recordings of an uninfected erythrocyte and a cell infected with a Plasmodium falciparum trophozoite (black and white circles, respectively). Notice that the infected cell has larger currents at each Vm. (B) Single plasmodial surface anion channel recordings in the cell-attached patch-clamp configuration. All traces were recorded from a single patch with imposed Vm values indicated to the right of each trace. For each trace, the closed channel level is indicated with horizontal dashes on each side. Channel openings are reflected by transitions away from this level; these transitions are upgoing at positive Vm and downgoing at negative Vm. Scale bar represents 100 ms (horizontal) and 2 pA (vertical). Notice that plasmodial surface anion channel openings are more frequent at negative Vm, consistent with greater absolute magnitudes of whole-cell currents at negative Vm in (A). Recordings were obtained with symmetrical molar salt solutions as described previously [15].
I: Current; Vm: Membrane potential.
The second patch-clamp configuration, the cell-attached configuration, revealed single-channel molecule activity on infected cells that was never detected on uninfected cells (Figure 1B). We termed this molecular activity the plasmodial surface anion channel, or PSAC. Several findings suggested that 1000–2000 copies of this channel type account for the increased permeability detected in our whole-cell recordings. First, single-molecule recordings of PSAC revealed opening events that were more abundant and lasted for longer durations at negative membrane potentials than at positive membrane potentials (bottom vs top traces in Figure 1B). This voltage-dependence matched that seen in our inward-rectifying whole-cell currents, suggesting a common underlying mechanism. Second, the power spectrum of single PSAC recordings, a measure of the frequency content of transitions between open and closed channel states (known as ‘gating’), paralleled the spectrum calculated from our whole-cell recordings. Parallel power spectra provide quantitative evidence against significant contributions from channels with different gating phenotypes. Third, single-channel studies with various inhibitors – furosemide, phloridzin, NPPB, dantrolene and dantrolene derivatives [14–19] – revealed inhibition that matched the effects of these compounds on whole-cell currents. Lastly, PSAC activity was not seen in cell-attached patch clamp of either uninfected erythrocytes or cells infected with the distantly related parasite Babesia divergens [20], consistent with significantly smaller whole-cell currents on those cells. These studies strongly suggested that PSAC is the primary conductive pathway for Cl− flux in the infected erythrocyte membrane, at least under the various physiological and hypertonic recording conditions we have employed (see discussion of technical issues below).
As each of the above inhibitors also abrogates uptake of organic solutes at concentrations that are effective in patch clamp, these studies also implicated PSAC as the primary mechanism for uptake of most small solutes with increased permeability after infection. (A notable exception is the increased permeability of divalent cations such as Ca2+ [21,22]. Since Ca2+ uptake is insensitive to furosemide [23], it may be mediated by an unrelated mechanism.) As described below, the functional properties of two new PSAC mutants provide additional independent evidence for this channel’s central role because each mutant exhibits changes in single PSAC recordings and in macroscopic transport of various organic solutes whose permeabilities increase after infection.
Unusual functional properties of PSAC
The plasmodial surface anion channel has a number of unique functional properties that warrant intensive study as a model microbe-associated ion channel. Some of these properties are absent from channels in higher organisms, suggesting that they have evolved to meet the unique needs of the intracellular malaria parasite. For example, while PSAC can transport solutes that carry net positive or negative charge and may be larger than 600 Da in size, it efficiently excludes the small Na+ ion (23 Da and a naked ionic radius of ~1.1 Å),), whose permeability has been estimated at less than 10−5–10−3.5 relative to Cl− [24,25]. Other broad permeability channels lack this ability to exclude individual small ions; our survey of published studies of such channels found permeability ratios for those channels no lower than 0.1 for common inorganic ions such as Na+ and K+ relative to the most permeant solute. This unique characteristic has physiological consequences and is essential for parasite survival; without stringent Na+ exclusion by PSAC, infected cells would incur net Na+Cl− uptake and undergo osmotic lysis in the bloodstream. There are other examples of PSAC’s unusual selectivity profile: while its ability to transport both physiological and nonphysiological amino acids suggests poor selectivity, the channel can distinguish between proline and hydroxyproline and transport these congeners by distinct mechanisms [26]. Although the structural basis of this channel’s unusual selectivity filter(s) remains largely unexplored, one study used N-hydroxysulfosuccinimide esters to identify multiple amines at PSAC extracellular face; these amines are positively charged at physiological pH values and appear to contribute to Na+ exclusion through electrostatic repulsion [25].
Another puzzling functional property is PSAC’s small single-channel conductance in patch-clamp studies (<3 pS in isotonic Cl− solutions). Single-channel conductance is a quantitative measure of how many ions are transported through an open channel per unit time. Based on comparisons with other broad permeability channels (e.g., bacterial porins, which usually have conductances >1000 pS [27,28]), we expected PSAC to have a larger conductance because its ability to transport solutes having large molecular volumes suggests that it must have a wide aqueous pore; large pores generally produce high rates of ion flux. Thus, these functional studies suggest that PSAC must use as yet undescribed mechanisms to limit rates of ion and solute flux; we imagine that this relatively low throughput somehow permits the channel to exclude unwanted small solutes such as Na+.
Other proposed channels
Since the initial detection of PSAC activity approximately 10 years ago [14], our recording conditions have detected only two reproducible channel activities on infected erythrocyte membranes despite intensive study by multiple workers in our group: PSAC and the endogenous Ca2+-activated K+ channel [29]. It is important to recognize that cell-attached recordings designed to identify rare single-channel activity are limited by the unknown lateral distribution of functional channels on the membrane of interest. With this caveat in mind, four other groups have performed patch-clamp studies of infected cells and reported their findings. These studies have achieved universal consensus that infection with Plasmodium falciparum increases the host membrane anion permeability and that ion channels, as opposed to nonspecific membrane leaks, mediate these increases. The magnitudes of whole-cell currents in each group’s studies were similar to those we obtained, suggesting that each group is observing the same basic phenomenon. However, there are some fundamental differences in the results obtained and the interpretations made by each group, as reviewed here.
The Thomas group (Roscoff, France) has used cell-attached and whole-cell patch-clamp configurations to report three distinct ion channels on infected erythrocytes [30,31]: an inward-rectifying channel (12–18 pS, referred to as IRC), a small conductance channel (SCC) with similar voltage dependence (4–5 pS) and a less abundant large conductance, outward-rectifying ion channel. Their studies suggest that the IRC and the SCC both contribute significantly to the whole-cell current, but that the IRC is the greater contributor in physiological saline [32]. As each of these channel activities was seen in their experiments using uninfected erythrocytes stimulated with PKA along with high concentrations of ATP and theophylline added to the pipette [31], the Thomas group proposed that the parasite-induced permeability of organic solutes results from phosphorylation of one or more of these endogenous ion channels. A more recent study from this group reported a parasite-encoded regulatory subunit of PKA that modulates infected erythrocyte transport properties [33].
The group of Huber and Lang (Tübingen, Germany) has primarily used whole-cell patch-clamp to propose at least four distinct ion channels that each differ from PSAC and the channels proposed by the Thomas group. Each of these channels represents an endogenous ion channel that is activated by the oxidative stress resulting from the intracellular parasite’s metabolic activity [34]. Three of these channels are anion selective, with an outward rectifying channel presumed to mediate organic solute transport [35] and an inward-rectifying channel absent in mice that lack the ClC-2 chloride channel [36]. The fourth channel proposed by this group is a nonselective cation channel [37].
A single study from the De Jonge group (Rotterdam, The Netherlands) examined the transport properties of infected and uninfected erythrocytes from both healthy donors and cystic fibrosis (CF) patients who carry a homozygous F508 deletion in the CFTR chloride channel gene. This group reported ATP-dependent currents that do not rectify on uninfected erythrocytes from healthy volunteers but are absent from CF erythrocytes. Cell shrinkage or infection with P. falciparum induced an inward-rectifying current in normal erythrocytes, but not in CF erythrocytes in their hands. The authors suggested that the parasite activates an endogenous anion channel that is distinct from CFTR, but is dependent on wild-type CFTR for its activity. Osmotic lysis studies revealed no differences in parasite-induced organic solute permeability between normal and CF erythrocytes, suggesting that organic solute transport occurs via mechanisms not detected in their patch-clamp studies.
Henry Staines (London, UK) has worked closely with the Thomas and Lang groups and has attempted to reconcile the differences between those results. His studies suggest that components of serum may contribute to the activation of multiple distinct human channels after infection [38]. Although they have not used electrophysiological methods, analyses by Ginsburg and Stein also appear to suggest the involvement of distinct ion channels for at least two subsets of solutes with increased permeability after infection [39].
