Abstract
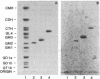
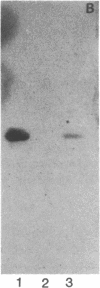
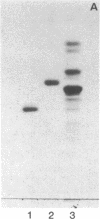
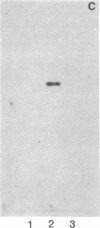
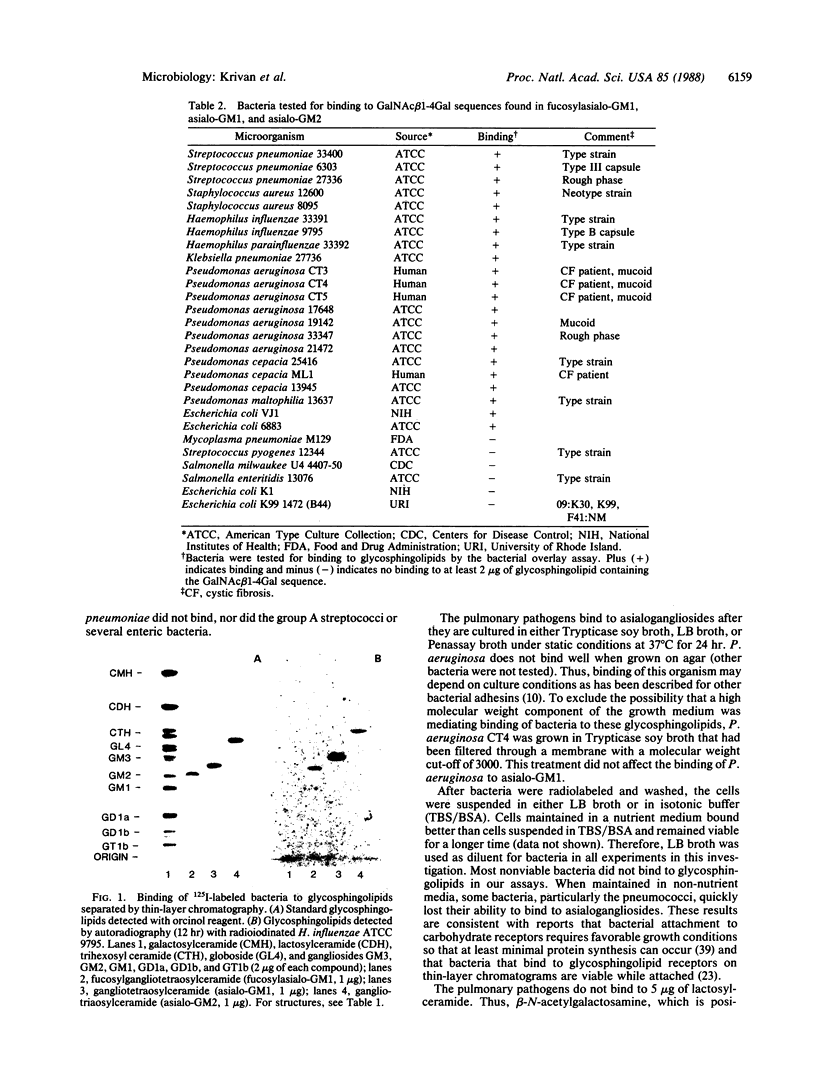
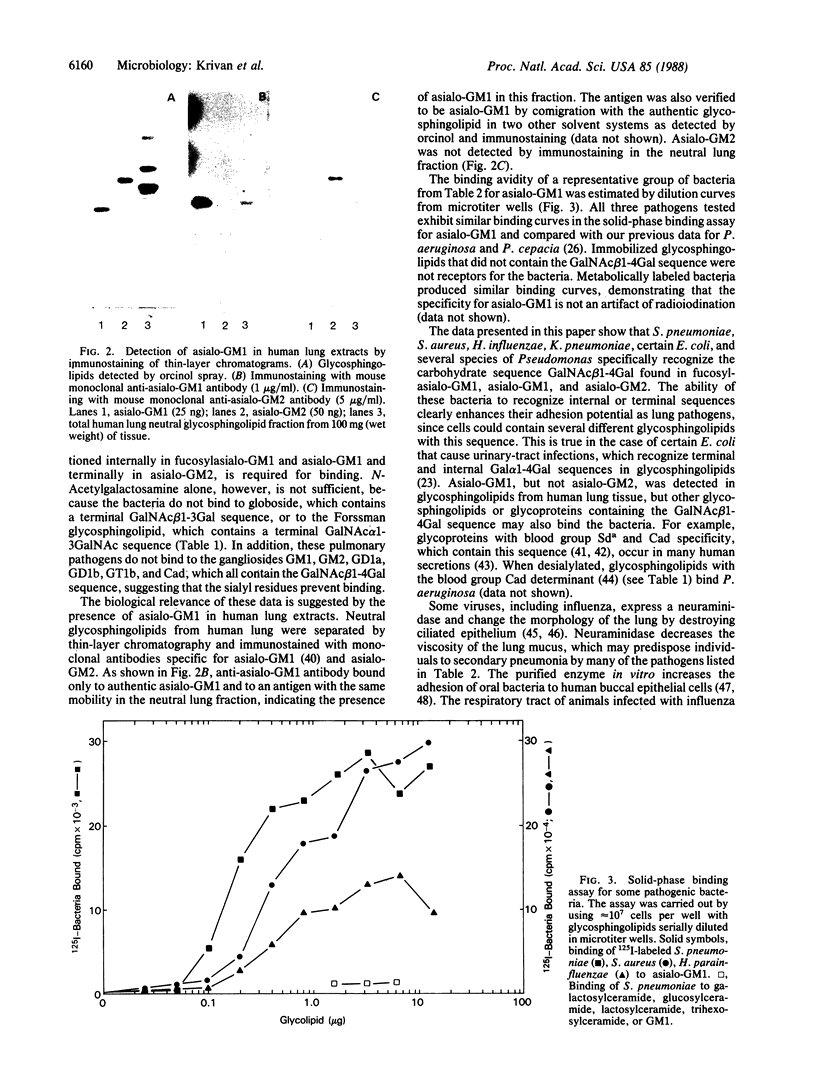
Pneumonia is one of the most common causes of death from infectious disease in the United States. To examine the possible role of carbohydrates as adhesion receptors for infection, several pulmonary pathogenic bacteria were studied for binding to glycosphingolipids. Radiolabeled bacteria were layered on thin-layer chromatograms of separated glycosphingolipids, and bound bacteria were detected by autoradiography. The classic triad of infectious bacteria found in cystic fibrosis, Pseudomonas aeruginosa, Haemophilus influenzae, and Staphylococcus aureus, along with other bacteria commonly implicated in typical pneumonia, such as Streptococcus pneumoniae, Klebsiella pneumoniae, and certain Escherichia coli, bind specifically to fucosylasialo-GM1 (Fuc alpha 1-2Gal beta 1-3GalNAc beta 1-4Gal beta 1-4Cer), asialo-GM1 (Gal beta 1-3GalNAc beta 1-4Gal beta-1-4Galc beta 1-1Cer), and asialo-GM2 (GalNAc beta 1-4Gal beta 1-4Glc beta 1-1Cer). Bacteria maintained in nutrient medium bind better than the same cells suspended in buffer. They do not bind to galactosylceramide, glucosylceramide, lactosylceramide, trihexosylceramide, globoside, paragloboside, Forssman glycosphingolipid, or several other glycosphingolipids tested, including the gangliosides GM1, GM2, GM3, GD1a, GD1b, GT1b, and Cad. The finding that these pathogens do not bind to lactosylceramide suggests that beta 1-4-linked GalNAc, which is positioned internally in fucosylasialo-GM1 and asialo-GM1 and terminally in asialo-GM2, is required for binding. beta-N-Acetylgalactosamine itself, however, is not sufficient for binding, as the bacteria did not bind to globoside, which contains the terminal sequence GalNAc beta 1-3Gal. These data suggest that these bacteria require at least terminal or internal GalNAc beta 1-4Gal sequences unsubstituted with sialyl residues for binding. Other bacteria, including Mycoplasma pneumoniae, Streptococcus pyogenes, Salmonella species, and some E. coli, do not bind to the GalNAc beta 1-4Gal sequence. The biological relevance of these data is suggested by our finding that substantial amounts of asialo-GM1 occur in human lung tissue.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aly R., Shinefield H. I., Strauss W. G., Maibach H. I. Bacterial adherence to nasal mucosal cells. Infect Immun. 1977 Sep;17(3):546–549. doi: 10.1128/iai.17.3.546-549.