Abstract
Apoptosis-inducing factor (AIF) is critical for poly(ADP-ribose) polymerase-1 (PARP-1)-dependent cell death (parthanatos). The molecular mechanism of mitochondrial AIF release to the nucleus remains obscure, although a possible role of calpain I has been suggested. Here we show that calpain is not required for mitochondrial AIF release in parthanatos. Although calpain I cleaved recombinant AIF in a cell free system, in intact cells under conditions where endogenous calpain was activated by either NMDA or MNNG administration, AIF was not cleaved, and it was released from mitochondria to the nucleus in its 62 kDa uncleaved form. Moreover, NMDA administration under conditions that failed to activate calpain still robustly induced AIF nuclear translocation. Inhibition of calpain with calpastatin or genetic knockout of the regulatory subunit of calpain failed to prevent NMDA- or MNNG-induced AIF nuclear translocation and subsequent cell death, respectively, which was markedly prevented by the PARP-1 inhibitor DPQ. Our study clearly shows that calpain activation is not required for AIF release during parthanatos, suggesting that other mechanisms rather than calpain are involved in mitochondrial AIF release in parthanatos.
Keywords: Apoptosis-inducing factor, calpain, parthanatos, poly(ADP-ribose) polymerase-1
Introduction
Apoptosis-inducing factor (AIF) is a mitochondrial protein critical for cell survival; but similar to cytochrome c, AIF is also a cell death effector. On release from the mitochondria, AIF moves to the nucleus and in a series of biochemical events, triggers DNA fragmentation and nuclear condensation resulting in cell death. AIF-mediated cell death has been implicated in a variety of pathologic insults including, glutamate excitotoxicity, stroke, trauma, cerebral hypoxia/ischemia and MPTP toxicity (Zhang et al. 1994; Eliasson et al. 1997; Endres et al. 1997; Cosi and Marien 1999; Mandir et al. 1999; Mandir et al. 2000; Plesnila et al. 2004; Wang et al. 2004; Culmsee et al. 2005). Several mechanisms for the release of AIF from mitochondria under different cell death stimuli or experimental conditions are described including caspases, calpains, capthepsins and poly(ADP-ribose) polymerase-1 (PARP-1) activation. In PARP-1-dependent cell death, AIF is the commitment point for cell death (Yu et al. 2002). This form of cell death has recently been designated parthanatos to distinguish it from other forms of cell death such as apoptotic caspase-dependent cell death and necrotic programmed cell death (Andrabi et al. 2006; Yu et al. 2006; Andrabi et al. 2008; David et al. 2009; Wang et al. 2009).
In parthanatos PARP-1 activation leads to the formation of poly(ADP-ribose) polymer (PAR) that translocates from the nucleus to the mitochondria resulting in the release of AIF and subsequent cell death (Andrabi et al. 2006; Yu et al. 2006). Neutralizing antibodies to PAR or catabolism of PAR by over-expression of poly(ADP-ribose) glycohydrolase prevents AIF translocation to the nucleus and cell death (Andrabi et al. 2006). Pharmacologic inhibition or genetic deletion of PARP-1 also prevents AIF translocation to the nucleus and cell death in a variety of experimental paradigms (Yu et al. 2002). Thus, PARP-1 activation and PAR polymer formation are essential for AIF nuclear translocation in parthanatos.
Parthanatos plays a prominent and primary role in a variety of cell injury paradigms including glutamate excitotoxicity, stroke, trauma, cerebral hypoxia/ischemia and MPTP intoxication model of Parkinson’s disease (Andrabi et al. 2008; Wang et al. 2009). Moreover, parthanatos also plays a prominent role in cellular injury outside the nervous system including ischemia/reperfusion injury, myocarditis, heart failure, circulatory shock, diabetes and diabetic complications to atherosclerosis, arthritis, and colitis (Pacher and Szabo 2008). Understanding the molecular mechanisms governing mitochondrial AIF release under different cell death stimuli or experimental conditions are important for developing new therapeutic approaches for a variety of neurologic disorders and a variety of degenerative diseases. Recently, it was reported that calpain, a calcium-dependent intracellular cysteine protease, may play an important role in postischemic neuronal cell death via proteolysis of a wide variety of substrates (Bevers and Neumar 2008). Two major isoforms of brain calpains are calpain I (μ-calpain) and calpain II (m-calpain). It was also suggested that the cleavage of AIF by calpain I is required for AIF release from mitochondria (Polster et al. 2005; Cao et al. 2007; Moubarak et al. 2007). However, it is unknown whether calpain plays a role in AIF release during parthanatos. Here we investigate and demonstrate that caplain activation is not required for the release of AIF during parthanatos.
Materials and methods
Cell culture, adenovirus transduction and cytotoxicity
Capn4 PZ/PZ, Capn 4 P/P and Capn4 P/P;R MEF cells were cultured in Dulbecco’s Modified Eagle’s Medium (Invitrogen) supplemented with 10% Fetal Bovine Serum (HyClone), 100 µg/ml streptomycin, and 100 U/ml penicillin (Invitrogen) (Tan et al. 2006). Primary neuronal cultures from cortex were prepared as described previously (Dawson et al. 1993) and neurons were transduced by adenovirus carrying calpastatin-GFP or GFP (kindly provided by Dr. Ruth S. Slack, University of Ottawa, Canada). PARP-1 dependent cell death was induced by either 500 µM N-Methyl-N'-Nitro-N-Nitrosoguanidine (MNNG) (Sigma) for 15 min or N-methyl D-aspartate (NMDA) (Sigma) as indicated. Viability was assessed 24 h after treatment by Hoechst 33342 (7 µM, for total nuclei) and propidium iodide (2 µM, for dead cell nuclei) double staining.
AIF expression and purification
Mouse cDNAs encoding 67 kDa precursor AIF (1–612 aa), 62 kDa mature AIF (54–612) and 57 kDa truncated AIF (104–612) were subcloned into pGex-6P-1 vector (GE Health Care). The proteins were expressed and purified from E.coli using glutathione-sepharose affinity purification. In some cases the GST-tag was removed by incubation with Precision protease.
Preparation of whole cell lysate and subcellular fractionation
For whole cell lysate, cortical neurons and MEFs, which were treated with either NMDA or MNNG as indicated, were lysed in modified RIPA buffer [50 mM Tris, pH 7.4, 1% Igepal, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, and a proteinase inhibitor cocktail (Roche Molecular Biochemicals)]. After centrifugation at 15,000 g for 15 min at 4°C, the whole cell lysate was collected from the resulting supernatant (Wang et al. 2007). For subcellular fractions, nuclear subcellular fractionation and post-nuclear subcellular fractionation, which includes mitochondria and cytosol, were prepared as described previously (Wang et al. 2004). The protein content of both whole cell lysate and subcellular fractions were determined by the Bradford method using BSA as the standard (Bradford 1976).
Immunoblotting
The proteins were separated on denaturing polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membrane. The membrane was blocked and incubated overnight with primary antibodies: anti-AIF (JHU, (Yu et al. 2002)), anti-manganese superoxide dismutase (MnSOD) (JHU, (Yu et al. 2002)), anti-calpastatin (Sigma), anti-spectrin (Chemicon), anti-GFP (Rockland), anti-actin (Sigma), anti-core histone (USBiological) and anti-m-calpain (Tan et al. 2006) at 4°C, followed by donkey anti-mouse, goat anti-rabbit or chicken anti-sheep IgG conjugated to HRP for 1 h at room temperature. After washing, the immune complexes were detected by the SuperSignal West Pico Chemiluminescent Substrate (Pierce).