While the independent groups do not fully agree with the findings from any other laboratory, consideration of the various findings has led to two broad hypotheses about the possible underlying mechanisms. The first proposes a single parasite-encoded ion channel for most permeant solutes and is supported by data from our group. The in vitro selection of two independent PSAC mutants, described below, may represent the strongest evidence for this hypothesis. The second hypothesis suggests that two or more distinct ion channels endogenous to the erythrocyte membrane are activated by the parasite; proponents of this hypothesis generally agree that multiple channel types are required to account for both organic and inorganic solute transport after infection. Universal consensus on which hypothesis is correct, or if a hybrid model combining features from both is required, will almost certainly require definitive identification of the responsible ion channel gene(s).
At present, the channel gene or genes continue to resist identification despite the best efforts of many laboratories. One reason may be that the low abundance of each putative ion channel: the upper estimate seems to be approximately 1000–2000 functional copies/cell [14], a value much lower than that of Band 3 glycophorins and other known proteins in the red cell membrane. Another reason has been that there are no clear orthologs of known anion channels in the parasite genome [40], limiting the utility of informatic approaches to gene identification and possibly favoring the modified human channel hypothesis. It appears that new approaches and/or new research tools may be needed to identify the elusive genes. We believe the recent identification of parasite mutants exhibiting altered solute transport may provide such new handles for gene identification (described below).
What are the physiological roles of these channels?
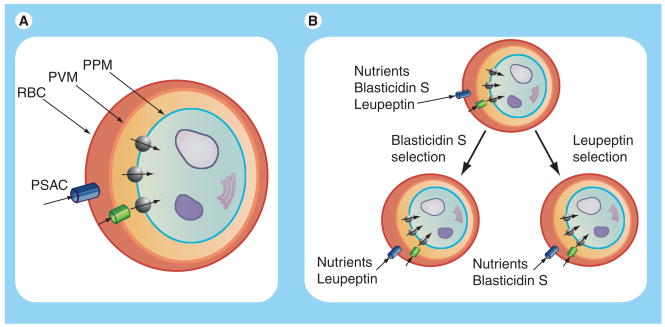
An important unanswered question is the physiological role served by increased host membrane permeability. By localizing PSAC activity to the host membrane [14], we could formalize general speculations about a role in nutrient acquisition into a model of sequential uptake of essential nutrients via PSAC at the host membrane (Figure 2A), the PVM channel at the parasitophorous vacuolar membrane surrounding the intracellular parasite [41,42] and specific transporters for each nutrient at the parasite plasma membrane [43–47]. According to our model, soluble metabolic waste products may also exit infected cells by movement through these channels and transporters in the opposite direction. Uncharged nutrients are present at higher concentrations in serum and will generally enter the cell. As they are continuously generated through parasite metabolism, waste products such as lactate− are above their equilibrium concentration in parasite cytosol and can be removed via passive efflux. A near-comprehensive list of nutrients required for in vitro propagation of parasite cultures has been developed [48]. The substantial permeability of most of these nutrients through PSAC is strong circumstantial evidence for this proposed role [49]; nevertheless, direct experimental evidence for this proposal is missing.
Figure 2. Sequential diffusive pathway for nutrient acquisition by intracellular malaria parasites (A) and a model for plasmodial surface anion channel-mediated drug resistance (B).
(A) Nutrients and toxins may enter infected cells by passive uptake via PSAC on the erythrocyte membrane, a large-conductance nonselective channel on the PVM and multiple distinct carriers on the PPM. Metabolic waste products generated in the parasite cytosol (blue) exit the infected cell complex by flux in the opposite direction. According to our proposed model, various solutes share a single ion channel at the erythrocyte membrane and at the PVM, but use separate and specific transporters at the PPM. A nucleus and some organelles are shown within the parasite cytosol to exemplify the metabolic activity of the intracellular parasite. (B) In vitro selection with either leupeptin or blasticidin S generates separate PSAC mutants that have reduced uptake of the applied toxin but only modestly compromised uptake of nutrients and the other toxin.
PPM: Parasite plasma membrane; PVM: Parasitophorous vacuolar membrane; PSAC: Plasmodial surface anion channel; RBC: Red blood cell membrane.
Direct evidence for other proposed roles is also missing. These other possibilities include infected erythrocyte volume regulation [50], modification of host cytosolic ion concentrations to create a high Na+/low K+ environment suitable for parasite growth [51,52], erythrocyte apoptosis [53] and timed osmotic lysis of infected cells to permit release of daughter parasites at the end of the intraerythrocytic cycle [24]. Another formal possibility, that the channels serve no essential role and are only an epiphenomenon of parasite invasion, seems unlikely to us because of conservation of this activity in other plasmodial species [54] and because of the inability of in vitro selection protocols to generate a complete functional knockout, as described below.
Two novel permeability mutants & their implications for parasite biology
A recent breakthrough has been the generation of two separate parasite mutants that exhibit altered PSAC activity [55,56]. Both of these mutants were generated with selective pressure applied to wild-type parasite cultures. One used 2.5 μg/ml blasticidin S, a toxin made by Streptomyces bacteria that kills cells by inhibiting protein translation on ribosomes [57]. The other mutant was generated by selection with 50 μM leupeptin, another bacterial toxin that is a potent and relatively nonspecific calpain protease inhibitor [58,59]. Importantly, both toxins must cross multiple membranes to reach their targets within the intraerythrocytic parasite. When either toxin is added to parasite cultures at its respective concentration, it produces microscopic clearance of parasite cultures with 3–4 days. Nevertheless, resistant parasites frequently grow out of these cultures if they are maintained under selective pressure for extended periods of time (typically ≥ 2 months). Interestingly, each mutant can only be generated from a single and distinct wild-type parasite isolate. While blasticidin S resistance could only be acquired by the FCB laboratory isolate, leupeptin resistance was possible with only the HB3 parasite. Moreover, the two mutants exhibit limited or no cross-resistance as each is rapidly killed by addition of the other toxin. Standard limiting dilution culturing of these resistant parasites has been used to generate clonal cultures of each mutant; the blasticidin S- and leupeptin-resistant clones are here referred to as FCB-br1 and HB3-leuR1, respectively. These clones can be propagated indefinitely in the presence of the toxin used for selection and have been made broadly available to groups interested in membrane transport.
Functional studies with each resistant parasite revealed marked changes in PSAC activity. Both exhibited changes in organic solute permeability, PSAC single-channel properties and pharmacology. Both could be easily distinguished from wild-type channels in both macroscopic and single-channel molecule studies. The two mutants could also be easily distinguished from each other as the changes selected by blasticidin S and by leupeptin appear to be distinct. For example, FCB-br1 exhibits a 16-fold reduction in sorbitol permeability, a novel subconductance state in single-channel recordings, and an approximately 40-fold decrease in furosemide inhibitory affinity [55]. By contrast, HB3-leuR1 has only a 2.4-fold reduction in sorbitol permeability, altered gating without subconductance states, and more marked changes in inhibition by phloridzin [56]. These functional differences suggest that blasticidin S and leupeptin select for separate mutations that lead to distinct changes in PSAC; this is consistent with the observed lack of cross-resistance to killing by these toxins.
These studies lead to a model of acquired resistance as illustrated in Figure 2B. Our studies strongly suggest that both blasticidin S and leupeptin enter infected cells primarily via PSAC and that changes in PSAC contribute to resistance by decreasing toxin permeability. Decreased permeability reduces toxin uptake to a level that permits survival and propagation of parasites in culture. At the same time, the altered PSAC must still be able to serve whatever essential roles the channel plays for the parasite; if the role is in nutrient uptake as described above, then key nutrient solutes may have somewhat reduced permeabilities, but these must be sufficient to sustain continuous parasite growth. The slower growth rate of the blasticidin S mutant is consistent with a fitness cost associated with reducing nutrient permeability. As such, our studies suggest that a balance is struck between reducing toxin uptake and maintaining uptake of essential solutes. Consistent with this hypothesis, we found that a mere doubling of either blasticidin S or leupeptin concentration was sufficient to kill the corresponding resistant isolate. More extreme mutants, such as mutations or changes that produce functional ‘knockouts’ of the channel, might have conferred significantly greater levels of toxin resistance, but have not been generated despite multiple attempts. This observation suggests an essential role for the channel; that role can apparently be served adequately by the mutant channels on each resistant isolate.