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson B., Eriksson B., Falsen E., Fogh A., Hanson L. A., Nylén O., Peterson H., Svanborg Edén C. Adhesion of Streptococcus pneumoniae to human pharyngeal epithelial cells in vitro: differences in adhesive capacity among strains isolated from subjects with otitis media, septicemia, or meningitis or from healthy carriers. Infect Immun. 1981 Apr;32(1):311–317. doi: 10.1128/iai.32.1.311-317.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barondes S. H. Lectins: their multiple endogenous cellular functions. Annu Rev Biochem. 1981;50:207–231. doi: 10.1146/annurev.bi.50.070181.001231. [DOI] [PubMed] [Google Scholar]
- Baseman J. B., Cole R. M., Krause D. C., Leith D. K. Molecular basis for cytadsorption of Mycoplasma pneumoniae. J Bacteriol. 1982 Sep;151(3):1514–1522. doi: 10.1128/jb.151.3.1514-1522.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard D., Cartron J. P., Fournet B., Montreuil J., van Halbeek H., Vliegenthart J. F. Primary structure of the oligosaccharide determinant of blood group Cad specificity. J Biol Chem. 1983 Jun 25;258(12):7691–7695. [PubMed] [Google Scholar]
- Blanchard D., Piller F., Gillard B., Marcus D., Cartron J. P. Identification of a novel ganglioside on erythrocytes with blood group Cad specificity. J Biol Chem. 1985 Jul 5;260(13):7813–7816. [PubMed] [Google Scholar]
- Bock K., Breimer M. E., Brignole A., Hansson G. C., Karlsson K. A., Larson G., Leffler H., Samuelsson B. E., Strömberg N., Edén C. S. Specificity of binding of a strain of uropathogenic Escherichia coli to Gal alpha 1----4Gal-containing glycosphingolipids. J Biol Chem. 1985 Jul 15;260(14):8545–8551. [PubMed] [Google Scholar]
- Brennan M. J., Joralmon R. A., Cisar J. O., Sandberg A. L. Binding of Actinomyces naeslundii to glycosphingolipids. Infect Immun. 1987 Feb;55(2):487–489. doi: 10.1128/iai.55.2.487-489.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg S., Gerlach G. F. Enterobacterial fimbriae. J Bacteriol. 1987 Mar;169(3):934–938. doi: 10.1128/jb.169.3.934-938.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Graaf F. K., Mooi F. R. The fimbrial adhesins of Escherichia coli. Adv Microb Physiol. 1986;28:65–143. doi: 10.1016/s0065-2911(08)60237-4. [DOI] [PubMed] [Google Scholar]
- Donald A. S., Soh C. P., Watkins W. M., Morgan W. T. N-Acetyl-D-galactosaminyl-beta-(1 goes to 4)-d-galactose: a terminal non-reducing structure in human blood group Sda-active Tamm-Horsfall urinary glycoprotein. Biochem Biophys Res Commun. 1982 Jan 15;104(1):58–65. doi: 10.1016/0006-291x(82)91940-4. [DOI] [PubMed] [Google Scholar]
- Eickhoff T. C. Respiratory tract infections. Goals for 1995. Am J Med. 1985 Jun 28;78(6B):58–62. doi: 10.1016/0002-9343(85)90365-1. [DOI] [PubMed] [Google Scholar]