Immunofluorescence
Cells were fixed with 4% paraformaldehyde, permeabilized with 0.05% Triton X-100 and blocked with 3% bovine serum albumin in PBS. AIF was visualized by immunofluorescence using anti-AIF antibody and Cy3 donkey anti-rabbit IgG. The nucleus was stained with 4',6-Diamidino-2-phenylindole (DAPI). Immunofluorescence analysis was carried out using a LSM510 confocal laser scanning microscope (Carl Zeiss).
In vitro calpain cleavage assay
As described previously (Polster et al. 2005), GST-tagged precursor AIF (GST-AIF67) or mature AIF (AIF62) (10 ng/µl) was incubated with 0–5 units/ml of calpain I (Calbiochem) in 20 µl of reaction buffer containing 30 mM Tris-HCl, pH 7.5, 200 µM CaCl2, and 1.5 mM dithiothreitol, in the presence or absence of EGTA as indicated, for 30 min at 30 °C. All reactions were terminated by boiling in SDS-PAGE sample buffer for 5 min. Proteins were separated by 7% SDS-PAGE and assessed by immunoblotting.
Calpain activity assay
Calpain activity was determined by the cleavage of a specific fluorescent calpain I substrate H-E(EDANS)PLF-AERK(DABCYL)-OH (Calbiochem) as described previously (Cuerrier et al. 2005; Cao et al. 2007). In brief, 30 µg whole cell lysates were incubated with 10 µM calpain substrate in the reaction buffer containing 20 mM HEPES, pH 7.6, 1 mM EDTA, 50 mM NaCl, 0.1% 2-mercaptoethanol, 5 mM CaCl2 at 37 °C for 30 min. Native calpain I from human erythrocytes (Calbiochem) with 0–5 units/ml was applied to generate the standard curve. Calpain activity was measured by detecting the increase in fluorescence using excitation/emission wavelength of 335/500 nm.
Experimental Stroke
Male C57BL/6 mice were anesthetized with isoflurane and subjected to sham surgery or 90 min of middle cerebral artery occlusion by the intraluminal filament technique (Cao et al. 2007). At 24 hours of reperfusion, the brain was harvested and nuclear and mitochondrial subcellular fractions from the middle cerebral artery region were probed for AIF by immunoblotting as described (Cao et al. 2007). These procedures were approved by the Johns Hopkins University Animal Care and Use Committee.
Statistical analysis
Statistical evaluation was carried out by Student’s t-test between two groups and by one-way analysis of variance (ANOVA) with GraphPrism software within multiple groups. Data were given as means ± SEM and p < 0.05 was considered significant.
RESULTS
AIF is cleaved by calpain I in vitro
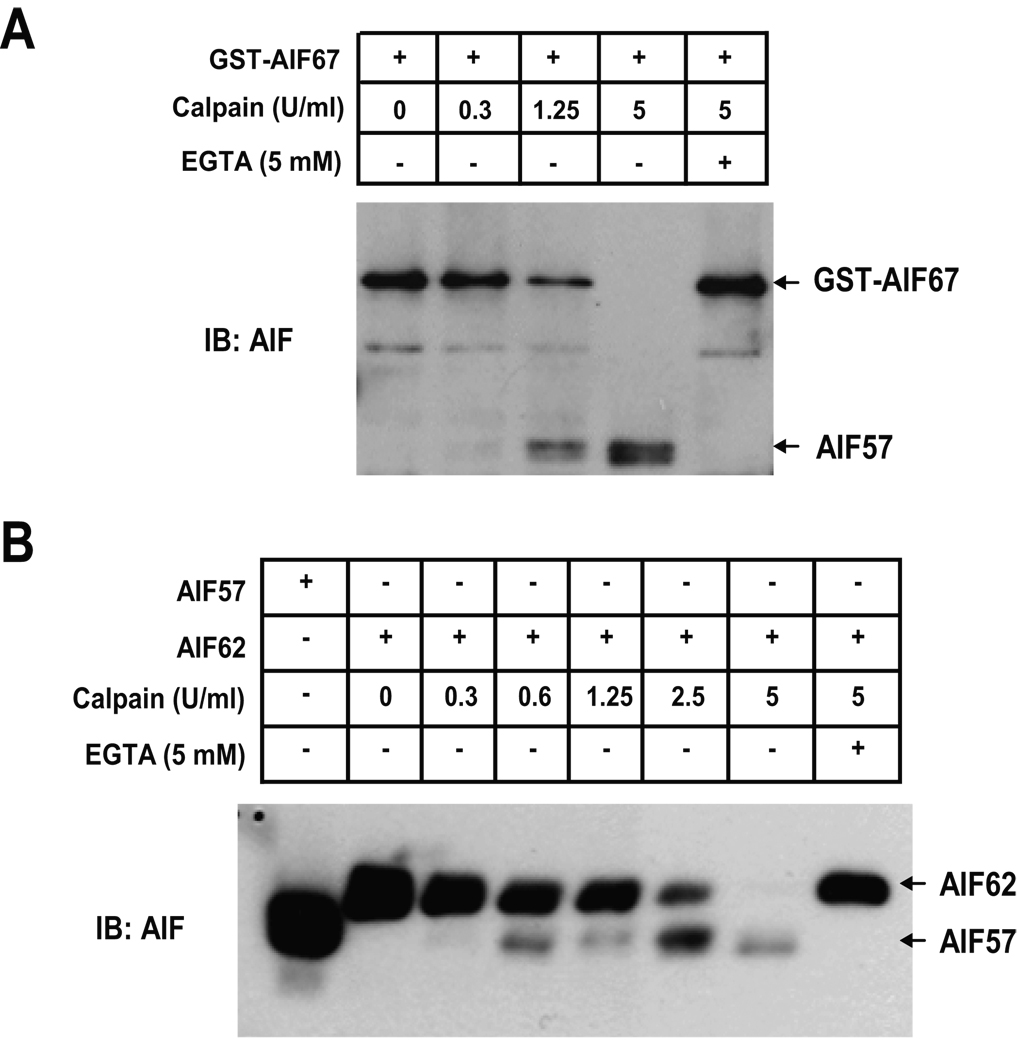
To confirm that AIF can be cleaved by calpain I, recombinant GST-tagged precursor AIF (GST-AIF67) was incubated with increasing concentrations of calpain I (0.3–5 U/ml) for 30 minutes. 1.25 U/ml of calpain I partially cleaves GST-AIF67 to a smaller 57 kDa cleavage product. 5 U/ml of calpain I completely cleaves GST-AIF67. Chelation of calcium by EGTA (5 mM), which inhibits calpain, prevents the cleavage of GST-AIF67 by calpain I (Fig. 1A). Since it was suggested that AIF is processed in cells to a mature 62 kDa (AIF62) form consisting of amino acids 54–612, the ability of calpain to cleave AIF62 was next assessed (Fig. 1B). 0.6 U/ml of calpain I partially cleaves recombinant AIF62 into AIF57. Increasing concentrations of calpain I results in increased cleavage of recombinant AIF62. 5 U/ml completely cleaves AIF62 into AIF57 (Fig. 1B). These data clearly demonstrate that calpain I is able to cleave AIF in vitro, which is identical to the observation reported previously (Polster et al. 2005).