What is the experimental evidence for this model of resistance? Blasticidin S and leupeptin are bulky organic solutes (>420 Da each) that carry net positive charge; as a consequence, their toxicities against many cell types are limited by their poor membrane permeability [60–63]. We confirmed this experimentally in the human erythrocyte system with a semiquantitative leupeptin uptake assay [56]. This assay revealed that uninfected human erythrocytes have a low leupeptin permeability that increases significantly after infection with P. falciparum. This increase was shown to be PSAC mediated because it was largely abrogated by 2-butyl-5-imino-6-([5-{4-nitrophenyl}-2-furyl]methylene)-5,6-dihydro-7H-(1,3,4)thiadiazolo(3,2-a)pyrimidin-7-one (NPF-1), a specific PSAC inhibitor. These experiments, when performed with HB3-leuR1, revealed that leupeptin permeability is significantly reduced (p = 2 × 10−5), providing compelling evidence for the model in Figure 2B. Although direct blasticidin S uptake measurements have not been carried out to confirm this mechanism for the FCB-br1 mutant, we are currently working on a protein translation assay that may further support this model.
The proposed model is also supported by an independent set of experiments that examined parasite growth inhibition by these toxins in the presence of PSAC inhibitors. If PSAC is involved in uptake of these toxins, specific PSAC inhibitors should reduce toxin uptake and thereby increase the toxin’s IC50 value for parasite killing. This experiment is complicated by the fact that PSAC inhibitors also kill parasites. Nevertheless, it is possible to test this expectation with isobologram analysis of parasite killing by different mixtures of the toxin with a known PSAC inhibitor. These studies determined that leupeptin toxicity is significantly reduced by addition of the PSAC inhibitor NPF-1. Similar results have also been obtained with blasticidin S, suggesting that it too enters infected cells via PSAC [Desai S, Unpublished Data].
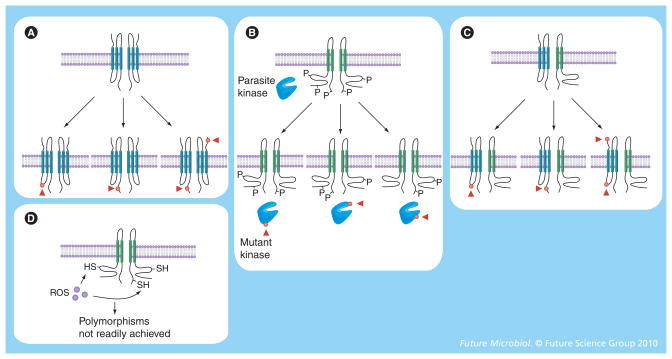
The generation of distinct PSAC mutants through in vitro selection with antiplasmodial toxins has several implications for our understanding of parasite biology. First, it contributes to the debate about whether these channels are parasite encoded or modified human proteins (Figure 3). These mutants support the parasite-encoded channel hypothesis as the mutant parasites can be passaged through different donors’ red cells without loss of the altered phenotype. If the channel is parasite encoded, the mutant phenotype can be conservatively attributed to one or more single-nucleotide polymorphisms (SNPs) in the channel gene(s) (red dots and arrowheads in bottom row of channels, Figure 3A). SNPs may also account for differences in PSAC behavior detected through comparisons of divergent laboratory isolates [15,64].
Figure 3. Possible mechanisms for functional polymorphisms in plasmodial surface anion channel activity.
In each panel, a ‘wild-type’ channel is shown in the top bilayer, while mutant or polymorphic channels are shown on the lower membrane. Parasite-encoded channel subunits or enzymes are shown in blue, while human proteins are shown in green. Polymorphic residues are shown as red dots (plus arrowheads) on the channel protein or on modifying enzymes and can arise only on parasite-encoded proteins. (A) A parasite-encoded ion channel model can accrue multiple distinct single-nucleotide polymorphisms and yield a spectrum of channel phenotypes. (B) A human ion channel activated by a specific parasite enzyme (e.g., a kinase that phosphorylates multiple sites on the channel protein) can produce distinct patterns of activation if mutations in the enzyme yield differing affinities for the sites on the human target protein. (C) An ion channel consisting of both parasite and human subunits can accrue mutations via single-nucleotide polymorphisms in the parasite subunit. (D) Nonspecific activation of a human channel (e.g., via the action of ROS) cannot easily yield heritable changes in channel activity.
P: Site of phosphorylation; ROS: Reactive oxygen species; SH: A thiol that can be oxidized to a disulfide, presented as an example target of ROS generated by parasite oxidative stress.
Heritable changes in infection-induced channel activity may also be consistent with the modified human channel hypothesis, although there are some important caveats. One scenario is that activation of human channel(s) is through the direct action of one or more parasite-encoded enzymes (Figure 3B). For example, a parasite-encoded kinase may activate a quiescent human channel, as has been reported in a number of other systems. We envision that activation of the wild-type channel would require phosphorylation at multiple sites on the human protein (represented as ‘P’, Figure 3B). This may allow selection of channel mutants through SNPs in the putative parasite kinase that affect its enzymatic specificity and yield new permutations of phosphorylations on the target human protein (Figure 3B, bottom channels). Enzymatic activation permits differing channel activities to be heritable because changes in the parasite kinase gene accrued during in vitro selection of mutants can be passed to progeny parasites. This model can also account for functional polymorphisms in PSAC activity between laboratory isolates. Whether a kinase or some other type of parasite enzyme mediates this activation, this model is somewhat less appealing than a parasite-encoded channel because it requires action at multiple sites on the human channel and the ability to modify the relative affinities for these sites through SNPs in the parasite enzyme.
Figure 3C shows another model that can account for heritable differences in channel activity, although there are presently no data to support this model. Here, the channel is made of at least two subunits, one contributed by the parasite and one from the host. In this model, SNPs in the parasite subunit could produce functional polymorphisms in PSAC activity as observed. It is difficult to imagine how nonenzymatic activation of channels consisting of only human subunits (e.g., parasite-induced oxidative stress [65]) could produce heritable changes in channel activity even if there are multiple sites of action on the protein. This difference between nonenzymatic and enzymatic activation of channels results from the biochemical observation that nonspecific soluble modulators generally cannot alter their relative effects on multiple target sites to produce distinct and heritable patterns of activation (Figure 3D).
In vitro selection of permeability mutants also contributes to the debate on the number of distinct ion channels required to mediate the uptake of various solutes [66]. While our early studies supported a single-channel model by demonstrating concordant dose responses for inhibition of each solute’s uptake by furosemide, phloridzin and dantrolene [15,16,18], these findings were not universally compelling because these inhibitors may be nonspecific. More specific inhibitors have been identified and also produce quantitatively parallel effects on organic solute uptake and single PSAC patch-clamp recordings [Desai S, Manuscript Submitted], increasing confidence in this line of reasoning. The recently identified channel mutants provide independent evidence for a single-channel model. Notably, we determined that the cloned FCB-br1 mutant exhibits reduced permeability of not only Cl−, but also of sorbitol, proline, phenyltrimethylammonium (an organic cation) and an N-hydroxysuccinimide ester [55]. Such universal changes in solute permeability would not be expected with distinct channels for uptake of separate solutes and, therefore, support a single, shared ion channel.
Another important implication of these mutants is that they represent a new antimalarial drug resistance mechanism based on reduced passive uptake at the erythrocyte membrane. Previously known mechanisms include active extrusion of the drug from its site of action [67,68], mutations in the target gene [69,70] and upregulated expression of the target to compensate for inhibition by the drug [71–73]. The addition of a new resistance mechanism is discouraging for groups developing new antimalarial drugs. However, it is possible to predict which anti-malarial compounds are vulnerable to resistance via changes in PSAC. Only compounds whose uptake at the erythrocyte membrane is primarily PSAC mediated are susceptible; compounds that enter via diffusion through lipid membranes are not. While drug uptake has historically been assumed to involve movement through the lipid phase, evidence supporting channel- or transporter-mediated uptake is accumulating rapidly in other systems [74]. One approach to determining if a compound enters infected cells via PSAC would be to synthesize a radiolabeled derivative and examine uptake in the absence and presence of PSAC inhibitors; unfortunately, such syntheses are often not feasible or may be very costly. An alternative approach for antimalarial compounds having in vitro activity is to determine if the compound’s IC50 increases in the presence of a known PSAC inhibitor. If it does, as described above for blasticidin S and leupeptin, then our findings suggest resistance via this mechanism is possible. Large-scale administration to malaria patients may indeed select for additional novel PSAC mutants in the clinical setting.
Technical issues & limitations of erythrocyte patch clamp
Many of the advances in our mechanistic understanding of the parasite-induced changes in erythrocyte permeability have resulted from the addition of patch-clamp methods to our armamentarium. Soon after patch clamp was first used to detect single ion channel molecules [11], it was recognized that the various configurations of this method together provide kinetic and biophysical insights into the inner workings of ion channel molecules simply not possible with other methods. For developing patch clamp as now used by countless laboratories, Erwin Neher and Bert Sakmann were awarded the 1991 Nobel Prize in Physiology or Medicine.