- Fader R. C., Avots-Avotins A. E., Davis C. P. Evidence for pili-mediated adherence of Klebsiella pneumoniae to rat bladder epithelial cells in vitro. Infect Immun. 1979 Aug;25(2):729–737. doi: 10.1128/iai.25.2.729-737.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Frazier W., Glaser L. Surface components and cell recognition. Annu Rev Biochem. 1979;48:491–523. doi: 10.1146/annurev.bi.48.070179.002423. [DOI] [PubMed] [Google Scholar]
- Gillard B. K., Jones M. A., Marcus D. M. Glycosphingolipids of human umbilical vein endothelial cells and smooth muscle cells. Arch Biochem Biophys. 1987 Aug 1;256(2):435–445. doi: 10.1016/0003-9861(87)90600-x. [DOI] [PubMed] [Google Scholar]
- Hakomori S. I., Siddiqui B. Isolation and characterization of glycosphingolipid from animal cells and their membranes. Methods Enzymol. 1974;32:345–367. doi: 10.1016/0076-6879(74)32036-8. [DOI] [PubMed] [Google Scholar]
- Hansson G. C., Karlsson K. A., Larson G., Strömberg N., Thurin J. Carbohydrate-specific adhesion of bacteria to thin-layer chromatograms: a rationalized approach to the study of host cell glycolipid receptors. Anal Biochem. 1985 Apr;146(1):158–163. doi: 10.1016/0003-2697(85)90410-5. [DOI] [PubMed] [Google Scholar]
- JANSSEN R. J., CHAPPELL W. A., GERONE P. J. SYNERGISTIC ACTIVITY BETWEEN PR8 INFLUENZA VIRUS AND STAPHYLOCOCCUS AUREUS IN THE GUINEA PIG. Am J Hyg. 1963 Nov;78:275–284. [PubMed] [Google Scholar]
- Jackson G. G., Muldoon R. L. Viruses causing common respiratory infections in man. V. influenza A (Asian). J Infect Dis. 1975 Mar;131(3):308–357. doi: 10.1093/infdis/131.3.308. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Isaacson R. E. Proteinaceous bacterial adhesins and their receptors. Crit Rev Microbiol. 1983;10(3):229–260. doi: 10.3109/10408418209113564. [DOI] [PubMed] [Google Scholar]
- Krivan H. C., Ginsburg V., Roberts D. D. Pseudomonas aeruginosa and Pseudomonas cepacia isolated from cystic fibrosis patients bind specifically to gangliotetraosylceramide (asialo GM1) and gangliotriaosylceramide (asialo GM2). Arch Biochem Biophys. 1988 Jan;260(1):493–496. doi: 10.1016/0003-9861(88)90473-0. [DOI] [PubMed] [Google Scholar]
- Lampe R. M., Mason E. O., Jr, Kaplan S. L., Umstead C. L., Yow M. D., Feigin R. D. Adherence of Haemophilus influenzae to buccal epithelial cells. Infect Immun. 1982 Jan;35(1):166–172. doi: 10.1128/iai.35.1.166-172.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani J. L., Nilsson B., Brockhaus M., Zopf D., Steplewski Z., Koprowski H., Ginsburg V. A monoclonal antibody-defined antigen associated with gastrointestinal cancer is a ganglioside containing sialylated lacto-N-fucopentaose II. J Biol Chem. 1982 Dec 10;257(23):14365–14369. [PubMed] [Google Scholar]