Fig. 1. Calpain cleaves recombinant AIF in vitro.
A, GST-AIF67 (10 ng/µl) was incubated with 0–5 U/ml calpain I as indicated, in the absence or presence of 5 mM EGTA. B, Purified mature AIF62 was incubated with 0–5 U/ml calpain I as indicated, in the absence or presence of 5 mM EGTA. Purified AIF57 was used as a molecular marker. Protein was detected using the antibody against AIF.
Characterization of calpain activation following NMDA administration in primary cortical cultures
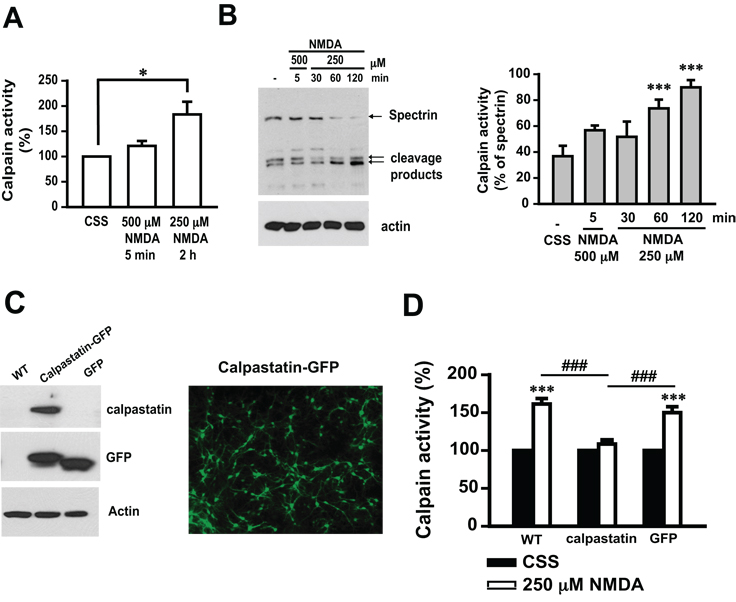
Glutamate kills neurons through excitotoxic mechanisms (Szatkowski and Attwell 1994; Zhang et al. 1994; Mandir et al. 2000; Sattler and Tymianski 2001). The majority of glutamate toxicity is mediated through activation of NMDA receptors (Szatkowski and Attwell 1994; Sattler and Tymianski 2001; Wang et al. 2004). The length and strength of stimulation determine the cell death cascades that are activated (Meli et al. 2004). Exposure to NMDA for 5 min at 500 µM induces cell death 24 h later that is primarily mediated by parthanatos (Yu et al. 2002; Andrabi et al. 2006). Whereas, cell death induced by prolonged administration of NMDA at lower concentrations occurs through activation of multiple cell death cascades including calpain activation. Accordingly, in this study calpain activation was monitored after exposure to 500 µM NMDA for 5 min and exposure to 250 µM NMDA for 2 h using a fluorogenic calpain substrate activity assay (Fig 2A). 250 µM NMDA for 2 h results in nearly a two-fold increase in calpain activity, whereas 500 µM NMDA for 5 min fails to induce significant calpain activity 2 h after the initial treatment (Fig. 2A). Calpain activation was also monitored by the cleavage of its endogenous substrate spectrin (Moubarak et al. 2007; Wang et al. 2008). 500 µM NMDA for 5 min fails to elicit spectrin cleavage (assayed 2 h later), whereas 250 µM NMDA led to marked spectrin cleavage, with the maximal effect at 2 h (Fig. 2B). The faster migrating of the two indicated spectrin fragments corresponds to a calpain-specific 145 kDa fragment (Wang et al. 2008). Only the 145 kDa species increases in the lower NMDA concentration; but about the same amount of each fragment (150/145) is seen in the control and higher NMDA concentration condition, which is consistent with the results of fluorogenic calpain activity assay (Fig. 2D). Adenoviral mediated over-expression of calpastatin (Ad.calpastatin) (Fig. 2C), a naturally expressed calpain-specific peptide inhibitor, attenuates 250 µM NMDA-induced calpain activity compared to non-transduced and control Ad.GFP transduced cultures (Fig. 2D). Therefore, the administration of NMDA at 500 µM for 5 min initiates a calpain-independent pathway, whereas the prolonged administration of NMDA at 250 µM for 2 h leads to calpain activation.
Fig. 2. NMDA treatment induces calpain activation in cortical neurons.
A, Fluorogenic analysis of calpain activity was performed in the whole cell lysate from cells treated with Controlled Salt Solution (CSS) or NMDA under the indicated conditions. The calpain activity in control cells treated with CSS only is regarded as 100%. B, Spectrin immunoblotting analysis (left) was performed on whole cell lysate from cells treated with 500 µM or 250 µM NMDA for the indicated times. Arrows indicate full-length spectrin (240 kDa) and calpain-dependent cleavage products (150 and 145 kDa). The calpain activity is quantified and presented as the percentage of cleavage products in total spectrin (right). n = 8. C, The expression of calpastatin and/or GFP in cortical neurons was assessed by immunoblotting (left) and fluorescence microscopy (right). For immunoblotting, whole cell lysate was prepared from cells transduced with Ad.calpastatin or Ad.GFP for 48 h. D, Calpastatin inhibited calpain activity induced by NMDA (250 µM for 2 h). Data shown in A, B and D represent the mean ± SEM of at least three independent experiments. ***p < 0.001, *p < 0.05 as compared with cells treated with CSS, ### p < 0.001 as compared with cells treated with NMDA.
NMDA-induced AIF release does not require calpain activation
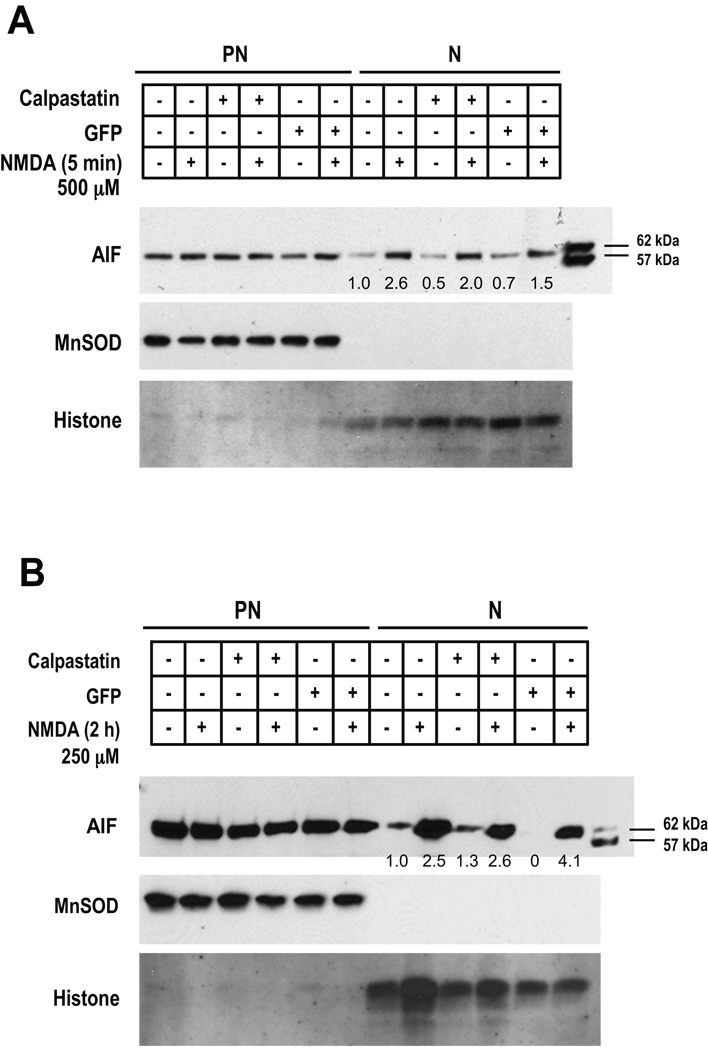
To test whether calpain is involved in mitochondrial AIF release in parthanatos, primary cortical neurons were treated with NMDA (500 µM, 5 min) under conditions where calpain is not activated and AIF translocation was monitored 2 h later by immunoblotting analysis of postnuclear (mitochondria and cytosol) and nuclear fractions. NMDA administration under these conditions robustly induces AIF nuclear translocation in cortical neurons, which is 2.6-fold higher than that in non-treated cells (Fig. 3A). Calpastatin, the calpain inhibitor, did not prevent NMDA-induced mitochondrial AIF release (Fig. 3A). It has been suggested that AIF needs to be cleaved to the 57 kDa form to be released from the mitochondria (Susin et al. 1999; Polster et al. 2005; Cao et al. 2007; Moubarak et al. 2007). Accordingly, in monitoring AIF release we included recombinant AIF62 and AIF57 as molecular markers to identify the form of AIF released (Fig. 3A). We observed that only AIF62 is released.