As with any other basic research method, there are technical issues that must be considered in the design and interpretation of patch-clamp experiments. Some are especially important for studies of human erythrocytes, which are small, deformable cells with special membrane properties that adversely affect the reproducibility and reliability of acquired recordings. Varying perspectives on the importance of these technical issues may be a major contributor to the discrepant results obtained in studies of uninfected and P. falciparum-infected erythrocytes. We review these technical issues here, attempting to make these issues accessible to nonelectrophysiologists.
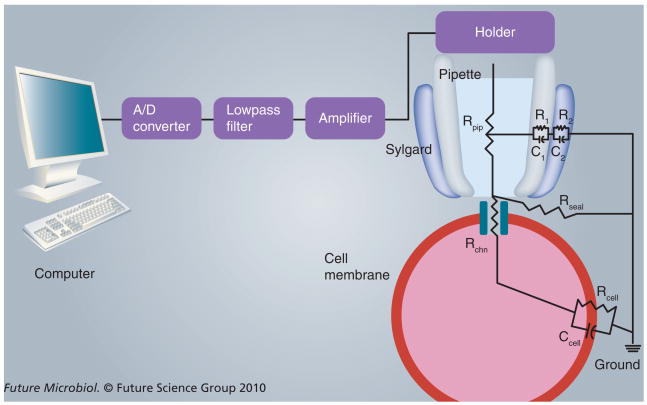
One of the most important concepts in patch clamp is the recognition that all recordings contain both a ‘signal’ of interest (i.e., the channel being recorded) and ‘noise’ from various sources. Distinguishing between these components is critical for evaluation of recordings; such analyses are often summarized with a single statistic known as the signal-to-noise ratio. Faithful reproduction of the signal and minimization of noise are the most important goals of good patch-clamp design. Proper design can be achieved and quantitatively assessed through consideration of the equivalent electrical circuit for the patch-clamp instrumentation and cell under study. A simplified equivalent circuit for a cell-attached recording that emphasizes key sites where major errors can arise is shown in Figure 4. The major elements, with an eye toward the types of interventions that yield improved signal-to-noise ratios, will be discussed in this section.
Figure 4. Key instrumentation in a patch-clamp set-up and an equivalent circuit for a pipette sealed on a cell membrane patch containing a single ion channel molecule.
While a gap is shown between the pipette tip and the cell membrane to show the effect of finite Rseal, useful patch-clamp recordings require isolation of the membrane patch under the annulus of the pipette through covalent interactions between the pipette tip and cell surface. See the text for discussion of each component in this drawing.
A/D: Analog-to-digital; Ccell: Equivalent total capacitance of the cell membrane; Rcell: Equivalent total resistance of the cell membrane; Rchn: Resistance of a single channel molecule; Rpip: Pipette resistance; Rseal: Seal resistance.
The patch-clamp amplifier detects the small current resulting from ion movement across the biological membrane under study; it then amplifies this current into a signal that can be recognized by other instrumentation and eventually visualized and stored on a computer. If a single PSAC molecule is being studied, the current that must be detected and captured is approximately 109-fold smaller than the current flowing through an illuminated 60 W light bulb. Modern amplifiers are sophisticated instruments with multiple stages of amplification designed to introduce as little noise into the recording as possible; we use the Axopatch 200B amplifier (Molecular Devices, CA, USA) because it achieves the lowest open-circuit noise amongst the major commercial models with root mean squared (rms) values of less than 60 fA at 5-kHz bandwidth. As our cell-attached recordings on infected erythrocytes often achieve total noise as low as 120 fA rms, the amplifier alone may contribute as much as half of all the noise in our recordings. Future improvements in amplifier design should permit some improvement in recording quality.
A second instrument, the analog-to-digital (A/D) converter, converts the continuous signal coming from the amplifier into discrete digital values, as required for input into modern computers and other storage devices. Two critical factors here are the converter resolution, measured in bits, and the data acquisition software’s sampling rate because these together determine how faithfully the digital signal reproduces the time-variant ion channel currents. 16-bit converters have become affordable for most laboratories and should be used for all patch-clamp recordings. Since data storage space on computer hard drives has become inexpensive, we generally set the sampling rate to 10 μs (100 kHz). The Nyquist sampling theorem states that the sampling frequency should be at least three- to five-times the rate of the highest desired frequency in the time-variant signal being recorded. 100 kHz is well beyond this threshold; such ‘oversampling’ may allow for extraction of additional information in downstream analyses.
The amplifier, converter and other instrumentation are susceptible to introduction of noise. Much of this noise results from electrical noise (typically 60 Hz noise in the USA) that bleeds into the signal from the laboratory power supply, which often incurs sags and surges from power-hungry appliances such as centrifuges and freezers. To minimize this source of noise, we use an isolated power supply (Symmetra® RM; APC, RI, USA) that exclusively powers critical patch-clamp instrumentation. We also use halogen lighting in our patch-clamp rooms because fluorescent tube lights generate significant 60 Hz noise and can contaminate the signal. Our patch-clamp rooms are small cubicles designed for a single patch-clamp rig, further reducing both mechanical and electrical noise.
The next major site in the patch-clamp circuit is the patch pipette and holder, which can introduce noise into the recordings through multiple mechanisms as described in greater detail by Levis and Rae [75]. The holder can behave as an antenna and pick up 60 Hz noise; we find the HL-U holder (Axon Instruments, CA, USA) adequate as long as it is kept clean by washing in ethanol on a regular basis. The patch-clamp pipette is filled with a salt solution and connected to this holder, where an Ag–AgCl wire transduces the ionic current in the aqueous solution into an electron-based current recognized by the amplifier. The Ag–AgCl junction should be checked regularly and anodized to maintain a uniform oxidized coating.
For infected erythrocyte studies, we find that the optimal patch pipette geometry balances a number of competing factors. A very small opening at the tip (100–200 nm diameter, considered excessively small for other cell types) is desirable because it permits the highest possible seal resistances (Rseal, discussed below); however, it is often difficult to fill these narrow inner geometry pipettes with recording solution as air bubbles are easily retained. Small inside diameters also tend to increase pipette resistance (Rpip), which introduces noise into the recordings and also compromises the signal through voltage-divider considerations. We have found that hypertonic pipette solutions, as discussed below, significantly reduce Rpip. Filling of these small geometry pipettes is a two-step process: we first fill the tip with saline under large negative pressures and then backfill with a narrow bore syringe prepared from a 1-ml tuberculin syringe by melting. With practice, even our small-tipped pipettes can be routinely filled without introduction of air bubbles. Submersion of the patch-pipette into the bath solution, as required to access the cells being studied, also introduces noise into the recording because of capacitive transfer across the patch pipette wall. As described previously, we fabricate our pipettes from quartz, which has a lower dissipation factor than borosilicate glass used in most laboratories [75]. As quartz does not melt below 1600°C, switching to quartz requires investment in a laser-based pipette puller (P2000, Sutter Instruments, CA, USA), but our experience suggests that this is worthwhile. After the pipette is pulled, we coat the pipette as close to the tip as possible with the silicone polymer Sylgard® 184 (Dow Corning, MI, USA) to further reduce capacitive noise and to minimize wetting of the outside of the pipette. The thin film of saline on the pipette exterior leads to a cable-like resistor-capacitor circuit to ground and can, without the Sylgard coating, introduce significant amounts of noise (circuit elements R1, C1, R2 and C2 in Figure 4). Despite these various precautions, we find it essential to raise the pipette with the attached cell after seal formation to minimize this unwanted circuit route; we raise the pipette tip to as near the air–saline interface as possible without loss of the seal.
The next major element in the circuit is Rseal, which describes how well the pipette tip is sealed on the surface of the biological membrane. Although it has been nearly 30 years since the first gigaohm seals were formed [13], the molecular basis of high-resistance seals between glass and membrane remain poorly understood. We know that there must be covalent bonding at this interface because calculations based on the geometry of the pipette tip annulus, area of contact with the underlying lipid bilayer and the resistivity of the bathing solution indicate that the gap between the pipette and the cell is only angstroms wide with a good seal. Recognition that seal formation requires a ‘chemical reaction’ makes some of the precautions required for gigaseal formation seem more rational. We have found that it is important to use pipettes immediately after fabrication, filter all solutions to remove debris, apply positive pressure to the pipette as it crosses the air–water interface and use each pipette only once. In addition, we insist on raising the cell off the dish bottom as the seal is forming because this reduces mechanical vibrations and facilitates seal improvement. With practice, we routinely obtain seal resistances over 100 GΩ, as generally required for unambiguous detection of PSAC without spurious seal breakdown [15]. Values approaching 1 TΩ have been obtained on human erythrocytes. Some workers have argued that such high seal resistances may create a selection bias and prevent detection of other channels present on infected erythrocytes. Such arguments are baseless when one recognizes that the seal forms between pipette and lipid and that channel molecules are much smaller than the area of the membrane patch under study. (Hypothetical channels that never close may be incorrectly attributed to leaks and therefore rejected by an insistence on high seal resistances, but such channels cannot be gainfully studied with cell-attached patch clamp at any value of Rseal.) Instead, workers should strive to obtain as high a seal resistance as possible because this improves detection of all ion channels.