- Nugent K. M., Pesanti E. L. Tracheal function during influenza infections. Infect Immun. 1983 Dec;42(3):1102–1108. doi: 10.1128/iai.42.3.1102-1108.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington J. E. Treating respiratory infections in the era of cost control. Am Fam Physician. 1986 Feb;33(2):153–160. [PubMed] [Google Scholar]
- Plotkowski M. C., Puchelle E., Beck G., Jacquot J., Hannoun C. Adherence of type I Streptococcus pneumoniae to tracheal epithelium of mice infected with influenza A/PR8 virus. Am Rev Respir Dis. 1986 Nov;134(5):1040–1044. doi: 10.1164/arrd.1986.134.5.1040. [DOI] [PubMed] [Google Scholar]
- Pseudomonas aeruginosa infections: persisting problems and current research to find new therapies. Ann Intern Med. 1975 Jun;82(6):819–831. doi: 10.7326/0003-4819-82-6-819. [DOI] [PubMed] [Google Scholar]
- Ramphal R., Pyle M. Adherence of mucoid and nonmucoid Pseudomonas aeruginosa to acid-injured tracheal epithelium. Infect Immun. 1983 Jul;41(1):345–351. doi: 10.1128/iai.41.1.345-351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Pyle M. Evidence for mucins and sialic acid as receptors for Pseudomonas aeruginosa in the lower respiratory tract. Infect Immun. 1983 Jul;41(1):339–344. doi: 10.1128/iai.41.1.339-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Small P. M., Shands J. W., Jr, Fischlschweiger W., Small P. A., Jr Adherence of Pseudomonas aeruginosa to tracheal cells injured by influenza infection or by endotracheal intubation. Infect Immun. 1980 Feb;27(2):614–619. doi: 10.1128/iai.27.2.614-619.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauvala H. Gangliosides of human kidney. J Biol Chem. 1976 Dec 10;251(23):7517–7520. [PubMed] [Google Scholar]
- Rauvala H. Isolation and partial characterization of human kidney gangliosides. Biochim Biophys Acta. 1976 Feb 23;424(2):284–295. doi: 10.1016/0005-2760(76)90196-x. [DOI] [PubMed] [Google Scholar]
- Saito T., Hakomori S. I. Quantitative isolation of total glycosphingolipids from animal cells. J Lipid Res. 1971 Mar;12(2):257–259. [PubMed] [Google Scholar]
- Sanford B. A., Davison V. E., Ramsay M. A. Staphylococcus aureus adherence to influenza A virus-infected and control cell cultures: evidence for multiple adhesins. Proc Soc Exp Biol Med. 1986 Jan;181(1):104–111. doi: 10.3181/00379727-181-42230. [DOI] [PubMed] [Google Scholar]
- Saunders J. M., Miller C. H. Neuraminidase-activated attachment of Actinomyces naeslundii ATCC 12104 to human buccal epithelial cells. J Dent Res. 1983 Oct;62(10):1038–1040. doi: 10.1177/00220345830620100501. [DOI] [PubMed] [Google Scholar]