Fig. 3. Calpastatin fails to prevent NMDA-induced AIF nuclear translocation in cortical neurons.
Wild-type cortical neurons and neurons transduced by Ad.calpastatin or Ad.GFP were treated with 500 µM NMDA for 5 min or 250 µM NMDA for 2 h. Two hours after the treatment, post-nuclear (PN) and nuclear subcellular (N) fractions were isolated. The integrities of nuclear and postnuclear fractions were monitored by MnSOD and histone immunoreactivity, respectively. A, Representative immunoblots of AIF show nuclear translocation induced by 500 µM NMDA for 5 min. B, Representative immunoblots of AIF nuclear translocation caused by 250 µM NMDA for 2 h. The quantitative values indicated in A & B represent the relative intensity of AIF nuclear translocation normalized to histone. The intensity of AIF in non-transduced cells without NMDA treatment is regarded as 1. Recombinant AIFs, 62 kDa and 57 kDa, serve as molecular markers.
To study whether calpain cleaves AIF and mediates its release from mitochondria and translocation to the nucleus under conditions where calpain is activated by NMDA treatment (250 µM NMDA for 2 h), AIF nuclear translocation was monitored. As Fig. 3B shows, NMDA treatment substantially induces AIF translocation to the nucleus in cortical neurons. Surprisingly AIF translocation to the nuclear fraction by 250 µM NMDA for 2 h, is also not prevented by calpastatin (Fig. 3B), which clearly reduced NMDA-induced calpain activity under these conditions (Fig. 2D). In Ad.calpastatin-transduced cortical neurons, NMDA administration causes about 2.6-fold increase of AIF nuclear translocation which is comparable to that observed in non-transduced cells (Fig. 2D). The integrity of the nuclear fraction was monitored by immunoblotting analysis of the mitochondrial marker MnSOD, and the integrity of the postnuclear fraction was monitored by immunoblotting analysis of the nuclear marker histone (Fig. 3A, 3B).
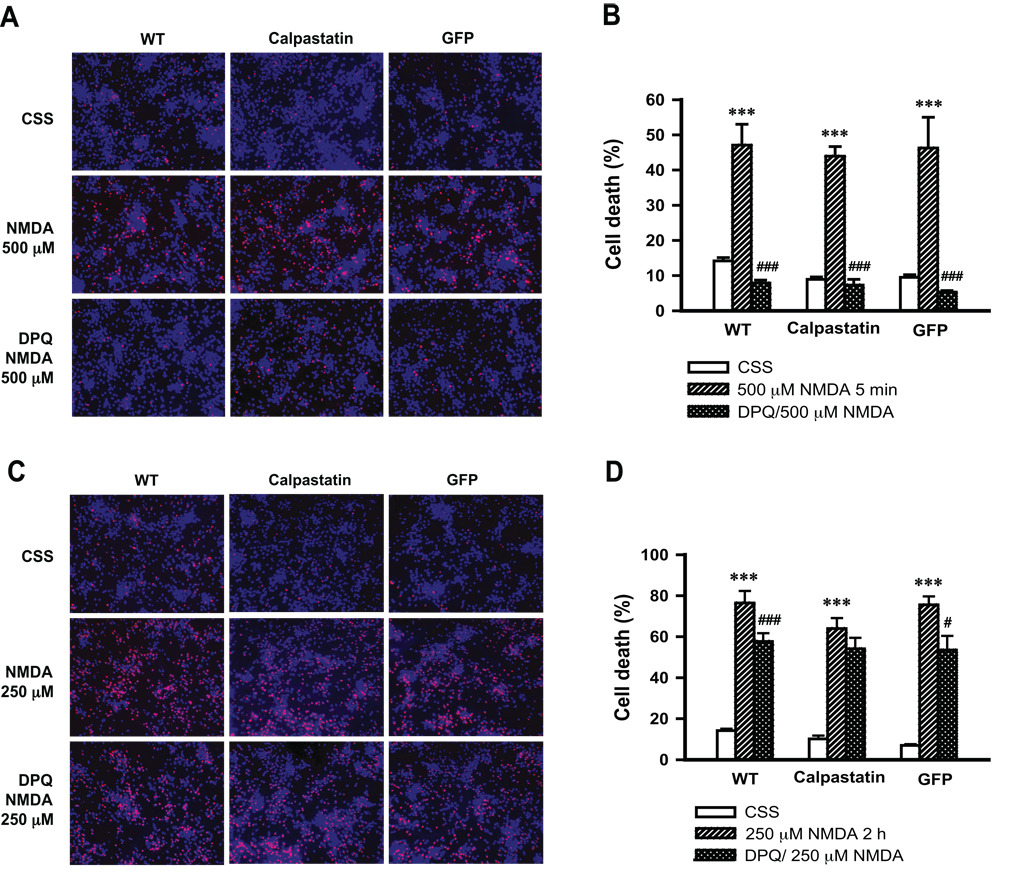
To confirm that administration of 500 µM NMDA for 5 min results in parthanatos, cell death was monitored in the absence and presence of the PARP inhibitor, 3,4-dihydro-5-[4-(1-piperidinyl)butoxyl]-1(2H)-iso-quinolinone (DPQ). Exposure to 500 µM NMDA for 5 min induces approximately 50% cell death (Fig. 4A, 4B). This effect is markedly inhibited by 30 µM DPQ (Fig. 4A, 4B). Transduction of primary cortical neurons with Ad.calpastatin fails to protect neurons from NMDA (500 µM, 5 min) toxicity. Similar results are seen in non-transduced cortical neurons or those transduced with Ad.GFP; and in these, as well as Ad.calpastatin transduced cells, DPQ protects against NMDA toxicity (Fig. 4A, 4B). On the other hand, 250 µM NMDA for 2 h results in about 80% cell death, which is blocked partially by DPQ (Fig. 4C, 4D). However, transduction of neurons by Ad.calpastatin has no clear protective effect (P > 0.05) (Fig. 4C, 4D). These results taken together indicate that calpain activation is not required for NMDA-induced AIF translocation. Moreover, calpain activation does not play a role in NMDA-induced parthanatos.