The ion channel molecule in Figure 4 is represented as a time-varying resistor, Rchn. When the channel is closed, Rchn may be infinite; when open, it is limited by the rate of ion flux through the channel. Faithful measurement of Rchn as a function of time, applied voltage and modulators (e.g., inhibitors) added to the recording solution is the primary goal of patch-clamp experiments. Since PSAC has a low Cl− ion throughput rate, we have found it necessary to use hypertonic Cl− salt solutions to increase the signal in our studies [14,15]. Use of hypertonic pipette solutions also reduces noise in the recordings by lowering Rpip.
Our use of hypertonic solutions to study transport on intact cells has been criticized as nonphysiological; we agree that the physiological relevance of any patch-clamp recording needs to be critically considered. To examine possible effects of nonphysiological solutions on the channel, we performed patch clamp in solutions with osmolarities between 300 and approximately 4000 mOsm/l by adding increasing concentrations of choline chloride to a buffered isotonic NaCl solution. These studies determined that the transport rate increases as the solution osmolarity increases, but that PSAC’s gating and voltage dependence are not measurably affected [15]. The channel’s pharmacology is also not affected by raising the salt concentration because the dose response for inhibition by furosemide, phloridzin and dantrolene are quantitatively similar in molar salt solution and in more physiological solutions [15,16,18]. Moreover, whole-cell measurements of PSAC activity can be reliably made in physiological saline because this configuration sums the current flowing through a population of channel molecules present on the cell surface. These whole-cell currents have voltage-dependence and gating power spectra indistinguishable from our single-channel recordings in molar salt solutions, further allaying concerns regarding the hypertonic conditions.
Another important consideration for patch-clamp of human erythrocytes is perfusion of the bath solution around a clamped erythrocyte. Our studies demonstrate that perfusion conditions tolerated by other cell types create unacceptable mechanical stress on the seal with an erythrocyte. This stress reduces Rseal, creating leak currents that may be erroneously attributed to channel activity. This is especially problematic with the whole-cell patch-clamp configuration, where it is difficult to track changes in Rseal over the course of an experiment. To examine the basis of these perfusion-induced errors, we recorded video files of clamped erythrocytes and found that even slow rates of perfusion under standard conditions cause the cell to flail around the pipette tip, creating substantial mechanical shearing of contact area between the pipette and cell. These movie files are available as supplementary files in [19]; they show that perfusion artifacts may result from the smaller size and greater deformability of human erythrocytes. Nevertheless, it is very useful to be able to change the bath solution around a clamped cell. For example, perfusion allows one to apply increasing concentrations of an inhibitor and determine its dose response on a single cell. To address this need, a slower-than-gravity perfusion system has been developed; this system utilizes a modified chamber design that permits rapid solution changes around a small cell without turbulence [19].
Attention to these multiple technical issues is critical for obtaining reliable and reproducible recordings. When these guidelines are followed, it is possible to obtain single-channel recordings on infected erythrocytes that correlate with macroscopic transport assays (e.g., osmotic fragility and tracer flux measurements for organic solutes). Systematic molecular characterizations of mutant PSAC activities and of variations in channel behavior between geographically divergent isolates have also been possible only because of rigorous standards for patch clamp [15,55,56,64].
In our own work, we have insisted as much as possible on independent corroboration of patch-clamp results with other transport assays, which have included a quantitative osmotic lysis assay and tracer flux [76]. The channel mutants are an ideal example of this strategy because parasites carrying these mutant channels have demonstrated changes in both organic and inorganic solute permeabilities, which have been quantitatively examined with multiple independent transport assays.
Another potential problem with patch-clamp studies is that large amounts of data can be collected in a short amount of time. For example, our data-acquisition rate corresponds to 100,000 data points for every second of recording. Since recordings on a single cell can be made for 1 h or more and because it is not always obvious which recordings or parts of a recording are of high or low quality, it is possible to bias one’s analysis of the data unknowingly. To avoid such biases, we have incorporated population analyses of single-channel recordings by developing scripts that calculate dwell-time distributions, all points histograms, power spectral analyses and other quantitative measures [16,77]. Such an approach has allowed us to include hundreds of seconds of single-channel recording in analyses of inhibitor studies. We have also used this approach to identify individual traces that match the population mean; publication of these ‘average’ traces has helped prevent selection biases in our studies.
In the absence of a standardized experimental protocol, it may help if workers agree on the reporting of key experimental parameters necessary for the full evaluation of data presented in manuscripts. We suggest that each study should, at a minimum, itemize:
Amplifier and A/D converter make and model
Glass composition from which patch pipettes are fabricated
Data sampling rate and filter frequencies
Seal resistances and other measures of recording durability
Solution compositions and whether perfusion has been used
Statistical analysis of reproducibility
Inclusion of these parameters in manuscripts will permit consideration of signal quality and sources of noise as described above.
Conclusion
The increased permeability of erythrocytes infected with malaria parasites is an important example of how intracellular parasites modify their host cells. Electrophysiological methods have revealed that these changes result from the action of ion channels induced by the parasite, but important questions remain to be answered. One particular ion channel, PSAC, appears to mediate the transport of both organic and inorganic solutes based on patch-clamp detection on erythrocytes infected with plasmodia and absence from uninfected cells and from cells infected with B. divergens, quantitatively concordant pharmacology for inhibition of each solute’s uptake, marked changes in PSAC activity that parallel changes in organic solute permeability after selection of mutants resistant to blasticidin S or leupeptin, and parasite isolate-specific differences in channel properties that can be detected in transport assays that examine flux of distinct solutes [64]. Our studies to date suggest that PSAC is parasite encoded; evidence for unrelated ion channels from other groups instead supports upregulation of human ion channels.
In either case, conservation of the activity on other plasmodial species, in vitro growth inhibition by available inhibitors and the apparent inability of stringent selection conditions to generate complete loss-of-function mutants all suggest that the channel serves an essential role for the intracellular parasite. However, the exact role is not yet well established. Recently generated channel mutants suggest that PSAC may also mediate uptake of bulky antimalarial agents. This may relax concerns about membrane permeability of drug leads acting against intracellular parasite activities and be, at one level, desired by drug discovery programs. However, the ability of malaria parasites to acquire resistance to such agents by altering the channel’s selectivity for permeating solutes suggests this is a dangerous approach. Thus, the increased permeability of infected cells may be both an important drug target and a central site for acquired resistance to some antimalarials.
Future perspective
Fundamental questions to be addressed in the coming years include: what is the genetic basis of PSAC and other ion channels proposed for the infected erythrocyte membrane? How are the encoded proteins trafficked to and activated in the erythrocyte membrane? Which channel(s) are responsible for the increased permeability of inorganic and organic solutes as known for decades through macroscopic transport assays? If a single ion channel mediates these permeability changes, what is the molecular and structural basis of the channel’s unusual ability to distinguish between similar solutes? How does it mediate the uptake of diverse solutes but maintain very low permeability to Na+? Do these channels serve an essential role or roles for the intracellular parasite? If so, what are these roles? Can these channels serve as antimalarial targets for drug and/or vaccine development?
Systematic but incremental progress is being made towards answering these questions. We expect that the next decade of research in this field will provide conclusive answers to these questions even though each is intensely debated at present. An important question is whether the channels are parasite encoded or host derived. Identification of functional polymorphisms between laboratory isolates and generation of mutants through in vitro selection suggest that PSAC is parasite encoded; at the same time, detection of essentially identical activity on uninfected erythrocytes suggests that the other proposed channels are human channels, possibly upregulated by the intracellular parasite. It seems that definitive resolution of this fundamental debate will only be possible when the responsible gene(s) are identified.