- Selinger D. S., Reed W. P. Pneumococcal adherence to human epithelial cells. Infect Immun. 1979 Feb;23(2):545–548. doi: 10.1128/iai.23.2.545-548.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N. Bacterial lectins, cell-cell recognition and infectious disease. FEBS Lett. 1987 Jun 15;217(2):145–157. doi: 10.1016/0014-5793(87)80654-3. [DOI] [PubMed] [Google Scholar]
- Siddiqui B., Hakomori S. A ceramide tetrasaccharide of human erythrocyte membrane reacting with anti-type XIV pneumococcal polysaccharide antiserum. Biochim Biophys Acta. 1973 Dec 13;330(2):147–155. doi: 10.1016/0005-2736(73)90219-8. [DOI] [PubMed] [Google Scholar]
- Siddiqui B., Hakomori S. A revised structure for the Forssman glycolipid hapten. J Biol Chem. 1971 Sep 25;246(18):5766–5769. [PubMed] [Google Scholar]
- Sweet C., Smith H. Pathogenicity of influenza virus. Microbiol Rev. 1980 Jun;44(2):303–330. doi: 10.1128/mr.44.2.303-330.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadano K., Ishizuka I. Isolation and characterization of the sulfated gangliotriaosylceramide from rat kidney. J Biol Chem. 1982 Feb 10;257(3):1482–1490. [PubMed] [Google Scholar]
- WELLS M. A., DITTMER J. C. THE USE OF SEPHADEX FOR THE REMOVAL OF NONLIPID CONTAMINANTS FROM LIPID EXTRACTS. Biochemistry. 1963 Nov-Dec;2:1259–1263. doi: 10.1021/bi00906a015. [DOI] [PubMed] [Google Scholar]
- Wood R. E., Boat T. F., Doershuk C. F. Cystic fibrosis. Am Rev Respir Dis. 1976 Jun;113(6):833–878. doi: 10.1164/arrd.1976.113.6.833. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Bass J. A., Johanson W. G., Jr, Straus D. C. Role of adherence in the pathogenesis of Pseudomonas aeruginosa lung infection in cystic fibrosis patients. Infect Immun. 1980 Dec;30(3):694–699. doi: 10.1128/iai.30.3.694-699.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Straus D. C., Johanson W. G., Jr, Berry V. K., Bass J. A. Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect Immun. 1980 Sep;29(3):1146–1151. doi: 10.1128/iai.29.3.1146-1151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y., Ebisu S., Okada H. Eikenella corrodens adherence to human buccal epithelial cells. Infect Immun. 1981 Jan;31(1):21–27. doi: 10.1128/iai.31.1.21-27.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young W. W., Jr, Borgman C. A. Short-bed, continuous development, thin-layer chromatography of glycosphingolipids. Methods Enzymol. 1987;138:125–132. doi: 10.1016/0076-6879(87)38011-5. [DOI] [PubMed] [Google Scholar]
- Yu C., Lee A. M., Roseman S. The sugar-specific adhesion/deadhesion apparatus of the marine bacterium Vibrio furnissii is a sensorium that continuously monitors nutrient levels in the environment. Biochem Biophys Res Commun. 1987 Nov 30;149(1):86–92. doi: 10.1016/0006-291x(87)91608-1. [DOI] [PubMed] [Google Scholar]