Fig. 4. Overexpression of calpastatin fails to protect cortical neurons from NMDA-induced cell death.
Wild-type cortical neurons (WT) and neurons transduced by Ad.calpastatin or Ad.GFP, were pretreated with or without 30 µM DPQ for 30 min, then exposed to 500 µM NMDA for 5 min (A & B) or 250 µM NMDA for 2 h (C & D). Cell viability was assessed 24 h after treatment by Hoechst 33342 (7 µM, for total nuclei) and propidium iodide (2 µM, for dead cell nuclei) double staining. Representative images of NMDA-induced cell death are shown in A & C, and quantification of NMDA-induced cell death is shown in B & D. Data shown in B and D represent the mean ± SEM of at least three independent experiments. ***p < 0.001 as compared with cells treated with Controlled Salt Solution (CSS); #p < 0.05, ### p < 0.001 as compared with cells treated with NMDA.
MNNG-induced AIF release is calpain independent
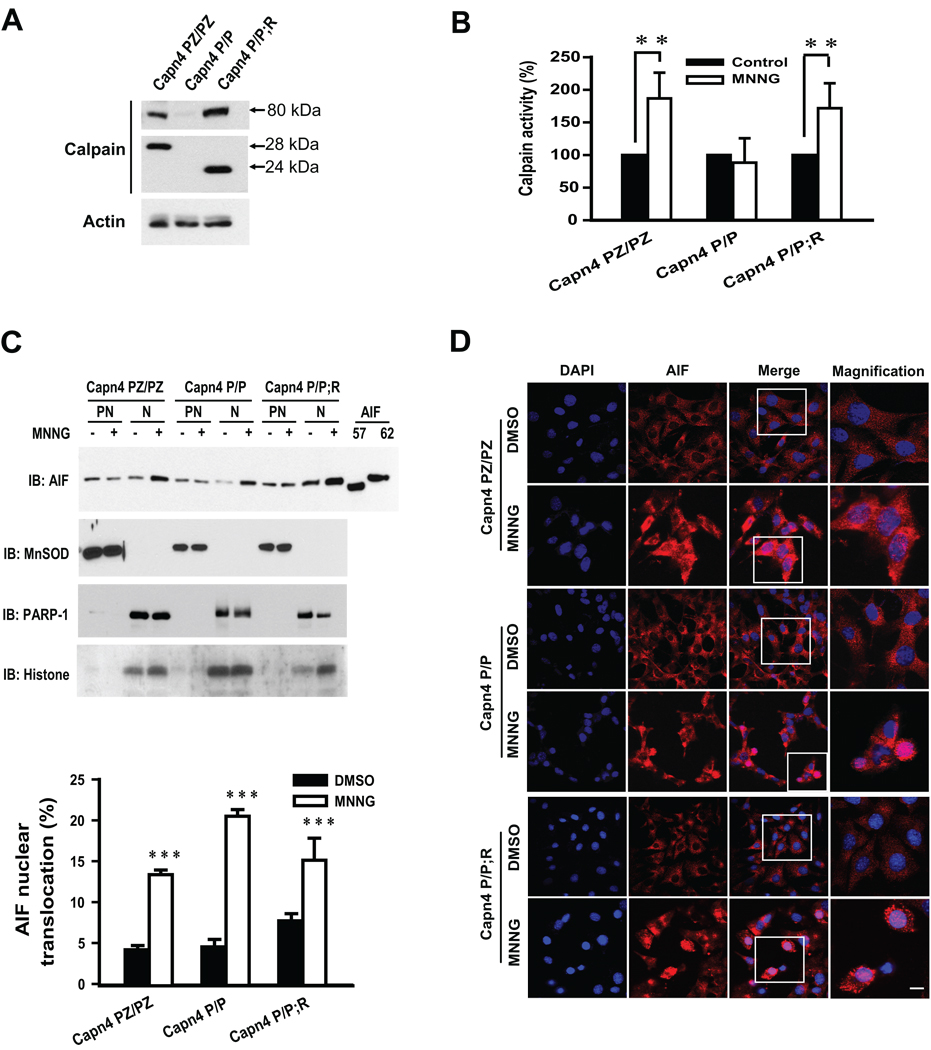
MNNG damages DNA resulting in DNA strand nicks and breaks, activation of PARP-1 and parthanatos (Yu et al. 2002). To further determine if calpain activation is required for AIF release following MNNG treatment, AIF release was monitored in Capn4 knockout mouse embryonic fibroblasts (MEF). Capn4 encodes the regulatory subunit of calpain I and II (μ-calpain and m-calpain, respectively) and the absence of Capn4 leads to complete loss of calpain I and II activity (Arthur et al. 2000; Tan et al. 2006). Capn4 PZ/PZ MEFs are homozygous for the intact floxed allele, and display wild type levels of calpain I and II activity. Capn4 P/P MEFs are homozygous for the Cre-excised allele and are devoid of calpain I and II activity because they lack the small subunit. Capn4 P/P;R MEFs are Capn4 P/P cells rescued for calpain I and II activity with lentivirus expressing a 24 kDa N-terminally truncated Capn4 encoded protein (Tan et al. 2006). The Capn4 PZ/PZ MEFs express the m-80 and 28 kDa subunits, whereas no 28 kDa subunit and very little m-80 are observed in Capn4 P/P MEFs. Capn4 P/P;R MEFs express the m-80 and the active N-terminally truncated 24 kDa Capn4 protein (Fig. 5A). The Capn4 MEFs were treated with MNNG (500 µM, 15 min) under conditions that induce cell death primarily through parthanatos. Two hours later calpain activity was monitored in Capn4 PZ/PZ, Capn4 P/P and Capn4 P/P;R MEFs. As shown in Fig. 5B, calpain activity in Capn4 PZ/PZ MEF is significantly increased by MNNG treatment (Fig. 5B). However, MNNG fails to induce calpain activity in Capn4 P/P MEFs (Fig. 5B). The calpain activity is clearly rescued in Capn4 P/P;R MEFs.
Fig. 5. MNNG-induced AIF nuclear translocation in Capn4 knockout MEFs.
A, Immunoblotting analysis of m-calpain 80 kDa catalytic and 28 or 24 kDa regulatory subunits in Capn4 PZ/PZ (homozygous for the floxed allele), Capn4 P/P (homozygous for Cre-excised allele) and Capn4 P/P;R (Capn4 P/P rescued with lentivirus expressing the small active 24 kDa N-terminally truncated regulatory subunit) MEFs. B, Calpain activity in Capn4 PZ/PZ, Capn4 P/P and Capn4 P/P;R MEFs was determined 2 h after 15 min 500 µM MNNG treatment. ***p < 0.01. C, Representative immunoblots of MNNG (500 µM, 15 min)-caused AIF nuclear translocation in Capn4 PZ/PZ, Capn4 P/P and Capn4 P/P;R MEFs (upper panel). PN, post-nuclear fraction; N, nuclear fraction. MnSOD was used as a marker of the mitochondrial, postnuclear fraction. Histion and PARP-1 were used as markers of the nuclear fractions. Quantification of AIF nuclear translocation is shown (lower panel). ***p < 0.001. D, Confocal immunofluorescence images of AIF nuclear translocation in Capn4 PZ/PZ, Capn4 P/P and Capn4 P/P;R MEFs induced by MNNG treatment (500 µM, 15 min). Pink indicates the colocalization of Cy3-stained AIF and DAPI-stained nuclei. Scale bar = 10 µm.