We believe that molecular and genetic approaches hold the greatest promise for identification of these elusive genes. For example, molecular biological studies with the newly identified channel mutants represent a new direction with conceptual advantages over brute-force biochemical approaches. DNA and RNA microarray experiments with these mutants and their closely related wild-type parental isolates are being aggressively pursued in our laboratory. These have the potential to identify mutations in the channel gene that account for the functional changes detected in transport studies and mediate in vitro toxin resistance. We are also using next-generation whole-genome sequencing as another powerful tool to characterize the molecular basis of altered permeability [78]. Classical genetic approaches based on functional polymorphisms in transport activity induced by separate laboratory isolates are also being used and may also contribute significantly [64]. Given the various complexities we and others have identified for these transport changes, definitive identification of the channel’s gene(s) is likely to require a convergence of these and other approaches.
When strong candidate genes are identified, a number of new directions will become available to help address the fundamental questions listed at the start of this section. Biochemical studies with specific antibodies will be necessary to localize candidate proteins to the host erythrocyte membrane and to map the membrane topology; these antibodies may also be used in immunoprecipitation experiments to identify interacting proteins as possible channel subunits or accessory proteins. DNA transfection experiments can also be initiated when a candidate gene is identified. These experiments can determine whether molecular knockouts are viable and permit creation of site-directed mutants that shed light on how the channel is trafficked to the host membrane. Heterologous expression of the candidate gene(s) will also be necessary; functional reconstitution of the activity in a system amenable to rigorous transport studies (e.g., Xenopus oocytes or baculovirus-transfected insect cells) would definitively implicate the gene(s) in encoding PSAC activity.
This field is ideally suited to build from early observations made with traditional transport methods to detailed molecular mechanisms that can be tested with state-of-the-art methods ranging from electrophysiology to molecular and chemical biology. The addition of new tools makes this is an exciting time for membrane transport studies in malaria parasites.
Executive summary
Broad range of solutes with increased permeability identified with classical transport methods
Plasmodium-infected erythrocytes have increased permeability to sugars, amino acids, purines, some vitamins, organic and inorganic anions, and some monovalent cations.
A number of nonspecific and specific inhibitors have been identified.
Molecular mechanism revealed with patch-clamp studies
Cell-attached and whole-cell patch-clamp configurations can provide detailed mechanistic insights that are not possible with macroscopic transport measurements.
Our studies suggest the plasmodial surface anion channel (PSAC) is the predominant conductive pathway in the infected erythrocyte membrane.
Pharmacological studies provided quantitative correlations between single-channel patch clamp and macroscopic organic solute transport, implicating a single shared ion channel.
Unusual functional properties of PSAC
Na+ has very low permeability in spite of high permeability of larger solutes that may be charged or uncharged.
The channel is able to distinguish between structurally similar solutes.
The channel shows surprisingly low single-channel conductance.
A number of other channels have been reported on infected erythrocytes
These channels are presumed to be human channels modified and/or upregulated by the parasite.
Two main hypotheses exist: a single parasite-encoded channel shared by diverse solutes or parasite-induced activation of multiple human channels with distinct solute preferences.
Physiological role of these channels remains unknown
The leading hypothesis is a role in the sequential uptake of nutrients, which must cross three membranes to reach the intracellular parasite.
Other proposals include volume regulation, altered ionic composition of erythrocyte cytosol, apoptosis and cell-cycle regulation.
Two novel PSAC mutants & their implications for parasite biology
PSAC mutants were generated by in vitro selection with blasticidin S or leupeptin, charged, bulky toxins that resemble solutes with known PSAC permeability.
These mutants exhibit markedly altered single-channel properties, pharmacology and organic solute permeability.
Mutants appear to resist toxin-mediated killing by reducing toxin uptake while maintaining sufficient nutrient uptake. This is supported by leupeptin permeability measurements and by effects of PSAC inhibitors on toxin IC50 values.
Mutants provide evidence for a parasite-encoded channel, but other explanations are possible.
Mutants also support a single, shared ion channel because each mutant exhibits parallel changes in the uptake of diverse solutes.
Permeability mutants represent a novel antimalarial drug resistance mechanism in Plasmodium falciparum based on reduced passive uptake at the erythrocyte membrane. An assay for evaluating susceptibility to this resistance mechanism has been developed.
Technical issues in patch clamp of human erythrocytes
Small size, deformability and unusual membrane properties make erythrocytes more difficult to patch.
Careful attention to a number of experimental conditions is essential for reproducibility and analysis of patch-clamp data.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This research was funded by the Intramural Research Program of the NIH, NIAID. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
David A Hill, Email: davhill@mail.med.upenn.edu, University of Pennsylvania, Department of Pathobiology, Philadelphia, PA, USA, Tel.: +1 215 898 6268, Fax: +1 215 746 2295.
Sanjay A Desai, Email: sdesai@niaid.nih.gov, Laboratory of Malaria & Vector Research, NIAID/NIH, Room 3W-01, 12735 Twinbrook Parkway, Rockville, MD 20852-8132, USA, Tel.: +1 301 435 7552, Fax: +1 301 402 2201.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434(7030):214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallup JL, Sachs JD. The economic burden of malaria. Am J Trop Med Hyg. 2001;64(1–2):85–96. doi: 10.4269/ajtmh.2001.64.85. [DOI] [PubMed] [Google Scholar]
- 3.Fegan GW, Noor AM, Akhwale WS, Cousens S, Snow RW. Effect of expanded insecticide-treated bednet coverage on child survival in rural Kenya: a longitudinal study. Lancet. 2007;370(9592):1035–1039. doi: 10.1016/S0140-6736(07)61477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hastings IM, Korenromp EL, Bloland PB. The anatomy of a malaria disaster: drug policy choice and mortality in African children. Lancet Infect Dis. 2007;7(11):739–748. doi: 10.1016/S1473-3099(07)70214-1. [DOI] [PubMed] [Google Scholar]
- 5.Overman RR. Reversible cellular permeability alterations in disease In vivo studies on sodium, potassium and chloride concentrations in erythrocytes of the malarious monkey. Am J Physiol. 1948;152:113–121. doi: 10.1152/ajplegacy.1947.152.1.113. [DOI] [PubMed] [Google Scholar]
- 6.Ginsburg H, Kutner S, Krugliak M, Cabantchik ZI. Characterization of permeation pathways appearing in the host membrane of Plasmodium falciparum infected red blood cells. Mol Biochem Parasitol. 1985;14(3):313–322. doi: 10.1016/0166-6851(85)90059-3. [DOI] [PubMed] [Google Scholar]
- 7.Homewood CA, Neame KD. Malaria and the permeability of the host erythrocyte. Nature. 1974;252(5485):718–719. doi: 10.1038/252718a0. [DOI] [PubMed] [Google Scholar]
- 8.Kirk K, Horner HA, Elford BC, Ellory JC, Newbold CI. Transport of diverse substrates into malaria-infected erythrocytes via a pathway showing functional characteristics of a chloride channel. J Biol Chem. 1994;269(5):3339–3347. [PubMed] [Google Scholar]
- 9.Kutner S, Breuer WV, Ginsburg H, Cabantchik ZI. On the mode of action of phlorizin as an antimalarial agent in in vitro cultures of Plasmodium falciparum. Biochem Pharmacol. 1987;36(1):123–129. doi: 10.1016/0006-2952(87)90389-3. [DOI] [PubMed] [Google Scholar]
- 10.Kirk K, Horner HA, Spillett DJ, Elford BC. Glibenclamide and meglitinide block the transport of low molecular weight solutes into malaria-infected erythrocytes. FEBS Lett. 1993;323(1–2):123–128. doi: 10.1016/0014-5793(93)81462-9. [DOI] [PubMed] [Google Scholar]
- 11▪.Neher E, Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976;260(5554):799–802. doi: 10.1038/260799a0. First use of patch-clamp methods to detect currents through single ion channel molecules. [DOI] [PubMed] [Google Scholar]
- 12.Neher E, Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci USA. 1982;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13▪▪.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391(2):85–100. doi: 10.1007/BF00656997. Important for understanding and implementing the various patch-clamp configurations. [DOI] [PubMed] [Google Scholar]
- 14▪▪.Desai SA, Bezrukov SM, Zimmerberg J. A voltage-dependent channel involved in nutrient uptake by red blood cells infected with the malaria parasite. Nature. 2000;406(6799):1001–1005. doi: 10.1038/35023000. First use of patch clamp to explore increased erythrocyte permeability after infection with malaria parasites. [DOI] [PubMed] [Google Scholar]
- 15.Alkhalil A, Cohn JV, Wagner MA, Cabrera JS, Rajapandi T, Desai SA. Plasmodium falciparum likely encodes the principal anion channel on infected human erythrocytes. Blood. 2004;104:4279–4286. doi: 10.1182/blood-2004-05-2047. [DOI] [PubMed] [Google Scholar]
- 16.Desai SA, Alkhalil A, Kang M, Ashfaq U, Nguyen ML. PSAC-independent phloridzin resistance in Plasmodium falciparum. J Biol Chem. 2005;280(17):16861–16867. doi: 10.1074/jbc.M414629200. [DOI] [PubMed] [Google Scholar]
- 17.Kang M, Lisk G, Hollingworth S, Baylor SM, Desai SA. Malaria parasites are rapidly killed by dantrolene derivatives specific for the plasmodial surface anion channel. Mol Pharmacol. 2005;68(1):34–40. doi: 10.1124/mol.104.010553. [DOI] [PubMed] [Google Scholar]
- 18.Lisk G, Kang M, Cohn JV, Desai SA. Specific inhibition of the plasmodial surface anion channel by dantrolene. Eukaryot Cell. 2006;5(11):1882–1893. doi: 10.1128/EC.00212-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lisk G, Desai SA. Improved perfusion conditions for patch-clamp recordings on human erythrocytes. Biochem Biophys Res Commun. 2006;347(1):158–165. doi: 10.1016/j.bbrc.2006.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alkhalil A, Hill DA, Desai SA. Babesia and plasmodia increase host erythrocyte permeability through distinct mechanisms. Cell Microbiol. 2007;9(4):851–860. doi: 10.1111/j.1462-5822.2006.00834.x. [DOI] [PubMed] [Google Scholar]
- 21.Tanabe K, Mikkelsen RB, Wallach DF. Calcium transport of Plasmodium chabaudi-infected erythrocytes. J Cell Biol. 1982;93(3):680–684. doi: 10.1083/jcb.93.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desai SA, McCleskey EW, Schlesinger PH, Krogstad DJ. A novel pathway for Ca++ entry into Plasmodium falciparum-infected blood cells. Am J Trop Med Hyg. 1996;54(5):464–470. doi: 10.4269/ajtmh.1996.54.464. [DOI] [PubMed] [Google Scholar]
- 23.Staines HM, Chang W, Ellory JC, Tiffert T, Kirk K, Lew VL. Passive Ca2+ transport and Ca2+-dependent K+ transport in Plasmodium falciparum-infected red cells. J Membr Biol. 1999;172(1):13–24. doi: 10.1007/s002329900579. [DOI] [PubMed] [Google Scholar]
- 24.Staines HM, Ellory JC, Kirk K. Perturbation of the pump-leak balance for Na+ and K+ in malaria-infected erythrocytes. Am J Physiol Cell Physiol. 2001;280(6):C1576–C1587. doi: 10.1152/ajpcell.2001.280.6.C1576. [DOI] [PubMed] [Google Scholar]
- 25.Cohn JV, Alkhalil A, Wagner MA, Rajapandi T, Desai SA. Extracellular lysines on the plasmodial surface anion channel involved in Na+ exclusion. Mol Biochem Parasitol. 2003;132(1):27–34. doi: 10.1016/j.molbiopara.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Bokhari AA, Solomon T, Desai SA. Two distinct mechanisms of transport through the plasmodial surface anion channel. J Membr Biol. 2008;226(1–3):27–34. doi: 10.1007/s00232-008-9136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rostovtseva TK, Nestorovich EM, Bezrukov SM. Partitioning of differently sized poly(ethylene glycol)s into OmpF porin. Biophys J. 2002;82(1):160–169. doi: 10.1016/S0006-3495(02)75383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phale PS, Philippsen A, Widmer C, Phale VP, Rosenbusch JP, Schirmer T. Role of charged residues at the OmpF porin channel constriction probed by mutagenesis and simulation. Biochemistry. 2001;40(21):6319–6325. doi: 10.1021/bi010046k. [DOI] [PubMed] [Google Scholar]
- 29.Christophersen P. Ca2+-activated K+ channel from human erythrocyte membranes: single channel rectification and selectivity. J Membr Biol. 1991;119(1):75–83. doi: 10.1007/BF01868542. [DOI] [PubMed] [Google Scholar]
- 30.Bouyer G, Egee S, Thomas SL. Three types of spontaneously active anionic channels in malaria-infected human red blood cells. Blood Cells Mol Dis. 2006;36(2):248–254. doi: 10.1016/j.bcmd.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Egee S, Lapaix F, Decherf G, et al. A stretch-activated anion channel is up-regulated by the malaria parasite Plasmodium falciparum. J Physiol. 2002;542(Pt 3):795–801. doi: 10.1113/jphysiol.2002.022970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouyer G, Egee S, Thomas SL. Toward a unifying model of malaria-induced channel activity. Proc Natl Acad Sci USA. 2007;104(26):11044–11049. doi: 10.1073/pnas.0704582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merckx A, Nivez MP, Bouyer G, et al. Plasmodium falciparum regulatory subunit of cAMP-dependent PKA and anion channel conductance. PLoS Pathog. 2008;4(2):e19. doi: 10.1371/journal.ppat.0040019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huber SM, Duranton C, Lang F. Patch-clamp analysis of the “new permeability pathways” in malaria-infected erythrocytes. Int Rev Cytol. 2005;246:59–134. doi: 10.1016/S0074-7696(05)46003-9. [DOI] [PubMed] [Google Scholar]
- 35.Duranton C, Huber SM, Tanneur V, et al. Organic osmolyte permeabilities of the malaria-induced anion conductances in human erythrocytes. J Gen Physiol. 2004;123(4):417–426. doi: 10.1085/jgp.200308919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huber SM, Duranton C, Henke G, et al. Plasmodium induces swelling-activated ClC-2 anion channels in the host erythrocyte. J Biol Chem. 2004;279(40):41444–41452. doi: 10.1074/jbc.M407618200. [DOI] [PubMed] [Google Scholar]
- 37.Duranton C, Huber S, Tanneur V, et al. Electrophysiological properties of the Plasmodium falciparum-induced cation conductance of human erythrocytes. Cell Physiol Biochem. 2003;13(4):189–198. doi: 10.1159/000072421. [DOI] [PubMed] [Google Scholar]
- 38.Staines HM, Ashmore S, Felgate H, Moore J, Powell T, Ellory JC. Solute transport via the new permeability pathways in Plasmodium falciparum-infected human red blood cells is not consistent with a simple single-channel model. Blood. 2006;108(9):3187–3194. doi: 10.1182/blood-2006-02-001693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ginsburg H, Stein WD. The new permeability pathways induced by the malaria parasite in the membrane of the infected erythrocyte: comparison of results using different experimental techniques. J Membr Biol. 2004;197(2):113–134. doi: 10.1007/s00232-003-0646-7. [DOI] [PubMed] [Google Scholar]
- 40.Gardner MJ, Hall N, Fung E, et al. Genome sequence of the human malaria parasite. Plasmodium falciparum Nature. 2002;419(6906):498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desai SA, Krogstad DJ, McCleskey EW. A nutrient-permeable channel on the intraerythrocytic malaria parasite. Nature. 1993;362(6421):643–646. doi: 10.1038/362643a0. [DOI] [PubMed] [Google Scholar]
- 42.Desai SA, Rosenberg RL. Pore size of the malaria parasite’s nutrient channel. Proc Natl Acad Sci USA. 1997;94(5):2045–2049. doi: 10.1073/pnas.94.5.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woodrow CJ, Penny JI, Krishna S. Intraerythrocytic Plasmodium falciparum expresses a high affinity facilitative hexose transporter. J Biol Chem. 1999;274(11):7272–7277. doi: 10.1074/jbc.274.11.7272. [DOI] [PubMed] [Google Scholar]
- 44.Elliott JL, Saliba KJ, Kirk K. Transport of lactate and pyruvate in the intraerythrocytic malaria parasite, Plasmodium falciparum. Biochem J. 2001;355(Pt 3):733–739. doi: 10.1042/bj3550733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saliba KJ, Kirk K. H+-coupled pantothenate transport in the intracellular malaria parasite. J Biol Chem. 2001;276(21):18115–18121. doi: 10.1074/jbc.M010942200. [DOI] [PubMed] [Google Scholar]