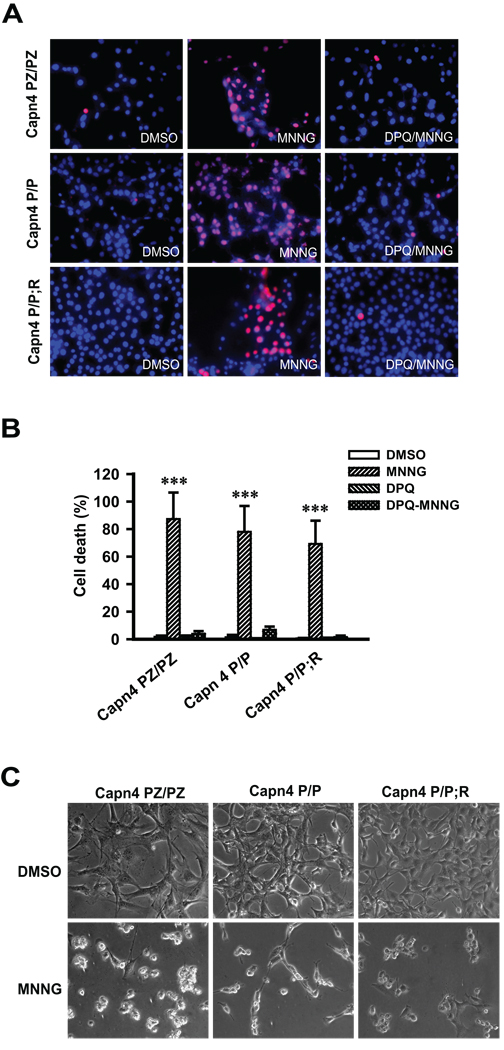
Next AIF translocation was monitored by immunoblotting analysis of postnuclear and nuclear subcellular fractions of the Capn4 MEFs 2 h after 500 µM MNNG treatment for 15 min. MNNG administration clearly induces AIF nuclear translocation in Capn4 knockout (Capn4 P/P) MEFs (Fig. 5C upper panel). Quantitative data show that approximately 15–20 % of the total AIF translocates to nucleus (Fig. 5C lower panel). A similar amount of AIF nuclear translocation is also observed in Capn4 PZ/PZ and Capn4 P/P;R MEFs after MNNG treatment (Fig. 5C). However, the levels of AIF in the postnuclear fractions are not obviously decreased after MNNG treatment, which may due to the abundant amount of AIF in the postnuclear fractions. Recombinant AIF62 and AIF57 were included as molecular markers to identify the form of AIF released. The integrities of nuclear and postnuclear fractions were monitored by the mitochondrial marker MnSOD and nuclear markers PARP-1(data not shown) and histone, respectively. The levels of PARP-1 do not change in response to parthanatos (data not shown). Only AIF62 is detected in both nuclear and postnuclear fractions (Fig. 5C). Moreover, confocal image analysis shows that MNNG efficiently induces AIF translocation in Capn4 knockout MEF (Capn4 P/P) as well as in Capn4 PZ/PZ and Capn4 P/P;R MEFs (Fig. 5D). To confirm that MNNG (500 µM, 15 min) induces primarily parthanatos, cell death was monitored in the Capn4 MEFs in the presence and absence of DPQ (30 µM). Under these conditions MNNG cell death occurs primarily by parthanatos because the cell death is nearly completely attenuated by DPQ in all three genotypes of Capn4 MEFs (Fig. 6). Over-expression of calpastatin also fails to block MNNG-induced cell death in HeLa cells (data not shown). These results taken together indicate that MNNG-induced AIF translocation and cell death does not require calpain activation.
Fig. 6. MNNG caused cell death in Capn4 knockout MEFs.
A, Capn4 PZ/PZ, Capn4 P/P and Capn4 P/P;R MEFs were treated with DMSO, MNNG (500 µM, 15 min) or DPQ (30 µM) + MNNG (500 µM, 15 min). Cell viability was assessed 24 h after treatment by Hoechst 33342 (7 µM, for total nuclei) and propidium iodide (2 µM, for dead cell nuclei) double staining. B, Quantification of MNNG-induced cell death. C, Representative transmission images of Capn4 MEFs 24 h after DMSO or MNNG treatment.
Uncleaved AIF62 translocates to nucleus in vivo after middle cerebral artery occlusion
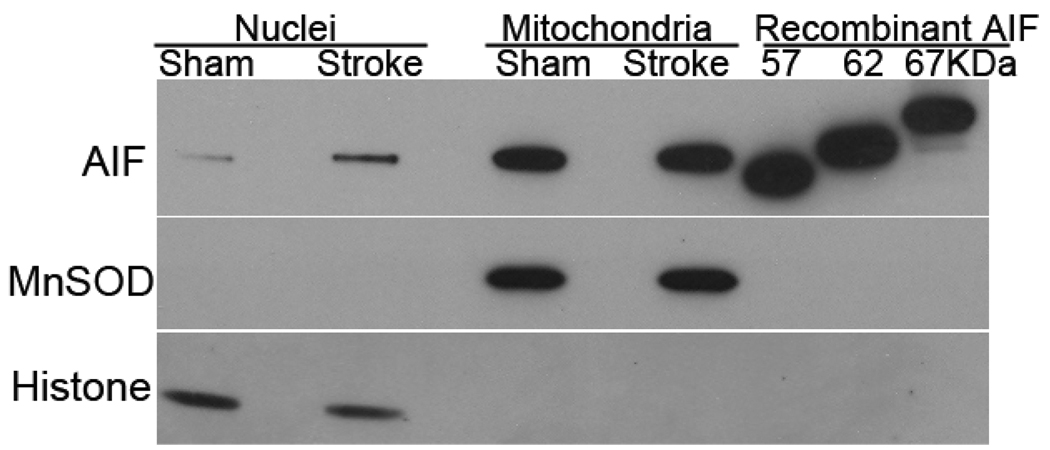
Injury from focal cerebral ischemia partly depends on activation of NMDA receptors. To evaluate which form of AIF translocates to the nucleus and whether calpain is involved in AIF cleavage and release in mice after transient focal ischemia, immunoblots were run on nuclear and mitochondrial fractions obtained 24 hours after middle cerebral artery occlusion. Using an antibody that recognized the 57, 62, and 67 kDa recombinant AIF, the mitochondria fraction of brain tissue of mice that had undergone sham surgery or stroke showed a band that was close to 62 kDa form of AIF (Fig. 7). As expected, AIF in the nuclear fraction was greater in tissue from the post-ischemic brain. However, only a single band was visible and that band showed the same migration as the mitochondrial 62 kDa band. Thus, nuclear accumulation of truncated 57 kDa AIF was not evident after transient focal cerebral ischemia, indicating that calpain is not required for AIF release in vivo.
Fig. 7. 62 kDa AIF translocates following experimental stroke.