- 46.Lehane AM, Saliba KJ, Allen RJ, Kirk K. Choline uptake into the malaria parasite is energized by the membrane potential. Biochem Biophys Res Commun. 2004;320(2):311–317. doi: 10.1016/j.bbrc.2004.05.164. [DOI] [PubMed] [Google Scholar]
- 47.El BK, Zufferey R, Witola WH, Carter NS, Ullman B, Ben MC. The plasma membrane permease PfNT1 is essential for purine salvage in the human malaria parasite Plasmodium falciparum. Proc Natl Acad Sci USA. 2006;103(24):9286–9291. doi: 10.1073/pnas.0602590103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Divo AA, Geary TG, Davis NL, Jensen JB. Nutritional requirements of Plasmodium falciparum in culture I Exogenously supplied dialyzable components necessary for continuous growth. J Protozool. 1985;32(1):59–64. doi: 10.1111/j.1550-7408.1985.tb03013.x. [DOI] [PubMed] [Google Scholar]
- 49.Desai SA. Targeting ion channels of Plasmodium falciparum-infected human erythrocytes for antimalarial development. Curr Drug Targets Infect Disord. 2004;4(1):79–86. doi: 10.2174/1568005043480934. [DOI] [PubMed] [Google Scholar]
- 50.Mauritz JM, Esposito A, Ginsburg H, Kaminski CF, Tiffert T, Lew VL. The homeostasis of Plasmodium falciparum-infected red blood cells. PLoS Comput Biol. 2009;5(4):e1000339. doi: 10.1371/journal.pcbi.1000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brand VB, Sandu CD, Duranton C, et al. Dependence of Plasmodium falciparum in vitro growth on the cation permeability of the human host erythrocyte. Cell Physiol Biochem. 2003;13(6):347–356. doi: 10.1159/000075122. [DOI] [PubMed] [Google Scholar]
- 52.Saliba KJ, Martin RE, Broer A, et al. Sodium-dependent uptake of inorganic phosphate by the intracellular malaria parasite. Nature. 2006;443(7111):582–585. doi: 10.1038/nature05149. [DOI] [PubMed] [Google Scholar]
- 53.Lang F, Lang PA, Lang KS, et al. Channel-induced apoptosis of infected host cells-the case of malaria. Pflugers Arch. 2004;448(3):319–324. doi: 10.1007/s00424-004-1254-9. [DOI] [PubMed] [Google Scholar]
- 54.Lisk G, Desai SA. The plasmodial surface anion channel is functionally conserved in divergent malaria parasites. Eukaryot Cell. 2005;4(12):2153–2159. doi: 10.1128/EC.4.12.2153-2159.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55▪.Hill DA, Pillai AD, Nawaz F, et al. A blasticidin S-resistant Plasmodium falciparum mutant with a defective plasmodial surface anion channel. Proc Natl Acad Sci USA. 2007;104(3):1063–1068. doi: 10.1073/pnas.0610353104. First description of a permeability mutant in Plasmodium falciparum-infected erythrocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56▪.Lisk G, Pain M, Gluzman IY, et al. Changes in the plasmodial surface anion channel reduce leupeptin uptake and can confer drug resistance in P. falciparum-infected erythrocytes. Antimicrob Agents Chemother. 2008;52(7):2346–2354. doi: 10.1128/AAC.00057-08. Evidence for altered toxin permeability at the erythrocyte membrane as a new drug resistance mechanism in malaria parasites. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamaguchi H, Yamamoto C, Tanaka N. Inhibition of protein synthesis by blasticidin S. I. Studies with cell-free systems from bacterial and mammalian cells. J Biochem (Tokyo) 1965;57(5):667–677. [PubMed] [Google Scholar]
- 58.Aoyagi T, Takeuchi T, Matsuzaki A, Kawamura K, Kondo S. Leupeptins, new protease inhibitors from actinomycetes. J Antibiot (Tokyo) 1969;22(6):283–286. doi: 10.7164/antibiotics.22.283. [DOI] [PubMed] [Google Scholar]
- 59.Maeda K, Kawamura K, Kondo S, Aoyagi T, Takeuchi T. The structure and activity of leupeptins and related analogs. J Antibiot (Tokyo) 1971;24(6):402–404. doi: 10.7164/antibiotics.24.402. [DOI] [PubMed] [Google Scholar]
- 60.Contreras A, Carrasco L. Selective inhibition of protein synthesis in virus-infected mammalian cells. J Virol. 1979;29(1):114–122. doi: 10.1128/jvi.29.1.114-122.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iordanov MS, Pribnow D, Magun JL, et al. Ribotoxic stress response: activation of the stress-activated protein kinase JNK1 by inhibitors of the peptidyl transferase reaction and by sequence-specific RNA damage to the α-sarcin/ricin loop in the 28S rRNA. Mol Cell Biol. 1997;17(6):3373–3381. doi: 10.1128/mcb.17.6.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ishiguro J, Miyazaki M. Characterization of blasticidin S-resistant mutants of Saccharomyces cerevisiae. Curr Genet. 1985;9(2):179–181. doi: 10.1007/BF00436968. [DOI] [PubMed] [Google Scholar]
- 63.Mehdi S. Cell-penetrating inhibitors of calpain. Trends Biochem Sci. 1991;16(4):150–153. doi: 10.1016/0968-0004(91)90058-4. [DOI] [PubMed] [Google Scholar]
- 64.Alkhalil A, Pillai AD, Bokhari AA, Vaidya AB, Desai SA. Complex inheritance of the plasmodial surface anion channel in a Plasmodium falciparum genetic cross. Mol Microbiol. 2009;72(2):459–469. doi: 10.1111/j.1365-2958.2009.06661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65▪.Huber SM, Uhlemann AC, Gamper NL, Duranton C, Kremsner PG, Lang F. Plasmodium falciparum activates endogenous Cl− channels of human erythrocytes by membrane oxidation. EMBO J. 2002;21(1–2):22–30. doi: 10.1093/emboj/21.1.22. First evidence for P. falciparum activation of a human ion channel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66▪▪.Staines HM, Alkhalil A, Allen RJ, et al. Electrophysiological studies of malaria parasite-infected erythrocytes: current status. Int J Parasitol. 2007;37(5):475–482. doi: 10.1016/j.ijpara.2006.12.013. Consensus report on parasite-induced erythrocyte permeability changes co-written by the major groups in this field. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fidock DA, Nomura T, Wellems TE. Mutations in the P falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol Cell. 2000;6:861–871. doi: 10.1016/s1097-2765(05)00077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duraisingh MT, Cowman AF. Contribution of the pfmdr1 gene to antimalarial drug-resistance. Acta Tropica. 2005;94(3):181–190. doi: 10.1016/j.actatropica.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 69.Peterson DS, Walliker D, Wellems TE. Evidence that a point mutation in dihydrofolate reductase–thymidylate synthase confers resistance to pyrimethamine in falciparum malaria. Proc Natl Acad Sci USA. 1988;85(23):9114–9118. doi: 10.1073/pnas.85.23.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Triglia T, Menting JG, Wilson C, Cowman AF. Mutations in dihydropteroate synthase are responsible for sulfone and sulfonamide resistance inPlasmodium falciparum. Proc Natl Acad Sci USA. 1997;94(25):13944–13949. doi: 10.1073/pnas.94.25.13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kidgell C, Volkman SK, Daily J, et al. A systematic map of genetic variation in Plasmodium falciparum. PLoS Pathog. 2006;2(6):e57. doi: 10.1371/journal.ppat.0020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singh A, Rosenthal PJ. Selection of cysteine protease inhibitor-resistant malaria parasites is accompanied by amplification of falcipain genes and alteration in inhibitor transport. J Biol Chem. 2004;279(34):35236–35241. doi: 10.1074/jbc.M404235200. [DOI] [PubMed] [Google Scholar]
- 73.Thaithong S, Ranford-Cartwright LC, Siripoon N, et al. Plasmodium falciparum: Gene mutations and amplification of dihydrofolate reductase genes in parasites grown in vitro in presence of pyrimethamine. Exp Parasitol. 2001;98(2):59–70. doi: 10.1006/expr.2001.4618. [DOI] [PubMed] [Google Scholar]
- 74.Dobson PD, Kell DB. Carrier-mediated cellular uptake of pharmaceutical drugs: an exception or the rule? Nat Rev Drug Discov. 2008;7(3):205–220. doi: 10.1038/nrd2438. [DOI] [PubMed] [Google Scholar]
- 75▪.Levis RA, Rae JL. The use of quartz patch pipettes for low noise single channel recording. Biophys J. 1993;65(4):1666–1677. doi: 10.1016/S0006-3495(93)81224-4. Rigorous discussion of noise considerations in patch clamp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wagner MA, Andemariam B, Desai SA. A two-compartment model of osmotic lysis in Plasmodium falciparum-infected erythrocytes. Biophys J. 2003;84(1):116–123. doi: 10.1016/S0006-3495(03)74836-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Desai SA. Open and closed states of the plasmodial surface anion channel. Nanomedicine. 2005;1(1):58–66. doi: 10.1016/j.nano.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 78.MacLean D, Jones JD, Studholme DJ. Application of ‘next-generation’ sequencing technologies to microbial genetics. Nat Rev Microbiol. 2009;7(4):287–296. doi: 10.1038/nrmicro2122. [DOI] [PubMed] [Google Scholar]