Immunoblots of nuclear and mitochondrial fractions of brain tissue from mice subjected to sham surgery or 90 minutes of middle cerebral artery occlusion and 24 hours of reperfusion. AIF increased in the nuclear fraction after stroke, but the AIF antibody, which recognized recombinant 57, 62, and 67 kDa AIF, recognized only a single AIF close to 62 kDa in the nucleus and mitochondria after sham surgery or stroke. Manganese superoxide dismutase (MnSOD) and histone antibodies were used to assess the integrity of the nuclear and mitochondrial fractions.
DISCUSSION
The major finding of this study is that calpain activation is not required for mitochondrial AIF release during parthanatos. Although calpain could cleave recombinant AIF in a cell free system, in intact cells under conditions where endogenous calpain was activated by either NMDA or MNNG administration, AIF was not cleaved. NMDA- and MNNG-induced translocation of AIF was not prevented by the calpain inhibitor calpastatin. In Capn4 knockout MEFs, which lacked calpain activity, AIF was still released following MNNG administration. Inhibition of calpain with calpastatin or genetic knockout of the regulatory subunit of calpain, which eliminates both calpain I and II activity (Tan et al. 2006), failed to prevent NMDA- or MNNG-induced cell death, respectively. Furthermore, the AIF that was released and translocated to the nucleus was the uncleaved 62 kDa form consistent with lack of involvement of calpain in the release of AIF.
A large body of evidence indicates that PARP-1 activation plays a pivotal role in multiple injury paradigms via PAR polymer generation and mitochondrial AIF release (Yu et al. 2002; Andrabi et al. 2006; Yu et al. 2006; Wang et al. 2009). Understanding the mechanism underlying PAR-mediated AIF release could help to develop therapeutic approaches for the treatment of a variety of neurologic disorders. Although an essential role of calpain has been suggested in other forms of cell death, here we exclude the involvement of calpain in parthanatos.
Prior reports suggested that release of AIF from mitochondria requires cleavage by calpains or capthepsins (Otera et al. 2005). In calcium-induced permeability transition, calpain-sensitive release of AIF from isolated liver mitochondria was observed (Polster et al. 2005). Similar to prior reports (Polster et al. 2005), we were able to observe cleavage of recombinant AIF by calpain I in a cell free system, however we did not observe calpain-dependent release of AIF from mitochondria in intact cells. The difference in observations may be due to the use of isolated mitochondrial preparations versus whole cell preparations. Our results differ from those of Cao et al. in which the authors suggested that calpain I activity is required for AIF translocation following ischemic neuronal injury (Cao et al. 2007). That study found that following ischemic injury, AIF was cleaved from the 62 kDa to the 57 kDa form and translocated from the mitochondria to the nucleus in a calpain-dependent manner (Cao et al. 2007). Thus under specific experimental conditions calpain apparently can play a role in AIF release through AIF cleavage. However, in the present study, we do not observe a 57 kDa AIF product in the nucleus after transient focal cerebral ischemia, rather only the 62 kDa AIF is seen in the mitochondria of brain tissue. This may be due in part to different mechanisms in delayed hippocampal neuronal death after brief global ischemia from those involved in prolonged focal ischemia. Taken together these data indicated that truncation of AIF to the 57 kDa form does not appear to be required in all types of ischemic injury.
Our results indicate that calpain activation is not necessary for AIF release and cleavage of AIF in parthanatos. Taken together these different observations indicate that there are different mechanisms for AIF release that are dependent on the specific conditions and signaling pathways involved. An intriguing question is why under ischemic conditions calpain activation results in AIF cleavage and translocation, while under conditions of parthanatos, calpain activation does not result in AIF cleavage, nor does it regulate AIF translocation.
Sequential activation of PARP-1, calpains and Bax is thought to play a role in MNNG-induced necrotic programmed cell death (Moubarak et al. 2007). Necrotic programmed cell death differs from parthanatos in that parthanatos does not require the absence of glucose, suggesting there are different signaling events in these two forms of cell death. The difference is likely due to a reliance on the glycolytic pathway in necrotic programmed cell death. Ischemic forms of neuronal injury are also thought to resort to utilization of the glycolytic pathway (Cao et al. 2007), thus necrotic programmed cell death and ischemic injury may utilize similar pathways to release AIF, which differ from those that do not shift to the glycolytic pathway. Why glycolysis may contribute to calpain-dependent cleavage and release of AIF is not known.
AIF, contains a hydrophobic segment at the N-terminus, which is thought to be primarily located in the mitochondrial intermembrane space as an insoluble form (Otera et al. 2005). Although, to date, no specific amino acid sequence has been identified to be uniquely recognized by calpain, calpain is thought to remove the hydrophobic domain of AIF, which anchors AIF in the mitochondrial inner membrane, thereby processing it into a 57 kDa soluble form. In the present study, only uncleaved 62 kDa AIF was observed to be released from the mitochondria independent of calpain activation. This suggests that a second pool of AIF may present in a different submitochondrial compartment and different mechanisms might be involved in mitochondrial AIF release due to its localization.
In summary we show that AIF release in parthanatos is distinct from previously defined cleavage and release of AIF by calpain in ischemic injury and programmed necrotic cell death. In parthanatos, AIF release does not require cleavage or calpain activation. Parthanatos involves PAR as a cell death signal (Andrabi et al. 2006) and AIF release requires PAR. How PAR induces AIF release is not yet known. Recent attention on the role of PAR as a signaling molecule suggests that PAR can posttranslationally modify proteins conferring changes in protein activity. The data presented in this study indicate that calpain is not a likely target for PAR-dependent release of AIF in parthanatos. Elucidating the mechanism by which PAR induces AIF release awaits further study and holds promise for identifying novel mechanisms of AIF release that could be therapeutic targets.
Acknowledgments
This work was supported by grants from the NIH (NS39148), the American Heart Association Postdoctoral Fellowship Award to YW, NSK, and the Canadian Institutes of Health Research. T.M.D. is the Leonard and Madlyn Abramson Professor of Neurodegenerative Disease at Johns Hopkins University.
Abbreviations
- AIF
apoptosis-inducing factor
- DAPI
4',6-Diamidino-2-phenylindole
- DPQ
3,4-dihydro-5-[4-(1-piperidinyl)butoxyl]-1(2H)-iso-quinolinone
- MEF
mouse embryonic fibroblasts
- MNNG
N-Methyl-N'-Nitro-N-Nitrosoguanidine
- MnSOD
manganese superoxide dismutase
- NMDA
N-methyl D-aspartate
- PAR
poly(ADP-ribose) polymer
- PARP-1
poly(ADP-ribose) polymerase-1
- SDS-PAGE
denaturing polyacrylamide gel electrophoresis
REFERENCES
- Andrabi SA, Dawson TM, Dawson VL. Mitochondrial and nuclear cross talk in cell death: parthanatos. Ann N Y Acad Sci. 2008;1147:233–241. doi: 10.1196/annals.1427.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrabi SA, Kim NS, Yu SW, Wang H, Koh DW, Sasaki M, Klaus JA, Otsuka T, Zhang Z, Koehler RC, Hurn PD, Poirier GG, Dawson VL, Dawson TM. Poly(ADP-ribose) (PAR) polymer is a death signal. Proc Natl Acad Sci U S A. 2006;103:18308–18313. doi: 10.1073/pnas.0606526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur JS, Elce JS, Hegadorn C, Williams K, Greer PA. Disruption of the murine calpain small subunit gene, Capn4: calpain is essential for embryonic development but not for cell growth and division. Mol Cell Biol. 2000;20:4474–4481. doi: 10.1128/mcb.20.12.4474-4481.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevers MB, Neumar RW. Mechanistic role of calpains in postischemic neurodegeneration. J Cereb Blood Flow Metab. 2008;28:655–673. doi: 10.1038/sj.jcbfm.9600595. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cao G, Xing J, Xiao X, Liou AK, Gao Y, Yin XM, Clark RS, Graham SH, Chen J. Critical role of calpain I in mitochondrial release of apoptosis-inducing factor in ischemic neuronal injury. J Neurosci. 2007;27:9278–9293. doi: 10.1523/JNEUROSCI.2826-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosi C, Marien M. Implication of poly (ADP-ribose) polymerase (PARP) in neurodegeneration and brain energy metabolism. Decreases in mouse brain NAD+ and ATP caused by MPTP are prevented by the PARP inhibitor benzamide. Ann N Y Acad Sci. 1999;890:227–239. doi: 10.1111/j.1749-6632.1999.tb07998.x. [DOI] [PubMed] [Google Scholar]
- Cuerrier D, Moldoveanu T, Davies PL. Determination of peptide substrate specificity for mu-calpain by a peptide library-based approach: the importance of primed side interactions. J Biol Chem. 2005;280:40632–40641. doi: 10.1074/jbc.M506870200. [DOI] [PubMed] [Google Scholar]
- Culmsee C, Zhu C, Landshamer S, Becattini B, Wagner E, Pellecchia M, Blomgren K, Plesnila N. Apoptosis-inducing factor triggered by poly(ADP-ribose) polymerase and Bid mediates neuronal cell death after oxygen-glucose deprivation and focal cerebral ischemia. J Neurosci. 2005;25:10262–10272. doi: 10.1523/JNEUROSCI.2818-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David KK, Andrabi SA, Dawson TM, Dawson VL. Parthanatos, a messenger of death. Front Biosci. 2009;14:1116–1128. doi: 10.2741/3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson VL, Dawson TM, Bartley DA, Uhl GR, Snyder SH. Mechanisms of nitric oxide-mediated neurotoxicity in primary brain cultures. J Neurosci. 1993;13:2651–2661. doi: 10.1523/JNEUROSCI.13-06-02651.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson MJ, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, Pieper A, Wang ZQ, Dawson TM, Snyder SH, Dawson VL. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med. 1997;3:1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- Endres M, Wang ZQ, Namura S, Waeber C, Moskowitz MA. Ischemic brain injury is mediated by the activation of poly(ADP-ribose)polymerase. J Cereb Blood Flow Metab. 1997;17:1143–1151. doi: 10.1097/00004647-199711000-00002. [DOI] [PubMed] [Google Scholar]
- Mandir AS, Przedborski S, Jackson-Lewis V, Wang ZQ, Simbulan-Rosenthal CM, Smulson ME, Hoffman BE, Guastella DB, Dawson VL, Dawson TM. Poly(ADP-ribose) polymerase activation mediates 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism. Proc Natl Acad Sci U S A. 1999;96:5774–5779. doi: 10.1073/pnas.96.10.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandir AS, Poitras MF, Berliner AR, Herring WJ, Guastella DB, Feldman A, Poirier GG, Wang ZQ, Dawson TM, Dawson VL. NMDA but not non-NMDA excitotoxicity is mediated by Poly(ADP-ribose) polymerase. J Neurosci. 2000;20:8005–8011. doi: 10.1523/JNEUROSCI.20-21-08005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meli E, Pangallo M, Picca R, Baronti R, Moroni F, Pellegrini-Giampietro DE. Differential role of poly(ADP-ribose) polymerase-1in apoptotic and necrotic neuronal death induced by mild or intense NMDA exposure in vitro. Mol Cell Neurosci. 2004;25:172–180. doi: 10.1016/j.mcn.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Moubarak RS, Yuste VJ, Artus C, Bouharrour A, Greer PA, Menissier-de Murcia J, Susin SA. Sequential activation of poly(ADP-ribose) polymerase 1, calpains, and Bax is essential in apoptosis-inducing factor-mediated programmed necrosis. Mol Cell Biol. 2007;27:4844–4862. doi: 10.1128/MCB.02141-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otera H, Ohsakaya S, Nagaura Z, Ishihara N, Mihara K. Export of mitochondrial AIF in response to proapoptotic stimuli depends on processing at the intermembrane space. Embo J. 2005;24:1375–1386. doi: 10.1038/sj.emboj.7600614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Szabo C. Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am J Pathol. 2008;173:2–13. doi: 10.2353/ajpath.2008.080019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesnila N, Zhu C, Culmsee C, Groger M, Moskowitz MA, Blomgren K. Nuclear translocation of apoptosis-inducing factor after focal cerebral ischemia. J Cereb Blood Flow Metab. 2004;24:458–466. doi: 10.1097/00004647-200404000-00011. [DOI] [PubMed] [Google Scholar]
- Polster BM, Basanez G, Etxebarria A, Hardwick JM, Nicholls DG. Calpain I induces cleavage and release of apoptosis-inducing factor from isolated mitochondria. J Biol Chem. 2005;280:6447–6454. doi: 10.1074/jbc.M413269200. [DOI] [PubMed] [Google Scholar]
- Sattler R, Tymianski M. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol Neurobiol. 2001;24:107–129. doi: 10.1385/MN:24:1-3:107. [DOI] [PubMed] [Google Scholar]
- Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- Szatkowski M, Attwell D. Triggering and execution of neuronal death in brain ischaemia: two phases of glutamate release by different mechanisms. Trends Neurosci. 1994;17:359–365. doi: 10.1016/0166-2236(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Tan Y, Dourdin N, Wu C, De Veyra T, Elce JS, Greer PA. Conditional disruption of ubiquitous calpains in the mouse. Genesis. 2006;44:297–303. doi: 10.1002/dvg.20216. [DOI] [PubMed] [Google Scholar]
- Wang H, Yu SW, Koh DW, Lew J, Coombs C, Bowers W, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Apoptosis-inducing factor substitutes for caspase executioners in NMDA-triggered excitotoxic neuronal death. J Neurosci. 2004;24:10963–10973. doi: 10.1523/JNEUROSCI.3461-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Shan P, Song Z, Dai T, Wang R, Chi Z. Mu-calpain mediates hippocampal neuron death in rats after lithium-pilocarpine-induced status epilepticus. Brain Res Bull. 2008;76:90–96. doi: 10.1016/j.brainresbull.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Wang Y, Luo W, Reiser G. Proteinase-activated receptor-1 and -2 induce the release of chemokine GRO/CINC-1 from rat astrocytes via differential activation of JNK isoforms, evoking multiple protective pathways in brain. Biochem J. 2007;401:65–78. doi: 10.1042/BJ20060732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Dawson VL, Dawson TM. Poly(ADP-ribose) signals to mitochondrial AIF: A key event in parthanatos. Exp Neurol. 2009 doi: 10.1016/j.expneurol.2009.03.020. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SW, Andrabi SA, Wang H, Kim NS, Poirier GG, Dawson TM, Dawson VL. Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proc Natl Acad Sci U S A. 2006;103:18314–18319. doi: 10.1073/pnas.0606528103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- Zhang J, Dawson VL, Dawson TM, Snyder SH. Nitric oxide activation of poly(ADP-ribose) synthetase in neurotoxicity. Science. 1994;263:687–689. doi: 10.1126/science.8080500. [DOI] [PubMed] [Google Scholar]